Abstract
Background: The 6-min walk test (6MWT) allows exercise tolerance to be assessed, and it has a significant prognostic value in COPD. The goal of this study was to analyse the determinants (obtained in routine practice) of a low 6-min walking distance (6MWD) and exercise-induced desaturation (EID) in COPD, including comorbidities.
Methods: Patients were recruited from the real-life French COPD cohort “Initiatives BPCO”. A low 6MWD was defined as <350 m. EID was defined by a minimum pulse oxygen saturation (SpO2)<90% and delta SpO2≥4% from baseline. Multivariate logistic regression analyses assessed the influence on 6MWD and EID of age, sex, obesity (body mass index, BMI >30 kg/m2), low BMI (BMI <18.5 kg/m2), modified Medical Research Council (mMRC) dyspnea scale, FEV1% pred, FVC % pred, hyperinflation and comorbidities including cardiovascular diseases.
Results: Among 440 patients with available 6MWT data, a 6MWD <350 m was found in 146 patients (33%), which was positively associated in multivariate analyses with age and mMRC and negatively with resting SpO2 and FVC % pred (rescaled r2=0.34), whereas no comorbidity was associated with a low 6WMD. EID was found in 155 patients (35%). This was positively associated with hypertension and negatively with age, obesity, FEV1% pred and resting SpO2 (rescaled r2=0.37).
Conclusion: 6MWD and EID exhibit different determinants in COPD with a minor impact of comorbidities limited to hypertension in EID and to obesity, which was unexpectedly associated with less EID. Other variables including age, routine resting lung function and SpO2 were weakly associated with 6MWD and EID. Altogether, these results suggest that 6MWT performance remains difficult to predict with routine clinical/functional parameters.
Keywords: 6-min walk test, distance, COPD, obesity, comorbidities, severity
Background
COPD is worldwide a leading cause of mortality and morbidity1,2 associated with progressive airflow limitation. Until recently, COPD severity was mainly defined by the level of post-bronchodilator FEV1.3 Dyspnea and exercise limitation are the predominant consequences of COPD in stable condition and appear now as major indexes of disease severity and prominent targets of treatment. The 6-min walk test (6MWT) is a widely used field test to assess exercise performance with an abundant literature supporting its clinical relevance.4,5 The distance walked at the end of the test (6MWD) is the primary outcome and a strong prognostic factor in COPD, either as a single parameter or as part of a multidimensional index.6–8 Several studies suggested the 350 m value as a relevant prognostic cutoff.6,9–11 The multidimensional BODE index (BMI, obstruction, dyspnea and exercise) also integrates an upper cutoff of 350 m for 6MWD,7 and a low baseline value of 6MWD is highly predictive of a worsening in BODE score at 2 to 3 years.12
Exercise-induced desaturation (EID) is common in COPD and routinely assessed during the 6MWT. Various definitions have been used to characterize significant EID. The most common definition is a pulse oxygen saturation (SpO2) drop >4% from baseline and/or a lowest SpO2<90% during the 6MWT.9,13 The predicting factors of EID in COPD are poorly known with conflicting results from different studies, usually demonstrating resting SpO2 and PaO2 as significant predictors.9,14–16 The negative prognostic value of EID during the 6MWT is confirmed by several studies.8,13,17
Comorbidities are frequent in COPD, with a significant impact on symptoms, morbidity and mortality.18 Among COPD comorbidities, cardiovascular diseases (eg, chronic heart failure [CHF] and coronary heart disease) and mood disorders (eg, depression and anxiety) could contribute to exercise limitation and then impact 6MWT performance.18,19 Recent results from the ECLIPSE study demonstrated that FEV1, modified Medical Research Council (mMRC) dyspnea score, COPD severity, depression and emphysema score on quantitative CT were associated with a 6MWD <350 m.10 Individual comorbidities were not specifically assessed, but patient-reported cardiovascular diseases were surprisingly not associated with a low 6MWD.
The purpose of the present study was to analyse the contribution of demographic data, symptoms, lung function, nutritional status and specifically comorbidities including cardiovascular diseases to the 6MWD and EID in a real-life cohort of COPD patients, using only usual parameters obtained in routine practice. A better knowledge of the correlates of 6MWT main variables could help to predict a low performance during the 6MWT and provide a better understanding of the determinants involved in exercise tolerance in COPD.
Methods
The Initiatives BPCO cohort
COPD subjects were recruited from the Initiatives BPCO cohort, a prospective real-life French cohort of clinically and spirometry-diagnosed COPD patients from 20 centers: 17 university hospitals, 2 general hospitals and 1 rehabilitation center. The details of this cohort have been previously described.20,21 In short, COPD subjects were recruited at stable condition with no history of exacerbation in the previous 4 weeks, and with a diagnosis of COPD based on a post-bronchodilator FEV1/FVC ratio <0.7.22 Exclusion criteria were a main diagnosis of bronchiectasis, asthma or other chronic respiratory diseases.
A standardized case report form was used to record demographic data, risk factors including tobacco history, respiratory symptoms, history of acute exacerbations of COPD during the previous year, health-related quality of life, anxiety and depression, spirometry and plethysmographic lung volumes according to American Thoracic Society/European Respiratory Society (ATS/ERS) standards,23 6MWT and comorbidities including sleep apnea syndrome, congestive HF, coronary artery disease, hypertension and diabetes mellitus. Physician investigators identified comorbidities from clinical assessment and patient files. Dyspnea was assessed by the mMRC scale24 ranging from 0 to 4. Health-related quality of life was evaluated by the Saint George’s Respiratory Questionnaire (SGRQ) ranging from 0 to 100. Anxiety and depression were assessed by the Hospital Anxiety Depression (HAD) questionnaire.25,26 The ADO score (age, dyspnea and obstruction) was calculated according to the original description.27 The 6MWT was performed by experienced respiratoy nurses according to ATS guidelines except for duplication, and the following variables were collected: end of 6MWT dyspnea assessed by a modified Borg scale from 0 to 10, baseline SpO2, SpO2 minimal value and distance. This study was conducted in accordance with the Declaration of Helsinki. The procedures of this study were approved by the Ethics Committee of Versailles (France). All subjects provided informed written consent.
