Summary
Objectives
Trichinellosis is a worldwide zooantroponosis caused by a nematode of the genus Trichinella that can pose a risk to human health. Among the species of Trichinella, T. Spiralis is the most common represented. The main source of human infection is the consumption of raw or undercooked meat (especially from pigs, wild boars and horses). Infection with Trichinella was one of the most frequent parasitic diseases in Italy until 1959 when obligatory screening for these parasites in slaughtered swines was introduced. As the last review on this topic was performed in 1989, the aim of our study was to describe the epidemiology of Trichinellosis in Italy from 1989 to 2017.
Study design
We performed a systematic research in Pubmed (MEDLINE).
Methods
We included in our review studies that were published in the peer reviewed literature using the MESH terms “Trichinellosis” and “Italy”. The only restrictions were the language (articles should be in English, Italian, Spanish or French) and the date of publication: from 1989 to March 2017. We excluded all the articles which referred to trichinellosis in the animals or which focused only on molecular biology of trichinella or on diagnostic techniques.
Results
We found 56 studies, but only 8 were considered eligible. During the study period, 764 cases of Trichinellosis occurred in Italy: 13.7% caused by T. Britovi and 84.4% by T. spiralis; in 14 cases the identification of the parasite was not performed. The outbreaks occurred in Umbria, Piedmont, Apulia (500 cases in 1990, by T. spiralis), Basilicata, Tuscany, Abruzzo, Emilia Romagna, Sardinia. In 2001 and in 2008 two outbreaks occurred in Lazio and Veneto respectively, but imported from abroad. The most important sources of infections were: horse meat (82.2%); wild boar meat (11.9%); pig meat (5.9%).
Conclusions
Trichinellosis is still present in Italy, but often forgotten by general practitioners and infectious diseases specialists. It’s pivotal to improve awareness about this parasitic disease in Physicians and veterinarians. A strict surveillance, especially on meat products from endemic countries or from wild animals is necessary to considerably reduce the risk of acquiring the infection.
Keywords: Trichinellosis, Epidemiology, Italy
Introduction
Trichinellosis is a worldwide antropozoonosis [1-3]: it is spread in a lot of Countries of Europe, South-East Asia, North and South America, New Zealand and North Africa. Outbreaks have been reported in 55 countries with an annual global average of 5,751 cases and five deaths [4]. According to the epidemiological data of the European Centre for Disease Prevention and Control (ECDC), Trichinellosis is most prevalent in eastern Europe but also in Italy and Spain where outbreaks have been reported in the past 10 years [5]. The parasite has a wide host spectrum due to its ability to virtually infect all mammals, birds and reptiles, depending on the involved Trichinella species [6]. Trichinella spiralis is the species that most adapted to domestic and wild swine but its life cycle could also include synanthropic rats. T. spiralis exhibits a wide and global distribution [7]. Trichinella britovi is the most widely distributed species within sylvatic life cycles of Europe, Asia, and Northern and Western Africa [8]. T. spiralis and T. britovi can also affect domestic pig populations mainly via extensive grazing systems or feed with scraps or carrion originating from sylvatic carnivores. Zoonotically, T. britovi is the second-most common species of Trichinella that may affect human health [7].
The incubation period for Trichinellosis symptoms is 7-21 days. Although an infected person may be asymptomatic, ingestion of a higher parasite load usually correlates with a shorter incubation period and with more severe symptoms. If clinical symptoms appear, they usually begin with several days of mild, non-bloody diarrhea, nausea, vomiting and abdominal discomfort. From 2 to 8 weeks later, host’s immunologic reaction to larval migration into tissues can result in persistent fever, sweating, chills, periorbital edema, urticarial rash and conjunctival or sub-nails hemorrhages. Long-term effects depend on parasite load and site of infection. Myalgia is often present, and cardiac manifestations (e.g. myocarditis) may rarely occur later especially in moderate and severe cases. Eosinophilia is often substantial and early appears in the infection [9].
