Abstract
Aquaculture is the fastest growing animal food production industry, now producing 50% of all food fish. However, aquaculture feeds remain dependent on fishmeal derived from capture fisheries, which must be reduced for continued sustainable growth. Purple phototrophic bacteria (PPB) efficiently yield biomass from wastewater with high product homogeneity, a relatively high protein fraction, and potential added value as an ingredient for fish feeds. Here we test bulk replacement of fishmeal with PPB microbial biomass in diets for Asian sea bass (Lates calcarifer), a high value carnivorous fish with high protein to energy requirement. Mixed culture PPB were grown in a novel 1 m3 attached photo-biofilm process using synthetic and real wastewater. Four experimental diets were formulated to commercial specifications but with the fishmeal substituted (0%, 33%, 66%, and 100%) with the synthetic grown PPB biomass and fed to a cohort of 540 juvenile fish divided amongst 12 tanks over 47 days. Weight and standard length were taken from individual fish at 18, 28, and 47d. No significant difference in survival was observed due to diet or other factors (94–100%). There was a negative correlation between PPB inclusion level and final weight (p = 5.94 × 10−5) with diet accounting for 4.1% of the variance over the trial (general linear model, R2 = 0.96, p = 1 × 10−6). Feed conversion ratio was also significantly influenced by diet (p = 6 × 10−7) with this factor accounting for 89% of variance. Specifically, feed conversion ratio (FCR) rose to 1.5 for the 100% replacement diet during the last sample period, approximately 1.0 for the partial replacement, and 0.8 for the nil replacement diet. However, this study demonstrates that bulk replacement of fishmeal by PPB is feasible, and commercially viable at 33% and 66% replacement.
Keywords: Fishmeal, Photobioreactor, Purple phototrophic bacteria, Single cell protein
Graphical abstract

Highlights
-
•
Purple phototrophic bacteria (PPB) were produced as a biofilm for harvesting and consistency.
-
•
PPB an effective bulk replacement for fishmeal for Barramundi at 66% substitution.
-
•
No change in mortality at all substitution rates.
-
•
Dose-response decrease in fish weight and yield for increased substitution rate.
1. Introduction
For over five decades, aquaculture has been the fastest growing animal food-production industry, with production expanding on average by 8.0% per year since 1961, and 47% of all food fish are now derived from aquaculture (FAO, 2018). However, there is heavy reliance on fishmeal and fish oil derived from capture fisheries to manufacture feeds for aquaculture species, however this is a limited resource with very strict quotas (Naylor et al., 2009). To reduce this pressure, the Food and Agriculture Organization (FAO) of the United Nations have charged the scientific community with the investigation of alternative protein sources that are simultaneously more environmentally friendly, low cost and may promote extra nutritional health benefits (FAO, 2016). Consequently, there has been an acceleration of research into alternative and sustainable protein sources for use in aquaculture feeds, including proteins derived from plants (Hardy, 2010), insects (Stamer, 2015) and microbial biomass (Gamboa-Delgado and Márquez-Reyes, 2016).
Microbial biomass, also referred to as single cell protein (SCP) has a range of advantages as a feed ingredient (Matassa et al., 2016). Conversion of organics, nitrogen and phosphorous to proteins is highly efficient compared to, for example, plant based alternatives (Matassa et al., 2016). Secondly, whereas plant and insect proteins are generally well digested, certain fractions may contain less digestible and anti-nutritional factors such as cellulose, chitin and phytate. Microbial proteins are generally well utilised by fish and crustaceans and comprise a protein content and amino acid profiles similar to fishmeal (Gamboa-Delgado and Márquez-Reyes, 2016). Whilst there has been substantial interest in proteins from algae and yeasts as ingredients for animal feeds (Matassa et al., 2015; Øverland and Skrede, 2017; Yaakob et al., 2014), microbial biomass from bacteria is regarded as one of the most promising vectors (Pikaar et al., 2017). Bacterial biomass is generally characterised by a protein content between 50 and 65% (Anupama and Ravindra, 2000), which is an important consideration when formulating feeds for popular aquaculture species such as salmon, trout and barramundi where final dietary requirement may exceed 50% protein (Gamboa-Delgado and Márquez-Reyes, 2018; Glencross, 2006). However, the majority of microbial SCP production relies on aerobic heterotrophs, which dissipate a substantial fraction of the energy during catabolism (Matassa et al., 2015), limiting biological yields and conversion ratios to below 50%.
Purple phototrophic bacteria (PPB), including Rhodopseudomonas sp. and Rhodobacter sp. are a phylogenetically diverse group of phototrophic anoxygenic microbes that avoid this dissipation through phototrophic growth and yields close to unity (Hülsen et al., 2018). They have been isolated from a vast variety of sources including soil, fresh and marine water and wastewaters (Zhang et al., 2003) and can be readily selected with infrared radiation (IR). IR is utilised as catabolic driver and enables direct and non-destructive, assimilative uptake of organics, nitrogen, and phosphorous (Hülsen et al., 2014). At the same time, PPBs exhibit high growth rates and contain >60% crude protein with additional, potentially beneficial compounds such as carotenoid pigments, polyhydroxyalkanoates and readily digestible cell walls (Kobayashi and Kobayashi, 1995). This makes PPB an ideal mediator for resource recovery from wastewater where IR allows for selective enrichment with little or no competition with other bacteria or algae that are unable to absorb wavelengths above 750 nm (Bidigare et al., 1990). As a result, PPB can dominate non-sterile systems in a mixed culture context, which enables consistent product quality. This offers the ability to generate PPB as a product stream, e.g. as feed or feed additive from industrial and potentially domestic wastewaters, with health controls or phase separation (Pikaar et al., 2017).
Whilst there are no studies on use of PPB as a bulk ingredient, there are a number of studies investigating PPB as feed supplement at very low inclusion rates in economically important species such as Nile tilapia, marble goby, barramundi and Tor tambroides (Banerjee et al., 2000; Chowdhury et al., 2016; Loo et al., 2015; Shapawi et al., 2012). These studies utilised axenic cultures (Rhodovulum, Marichromatium or Rhodospeudomonas sp.) grown on artificial media in the formulation of their diets. All studies, except Shapawi et al. (2012) reported significantly better survival and/or improved growth during the early life stages of the animals with some studies indicating unbalanced formulation for lipids or proteins (Banerjee et al., 2000; Chowdhury et al., 2016). Shapawi et al., (2012) only reported trends of increased growth, improved survival rate and better feed conversation ratio when fish were fed with 0.3% bacterial biomass. Loo et al. (2015) fed PPB directly to marble goby fries by mixing it with tank water or by feeding it to fish via rotifer and artemia, with both methods resulting in improved survival. This effect was confirmed in crucian carp at PPB addition rates of 0.1% (1:10,000 of the total food intake) (Kobayashi and Tchan, 1973) as well as in fry of loach, goldfish, carp, ark shell, and sweetfish that prey on phototrophic bacteria soon after hatching (Kobayashi and Kobayashi, 1995). These studies have demonstrated positive impacts of PPBs as a food supplement at rather low inclusion rates, utilising axenic cultures. They therefore represent more probiotic studies rather than a bulk feed substitute for fishmeal (whole or partial) and do not address the overall objective of fishmeal replacement with microbial protein. In contrast, the present study aims to evaluate the potential of a mixed PPB culture as a substitute for fishmeal in commercial aqua feeds. For this purpose, PPB grown on synthetic wastewater serve as proxy for PPB biomass generated on non-sterile sources. The main objectives were; (a) characterisation of the PPB product produced in a 1 m3 wastewater pilot fed with synthetic wastewater (and the comparison with PPB grown on real wastewater), (b) determination of the effects of 33%, 66% and 100% fishmeal replacement with this product on performance of the fish (barramundi, Lates calcarifer) in terms of growth, mortality, feed conversion and whole-body composition, relative to a fishmeal feed control and (c) to determine the potential value of the PPB product in aquaculture and the potential impacts on the PPB technology as a whole.