Selection of COPD subjects
Due to the real-life design of the Initiatives BPCO cohort, datasets do not have to be complete to include a patient. Demographic characteristics and spirometry only are mandatory at inclusion. At the time of the analysis, 1.194 COPD subjects were included in the Initiatives BPCO cohort. Because our goal was to study the impact of both lung function and comorbidities on exercise tolerance assessed by the 6MWT, we selected subjects with complete data for 6MWT, spirometry, plethysmography, comorbidities including coronary artery disease, CHF, thromboembolic history, diabetes, hypertension and exacerbation history during the previous year. A comparison between subjects with complete data and those with incomplete data was performed.
Statistical analyses
As 6MWT parameters did not exhibit a normal distribution according to the Kolmogorov–Smirnov test, nonparametric tests were used, and distributions were described using medians and IQRs.
Univariate analyses
The comparisons of qualitative and quantitative variables between 6MWD and EID categories were compared using Chi2 or Wilcoxon tests, respectively. The variables considered in the univariate analyses were age, sex, body mass index (BMI), mMRC dyspnea scale, FEV1% pred, forced vital capacity (FVC) % pred, lung volumes (residual volume (RV), total lung capacity (TLC), RV/TLC), SpO2 at rest, exacerbation frequency, chronic bronchitis, nutritional status and physician-diagnosed comorbidities.
Multivariate analyses
Variables explaining 6MWD and EID were explored using the LOGISTIC procedure from SAS® 9.2 statistical software, with significant levels for entry and stay of 15% and 10%, respectively, to introduce or remove a covariate. For each score, two stepwise ordinal logistic regressions were performed starting from no and all variables. The two stepwise analyses converged to the same model. Seventeen variables were used as covariates including lung function variables as continuous variables (FEV 1 % pred, FVC % pred, RV/TLC), comorbidities as individual variables and BMI as a categorical variable (low <18.5 kg/m2; obesity >30 kg/m2). The mMRC dyspnea scale was also used. The SGRQ and HAD scores were not entered in the models due to too many missing data. A multivariate analysis with the same 17 variables was performed for 6MWD in absolute value (meters).
Results
Patients
Among 1,194 COPD subjects included from January 2005 to October 2015, 440 subjects had complete data for the variables of interest. The characteristics of these patients are reported in Table 1. Compared to patients with incomplete data, subjects with complete data had a lower age (62 [56–70] vs 65 [58–73]; p<0.0001) and a lower prevalence of diabetes (10.2% vs 14.9%, p=0.02), with no other difference regarding clinical and lung function variables. Among the 440 subjects included in the analyses, all GOLD spirometry grades were represented (Table 1) with a median FEV1 of 51% pred and an mMRC score at 2 [1; 3]. A low BMI was found in 50 subjects and obesity in 83 subjects. Previously diagnosed comorbidities were found across all GOLD grades.
Table 1.
Clinical and lung function data
| Variable | N=440 |
|---|---|
| Comorbidities (present) | |
| Sex male/female | 72.7% (320)/ 27.3% (120) |
| Age | 62 [56–70] |
| Low BMI ≤18.5 kg/m2 | 9.1% (40) |
| Obesity BMI >30 kg/m2 | 18.9% (83) |
|
Smoking history (pack-years) Current/ex smokers |
38.0 [23.8–55.0] 31.2% (135)/65.8% (285) |
| Hypertension | 36.4% (160) |
| Ischemic heart disease | 13.4% (59) |
| Chronic heart failure | 12.5% (55) |
| Diabetes | 10.2% (45) |
| Mechanical limitation | 20.9% (92) |
| Sleep apnea | 8.4% (37) |
| FEV1 (L) | 1.41 [0.97–1.87] |
| FEV1% pred | 51 [36–68] |
| GOLD 2007 severity | |
| 1 2 3 4 |
8.4% (37) 42.5% (187) 32.7% (144) 16.4% (72) |
| FEV1/FVC | 0.51 [0.42–0.61] |
| FVC % pred | 86 [71–102] |
| RV % pred | 167 [131–208] |
| TLC % pred | 114 [103–128] |
| RV/TLC % | 55 [47–64] |
| mMRC (0–4) | 2 [1–2] |
| Chronic bronchitis | 66.6% (293) |
| Exacerbations/year | 1 [0–3] |
| BODE score | 3 [1–4] |
| ADO score | 3 [2–5] |
Note: Data reported as numbers (percentages) or median [IQRQ1–Q3].
Abbreviations: BMI, body mass index; RV, residual volume; TLC, total lung capacity; mMRC, modified Medical Research Council scale for dyspnea; BODE, body mass index, obstruction, dyspnea and exercise score; ADO, age, dyspnea and obstruction score.