The main source of human infection is the consumption of raw or undercooked meat, especially from pig, wild boar and horse [10, 11]. However, recently published studies described possible cases of human Trichinellosis linked also to other kinds of meat, such as beaver meat [12]. Infection with Trichinella species was one of the most frequent parasitic diseases in Italy until 1959, when obligatory screening for these parasites in slaughtered swine was introduced [13]. The last Guidelines for the surveillance, management, prevention and control of Trichinellosis reported (in the section related to the epidemiology in Italy) that only the sylvatic cycle (T. britovi) occurs among wildlife (e.g. red fox [Vulpes vulpes], wolf [Canis lupus], badger [Meles meles], marten [Martes martes], wild boar [Sus scrofa]) and the parasite is seldom transmitted to backyard or free-ranging pigs [14]. In the last 58 years, 13 backyard pigs were detected positive at the abattoir or were the source of infection for humans. Trichinella pseudospiralis has been documented once in two birds. Infections in humans have been documented from the consumption of pork from wild boar (Sus scrofa) or backyard and free-ranging pigs and meat from the red fox (Vulpes vulpes); however, the most important source of infection was horse meat imported from abroad [14]. The aim of our review was to describe the epidemiology of Trichinellosis in Italy, in order to continue the last review published in 1989 by De Carneri et al. [15].
Methods
In January-March 2017, we performed a systematic search for original peer-reviewed papers in the electronic database PubMed (MEDLINE). The key search Mesh terms were: “trichinellosis” AND “Italy”. Abstracts and full-text papers were reviewed. The inclusion criteria considered to include articles in the review were:
Type of article: original articles, but also letters to the editor if containing original data.
Language: articles should be written in English, Italian, Spanish or French.
Publication date: articles should be published in the period 1989-2017 (because the last review was written in 1989).
We excluded all the articles which referred to Trichinellosis in animals or which focused only on molecular biology of Trichinella or on diagnostic techniques.
When available, we collected data on: 1) laboratory methods used to identify the cases and the Trichinella species; 2) number of confirmed cases; 3) Trichinella species; 4) source of infection.
Studies were selected in a 2-stage process. Titles and abstracts from electronic searches were scrutinised by 2 reviewers independently (G.T. and N.N.) and full manuscripts and their citations list were analysed to retrieve missing articles and to select the eligible manuscripts according to the inclusion criteria. The level of agreement between the reviewers was high. Disagreements were resolved by consensus. Then, each article was further reviewed to identify the manuscripts suitable for our systematic review. The authors then extracted the data from included studies and collected them in a database for a unique analysis.
Results
The literature search yielded 56 publications. The titles, the abstracts and the full texts of these manuscripts were screened, resulting in 48 studies excluded because they didn’t satisfy the inclusion criteria (28 because they reported data before 1989, 19 because they described animal cases of trichinellosis or were molecular biology studies about innovative techniques for the identification of the parasite; one was excluded because it was in Polish). Finally, we identified 8 manuscripts [10, 16-22] (Fig. 1).
Fig. 1.
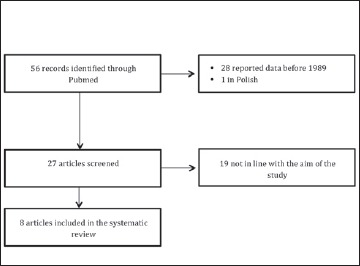
Flow diagram for identifying studies included in our review.
All the manuscripts reported data about human cases of trichinellosis, the geographical region and the year of the outbreak, and the source of infection. Two articles expressly reported the study design; 6 expressly reported the laboratory methods used to detect the parasite (ELISA or PCR).
The data collected from the 8 reviewed articles are shown in Table I (some original articles reported multiple data that we decided to report separately).
Tab. I.