2. Material and methods
2.1. Purple phototrophic bacteria biomass production
2.1.1. Inoculum
PPB inoculum (20 L) was enriched on domestic wastewater as described elsewhere (Hülsen et al., 2014).
2.1.2. Photo-anaerobic bioreactor (PAnB)
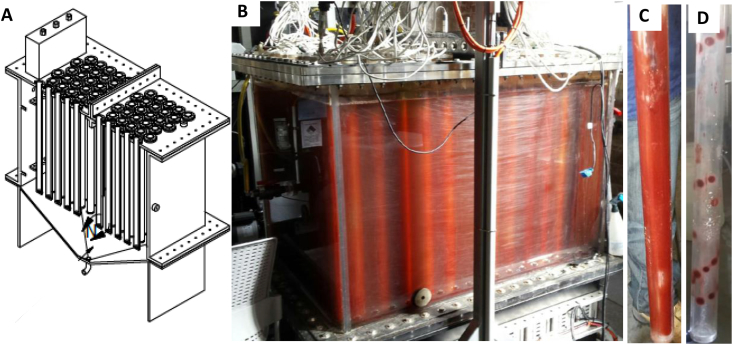
A 1000L (800L working volume) photo anaerobic bioreactor (PAnB) was used to grow PPB for the feed trial. The PAnB consisted of a high-density polyethylene (HDPE) base with a conical bottom flanged to a rectangular clear acrylic main tank (1164 mm × 1008 mm x 1007 mm). The reactor lid contained 100 sealed, hollow acrylic tubes with flanged connections (Diameter: 50 mm OD, Length: 905 mm) which were submerged in the reactor liquid. Each tube contained a rigid light bar with an aluminium triangle core with three sides, each with a 75 cm rigid, dimmable IR LED strip. Each triangular light bar contained 126 LEDs (10 W, IR 850 nm HK-R5050IR-30N-X, 12 V DC, Demo Photoelectric Tech (Wuxi) Co, LtD, China) to irradiate the PAnB from inside-out with 14–16 W m−2. The illuminated area to volume ratio was 14 m2 m−3. The PAnB was mixed via the conical bottom with two vacuum pumps, recycling headspace (62.3L min−1, Gast DOA-P725-BN, single head, 1.1 cfm, 220/240VAC, Cole Parmer, Australia). Fig. 1 provides a schematic and a picture of the unit.
Fig. 1.
Cross section of the PAB assembly with light tubes (A) and a picture of the reactor with PPB biofilm attached to the submerged tubes (B), fully covered tube (C), and tube with coverage only around the actual LED due to limited IR output (D).
2.1.3. Culture medium
PPB were grown with modified Ormerod medium (Ormerod et al., 1961) with the following modifications: biotin, (NH4)2SO4 and malic acid were substituted with: yeast extract (0.015 mg L−1), NH4Cl (50 mg L−1) and acetic acid (500 mgCOD L−1). The pH was set to 6.5. While synthetic wastewater was used to grow PPB for the feed trials, the system was further operated on-site at a wastewater treatment plant (Luggage Point, Queensland Urban Utilities) using domestic primary clarified wastewater in order to compare nutrient removal efficiency by PPB and to characterise the composition of the biomass grown on different sources.
2.1.4. PAnB operation
The PAnB was inoculated with enriched PPB and operated for 135 days on synthetic artificial wastewater including 24 harvesting events, approximately every 3 days followed by 70 days on domestic wastewater. After an initial start-up time of 1 week, PPB biomass attached to the submerged surfaces of the acrylic tubes and was harvested via manual scraping (see below) in intervals of 3–4 d. Consecutive batches were started by adding glacial acetic acid and NH4Cl to achieve a t = 0 concentration of 500 mgCOD L−1 (chemical oxygen demand) and 50 mgNH4N L−1, respectively. PAnB liquid from the previous batch was reused as inoculum (10 v/v%). The medium was refreshed every 3 weeks. For each batch, total chemical oxygen demand (TCOD), soluble chemical oxygen demand (SCOD), NH4-N, PO4-P were determined for t = 0 and t = end. The pH, NH4-N and temperature were monitored online (SensoLyt® 700 IQ/SET (pH), AmmoLyt Plus SET/Comp (NH4-N and temp), WTW IQ SensorNet, Royce Water Technologies Pty Ltd, Australia, respectively).
2.1.5. Biomass harvest, processing and characterisation
PPB biomass was harvested at the end of each batch by removing each tube and removing the biofilm manually. The harvested biomass was weighed and the total solid (TS) and volatile solid (VS) were determined. The collected biomass was stored in a freezer (−20 °C) before air drying in a commercial food dryer (DT6000 Food Lab™ Dehydrator, Sunbeam, Australia) at 45 °C until dry (2 × 19.5 h). The dried biomass was further processed in a mixer (Nutribullet 600, Australia) to produce a fine and homogenous powder. After collecting a total of 2.2 kg of dried biomass grown on synthetic wastewater, the bacterial biomass was analysed for proteins, fat, carbohydrates, ash, amino acids, total carotenoids, total chlorophyll, metals, poly-β-hydroxybutyrate (PHB), total nitrogen and phosphorous (Table 1, Table S1). Table 1 further contains comparative, compositional PPB data from Hülsen et al. (2018). Identical analyses were performed on PPB grown on domestic wastewater although only artificially fed PPB were fed to fish in this study to confirm proof-of-concept before testing PPB generated from other wastewater sources.
Table 1.
PPB biomass composition grown on different sources.
| Synthetic WW | Domestic WW | RM P1* | Pork P1* | Poultry P1-3* | ||
|---|---|---|---|---|---|---|
| TKN | g N kg−1 | 121 | 95 | 76 | 91 | 93 |
| TP | g P kg−1 | 19 | 10 | 11 | 15 | 11 |
| Protein | w/w% | 58 | 57.7 | 47 | 57 | 58 |
| Fat | w/w% | 3.4 | 11.5 | 15.7 | 10.3 | 24.9 |
| Carbohydrates** | w/w% | 18.8 | 14.9 | 18.6 | 19.8 | 29.9 |
| Ash and minerals | w/w% | 7.8 | 7.6 | 12.5 | 13.7 | 5 |
| Moisture | % | 12 | 8.3 | |||
| Gross Energy | MJ kg−1 | 21.3 | n/a | 23.7 | 19.3 | 22.4 |
| Total Carotenoids**** | g kg−1 | 6.8 ± 0.3 | 6.5 ± 0.2 | n/a | n/a | n/a |
| Alpha + beta carotene | ug 100g−1 | <5 | <5 | n/a | n/a | n/a |
| Total BChlorophyll**** | g kg−1 | 11.3 ± 0.8 | 13.2 ± 0.4 | n/a | n/a | n/a |
| PHB | w/w% | 2.5 | n/a | n/a | n/a | n/a |
| Amino acids*** | ||||||
| l-Alanine | g kg−1 | 43.9 | 46.6 | 19.9 | 20.9 | 25.4 |
| l-Arginine | g kg−1 | 28 | 22.6 | 16.3 | 15.5 | 20.7 |
| l-Aspartic Acid | g kg−1 | 42.6 | 50.2 | 25.7 | 26.1 | 32 |
| l-Cystine | g kg−1 | n/a | 3.6 | |||
| l-Glutamic Acid | g kg−1 | 49.9 | 55.6 | 25.6 | 31.6 | 37.3 |
| l-Glycine | g kg−1 | 28.4 | 27.2 | 12.9 | 13.3 | 16.2 |
| l-Histidine | g kg−1 | 13.5 | 10.2 | 5.8 | 5.1 | 8.2 |
| l-Isoleucine | g kg−1 | 24.9 | 25.1 | 13.7 | 13.6 | 16.7 |
| l-Leucine | g kg−1 | 45.9 | 44.8 | 24.5 | 23.4 | 30.8 |
| l-Lysine | g kg−1 | 28.4 | 34.4 | 12.7 | 14 | 18 |
| l-Methionine | g kg−1 | 9.1 | 9.4 | 6.5 | 6.4 | 8.9 |
| l-Phenylalanine | g kg−1 | 28.9 | 23.7 | 15.4 | 14.2 | 19.9 |
| l-Proline | g kg−1 | 22.1 | 24.8 | 11.4 | 12.3 | 14.6 |
| l-Serine | g kg−1 | 20.6 | 18.8 | 10.9 | 10.1 | 13.8 |
| l-Threonine | g kg−1 | 28.1 | 27.1 | 14.9 | 13.2 | 18.6 |
| l-Tyrosine | g kg−1 | 18.4 | 16.8 | 12.2 | 11.3 | 15.1 |
| l-Valine | g kg−1 | 33.4 | 33.8 | 17.7 | 18.4 | 22.5 |
| Total amino acids | g kg−1 | 466.1 | 474.7 | 246.1 | 249.4 | 318.7 |
Notes: *based on gTSS rather than gTS adapted from Hülsen et al. (2018) **calculated (100-protein + fat + ash + moisture). ***Amino acids from PPB biomass grown on synthetic wastewater (WW) analysed by Symbio and from domestic wastewater by Ridley. ****Total carotenoids and Bchlorophyll analysed at UQ. TKN: total Kjeldahl nitrogen, TP: total phosphorous, PHB: polyhydroxybutyrate.