6MWT
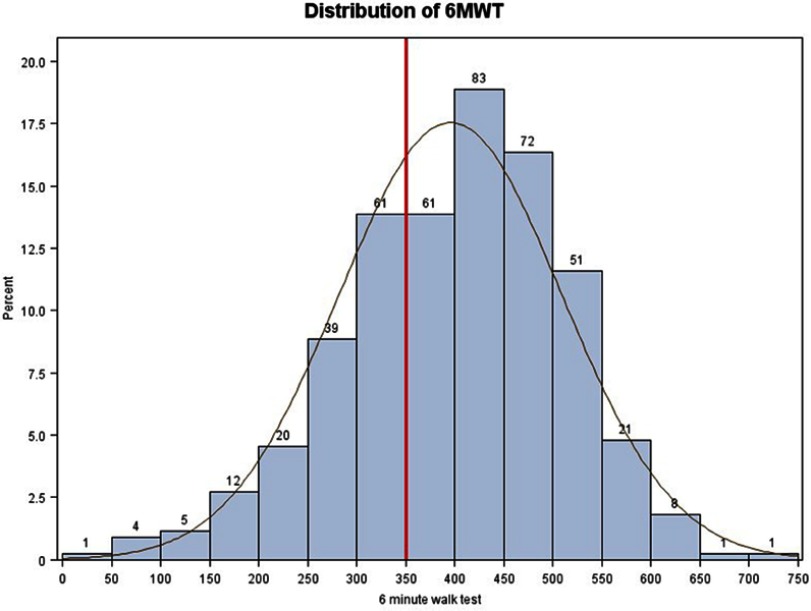
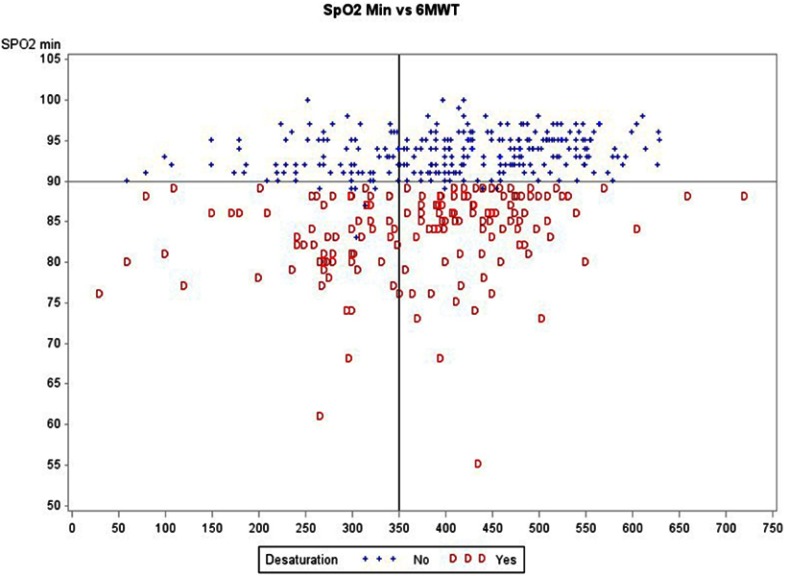
Median 6MWD was 404 m [318–480] and 146 patients (33%) walked a distance <350 m (Figure 1). End of 6MWT-related dyspnea was higher in those with a low 6MWD. Significant EID was found in 155 patients (35%). Of note, EID and low 6MWD were unrelated (Figure 2).
Figure 1.
Distribution of the 6MWD (m).
Abbreviations: 6MWD, 6-min walking distance; m, meters.
Figure 2.
Relationship between 6MWD and EID (SpO2 min).
Abbreviations: 6MWD, 6-min walking distance; EID, exercise induced desaturation.
Univariate associations with 6MWD and EID
Several categorical and continuous variables were related to 6MWD and EID (Tables 2 and 4, Tables S1 and S2) including age, mMRC stage, SGRQ global score, exacerbation frequency, FEV1% pred, FVC % pred and hyperinflation at rest (RV and RV/TLC). Baseline SpO2 was lower in patients with a low 6MWD or EID. The prevalence of cardiovascular comorbidities was similar in patients with low or preserved 6MWD, and between those with and without EID. Obesity was inversely associated with EID, whereas the prevalence of obesity was not significantly different in patients with and without a low 6MWD.
Table 2.
Univariate comparisons for low vs high 6MWD
| Variable | 6MWD ≤350 m N=146 |
6MWD >350 m N=294 |
p | Desaturation N=155 |
No desaturation N=285 |
p |
|---|---|---|---|---|---|---|
| Sex male/female | 70.5% (103)/29.5% (43) | 73.8% (217)/26.2% (77) | 0.469 | 69.0% (107)/31.0% (48) | 74.7% (213)/25.3% (72) | 0.199 |
| Age | 65 [58–74] | 60 [54–67] | <0.0001 | 60 [54–67] | 62 [56–71] | 0.0410 |
| BMI ≤18.5 | 10.3% (15) | 8.5% (25) | 0.543 | 11.6% (18) | 7.7% (22) | 0.175 |
| Obesity BMI >30 | 23.3% (34) | 16.7% (49) | 0.095 | 13.5% (21) | 21.8% (62) | 0.036 |
| Hypertension | 41.8% (61) | 33.7% (99) | 0.096 | 40.0% (62) | 34.4% (98) | 0.242 |
| Chronic heart failure | 16.4% (24) | 10.5% (31) | 0.078 | 11.0% (17) | 13.3% (38) | 0.474 |
| Ischemic heart disease | 16.4% (24) | 11.9% (35) | 0.189 | 10.3% (16) | 15.1% (43) | 0.161 |
| Diabetes | 11.0% (16) | 9.9% (29) | 0.721 | 9.0% (14) | 10.9% (31) | 0.542 |
| Exacerbations/year | 2 [0–3] | 1 [0–2] | 0.0021 | 1 [0–3] | 1 [0–2] | 0.0235 |
| mMRC stage | 2 [1–3] | 1 [1–2] | <0.0001 | 2 [1–3] | 1 [1–2] | <0.0001 |
| SGRQ global score | 53 [42–64] missing 19 |
39 [27–53] missing 46 |
<0.0001 | 49 [35–59] missing 20 |
42 [28–56] missing 45 |
0.0066 |
| BODE | 5 [3–6] | 2 [1–3] | <0.0001 | 4 [2–5] | 2 [1–4] | <0.0001 |
| ADO | 5 [4–6] | 3 [2–4] | <0.0001 | 4 [3–5] | 3 [2–4] | <0.0001 |
| HAD anxiety ≥10 | 26.7% (39) missing 18 |
23.8% (70) missing 50 |
0.414 | 25.2% (39) missing 22 |
24.6% (70) missing 46 |
0.864 |
| HAD depression ≥10 | 20.5% (30) missing 20 |
13.6% (40) missing 46 |
0.169 | 18.1% (28) missing 22 |
14.7% (42) missing 44 |
0.649 |
| FEV1% pred | 42 [31–54] | 56 [41–71] | <0.0001 | 38 [29–52] | 57 [43–71] | <0.0001 |
| FVC % pred | 74 [61–89] | 90 [76–106] | <0.0001 | 76 [62–91] | 89 [75–106] | <0.0001 |
| RV % pred | 175 [137–223] | 161 [131–199] | 0.0364 | 189 [147–238] | 156 [127–187] | <0.0001 |
| TLC % pred | 113 [98–127] | 115 [104–128] | 0.3958 | 118 [106–132] | 112 [100–124] | 0.0012 |
| RV/TLC % | 60 [52–67] | 53 [44–61] | <0.0001 | 61 [51–66] | 53 [46–61] | <0.0001 |
| SpO2 at rest | 95 [92–96] | 96 [94–97] | <0.0001 | 94 [92–96] | 96 [95–98] | <0.0001 |
Notes: Data reported as percentages (numbers) or median [IQR Q1–Q3]. Significant (P< 0.05) shown in bold.