Selected characteristics of the 8 studies included in the systematic review.
| Period of outbreak | Place of outbreak | Diagnosis technique | Identification of Trichinella spp. | Source of infection | Infected toll (number of confirmed cases) | Source |
|---|---|---|---|---|---|---|
| 1990 | Piedmont | n/s | Spiralis | Wild Boar | 11 | [16] |
| 1990 | Apulia | n/s | Spiralis | Horse (imported) | 500 | [16] |
| 1991 | Basilicata | n/s | Britovi | Pig | 6 | [16] |
| 1993 | Tuscany | n/s | Britovi | Pig | 4 | [16] |
| 1995 | Abruzzo | n/s | Britovi | Wild Boar | 23 | [16] |
| 1996 | Abruzzo | n/s | Britovi | Wild Boar | 10 | [16] |
| 1996 | Basilicata | n/s | Britovi | Pig | 3 | [16] |
| 1998 | Emilia Romagna | n/s | Spiralis | Horse (imported) | 92 | [16] |
| 2000 | Apulia | n/s | Spiralis | Horse (imported) | 36 | [16] |
| 2001 | Lazio (imported) | ELISA | -- | Pig | 7+1 | [21] |
| 2002 | Abruzzo | n/s | -- | Wild Boar | 2 | [17]* |
| 2005-2007 | Sardinia | PCR/ELISA | Britovi | Pig | 20 | [18, 20] |
| 2008 | Veneto (Imported) | ELISA | -- | Pig | 4 | [10] |
| 2008 | Piedmont | PCR/ELISA | Spiralis | Wild Boar | 6 | [19] |
| 2012 | Tuscany | PCR/ELISA | Britovi | Wild Boar | 34 | [17] |
| 2016 | Apulia | PCR/ELISA | Britovi | Wild Boar | 5 | [22] |
n/s: not specified
*: unpublished data of Pozio et al. reported in the article.
The total number of cases of human Trichinellosis in the last 25 years in Italy was 764 (Fig. 2).
Fig. 2.
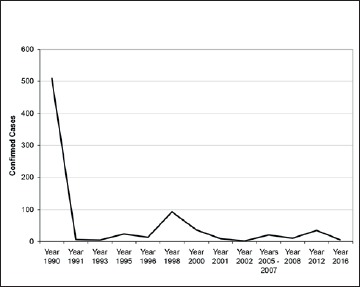
Number of confirmed trichinellosis cases in Italy reported from 1990 to 2016.
T. spiralis was responsible for 84.4% of the cases; T. Britovi was responsible for 13.7% of the cases, in 1.83% the laboratory identification of the parasite was not performed. The major sources of infections were: horsemeat (82.2%); wild boar fresh sausages (11.9%), meat from pigs slaughtered without any veterinary control (5.9%). The outbreaks occurred in Umbria, Piedmont, Apulia (500 cases in 1990, by T. spiralis), Basilicata, Tuscany, Abruzzo, Emilia Romagna, Sardinia. In 2001 and in 2008 two small epidemics happened in Lazio (N = 8) and Veneto (N = 4) respectively, but imported from abroad (Fig. 3).
Fig. 3.
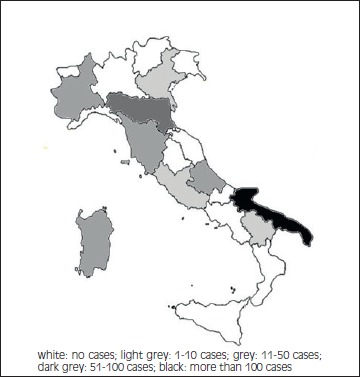
Choropleth map of Trichinellosis cases in Italy occurred from 1990 to 2016.