2.1.6. Analysis of biomass bacterial community composition
Microbial community composition of biomass grown on synthetic and domestic wastewater was determined by amplicon sequencing. DNA extraction and subsequent sequencing by Illumina Miseq Platform, using universal primer pair 926F (5′-AAACTYAAAKGAATTGACGG-3′) and 1392wR (5′-ACGGGCGGTGWGTRC-3′) primer sets and data analysis were performed on PPB biomass grown on both the synthetic and domestic wastewaters as previously described (Hülsen et al., 2018).
2.2. Principal study design – animal model
Juvenile barramundi were grown with complete or partial replacement of fishmeal by PPB in a series of iso-nitrogenous (∼56.3% CP) and iso-energetic (∼17.2 MJ kg−1) diets formulated with varying inclusion levels of PPB. Ethical approval for the feed trial in barramundi was granted by the University of Queensland Native and Exotic Wildlife and Marine Animals (NEWMA) Ethics Committee (permit SBS/130/17). The experiment was conducted in a re-circulating freshwater tank array fitted with continuous aeration, water temperature controlled and filtered through a mechanical and biological filtration system.
2.2.1. Diet formulation and preparation
PPB from synthetic wastewater was used to formulate nutritionally complete diets balanced on an equal protein and energy basis using standard raw materials used in commercial barramundi feeds (Ridley Aquafeeds, Australia) and with ingredient chemical composition described in Table 2A. Briefly, experimental diets were prepared with 30% fishmeal (control), or with substitution rates of 33%, 66% or 100% of the fishmeal component substituted with PPB, with adjustments in other components to maintain crude protein, phosphorous, and lipid levels (Table 2B), and amino acid balance based on pre-analysis of ingredients (see 2.3 Analytical Methods). Each raw material was pre-milled into a fine powder (<0.7 mm) and mixed in a 20 L Hobart planetary mixer A200 (Hobart, Ohio, USA) for 10 min to allow complete homogenisation. Lipid (fish oil 25%/75% poultry oil) was slowly added to the dry mash while mixing. Water was then added until an optimum dough consistency was achieved. The mash was then passed three times through a Hobart Tin chopper attachment fitted with a 2 mm tapered die mincing plate attached to a rotating knife (Hobart, Ohio, USA). Strands were then placed onto stainless steel mesh trays and steamed (100% humidity) at 105 °C for 2 min (fan speed 2) in a Cheftop Unox (XEVC-0511-E1R) combi oven. Steamed strands were then dried overnight at 65 °C (fan speed 4). The strands were first broken by hand and pulse-blended for 1 s five times. To produce 1.5–2 mm pellets, resulting mixed pellets were sieved through a 2 mm Endecotts test sieve shaker (Endecotts, London, England). Composition analysis of each diet after preparation was determined to confirm formulation levels (Table 2C).
Table 2.
A. Nutrient composition of key raw ingredients (%w/w); B. Formulation of the barramundi experimental diets (all values are g kg−1) C. Diet composition.
| Fish meal | PPB | Poultry meal | Blood meal | Maize pregel | Wheat gluten | Wholemeal flour | |
|---|---|---|---|---|---|---|---|
| A | |||||||
| Dry matter | 93.8 | 88 | 95.5 | 96.3 | 75.6 | 93.6 | 89.9 |
| Protein | 69.3 | 58 | 67.4 | 92.7 | 0.76 | 78.6 | 13.1 |
| Lipid | 9.6 | 3.4 | 13.5 | <0.1 | <0.1 | 0.4 | 1.4 |
| Ash | 13.1 | 7.8 | 13.1 | 1.8 | 0.3 | 0.9 | 1.0 |
| Ingredients | Diet 1 |
Diet 2 |
Diet 3 |
Diet 4 |
|---|---|---|---|---|
| Inclusion | Inclusion | Inclusion | Inclusion | |
| B | ||||
| Fish Meal (super prime) | 300 | 200 | 100 | 0 |
| Phototrophic purple bacteria (PPB) | 0 | 100 | 200 | 300 |
| Poultry offal meal | 240 | 240 | 240 | 240 |
| Blood meal | 100 | 112 | 124 | 137 |
| Maize Pregel starch (pre-cooked) | 100 | 78 | 55 | 34 |
| Wheat wholemeal plain flour | 100 | 100 | 100 | 100 |
| Premix vitamins & minerals | 3 | 3 | 3 | 3 |
| Mono sodium phosphate (MSP) | 0 | 6 | 11 | 16 |
| Methionine | 5 | 5 | 5 | 5 |
| Taurine | 2 | 2 | 2 | 2 |
| Lipid (fish oil 25%/75% poultry oil) | 50 | 54 | 60 | 63 |
| Dried fish soluble (DFS) | 20 | 20 | 20 | 20 |
| Wheat Gluten | 75 | 75 | 75 | 75 |
| Marker (Yttrium oxide) | 1 | 1 | 1 | 1 |
| Choline | 4 | 4 | 4 | 4 |
| Composition | Diet 1 | Diet 2 | Diet 3 | Diet 4 |
|---|---|---|---|---|
| C | ||||
| Dry matter (%) | 95.7 | 95.6 | 94.8 | 97 |
| Moisture (air) | 4.3 | 4.4 | 5.2 | 3 |
| Protein (%) | 56.2 | 56.2 | 55.5 | 57.3 |
| Lipid (%) | 11.9 | 13.3 | 11.8 | 13 |
| Ash (%) | 8 | 8.2 | 9.4 | 8.5 |
| Carbohydrate (%)* | 19.6 | 17.9 | 18.1 | 18.2 |
| PHB (gPHB g wet weight−1) | 0 | 0.25 | 0.49 | 0.46 |
| Gross energy (MJ kg−1)** | 17.2 | 17.4 | 16.7 | 17.5 |
| l-Alanine | 32.5 | 31.9 | 36.6 | 38.2 |
| l-Arginine | 26.3 | 24.3 | 26.2 | 25.6 |
| l-Aspartic Acid | 49.0 | 45.3 | 49.0 | 48.3 |
| l-Cystine | 7.6 | 6.4 | 7.1 | 6.8 |
| l-Glutamic Acid | 87.7 | 80.0 | 84.2 | 83.2 |
| l-Glycine | 29.4 | 28.1 | 32.4 | 30.1 |
| l-Histidine | 15.2 | 14.1 | 14.3 | 14.8 |
| l-Isoleucine | 20.3 | 18.7 | 19.5 | 19.6 |
| l-Leucine | 43.0 | 41.0 | 44.6 | 46.7 |
| l-Lysine | 39.4 | 35.4 | 37.0 | 36.8 |
| l-Methionine | 14.2 | 13.1 | 13.3 | 13.1 |
| l-Phenylalanine | 21.7 | 21.3 | 23.4 | 24.8 |
| l-Proline | 33.9 | 28.8 | 32.3 | 31.8 |
| l-Serine | 22.1 | 20.9 | 23.3 | 23.2 |
| l-Threonine | 20.5 | 19.6 | 21.5 | 22.5 |
| l-Tyrosine | 14.0 | 13.2 | 14.3 | 15.1 |
| l-Valine | 28.4 | 27.7 | 30.6 | 32.2 |
| Total Amino Acid | 505.4 | 469.6 | 509.6 | 512.9 |
Notes: *Carbohydrate based on the dry matter content of the sample minus the protein, lipid, and ash; **Gross energy or the caloric value of the feed was calculated following (Garling and Wilson, 1976) where proteins (solid TKN based), fats and carbohydrates contain on average 16.7, 37.7 and 16.7 MJ kg−1respectively.