Abbreviations: 6MWD, 6-min walking distance; ADO, age, dyspnea and obstruction score; BMI, body mass index; BODE, obstruction, dyspnea and exercise score; HAD, Hospital anxiety and depression scale; mMRC, modified Medical Research Council scale for dyspnea; RV, residual volume; SGRQ, Saint George's Respiratory Questionnaire; SpO2, transcutaneous oxygen saturation; TLC, total lung capacity.
Table 4.
Multivariate analysis for 6MWD in absolute value (m)
| Parameter | DF | Parameter estimate | SE | t value | Pr > |t| |
|---|---|---|---|---|---|
| Intercept | 1 | 100.46004 | 168.52394 | 0.60 | 0.5514 |
| Age | 1 | −3.22696 | 0.46779 | −6.90 | <0.0001 |
| Obesity | 1 | −24.05947 | 11.73060 | −2.05 | 0.0409 |
| Hypertension | 1 | −20.54332 | 9.78203 | −2.10 | 0.0363 |
| Diabetes | 1 | 33.89135 | 15.15119 | 2.24 | 0.0258 |
| FEV1% pred | 1 | 1.25108 | 0.25561 | 4.89 | <0.0001 |
| mMRC | 1 | −31.07953 | 4.65525 | −6.68 | <0.0001 |
| SpO2 at rest | 1 | 5.17758 | 1.73057 | 2.99 | 0.0029 |
Note: Significant (P< 0.05) shown in bold.
Abbreviations: 6MWD, 6-min walking distance; mMRC, modified Medical Research Council scale for dyspnea; SpO2, transcutaneous oxygen saturation.
Table S1.
Univariate logistic analysis for low 6MWD
| Effect | OR estimates | 95% Wald confidence limits | Wald Chi-square |
Pr > ChiSq | |
|---|---|---|---|---|---|
| Female vs male | 1.177 | 0.757 | 1.828 | 0.5227 | 0.4697 |
| Age | 1.056 | 1.034 | 1.079 | 24.4536 | <0.0001 |
| Low BMI yes vs no | 1.232 | 0.628 | 2.416 | 0.3690 | 0.5435 |
| Obesity yes vs no | 1.518 | 0.929 | 2.481 | 2.7719 | 0.0959 |
| Hypertension yes vs No | 1.414 | 0.940 | 2.126 | 2.7609 | 0.0966 |
| Ischemic heart disease yes vs No | 1.456 | 0.830 | 2.554 | 1.7137 | 0.1905 |
| Heart failure yes vs No | 1.669 | 0.940 | 2.965 | 3.0538 | 0.0805 |
| Diabetes yes vs No | 1.125 | 0.590 | 2.145 | 0.1273 | 0.7213 |
| OSAS yes vs No | 0.840 | 0.403 | 1.751 | 0.2167 | 0.6416 |
| Chronic bronchitis yes vs No | 1.192 | 0.779 | 1.825 | 0.6567 | 0.4177 |
| FEV1% pred | 0.966 | 0.955 | 0.977 | 35.1247 | <0.0001 |
| FEV/FVC | 0.073 | 0.015 | 0.360 | 10.3363 | 0.0013 |
| FVC % pred | 0.971 | 0.961 | 0.981 | 33.2866 | <0.0001 |
| RV (L) | 1.214 | 1.047 | 1.409 | 6.5540 | 0.0105 |
| TLC % pred | 1.004 | 0.990 | 1.019 | 0.3466 | 0.5560 |
| mMRC | 2.234 | 1.798 | 2.776 | 52.6517 | <0.0001 |
| Exacerbations/patient/year | 1.148 | 1.038 | 1.270 | 7.2482 | 0.0071 |
Note: Significant P-values shown in bold.
Abbreviations: BMI, body mass index; OSAS, obstructive sleep apnea syndrome; FEV1 % pred, forced expiratory volume in one second, percentage of predicted; FVC % pred, forced vital capacity, percentage of predicted; RV (L), residual volume in liters; TLC (L), total lung capacity in liters; TLC % pred, total lung capacity, percentage of predicted; mMRC, modified Medical Research Council dyspnea scale; 6MWD, 6-min walking distance.
Table S2.
Univariate logistic analysis for the presence of significant desaturations
| Effect | OR estimates | 95% Wald confidence limits | Wald Chi-square |
Pr > ChiSq | |
|---|---|---|---|---|---|
| Sex female vs male | 1.327 | 0.861 | 2.046 | 1.6424 | 0.2000 |
| Age/10 years | 0.840 | 0.690 | 1.024 | 2.9788 | 0.0844 |
| Low BMI yes vs no | 1.571 | 0.815 | 3.028 | 1.8185 | 0.1775 |
| Obesity yes vs no | 0.564 | 0.329 | 0.967 | 4.3370 | 0.0373 |
| Hypertension yes vs no | 1.272 | 0.850 | 1.905 | 1.3651 | 0.2427 |
| Ischemic heart disease yes vs no | 0.648 | 0.352 | 1.193 | 1.9404 | 0.1636 |
| Heart failure yes vs no | 0.801 | 0.436 | 1.472 | 0.5121 | 0.4742 |
| Diabetes yes vs no | 0.814 | 0.419 | 1.580 | 0.3712 | 0.5424 |
| OSAS yes vs no | 0.480 | 0.214 | 1.078 | 3.1579 | 0.0756 |
| Chronic bronchitis yes vs no | 1.084 | 0.714 | 1.643 | 0.1425 | 0.7058 |
| FEV1% pred | 0.952 | 0.940 | 0.964 | 58.3708 | <0.0001 |
| FEV1/FVC | 0.003 | <0.001 | 0.018 | 41.5437 | <0.0001 |
| FVC % pred | 0.973 | 0.964 | 0.983 | 29.4992 | <0.0001 |
| RV (L) | 1.346 | 1.158 | 1.563 | 15.0655 | 0.0001 |
| TLC (L) | 1.001 | 0.999 | 1.004 | 1.1197 | 0.2900 |
| RV/TLC % | 1.008 | 0.997 | 1.019 | 1.8886 | 0.1694 |
| mMRC | 1.490 | 1.231 | 1.804 | 16.7205 | <0.0001 |
| Exacerbations/patient/year | 1.110 | 1.005 | 1.226 | 4.2674 | 0.0388 |
Note: Significant P-values shown in bold.