Discussion
Our systematic review included 8 articles integrating all the available Italian data since 1990 till 2016 (the last published article on this topic, in 2017, reported data on an outbreak occurred in 2016) [22]. One of the most important limit of our review was the partially overlap between our results and those reported by Pozio et al. [16] that however was not a systematic review of the literature. According to our results, the number of reported cases of human Trichinellosis in the last 25 years in Italy was 764, but we can note a peak of incidence due to the outbreak in Apulia in 1990 (500 cases ascribed to horsemeat). T. spiralis was responsible for the 84.4% of the cases (but excluding the big epidemic occurred in 1990 this percentage dramatically falls), T. Britovi was responsible for 13.7% of the cases, in 1.8% the laboratory identification of the parasite was not performed. Scientific literature describes T. britovi as the most prevalent species, although cases from T. spiralis have also been reported [23]. In Italy, the most common etiological agent of infection is T. Britovi, which is maintained in nature by a sylvatic cycle in which the red fox (Vulpes vulpes) is the main reservoir [24].
The detection of specific anti-Trichinella antibodies in blood serum is of great diagnostic value and ELISA is the most commonly used approach for the detection of Trichinella infection in humans [7] whereas Real-time PCR is the most important and reliable assay for the identification of Trichinella species in muscle samples [25]. Only in some studies included in the review authors identified Trichinella species involved in the outbreak: this could be a limit for a complete epidemiological analysis of human Trichinellosis in Italy.
It is known that cultural traditional habits of eating such foods play a key role in the spread of the disease and were responsible for past outbreaks [16]. Moreover, political and economic changes, could be responsible of increased prevalence and incidence (as described in many former eastern European countries) especially because of a reduced efficacy of the veterinary control on susceptible production animals [7].
Our review has shown that the most important sources of infections were horsemeat (82.2%), wild boar fresh sausages (11.9%) and meat from pigs slaughtered without any veterinary control (5.9%), differently from that reported in the literature where the major source of infection is represented by insufficiently cooked pork products [26-31]. We have to consider that in our study 500 cases out of 764 was involved in Apulia-outbreak that was caused by horsemeat.
Our systematic review, therefore, adds some important information to the review of De Carneri et al. in 1989 [15]: before 1989, in Italy, the human infections were mostly caused by wild boar meat (11% of all cases) and by imported horse meat (43%), whereas the consumption of pork did not cause infection in men, as a result of a considerable reduction in foraging swine. Moreover T. spiralis was absent in Italy: only Trichinella species 3 (less pathogenic in men) was detected.
Our review demonstrated that after 1989, cases of pork-related Trichinellosis were identified, and that T. Spiralis resulted to be present in Italy (as demonstrated by the outbreaks occurred in 2008 [19], and in 1990 [16]).
The outbreaks occurred in Umbria, Piedmont, Apulia, Basilicata, Tuscany, Abruzzo, Emilia Romagna, Sardinia. In 2001 and in 2008 two small epidemics were described in Lazio and Veneto respectively, but imported from abroad. In 2001 the epidemics involved 7 immigrants from Eastern Europe who received a package containing smoked pork sausages as a present from their relatives. This unfortunate episode was similar to another one occurred in London among immigrants of another eastern European country after the consumption of infected sausages imported from their country of origin in December 1999 [21]. In 2008 a Romanian family living in Italy, during a visit to relatives and friends in Romania, ate ham produced from a pig slaughtered without any veterinary control [10]. As reported by R. Neghina in 2010, pork is the most frequent source of human Trichinellosis in Romania. “Pig’s alms,” a specific custom representing the thanksgiving meal offered to relatives, friends, or neighbors who participated in the slaughtering process may be a very good source of infection with Trichinella parasites, leading to unfortunate consequences, especially when animals are not veterinary tested [32].