2.2.2. Fish husbandry
Juvenile barramundi (n = 540) of approximately 25 mm were sourced from Mainstream Aquaculture hatchery (Melbourne, Vic, Australia), airfreighted to the University of Queensland and kept in a freshwater recirculating system composed of twelve 70L tank connected to two 300 L sumps, one fitted with two 300 μm filter socks to remove large organic matter before reaching a mechanical and biological filter (K1 micro bead filter media, Evolution Aqua). Water was pre-filtered through a carbon filter to remove any traces of chlorine and chloramine and any other contaminants. A heating/chilling unit (Teco TK3000) maintained the temperature constant at 28 ± 1 °C and the dissolved oxygen was maintained at >5 mg L−1 by means of an air compressor and in-tank airstones. Other water quality characteristics were analysed regularly, and water exchanges performed to maintain concentrations as follows: ammonia, (<0.25 mg L−1), nitrite (<0.5–1 mg L−1), nitrate (<20 mg L−1), pH (7.2–7.8), and general hardness 100–200 ppm. After a three weeks acclimatisation on a commercial feed, fish were starved for 24 h and then separated by size, with larger fish 6.11 ± 0.10 g fish−1 (mean ± S.D., n = 45) being randomly allocated to four tanks (one per diet), and medium sized fish 4.23 ± 0.21 g fish−1 (mean ± S.D; n = 45) being randomly allocated to 8 tanks (2 per diet). Each diet was randomly assigned two tanks with medium fish and one tank with large fish in a balanced block design. Fish were then reared over 47 d with initial, intermediate and final measurements of weight and length performed on individual fish. Fish losses were expressed as percentage survival. Each diet was fed manually to each tank twice daily (08:00 and 16:00) using a restrictive pair-fed feeding strategy (Glencross et al., 2003, Glencross et al., 2004) as opposed to apparent satiation or fixed ration (Burel et al., 1998; Farhangi, 2001; Robaina et al., 1995). Limiting-constraint feeding prevents confounding issue of unregulated feed intake due to nutrient-limited diets and allows for the standardisation of nutrient and energy utilisation of new ingredients in protein-replacement studies (Glencross et al., 2007). All diets were formulated to equivalent protein and energy levels, but feed was provided at a reduced proportion from the estimated requirements based on the diet with the lowest feed intake (e.g. usually 100% PPB diet 4). This strategy allows for the isolation of any particular effects caused by PPB protein.
Feed intake was recorded on a daily basis for each tank based on weight of feed provided (g) less uneaten feed collected, dried and weighed (g). Feed consumed was accounted for each diet and corrections applied to determine daily feed intake and feed conversion ratio (FCR) which is the ratio of the amount of feed consumed (dry weight in g) to fish biomass gain (live weight-gain in g). Weight gain was derived from final weight (g) minus initial weight (g).
At the end of the feeding trial (day 47) all fish were humanely killed by overdose with anaesthetic (AQUI-S) prior to weight and length measurements. Five fish from each tank were patted dry with paper towel to remove excess water and then frozen whole for total carcass composition analysis.
2.3. Analytical methods
2.3.1. PAnB and PPB biomass characterisation
The wet chemical analysis required for the PAnB operation (Table S2) and parts of the biomass characterisation (Table 1) are also described by Hülsen et al. (2018) and attached as supplementary materials (SM1). Pigment analysis (bacteriochlorophyll and total carotenoids) was performed as follows: Samples were frozen overnight (−80 °C). Then the temperature was raised to 4 °C, a small amount (<7 mg) of each dry sample (PPBs or diets) were weighed on a precision balance and the pigments extracted with acetone and methanol 7:2 while sonicating on ice for 10 min (modified from Bóna-Lovász et al. (2013); Van der Rest and Gingras (1974)). The process was repeated until a colourless pellet was obtained. The extracted pigments were detected at 475 nm (total carotenoids) and 771 nm (PPBs samples) and 750 nm (diets) for bacteriochlorophyll on the 0 to 1 absorbance scale using a Quartz cuvette. Pigment concentrations were calculated via the Beer-Lambert Law, assuming the Spirilloxanthin absorption coefficient (ε = 94000 M−1cm−1) for total carotenoids and using the Bacteriochlorophyll A absorption coefficient of 65300 M−1cm−1 respectively (Van der Rest and Gingras, 1974).
Gross energy was determined by bomb calorimetry or calculated following (Garling and Wilson, 1976), crude protein was calculated based on total TKN - NH4-N x 6.25 following Eding et al. (2006). Total lipid was determined by acid hydrolysis of the sample, then extraction of the total lipids in a soxhlet apparatus (FOSS Soxtec, Mulgrave, VIC, Australia). Ash content was determined gravimetrically following loss of mass after combustion in a muffle furnace at >550 °C. Moisture, lipid, nitrogen, and ash procedures were conducted by Symbio laboratories (Eight Mile Plains, QLD, 4113, Australia). Amino acid analysis from PPB grown on synthetic wastewater was performed by Symbio laboratories and the obtained values used to formulate the diets. Amino acid profiles for PPB grown on domestic wastewater and for all the manufactured diets were performed by Ridley Aquafeed (Narangba, Australia) by ultra-high-performance liquid chromatography (Acquity Arc, WATERS, Rydalmere, NSW, Australia). Samples were firstly hydrolysed in 6N HCL at 112 °C for 20 h, under nitrogen. Hydrolysed samples were then diluted and passed through a 0.2 μm syringe filter and then derivatized using the standard WATERS AccQ.Tag chemistry (Milford, MA, USA). Derivatives were analysed on an XBridge C18 column (2.5 μm, 3.0 mm × 150 mm) using the UHPLC quaternary pumping system, with WATERS eluents, LC-MS grade acetonitrile, and ultrapure water as mobile phase, which was set at a constant flow rate of 0.97 mL min−1 with column temperature set at 43 °C. Chromatograms were integrated and quantitated using the Empower v3 software (WATERS, MA, USA).
2.3.2. Diets and fish carcases proximate analysis
The diets, initial and final fish were processed and analysed for moisture, lipid, protein and amino acids following the methods described above. The diets were ground to a fine powder using a bench top grinder (Coffee & Spice, Breville, Australia). The whole, frozen fish were passed through an industrial food processor mincer twice (Hobart Tin chopper, Hobart) to obtain a homogeneous mixture. A sample was taken for dry matter analysis calculated by gravimetric analysis, following oven drying at 105 °C overnight and another sample was freeze dried for chemical analysis for 72 h at −106 °C and 0.2 hPa (CoolSafe™ Scanvac, Labogene).