Abbreviations: BMI, body mass index; OSAS, obstructive sleep apnea syndrome; FEV1 % pred, forced expiratory volume in one second, percentage of predicted; FVC % pred, forced vital capacity, percentage of predicted; RV (L), residual volume in liters; TLC (L), total lung capacity in liters; mMRC, modified Medical Research Council dyspnea scale.
Multivariate determinants of 6MWD and desaturations
The main determinants (as indicated by significant Wald Chi-square values) were different for 6MWD and EID (Table 3A and B). The mMRC scale was predictive of a low 6MWD only. The first functional covariate was FVC for 6MWD and FEV1 for EID. Baseline SpO2 was significantly associated with both 6MWD and EID. Age had a positive predictive value for a low 6MWD and negative for EID. Only hypertension appeared as a positive cardiovascular predictor of EID, whereas obesity was significantly associated with less EID. Altogether, considering all independent and significant determinants identified by our analyses, only a low-to-moderate proportion of the 6MWD and EID results were explained with a maximal rescaled r2 of 0.34 and 0.37, respectively. The prediction of 6MWD in absolute value (Table 4) retained age, FEV1% pred, mMRC and baseline SpO2, with obesity, hypertension and diabetes as additional factors of borderline significance. Adjusted r2 of the whole model was similar at 0.33.
Table 3.
(A) Multivariate analysis for low vs high 6MWD. (B) Multivariate analysis for the presence of desaturations
| OR estimates | |||
|---|---|---|---|
| Effect | Point estimate | 95% Wald confidence limits | |
| A | |||
| Age/10 years | 1.953 | 1.510 | 2.527 |
| FVC/10% pred | 0.794 | 0.707 | 0.890 |
| mMRC | 1.954 | 1.541 | 2.477 |
| SpO2 at rest | 0.857 | 0.787 | 0.933 |
| B | |||
| Age/10 years | 0.661 | 0.507 | 0.861 |
| Obesity yes vs no | 0.457 | 0.242 | 0.864 |
| Hypertension yes vs no | 1.690 | 1.020 | 2.800 |
| FEV1/10% pred | 0.674 | 0.590 | 0.770 |
| SpO2 at rest | 0.701 | 0.632 | 0.778 |
Abbreviations: 6MWD, 6-min walking distance; mMRC, modified Medical Research Council scale for dyspnea; SpO2, transcutaneous oxygen saturation.
Discussion
In this real-life cohort of COPD patients, a low 6MWD and/or EID occurred in approximately one-third of patients (33% and 35%, respectively). Low 6MWD and the presence of EID were unrelated, and their major determinants were different but with limited predictive values. Of note, the influence of comorbidities was low.
Determinants of a low 6MWD
Our results regarding the prevalence of a 6MWD <350 m are consistent with other COPD populations, especially the ECLIPSE cohort which exhibited a 6MWD <350m in 41% of the patients.10 The identification of determinants associated with decreased walking distance might be useful for prognosis assessment and to identify a risk of reduced physical activity,5 although the relationships between the amount or intensity of daytime activity and 6MWT performance remain variable among studies.28 Our results from a cohort of stable COPD patients show that age, FVC, mMRC scale and baseline SpO2 were the main determinants of a poor performance.29,30 Although very easy to obtain in clinical practice, these clinical and lung function correlates of 6MWD were relatively poor predictors (rescaled r2 0.34), insufficient to reliably estimate the presence of a low 6MWD in the individual patient. FEV1 and mMRC were significant predictors in the multivariate analyses of the Bergen cohort by Waatevik et al,31 but physical activity (>1–2 hrs per week) and comorbidities assessed by the Charlson index were additional predictive factors of a better 6MWD.
Contrary to what could be expected RV/TLC was unrelated to 6MWD, a finding suggesting a moderate relationship between resting and exercise-induced dynamic hyperinflation, mainly related to the level of expiratoy flow limitation and ventilatory pattern during the test. Interestingly, hyperinflation was also unrelated to EID, probably due to the major confounding effect and contribution of FEV1. Age represents a major confounder, as it is present in all reference equations. As a consequence, it was present in the multivariate 6MWD model.
Previous studies also focused on the impact of comorbidities. Waatevik et al demonstrated a mean 60 m reduction of 6MWD in patients with at least 2 comorbidities, using the Charlson comorbidity score.31 Impact of individual comorbidities was on the contrary rather modest in our model.