Health education of the general population is one of the most important way to prevent Trichinella infection [33, 34]. It is important to remember that the necessary temperature to kill the larvae is 77°C and it is achieved when the meat is no longer pink. Freezing temperatures of – 15°C for 20 to 30 days, – 23°C for 10 to 20 days, and – 29°C for 6 to 12 days are also effective, except for T. nativa which can infect for several days at these temperatures [35]. Non-commercial sources of pork, as from wild animals and small rural farms not using modern hog management practices, still represent a significant health problem. In all the articles inserted in our review, in fact, the meat that caused the infection was not controlled by a vet, but in the study conducted by Fichi et al. [17], the pigs used to prepare the sausages have been slaughtered in an official abattoir and resulted negative for Trichinella by artificial digestion: in this case the wild boar meat (without a veterinary control) shuffled with pig meat to prepare the sausages was the real cause of infection. Our review demonstrates that the circulation of Trichinella parasite is not an extinguished problem and that it is impossible to define a region with a negligible risk of acquiring the infection. Health personnel and veterinarians should be regularly trained about this parasitic disease often forgotten by general practitioners and infectious diseases specialists. Differential diagnosis of Trichinellosis is especially difficult for isolated cases and atypical clinical courses, and physicians practicing in non-endemic countries are usually unfamiliar with the disease and may thus experience problems in diagnosing Trichinellosis [7].
Figures and tables
Acknowledgements
Funding sources: this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of interest statement
None declared.
Authors’ contributions
GT had the idea of the study, collected data and wrote the article, NN provided support and suggestions.
References
- [1].Troiano G, Alfonzo MG, Mercurio I, Nante N. Trichinellosis: epidemiology and prevention. Prevention & Research 2016;5:70-4. doi: 10.11138/PER/2016.5.2.070. [Google Scholar]
- [2].Di Bari C, Santagada G, Pozio E, Schiraldi O. Epidemiological research on trichinellosis in Apulia and Basilicata (southern Italy). Eur J Epidemiol 1990;6:412-5. doi:10.1007/BF00151717. [DOI] [PubMed] [Google Scholar]
- [3].Ricchi E, Serafini A, Troiano G, Nante N, Petraglia F, Messina G. Food related risks during pregnancy: how much do women know about it? Epidemiology Biostatistics and Public Health 2016;13:e11868-1/6. doi: 10.2427/11868. [Google Scholar]
- [4].Rostami A, Gamble HR, Dupouy-Camet J, Khazan H, Bruschi F. Meat sources of infection for outbreaks of human trichinellosis. Food Microbiol 2017;64:65-71. doi: 10.1016/j.fm.2016.12.012. [DOI] [PubMed] [Google Scholar]
- [5].Messiaen P, Forier A, Vanderschueren S, Theunissen C, Nijs J, Van Esbroeck M, Bottieau E, De Schrijver K, Gyssens IC, Cartuyvels R, Dorny P, van der Hilst J, Blockmans D. Outbreak of trichinellosis related to eating imported wild boar meat, Belgium, 2014. Euro Surveill 2016;21(37). doi: 10.2807/1560-7917.ES.2016.21.37.30341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pinelli E, Mommers M, Kortbeek LM, Castagna B, Piergili-Fioretti D, Bruschi F. Specific IgG4 response directed against the 45-kDa glycoprotein in trichinellosis: a re-evaluation of patients 15 years after infection. Eur J Clin Microbiol Infect Dis 2007;26:641-5. doi: 10.1007/s10096-007-0349-6. [DOI] [PubMed] [Google Scholar]
- [7].Gottstein B, Pozio E, Nockler K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin Microbiol Rev 2009;22:127-45 doi: 10.1128/CMR.00026-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pozio E. World distribution of Trichinella spp. infections in animals and humans. Vet Parasitol 2007;149:3-21. doi:10.1016/j.vetpar.2007.07.002. [DOI] [PubMed] [Google Scholar]
- [9].McIntyre L, Pollock SL, Fyfe M, Gajadhar A, Isaac-Renton J, Fung J, Morshed M. Trichinellosis from consumption of wild game meat. CMAJ 2007;176:449-51. doi: 10.1503/cmaj.061530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Angheben A, Mascarello M, Zavarise G, Gobbi F, Monteiro G, Marocco S, Anselmi M, Azzini A, Concia E, Rossanese A, Bisoffi Z. Outbreak of imported trichinellosis in Verona, Italy, January 2008. Euro surveill 2008;13:pii=18891. [PubMed] [Google Scholar]
- [11].Heaton D, Huang S, Shiau R, Casillas S, Straily A, Kong LK, Ng V, Petru V. Trichinellosis outbreak linked to consumption of privately raised raw boar meat - California, 2017. MMWR Morb Mortal Wkly Rep 2018;67:247-9. doi: 10.15585/mmwr.mm6708a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bronstein AM, Lukashev AN. Possible case of trichinellosis associated with beaver (Castor fiber) meat. J Helminthol 2018:1-3. doi: 10.1017/S0022149X18000342. [DOI] [PubMed] [Google Scholar]
- [13].Frongillo RF, Baldelli B, Pozio E, Crapa G, Di Giuli C, Santirocchi M, Di Leonardo F. Report on an outbreak of trichinellosis in Central Italy. Eur J Epidemiol 1992;8:283-8. https://doi.org/10.1007/BF00144815. [DOI] [PubMed] [Google Scholar]
- [14].WHO/FAO/OIE. Guidelines for the surveillance, management, prevention and control of Trichinellosis 2007. Available at https://www.oie.int/doc/ged/d11245.pdf. Accessed on 02/03/2017.