2.4. Statistical analysis
2.4.1. Biomass characterisation
Measurement results for inputs are represented as averages, and variability in inputs are expressed as standard deviations in time-series measurements, given as ), where , is the average value for the data Xi, and is the standard deviation for the data. Measurement results for outputs and calculated parameters are represented as average values based on a two-tailed t-test (95% confidence, 5% significance threshold), represented as , where is the 95% confidence interval.
2.4.2. Fish performance analysis
The main explanatory variable (PPB inclusion) was continuous, rather than categorical, and hence mixed mode (categorical-continuous) linear modelling was used to assess its impact. This allowed for assessment of its impact vs other factors (tank number, initial fish size, age of fish etc). Effect of PPB inclusion on feed intake, daily gain, FCR, growth rate, and mortality between diets were examined using a generalized linear mixed regression model. Limits for all critical ranges were set at p < 0.05 (95% confidence interval) from three replicate tanks. An ANOVAN (n-way) analysis of variance (ANOVA) was used to test the effects of multiple factors on each tank and sample point using the ANOVAN function in MatLab (MathWorks, R2016b, also see SM1 for more details). Linear non-interaction and interaction models were tested, with the non-interaction model generally being used (as more parsimonious) where outcomes were comparable. Tank number was treated as categorical factor. Age of fish (time), start initial size (medium or large) and PPB fraction (diet type) were treated as primary continuous factors. Residual normality (noting n > 36) and heteroscedasticity (against explanatory variables) were tested and are given in the scripts. While linear modelling was used as the main statistical method for overall impact, comparison between pairs was done using an appropriate t-test.
3. Results
3.1. PPB biofilm harvesting and PPB biomass characteristics
A PPB biofilm was formed after 7 d post-inoculation (20 L) and harvesting was subsequently carried out every 3 d, reusing 10% (v/v) of the reactor liquid as inoculum. The biofilm was up to 3.0 mm thick (visual estimate), completely covered the irradiated surface (Fig. 1B and C) and was easy to remove from the tubes. Non-irradiated regions (due to non-functional LEDs) were immediately evident as no growth occurred (Fig. 1D). Approximately 1000 g wet biomass with 80–120 g L−1 dry solid was harvested per event with a peak sustained areal productivity (attached) of 6.0 g m2illuminated d−1 (or 22 tonnes ha−1 year−1) at 22 kWh kg−1. Although the primary purpose of the PPB reactor in this study was the production of PPB rather than optimising the COD, N and P removal and uptake efficiencies, the potential of simultaneous uptake of resources from artificial and wastewater sources has been shown. Representative time series of the artificial and the domestic wastewater reactor operation over 40 d (of the 135 d) and over 71 d respectively are shown Fig. S1. The COD and NH4-N removal for the synthetic medium and the domestic wastewater were similar (both around 300 mgSCOD L−1 and 14 mgNH4-N L−1 per batch (Table S2) although the TCOD, NH4-N and PO4-P removal rates of max 120, 8.0 and 1.0 mg L−1 d−1, were rather low which was likely caused by diffusion limitations. This is unfavourable for high rate systems, but we also note that this will ultimately depend on an optimised illuminated surface to volume ratio, which would further affect the mixing and likely increase the removal rates. Nevertheless, we consider the attached PPB growth as a successful proof of concept, which requires optimisation, especially with focus on the energy input.
The results further show the effect of attached PPB growth on the product consistency. Protein, amino acid, total carotenoid and bacteriochlorophyll contents in PPB biomass grown on synthetic and domestic wastewater were almost identical with slight variations in the fat and carbohydrate contents (Table 1). This indicates a degree of compositional independence from the feed matrix, which is crucial for product consistency on different growth media or wastewaters. This is further confirmed by the sequence-based analysis of the total harvested biomass. In both biofilms, grown on artificial and domestic wastewater, PPB were dominant (70% of sequences (Bradyrhizobiaceae, Rhodobacteraceae)) with Rhodopseudomonas sp and Rhodobacter sp accounting for up to up to 32% and 15%, (artificial medium) and 44% and 39% in domestic wastewater (Fig. S2). Table 1 also contains amino acid profiles of suspended PPB, grown on pork flush, poultry and red meat processing wastewater (Hülsen et al., 2018) and we hypothesise the general feasibility of attached PPB growth for these wastewaters and similar biomass compositions.
3.2. Description of feed processing and diet preparation
Moisture, crude lipid, crude protein and crude ash ranged from 3.0 to 5.2%, 11.8–13.3%, 55.5–57.3% and 8.0–9.4% respectively (Table 2C). Diets 3 and 4 containing the two highest inclusions of PPB had a notable smell and were much darker in colour (Fig. S3). The 2 mm fraction collected after the blending and sieving steps represented >93% of all gradients size. Pellets, regardless of the diet retained structural stability after 10 min immersion in water. Water from tanks receiving diet 4 and 3 were slightly more turbid than from diet 1 and 2 and this usually occurred for 10–15 min following feeding. The amino acid profiles of all feeds were determined, and are provided in Table 2C, with no significant correlation to PPB fraction in diet for all 17 amino acids (p > 0.05) [tested in Matlab but not reported here].
3.3. Fish growth performance
A summary of growth performance outcomes (daily growth rate, feed intake, feed conversion ratio (FCR), weight length gain and survival) for the four treatments are shown in Fig. 2 and Table 3. Feed intake was not significantly impacted by PPB inclusion suggesting that replacement of fishmeal with PPB protein did not affect palatability of the diets. Survival of barramundi fingerlings after 47 days was high (94%–100%) with no significant differences between diets or any other factors (p > 0.05). Three fish died in one tank in the initial period but, following this, only one further fish died in a different tank over the trial. All fish that died were abnormally small (below average weight of the cohort) and presented signs of aggression by others.
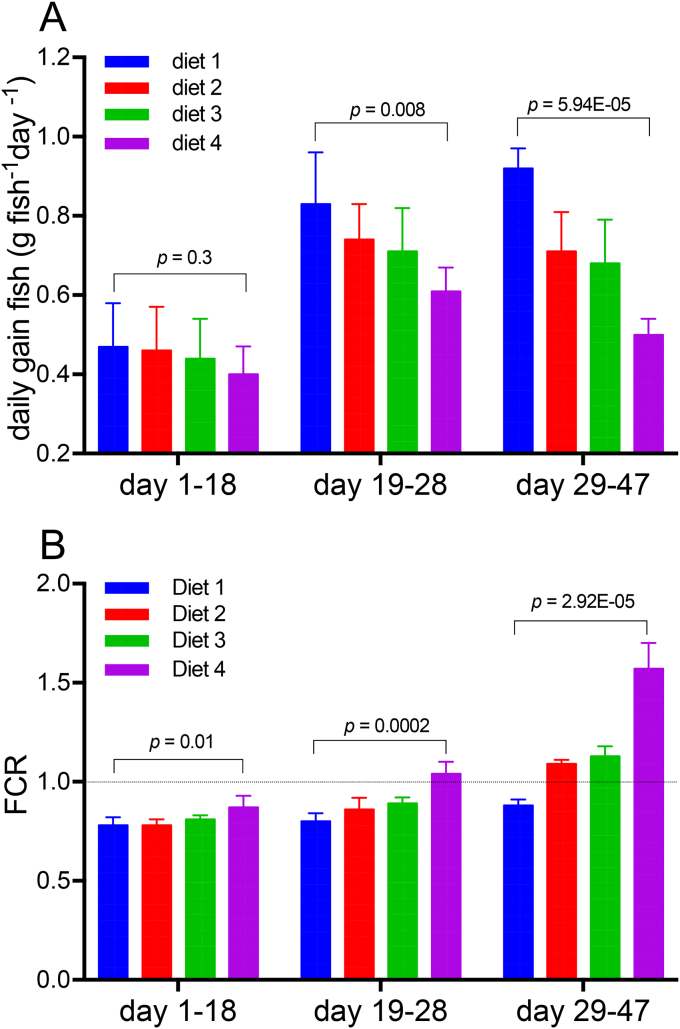
Fig. 2.