Unexpectedly, important comorbidities including CHF were unrelated to 6MWD. This result is in accordance with those of Spruit et al, in which pooled cardiovascular comorbidities were unrelated to the presence of a poor 6MWD.32 Conversely, a markedly lower 6MWD was recently found in COPD patients with a reduced (<50%) left ventricular ejection fraction (397 m vs 456 m) vs those with similar GOLD severity but preserved left ventricular contractility.33 A 30% reduction in cycle endurance time was also reported in COPD patients with coexisting CHF.34 CHF is a frequent comorbidity in COPD and contributes to dyspnea, although it is often difficult to determine its specific impact in individual patients. In our study, we cannot exclude an underdiagnosis of CHF, particularly diastolic or with preserved ejection fraction, especially because of the lack of systematic echocardiography. A recent study in the CHANCE cohort from Netherlands demonstrated a significant contribution of echocardiographic right ventricular systolic pressure, timed up and go test and quadriceps endurance work in the multivariate analysis of 6MWD.35 With the addition of GOLD stage, SGRQ activity, mMRC and resting lung function (FEV1, FEV1/FVC, diffusing capacity of the lung for carbon monoxide (DLCO) and PaO2), the model explained 72% of 6MWD variance. However, echocardiography and quadriceps assessment are not performed in routine practice for COPD evaluation. In addition, timed up and go test is rather considered as a surrogate than as a determinant of 6MWT. Peripheral artery disease was also associated with a lower 6MWD in the COSICONET cohort, as objectively defined by a low ankle-brachial index.36
One significant limitation of the present study regarding the 6MWD data is the lack of duplicate 6MWT. This was also the case in recent large-scale studies such as ECLIPSE.10 In clinical practice, a second test is rarely performed due to time constraints. In addition, the mean gain induced by the second test is around 26 m.5 When considering the distribution of 6MWD in our cohort, the risk of misclassification (ie, classification of a patient below the cutoff value of 350 m instead of above this cutoff value) would be limited to those who were in the 300–349 m group: as shown in Figure 2, this group contained only 61 patients. Even assuming a high (50%) rate of misclassification, this would affect <7% (30/440) of patients in our study and thus be of little influence on the multivariate analysis results. A second test is currently mainly recommended for comparison over time, according to the latest ATS/ERS technical standards.37
Determinants of EID
Although the criteria used to define significant EID in the literature are heterogeneous, recent studies concur in finding a frequency of 21–39% for these events.13,15 The proportion of EID was in the same range in our study. Several studies demonstrated the pejorative prognostic value of EID in COPD. The most extensive analysis was performed in the Bergen COPD cohort,13 using EID criteria similar to ours. Their evaluation also comprised a Charlson index, arterial blood gases and bioelectrical impedance. The HR for death was 2 among patients with EID and 2.4 after adjustment for clinical data (including Charlson index), FEV1, baseline PaO2 and 6MWD. Other recent studies demonstrated a significantly worse survival in COPD subjects with EID.8,38,39
A number of predictors of EID during the 6MWT have been described. Abnormal resting gas exchange is a major determinant in most studies. A baseline SpO2 value ≤95% appeared to have a high predictive value, together with female sex, a DLCO <50% pred, FEV1<45% pred and PaO2<10 kPa.14 A lower resting SpO2 threshold (93%) was found in the ECLIPSE cohort. Resting SpO2 was also present in our multivariate model with a significant OR (0.7).
Moderate-to-severe emphysema on quantified CT has also been found to be a significant predictor of EID,15 but at present quantitative CT is not routinely used to assess emphysema in COPD patients. Unexpectedly, obesity was negatively related to EID in our cohort. Obesity was found to be a positive predictive factor of EID in a recent multivariate analysis in the ECLIPSE cohort.15 Conversely, it was recently suggested that moderate obesity could improve dyspnea during exercise in COPD, by limiting the degree of dynamic hyperinflation during exercise.40 Thus, the relation between obesity, exercise hyperinflation and EIDs remains to be elucidated.
Age had a moderate negative predictive value (OR 0.66 per 10 years) of EID in our patients. This finding is opposite to the reduction of 6MWD with age, which may be explained by less desaturation due to lower distance.
The only cardiovascular comorbidity significantly associated with 6MWT variables was hypertension with a 1.6 OR for EID. Hypertension could be associated with subclinical left heart diastolic dysfunction. However, no difference in 6MWT-induced desaturation was found recently in COPD patients with HF and reduced left ventricular ejection fraction.33
In our patients, hyperinflation (RV/TLC) was not a significant determinant of 6MWT or desaturation, which was likely related to a major interaction with FEV1. Dynamic hyperinflation during exercise is currently considered as a major limiting factor, but recent data suggest a predominant role of ventilatory constraint per se (low inspiratory reserve volume), similar in patients with or without dynamic hyperinflation.41
One important strength of our study is the systematic assessment of clinical variables including comorbidities together with lung function parameters, including lung volumes. The present study has also some limitations. Most of our patients were recruited in university hospitals and therefore may not represent the global COPD population, with a lower proportion of spirometric stage I patients. The 6MWT was not performed in duplicate, which is also true for other large previous series including ECLIPSE.10 An average learning effect of 26 m has been previously observed.5 This small increment is unlikely to change the predictive value of our models if a second test had been performed in our cohort. A significant limitation of the present study is that the diagnosis of comorbidities was identified by physicians based on clinical evaluation and taking into account the medical history with no systematic assessment of the presence/absence and severity (for instance left ventricular ejection fraction, diastolic parameters and/or BNP measurement for CHF) of these comorbidities. However, it must be pointed out that the frequency of comorbidities in the present study is in line with other large observational studies in COPD. The Charlson index was unavailable but is obviously not used for the routine assessment of COPD patients. Furthermore, DLCO was not assessed in the present study, whereas it was found to be a significant predictor of 6MWD and desaturations in previous studies.14 It is, however, not a standard assessment tool in recent guidelines2 and clinical practice.
Conclusion
In conclusion, this study shows that, although related on a statistical ground, usual clinical data and resting lung function do not adequately predict either 6MWD or desaturations during the test. The present results also confirm the complexity of 6MWT determinants in COPD and suggest a low impact of common comorbidities, particularly clinically defined HF or coronary disease. In clinical practice, 6MWT performance remains difficult to predict with routine clinical and PFT parameters.
Acknowledgments
The Initiatives BPCO Study Group: Brinchault–Rabin G (Rennes), Burgel P.R (Paris), Caillaud D (Clermont-Ferrand), Carré P (Carcassonne), Chanez P (Marseille), Chaouat A (Vandœuvre les Nancy), Court-Fortune I (Saint-Etienne), Cuvelier A (Rouen), Escamilla R (Toulouse), Gut-Gobert C (Brest), Jebrak G (Paris), Lemoigne F (Nice), Nesme-Meyer P (Lyon), Perez T, Le Rouzic O and Tillie-Leblond I (Professor Tillie-Leblond passed away on March 13, 2013) (Lille), Perrin C (Cannes), Pinet C (Toulon), Raherison C (Bordeaux), Roche N (Paris), Surpas P (Bayere) and Zysman M (Nancy).