- [15].De Carneri I, Di Matteo L. Epidemiology of trichinellosis in Italy and in neighboring countries. Ann Ist Super Sanita 1989;25:625-33. [PubMed] [Google Scholar]
- [16].Pozio E, La Rosa G, Gomez Morales MA. Epidemiology of human and animal trichinellosis in Italy since its discovery in 1887. Parasite 2001;8(Suppl 2):S106-8. doi: 10.1051/parasite/200108s2106. [DOI] [PubMed] [Google Scholar]
- [17].Fichi G, Stefanelli S, Pagani A, Luchi S, De Gennaro M, Gómez-Morales MA, Selmi M, Rovai D, Mari M, Fischetti R, Pozio E. Trichinellosis outbreak caused by meat from a wild boar hunted in an Italian region considered to be at negligible risk for Trichinella. Zoonoses Public Health 2015;62:285-91. doi: 10.1111/zph.12148. [DOI] [PubMed] [Google Scholar]
- [18].Pozio E, Mesina P, Sechi F, Pira M, Liciardi M, Cossu P, Marucci G, Garippa G, Firinu A. Human outbreak of trichinellosis in the Mediterranean island of Sardinia, Italy. Vet Parasitol 2006;140(1-2):177-80. doi: 10.1016/j.vetpar.2006.03.012. [DOI] [PubMed] [Google Scholar]
- [19].Romano F, Motta A, Melino M, Negro M, Gavotto G, Decasteli L, Careddu E, Bianchi C, Bianchi DM, Pozio E. Investigation on a focus of human trichinellosis revealed by an atypical clinical case: after wild-boar (Sus scrofa) pork consumption in northern Italy. Parasite 2011;18:85-7. doi: 10.1051/parasite/2011181085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pozio E, Cossu P, Marucci G, Amati M, Ludovisi A, Morales MA, La Rosa G, Firinu T. The birth of a Trichinella britovi focus on the Mediterranean island of Sardinia (Italy). Vet Parasitol 2009;159:361-3. doi: 10.1016/j.vetpar.2008. [DOI] [PubMed] [Google Scholar]
- [21].IRCSS "Spallanzani" Team. Imported human outbreak of trichinellosis, Italy. Releve epidemiologique hebdomadaire 2001;76:97-8. Available at https://apps.who.int/iris/bitstream/handle/10665/231467/WER7613_97-98.PDF?sequence=1&isAllowed=y. Accessed on 02/03/2017. [PubMed] [Google Scholar]
- [22].Turiac IA, Cappelli MG, Olivieri R, Angelillis R, Martinelli D, Prato R, Fortunato F. Trichinellosis outbreak due to wild boar meat consumption in southern Italy. Parasit Vectors 2017;10:107 doi: 10.1186/s13071-017-2052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pozio E, Zarlenga DS. Recent advances on the taxonomy, systematics and epidemiology of Trichinella. Int J Parasitol 2005;35:1191-204. doi: 10.1016/j.ijpara.2005.07.012. [DOI] [PubMed] [Google Scholar]
- [24].Pozio E, Rinaldi L, Marucci G, Musella V, Galati F, Cringoli G, Boireau P, La Rosa G. Hosts and habitats of Trichinella spiralis and Trichinella britovi in Europe. Int J Parasitol 2009;39:71-9. doi: 10.1016/j.ijpara.2008.06.006. [DOI] [PubMed] [Google Scholar]