Performance parameters of Lates calcarifer juveniles fed different diets formulated with varying inclusions of fishmeal (FM) and purple phototrophic bacteria (PPB) (diet 1: 30%FM-0%PPB, diet 2: 20%FM-10%PPB, diet 3: 10%FM-20%PPB, diet 4: 0%FM-30%PPB). A. Fish daily growth rate (g fish−1day−1) determined for the three periods investigated: day 1–18, 19 to 28, and 29 to 47. Note the differences between diet 1 and 4 between day 19–28 and 29–47. B. Food conversion ratio (FCR) determined for those same periods, looking at any difference in FCR values between diets at the start, middle and end of the feed trial. The dot line indicates a FCR of 1. All data are given as the means with 95% CI from three replicate tanks. P-value indicates if the slope and intercept of the linear regression for a given time period are significantly different. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 3.
: Performance parameters of Lates calcarifer from each treatment (triplicate pooled mean ± 95%CI).
| Treatment | units | Diet 1 |
Diet 2 |
Diet 3 |
Diet 4 |
|
|---|---|---|---|---|---|---|
| 30-0 | 20-10 | 10-20 | 0-30 | |||
| Fish meal | (%) | 30 | 20 | 10 | 0 | Linear reg. |
| PPB | (%) | 0 | 10 | 20 | 30 | P-values |
| Initial weight | (g fish−1) | 4.8 ± 1.4 | 5.0 ± 1.3 | 4.9 ± 1.2 | 4.8 ± 1.4 | - |
| Final weight | (g fish−1) | 33.7 ± 5.2 | 30.0 ± 4.3 | 28.4 ± 5.0 | 25.7 ± 3.5 | 0.02 (-) |
| Weight gain | (g fish−1) | 29.0 ± 3.8 | 24.9 ± 3.0 | 23.5 ± 3.8 | 20.8 ± 2.1 | 0.004 (-) |
| Weight gain | (%) | 712 ± 90 | 611 ± 63 | 581 ± 34 | 544 ± 75 | 0.005 (-) |
| Initial length | (cm) | 7.1 ± 0.6 | 7.1 ± 0.6 | 7.1 ± 0.5 | 7.1 ± 0.5 | - |
| Final length | (cm) | 14.6 ± 0.9 | 13.5 ± 0.6 | 13.2 ± 0.7 | 12.8 ± 0.6 | 0.003 (-) |
| Length gain | (%) | 207.1 ± 8.0 | 191.2 ± 8.0 | 186 ± 3.0 | 181.3 ± 5.0 | 0.0003 (-) |
| Feed intake | (g fish−1 day−1) | 0.6133 ± 0.077 | 0.5870 ± 0.102 | 0.5973 ± 0.096 | 0.5907 ± 0.087 | - |
| Specific growth rate (%) | % (g d−1) | 4.2 ± 0.3 | 3.8 ± 0.2 | 3.7 ± 0.1 | 3.6 ± 0.3 | 0.005 (-) |
| Condition factor (CF) | 1.1 ± 0.02 | 1.2 ± 0.02 | 1.2 ± 0.03 | 1.2 ± 0.03 | 0.003 (+) | |
| survival | % | 96.3 ± 5.24 | 99.3 ± 1.44 | 100 | 94.8 ± 1.45 | - |
Notes: High 95%CI for initial and final weight because each diet has 2 tanks with medium fish and 1 tank with larger fish. Specific growth rate = (ln final mean weight – ln initial mean weight)/experimental days x 100. Condition factor (CF) = [fish weight/(total length)3]x100 at the start and at the end of the trial. P-values from linear regression (+) indicates positive correlation and (-) negative correlation. Further statistical tests are reported in the main text.
Fish in all tanks increased in weight over time (Fig. S4). During the 47 days of the trial, fish grew more than five-fold (Table 3), with fish fed control diet 1 increasing by 7.1 ± 0.9-fold, those fed diet 2 (30% FM replacement) by 6.1 ± 0.6-fold, fish fed diet 3 (66% FM replacement) increasing 5.8 ± 0.3-fold and fish fed diet 4 (100% FM replacement growing 5.4 ± 0.8-fold (Table 3). To predict weight, a simple linear model with explanatory factors age, initial size, and diet was as effective as a more complex interaction model (R2 = 0.96 vs R2 = 0.98), and the simpler model was used. Average weight was primarily driven by the age of fish (p = 5 × 10−23, 80% of variance), secondarily by initial size (p = 8 × 10−12, 12% of variance), and finally by diet (p = 1 × 10−06, 4.1% of variance). The model R2 was 0.96, indicating very little residual variance (probably due to the fact that each observation was averaged from 50 individual fish). Weight and length gain at the end of the trial were negatively correlated with increasing level of PPB with p-values of 0.0037 and 0.0003 respectively (Table 3).
Daily gain effectively removes the cumulative weight effect of time (i.e., fish get bigger over time), leaving analysis of the possibility that bigger or older fish grow faster or slower. There was no significant tank effect on the daily individual live-weight gain. Instead, daily gain was impacted positively by time (older fish grow faster (p = 4 × 10−5)), positively by initial size (p = 0.0029, big initial size results in higher daily gain), and negatively by PPB inclusion rate (p = 0.0001). R2 for the linear model was 0.62 (vs interaction model R2 = 0.7 – slight improvement). An R2 of 0.62 indicates that the main factors were highly explanatory, even with the cumulative growth effect of time removed. For the first 28 d of the trial there was no effect of PPB on daily weight gain (p = 0.3; Fig. 2A). From day 28 until day 47, PPB inclusion rate had a negative correlation with daily gain (p = 0.008 and 5.94 × 10−5 respectively) (Fig. 2A). Fish fed diet 4 (100% FM replacement) had the lowest gain at both sample times. As fish grew older from day 18 to day 28, the daily gain significantly increased in all diet treatments (p < 0.0001). At the end of the feed trial, daily weight gain slightly increased in fish fed diet 1 (0% FM replacement) but remained unchanged for fish fed diet 2 and 3 and decreased for fish fed diet 4 (Fig. 2A).
Feed conversion ratio varied substantially with time (p = 6 × 10−9) and diet (p = 6 × 10−7) (Fig. 2B), but tank allocation and initial size had no impact on FCR. Comparison of linear models and interaction models and removing non-significant factors resulted in two candidate models, each involving only time and diet. The best model was a two-factor model (diet and time*diet, R2 = 0.89), which was better than the two-parameter linear model (time and diet, R2 = 0.75), and not worse than the full 3-parameter interaction (R2 = 0.90, time alone not significant). This optimal model predicted PPB fraction in diet negatively affecting FCR (p = 4 × 10−7, fraction of variance 0.12) with a highly significant interaction between time*diet type (p = 7 × 10−15, fraction of variance 0.6). The primary (linear factor) indicates a slight decrease in FCR with increased PPB inclusion rate (i.e., beneficial impact), but the latter dominates, and is a detrimental impact. Overall, at an earlier time point, diet is less important to FCR, while at later times, diet has a strong impact in increasing FCR (detrimental). This can be effectively seen in Fig. 2B also, where only at later periods, and at higher dietary PPB inclusion rate, does the FCR rise substantially above 1.
Daily feed intake was not impacted by tanks or diet. However, it was slightly impacted by the initial size and, naturally, time (p = 4 × 10−15 of the fish (p = 6 × 10−6) with larger animals consuming more feed than smaller ones. There were no interactions between primary factors and, as expected, no differences observed in feed intakes between diets at any given time (restrictive pair-fed feeding strategy). Further analysis regressing daily intake vs fish weight (at tend) vs PPB fraction found that, apart from the strong correlation with fish weight (p = 1 × 10−18), there was a significant positive correlation with PPB inclusion (0.005, R2 = 0.9).