This work was funded by unrestricted grants from Boehringer Ingelheim France and Pfizer France until 2015 and is now funded by Boehringer Ingelheim France.
Abbreviation list
BMI, body mass index; COPD, chronic obstructive pulmonary disease; DLCO, diffusing capacity of the lung for carbon monoxide; FRC, functional residual capacity; HAD, Hospital Anxiety Depression score; IC, inspiratory capacity; mMRC, Modified Medical Research Council dyspnea scale; RV, residual volume; SpO2, oxygen saturation measured by pulse oximetry; TLC, total lung capacity.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Dr Thierry Perez reports personal fees from Boehringer Ingelheim, Novartis, GSK, and Chiesi, and grants from Astra Zeneca, outside the submitted work. Professor Gaétan Deslée reports grants and personal fees from BTG/PneumRx; grants from Nuvaira; personal fees from Chiesi, grants and personal fees from AstraZeneca, outside the submitted work. Professor Pierre Régis Burgel reports personal fees from Astra-Zeneca, Boehringer Ingelheim, Chiesi, GSK, Pfizer, Teva, Vertex, and Zambon, outside the submitted work. Professor Denis Caillaud has nothing to disclose. Dr Olivier Le Rouzic reports personal fees and non-financial support from AstraZeneca, Boehringer Ingelheim, Chiesi, Lilly, Novartis, and PulmonX; non-financial support from GlaxoSmithKlein,, outside the submitted work. Dr Maeva Zysman reports personal fees and non-financial support from Boehringer ingelheim, during the conduct of the study; grants and personal fees from Novartis, grants from Fondation pour la recherche medicale; grants from Astra Zeneca; personal fees from Chiesi, outside the submitted work. Dr Roger Escamilla has nothing to disclose. Dr Gilles Jebrak has nothing to disclose. Professor Pascal Chanez has nothing to disclose. Dr Isabelle Court-Fortune reports grants and personal fees from Boehringer Ingelheim, during the conduct of the study. Dr Graziella Brinchault-Rabin has nothing to disclose. Dr Pascale Nesme-Meyer reports personal fees from Boehringer Ingelheim, outside the submitted work. Mr Jean Louis Paillasseur reports grants from iBPCO association, during the conduct of the study. Professor Nicolas Roche reports grants from Boehringer Ingelheim (France), during the conduct of the study. The authors report no other conflicts of interest in this work.
Supplementary materials
References
- 1.Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and global analysis. Eur Respir J. 2006;28(3):523–532. doi: 10.1183/09031936.06.00124605 [DOI] [PubMed] [Google Scholar]
- 2.GOLD. Global strategy for the diagnosis, management and prevention of COPD, global initiative for chronic obstructive lung disease (GOLD);2019. [Update 2019] Available from: http://goldcopd.org. Accessed February 5, 2019
- 3.GOLD. Global strategy for the diagnosis, management and prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2010; 2010. Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed March 22, 2011.
- 4.Fotheringham I, Meakin G, Punekar YS, Riley JH, Cockle SM, Singh SJ. Comparison of laboratory- and field-based exercise tests for COPD: a systematic review. Int J Chron Obstruct Pulmon Dis. 2015;10:625–643. doi: 10.2147/COPD.S70518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh SJ, Puhan MA, Andrianopoulos V, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1447–1478. doi: 10.1183/09031936.00150414 [DOI] [PubMed] [Google Scholar]
- 6.Cote CG, Casanova C, Marin JM, et al. Validation and comparison of reference equations for the 6-min walk distance test. Eur Respir J. 2008;31(3):571–578. doi: 10.1183/09031936.00104507 [DOI] [PubMed] [Google Scholar]
- 7.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi: 10.1056/NEJMoa021322 [DOI] [PubMed] [Google Scholar]
- 8.Andrianopoulos V, Wouters EF, Pinto-Plata VM, et al. Prognostic value of variables derived from the six-minute walk test in patients with COPD: results from the ECLIPSE study. Respir Med. 2015;109(9):1138–1146. doi: 10.1016/j.rmed.2015.06.013 [DOI] [PubMed] [Google Scholar]
- 9.Crisafulli E, Iattoni A, Venturelli E, et al. Predicting walking-induced oxygen desaturations in COPD patients: a statistical model. Respir Care. 2013;58(9):1495–1503. doi: 10.4187/respcare.02321 [DOI] [PubMed] [Google Scholar]
- 10.Spruit MA, Watkins ML, Edwards LD, et al. Determinants of poor 6-min walking distance in patients with COPD: the ECLIPSE cohort. Respir Med. 2010;104(6):849–857. doi: 10.1016/j.rmed.2009.12.007 [DOI] [PubMed] [Google Scholar]
- 11.Celli B, Tetzlaff K, Criner G, et al. The 6 min-walk distance test as a chronic obstructive pulmonary disease stratification tool. Insights from the COPD biomarker qualification Consortium. Am J Respir Crit Care Med. 2016;194(12):1483–1493. doi: 10.1164/rccm.201508-1653OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casanova C, Aguirre-Jaime A, de Torres JP, et al. Longitudinal assessment in COPD patients: multidimensional variability and outcomes. Eur Respir J. 2014;43(3):745–753. doi: 10.1183/09031936.00096913 [DOI] [PubMed] [Google Scholar]
- 13.Waatevik M, Johannessen A, Gomez Real F, et al. Oxygen desaturation in 6-min walk test is a risk factor for adverse outcomes in COPD. Eur Respir J. 2016;48(1):82–91. doi: 10.1183/13993003.00975-2015 [DOI] [PubMed] [Google Scholar]