- [25].Cuttell L, Corley SW, Gray CP, Vanderlinde PB, Jackson LA, Traub RJ. Real-time PCR as a surveillance tool for the detection of Trichinella infection in muscle samples from wildlife. Vet parasitol 2012;188:285-93. doi: 10.1016/j.vetpar.2012.03.054. [DOI] [PubMed] [Google Scholar]
- [26].Clark PS, Brownsberger KM, Saslow AR, Kagan IG, Noble GR, Maynard JE. Bear meat trichinosis. Epidemiologic, serologic, and clinical observations from two Alaskan outbreaks. Ann Intern Med 1972;76:951-6. doi: 10.7326/0003-4819-76-6-951. [DOI] [PubMed] [Google Scholar]
- [27].Wilson R. Bear meat trichinosis. Profound serum protein alterations, minor eosinophilia, and response to thiabendazole. Ann Intern Med 1967;66:965-71. doi: 10.7326/0003-4819-66-5-965. [DOI] [PubMed] [Google Scholar]
- [28].Viallet J, MacLean JD, Goresky CA, Staudt M, Routhier G, Law C. Arctic trichinosis presenting as prolonged diarrhea. Gastroenterology 1986;91:938-46. https://doi.org/10.1016/0016-5085(86)90698-0. [DOI] [PubMed] [Google Scholar]
- [29].Margolis HS, Middaugh JP, Burgess RD. Arctic trichinosis: two Alaskan outbreaks from walrus meat. J Infect Dis 1979;139:102-5. doi: 10.1093/infdis/139.1.102. [DOI] [PubMed] [Google Scholar]
- [30].Ancelle T, Dupouy-Camet J, Bougnoux ME, Fourestie V, Petit H, Mougeot G, Nozais JP, Lapierre J. Two outbreaks of trichinosis caused by horsemeat in France in 1985. Am J Epidemiol 1988;127:1302-11. doi: 10.1093/oxfordjournals.aje.a114923. [DOI] [PubMed] [Google Scholar]
- [31].Pozio E, Cappelli O, Marchesi L, Valeri P, Rossi P. Third outbreak of trichinellosis caused by consumption of horse meat in Italy. Ann Parasitol Hum Comp 1988;63:48-53. doi: 10.1051/parasite/198863148. [DOI] [PubMed] [Google Scholar]
- [32].Neghina R. Trichinellosis, a Romanian never-ending story. An overview of traditions, culinary customs, and public health conditions. Foodborne Pathog Dis 2010;7:999-1003. doi: 10.1089/fpd.2010.0546. [DOI] [PubMed] [Google Scholar]
- [33].Clausen MR, Meyer CN, Krantz T, Moser C, Gomme G, Kayser L, Albrectsen J, Kapel CM, Bygbjerg IC. Trichinella infection and clinical disease. QJM 1996;89:631-6. doi: 10.1093/qjmed/89.8.631. [DOI] [PubMed] [Google Scholar]
- [34].Troiano G, Mercone A, Bagnoli A, Nante N. International Travelers' sociodemographic, health, and travel characteristics: an Italian study. Ann Glob Health 2017;83:380-5. doi: 10.1016/j.aogh.2016.12.004. [DOI] [PubMed] [Google Scholar]
- [35].Pozio E, La Rosa G. General introduction and epidemiology of trichinellosis. Southeast Asian J Trop Med Public Health. 1991;22 Suppl:291-4. [PubMed] [Google Scholar]