3.4. Carcass composition
Fish carcass compositions are presented in Table S3. Three samples were analysed per diet treatment, one for each replicate tank. Each sample was composed of five representative animals per tank. Differences in moisture, protein, lipid, ash, and carbohydrate were analysed by linear regression. Increasing level of PPB in the diets had a negative correlation with protein content in fish carcasses (p = 0.0220), with carcasses of fish fed diet 4 having the lowest protein content. Increasing level of PPB had a positive correlation on ash content of the carcass (p < 0.0032) with ash content maximum in carcasses of fish fed diet 4. Carcasses of fish fed diet 3 formulated with 66% PPB as fishmeal replacement had the highest level of lipid among all diets including control, though no statistically significant correlation was detected by regression. PPB concentration in diet had no effect on moisture or carbohydrate content in carcasses of animals fed the diets (p > 0.05).
Elemental analyses in diets and carcasses of fish fed the diets are presented in Table S1. Potassium, phosphorous, magnesium and calcium increased by 7%, 21%, 12% and 27% respectively from reference to complete fishmeal replacement, while sulphur and sodium decreased by 12 and 22%. Levels of calcium between diets did not show a particular trend but its levels in fish carcasses composition increased significantly (p = 1.20 × 10−4). Iron levels in diets increased by 37% in diets with 66% and 100% PPB inclusion but remained constant in fish carcasses. Potassium levels were fairly constant in diets and no differences in body compositions observed. Magnesium levels increased gradually in experimental diets (up to 48% in diet 4) so as its levels in fish carcasses (p = 7.06 × 10−3). Sodium increased in diets (up to 43% in diet 4) but decreased significantly in fish fed experimental diets (p = 6.88 × 10−4). Phosphorous level was maximum in diet 3 (23% higher than control diet 1) and increased significantly in carcases of fish fed increasing level of PPB (p = 1.61 × 10−4). As expected, sulphur levels increased along with FM replacement (up to 33% in diet 4) but its levels decreased significantly in carcasses of fish (p = 5.25 × 10−5). Concentrations of copper, iron, lead, and zinc remained constant in all fish carcasses tested.
4. Discussion
Fishmeal for aquaculture feed production in 2013 used 73% of total forage fish derived from the capture fisheries (Shepherd and Jackson, 2013) and recent data suggest that most of this fish is suitable for human food use (Cashion et al., 2017). Consequently, replacement or reduction of fishmeal as protein source in aquaculture feeds is critical to continued growth of fish farming as a foundation for future food security (Naylor et al., 2009; Troell et al., 2014). Microbial-derived proteins are of particular interest as they can be readily produced on inexpensive carbon sources and as byproducts of other processes such as brewing and waste treatment (Matassa et al., 2016; Øverland and Skrede, 2017). Here we show that bacterial biomass derived from synthetic or domestic wastewater using photoactive bioreactors can be substituted for fishmeal in diets for the popular tropical aquaculture fish barramundi (Asian sea bass, L. calcarifer), with moderate impact on main indicators (fish growth rate, FCR), and with commercially viable outcomes. Given this is a proof-of-concept study, with feed formulation not yet optimised for differences between PPB and fishmeal as primary protein source, there remains substantial scope to further improve these outcomes. These aspects are discussed further in this section.
4.1. PPB from water treatment is a consistent quality ingredient
PPB produced, using synthetic or domestic wastewater in photoactive bioreactors was consistent in terms of bacterial community, protein, lipid and carbohydrate content in agreement with previous research where biomass derived from bioreactors operating on wastewater streams from pork, dairy and poultry processing were compared (Hülsen et al., 2018). Consistent ingredient quality is critical for commercial feed production and is a common problem for formulation (Tangendjaja, 2015) so the product consistency across synthetic wastewater, domestic wastewater (this study) and disparate waste streams (Hülsen et al., 2018) is an advantage of the PPB biomass. Protein content of the PPB biomass was reasonably high (>57%) making it possible to substitute at quite high rates in feeds for carnivorous fish where dietary protein requirement may be between 40 and 55%. This compares favourably with other microbial protein sources such as yeast, fungi and microalgae where protein content is substantially less than 50% (Gong et al., 2018; Øverland and Skrede, 2017; Vidakovic et al., 2016).
4.2. PPB is effective as a partial fishmeal substitute in aqua feeds for farmed barramundi
Growth performance outcomes demonstrated progressive but fractional losses in fish performance indicators as PPB fraction (or protein input) increased, generally significant across all treatments, but not between neighbouring treatments. The main effect was on performance indicators growth, daily weight gain and FCR, rather than mortality and carcass composition. In terms of commercial viability, up to 66% of the fishmeal could be substituted with purple phototrophic bacteria without a major impact on the fish performance parameters for the first 28 d of the trial, noting that the impact is progressive after 28 d. It is important to consider that the Diet 4 in the present study contained zero fishmeal and the results are consistent with other studies where complete substitution was considered. For example, replacement of fishmeal with soybean and poultry meal in barramundi diets resulted reduced weight gain and increased feed conversion that was significant when replacement was 66% or 100% (Glencross et al., 2016). Indeed, over the duration of the trial FCR remained at around 1 for diets with 33% and 66% of FM replaced by PPB. The reason for the increased FCR and decreased daily weight gain at high replacement levels need to be further investigated. Similar impact on growth and feed conversion was noted in Arctic charr fed on diets with 40% FM replaced by intact baker's yeast (S. cerevisiae) or filamentous fungal biomass (Rhizopus oryzae) (Vidakovic et al., 2016). The negative effect of yeast inclusion on FCR and final weight was abrogated in Arctic charr by pre-lysing the yeast cells (Vidakovic et al., 2016) resulting in improved apparent digestibility of the protein and amino acids in the diet (Langeland et al., 2016). It is therefore possible that pre-lysis of PPB biomass may increase the performance at higher substitution rates and is worthy of further study, albeit that there would be additional processing cost. Other potential factors impacting final weight and FCR may include the presence of nucleic acids in the PPB biomass, as high levels would increase the energy cost for barramundi to metabolise them. While nucleotides are semi-essential nutrients in many animals including fish and shrimp (Burrells et al., 2001; Carver and Walker, 1995; Do et al., 2012; Hoang Do et al., 2013), nucleic acids need first to be processed via the salvage pathway prior to supplying to new genetic material during growth. Moreover, excess nucleotides need to be excreted and accrue a metabolic cost. As a consequence, levels must be optimised for each species and age as requirement decreases in older animals (Do et al., 2012; Li and Gatlin, 2006). Similar considerations apply to complex carbohydrates that could lead to gut irritation and lower digestibility. These will be tested in future digestibility experiments. We demonstrated successfully that the inclusion up to 33% FM replacement with PPB would have little or no impact on barramundi growth performance. Therefore, we can recover resources from wastewater, transform them into PPB biomass and convert it to a useful feed ingredient, reducing the reliance on wild caught fishmeal. In contrast, deleterious effects on growth, feed intake and feed conversion have been reported when microalgae were used at concentrations as low as 12% total inclusion (14.2% FM substitution) with Phaeodactylum tricornutum (Sorensen et al., 2016) and 20% total inclusion for defatted Nannochloropsis biomass (Sorensen et al., 2017).