- 14.Andrianopoulos V, Franssen FM, Peeters JP, et al. Exercise-induced oxygen desaturation in COPD patients without resting hypoxemia. Respir Physiol Neurobiol. 2014;190:40–46. doi: 10.1016/j.resp.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 15.Andrianopoulos V, Celli BR, Franssen FM, et al. Determinants of exercise-induced oxygen desaturation including pulmonary emphysema in COPD: results from the ECLIPSE study. Respir Med. 2016;119:87–95. doi: 10.1016/j.rmed.2016.08.023 [DOI] [PubMed] [Google Scholar]
- 16.Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med. 1980;93(3):391–398. [DOI] [PubMed] [Google Scholar]
- 17.Takigawa N, Tada A, Soda R, et al. Distance and oxygen desaturation in 6-min walk test predict prognosis in COPD patients. Respir Med. 2007;101(3):561–567. doi: 10.1016/j.rmed.2006.06.017 [DOI] [PubMed] [Google Scholar]
- 18.Fabbri LM, Luppi F, Beghe B, Rabe KF. Complex chronic comorbidities of COPD. Eur Respir J. 2008;31:204–212. doi: 10.1183/09031936.00114307 [DOI] [PubMed] [Google Scholar]
- 19.Maurer J, Rebbapragada V, Borson S, et al. Anxiety and depression in COPD current understanding, unanswered questions, and research needs. Chest. 2008;134(4):43S–56S. doi: 10.1378/chest.08-0342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgel PR, Nesme-Meyer P, Chanez P, et al. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009;135(4):975–982. doi: 10.1378/chest.08-2062 [DOI] [PubMed] [Google Scholar]
- 21.Perez T, Burgel PR, Paillasseur JL, et al. Modified medical research council scale vs Baseline Dyspnea index to evaluate dyspnea in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1663–1672. doi: 10.2147/COPD.S82408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO [DOI] [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 24.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical research council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George‘s respiratory questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. doi: 10.1164/ajrccm/145.6.1321 [DOI] [PubMed] [Google Scholar]
- 26.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- 27.Puhan MA, Garcia-Aymerich J, Frey M, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet. 2009;374(9691):704–711. doi: 10.1016/S0140-6736(09)61301-5 [DOI] [PubMed] [Google Scholar]
- 28.Watz H, Pitta F, Rochester CL, et al. An official European respiratory society statement on physical activity in COPD. Eur Respir J. 2014;44(6):1521–1537. doi: 10.1183/09031936.00046814 [DOI] [PubMed] [Google Scholar]
- 29.Mahler DA, Ward J, Waterman LA, Baird JCD. Longitudinal changes in patient-reported Dyspnea in patients with COPD. COPD. 2012;9:522–527. doi: 10.3109/15412555.2012.701678 [DOI] [PubMed] [Google Scholar]
- 30.Jones P, Miravitlles M, van der Molen T, Kulich K. Beyond FEV(1) in COPD: a review of patient-reported outcomes and their measurement. Int J Chron Obstruct Pulmon Dis. 2012;7:697–709. doi: 10.2147/COPD.S32675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waatevik M, Johannessen A, Hardie JA, et al. Different COPD disease characteristics are related to different outcomes in the 6 min walk test. COPD. 2012;9(3):227–234. doi: 10.3109/15412555.2011.650240 [DOI] [PubMed] [Google Scholar]
- 32.Spruit MA, Polkey MI, Celli B, et al. Predicting outcomes from 6 min walk distance in chronic obstructive pulmonary disease. J Am Med Dir Assoc. 2012;13(3):291–297. doi: 10.1016/j.jamda.2011.06.009 [DOI] [PubMed] [Google Scholar]
- 33.Mesquita R, Franssen FM, Houben-Wilke S, et al. What is the impact of impaired left ventricular ejection fraction in COPD after adjusting for confounders? Int J Cardiol. 2016;225:365–370. doi: 10.1016/j.ijcard.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 34.Oliveira MF, Arbex FF, Alencar MC, et al. Heart failure impairs muscle blood flow and endurance exercise tolerance in COPD. COPD 2016;13(4):407–415. doi: 10.3109/15412555.2015.1117435 [DOI] [PubMed] [Google Scholar]
- 35.McNamara RJ, Houben-Wilke S, Franssen FME, et al. Determinants of functional, peak and endurance exercise capacity in people with chronic obstructive pulmonary disease. Respir Med. 2018;138:81–87. doi: 10.1016/j.rmed.2018.03.032 [DOI] [PubMed] [Google Scholar]
- 36.Houben-Wilke S, Jorres RA, Bals R, et al. Peripheral artery disease and its clinical relevance in patients with chronic obstructive pulmonary disease in the COPD and systemic consequences-comorbidities network study. Am J Respir Crit Care Med. 2017;195(2):189–197. doi: 10.1164/rccm.201602-0354OC [DOI] [PubMed] [Google Scholar]
- 37.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. doi: 10.1183/09031936.00150314 [DOI] [PubMed] [Google Scholar]
- 38.Golpe R, Perez-de-Llano LA, Mendez-Marote L, Veres-Racamonde A. Prognostic value of walk distance, work, oxygen saturation, and dyspnea during 6 min walk test in COPD patients. Respir Care. 2013;58(8):1329–1334. doi: 10.4187/respcare.02290 [DOI] [PubMed] [Google Scholar]
- 39.Casanova C, Cote C, Marin JM, et al. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest. 2008;134(4):746–752. doi: 10.1378/chest.08-0520 [DOI] [PubMed] [Google Scholar]
- 40.Ora J, Laveneziana P, Wadell K, Preston M, Webb KA, O’Donnell DE. Effect of obesity on respiratory mechanics during rest and exercise in COPD. J Appl Physiol. 2011;111(1):10–19. doi: 10.1152/japplphysiol.01131.2010 [DOI] [PubMed] [Google Scholar]
- 41.Guenette JA, Webb KA, O’Donnell DE. Does dynamic hyperinflation contribute to dyspnoea during exercise in patients with COPD? Eur Respir J. 2012;40(2):322–329. doi: 10.1183/09031936.00157711 [DOI] [PubMed] [Google Scholar]