While the bulk properties (amino acid profile, metals profile etc.), were comparable across the four diets, PPB has a number of additional active compounds (vitamins, biopolymers (PHAs), carotenoids, and bacteriochlorophylls), which are important to commercial fish rearing. PPB contain a range of vitamins (e.g. B2, B12, E and niacin) (Sasaki et al., 1991) which are important for fish growth and are routinely added to commercial feed (vitamin premix), including in the current study. If substantially substituted by native compounds in PPB, this would allow reduction of premix from commercial feed formulations, with associated cost-saving. The same applies for carotenoids and bacteriochlorophyll, which in this study were present in the PPB product used to formulate the diets at 6.8 g kg−1 and 11.3 g kg−1 respectively. Interestingly, pigments were stable after the steaming and overnight drying steps of the pellets, their levels of detection in the diets were in accordance with the concentration of PPB biomass used in the formulation of the diets (Fig. S5). These pigments serve as anti-oxidants and play an important role on the coloration of fish skin/flesh (Sasaki et al., 1991), and are consequently added to diets for salmonids and marine shrimp (as astaxanthin) to improve meat colour (Torrissen and Christiansen, 1995; Wade et al., 2017).
4.3. Significance and application to wastewater treatment industry
The application of PPB biomass for fishmeal substitution adds value to the PPB biomass. This, primarily (but not limited to) concerns the value of bulk protein where fishmeal fluctuates in price according to grades and availability, in the last 5 years prices have ranged from 1,919 US$ metric ton−1 (01/2013) to 1,296.00 US$ metric ton-1 (04/2017) and last recorded at 1,604 US $ metric ton-1 (03/2018) or 2.5–3 US$ per kg crude protein (assuming 65% CP) (www.indexmundi.com/commodities/?commodity=fish-meal&months=120). Future prices are expected to increase due to overfishing and catch quota (Tveteras and Asche, 2008). Moreover, supply restriction during climatic events (for example fishery closure during El Niño events) are likely to increase in frequency and duration forcing prices higher (Ubilava, 2014). For the wastewater treatment industry, preferably agri-industry sources (e.g. food processors, abattoirs, or comparable industries) this means the production of PPB from their wastewaters will add an additional income stream. The PPB technology has the potential to remove organics, nitrogen and phosphorous from wastewater, lowering the discharge costs while simultaneously transforming these constituents into protein-rich biomass at yields around 0.8 gCOD−1 (Hülsen et al., 2018). General PPB applicability has been demonstrated in dairy, red meat, poultry processing wastewaters and pork flush with a characteristic profile of mixed culture PPB extensively discussed with respect to those protein sources (Hülsen et al., 2018). PPB from different sources has been found to be very consistent, almost independent from the wastewater source, as indicated with the comparison of PPB composition grown on synthetic and domestic wastewater (Table 1). This can provide feed manufacturers with a valuable ingredient stream, largely independent from seasonal variations. The areal productivity of 6 g m−2 d−1 is rather high compared to e.g. immobilised/attached algae biofilm productivities (e.g. 0.71 (Ozkan et al., 2012). and 0.58–2.57 g m−2 d−1 (Johnson and Wen, 2010)). 6 g m−2 d−1 is rather high compared to e.g. immobilised/attached algae biofilm productivities (e.g. 0.71 (Ozkan et al., 2012). and 0.58–2.57 g m−2 d−1 (Johnson and Wen, 2010)). The growth of PPB is naturally coupled with removal of carbon and nutrients through assimilative growth (Myung et al., 2004; Tadesse et al., 2003).
Although the attached reactor concept utilised to grow the PPB had low removal rates and required a high energy input, mainly for illumination, the concept of attached biofilms on IR illuminated submerged tubes for consistent biomass quality seems technically feasible. The tubes are by no means the final design and significantly higher illuminated surface to volume ratios can be easily achieved e.g. with smaller tube diameters or in a flat plate reactors (e.g. 67 m2 m−3 (Norsker et al., 2011)). This can further improve the mixing and the removal rates, which might also lead to increased attached areal productivities e.g. due to faster growth and more frequent harvesting rather than increased density. The logical step would be the use of outdoor cultures, which would harvest natural light, drastically increasing illumination intensity and reducing illumination costs to zero while sacrificing 12 h of illuminated operation due to night/day cycles.
4.4. PPB as aquaculture input: further research needs
Our results suggest that PPB up to 33% (∼10%) could offer an alternative to fishmeal with little or no detrimental effect on fish performance in barramundi, a tropical species with high dietary protein requirement. It may be possible to address the penalties in FCR and growth at higher inclusion levels by relatively inexpensive and simple ingredient pre-treatment (eg hydrolysis) as this improved digestibility and performance of baker's yeast in Arctic charr (Vidakovic et al., 2016), a fish which also has dietary protein requirement. In addition, the potential beneficial impact of vitamins and other compounds (including carotenoids, chlorophylls and biopolymers such as PHB) needs to be assessed to change the overall optimal formulation for commercial utilisation. It is noted that this study focused on manual preparation of relatively small amounts of feed (though still with a large number of individual animals). The encouraging results of the present study justify a larger trial, involving preparation of larger volumes of feed with a commercial extruder. Given the relatively high carotenoid content in PPB and its stability through feed processing demonstrated in this study, assessment of performance in feeds for high value crustaceans such as tiger prawns (P. monodon) that can utilise these carotenoids (Wade et al., 2017) to improve product quality should be a priority. There is clear potential here for added value beyond replacement of fishmeal with a sustainable waste treatment by-product.
Here we tested full and partial replacement of fishmeal with PPB in fast growing carnivorous species, the Asian sea bass or barramundi. Alternative species such as shrimp and prawns that are largely detritivorous (Bailey-Brock and Moss, 1992), and Tilapia that are omnivorous grazers may well thrive on microbial biomass and should be priorities for further study. Additionally, potential benefits of using PPB as whole feed (either directly in culture water or fed to rotifer or artemia) to survival and growth during early life stages of these animals should be considered.
Additionally, and most importantly, the PPB production costs need to be lowered. As discussed above, outdoor cultures and increased illuminated surface ratio are suitable approaches.
5. Conclusions
This represents proof of concept for the replacement of fishmeal using a mixed population PPB biofilm generated in a photo-anaerobic bioreactor (PAnB). Bulk characteristics and population were consistent on synthetic and actual wastewater. We encountered no significant increase in mortality associated with the use of PPB as a feed ingredient and demonstrated that up to 66% replacement of fishmeal with PPB has no significant adverse effects on fish performance for the first 28 days. However, full fishmeal replacement with bacterial biomass reduced growth and increased feed conversion ratio. Nevertheless, our study strongly supports further research to identify optimal opportunities for microbial protein substitutes for fishmeal for more cost effective and long-term sustainable aquaculture feeds.
Declaration of interest
None.
Acknowledgements
We would like to thank Emmanuelle Zoccola, Oleksandra Silayava and Grace Isdale for their help with fish metrics measurement, María Grassino for the pigment analysis by absorbance spectrometry and Justin Todhunter for assistance with the reactor start-up. We are also grateful to Ridley for suppling the raw materials used in this study. This work was funded by Australian Pork Limited and the Department of Agriculture and Forestry, Australia (Project No: 2014/534.05) as part of its Rural R&D for Profit programme.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.wroa.2019.100031.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Anupama, Ravindra P. Value-added food: single cell protein. Biotechnol. Adv. 2000;18(6):459–479. doi: 10.1016/s0734-9750(00)00045-8. [DOI] [PubMed] [Google Scholar]
- Bailey-Brock J.H., Moss S.M. Penaeid taxonomy, biology and zoogeography. Dev. Aquacult. Fish. Sci. 1992;23:9–27. [Google Scholar]
- Banerjee S., Azad S.A., Vikineswary S., Selvaraj O.S., Mukherjee T.K. Phototrophic bacteria as fish feed supplement. Asian-Australas. J. Anim. Sci. 2000;13(7):991–994. [Google Scholar]
- Bidigare R.R., Ondrusek M.E., Morrow J.H., Kiefer D.A. 1990. In Vivo Absorption Properties of Algal Pigments; pp. 290–302. [Google Scholar]
- Bóna-Lovász J., Bóna A., Ederer M., Sawodny O., Ghosh R. A rapid method for the extraction and analysis of carotenoids and other hydrophobic substances suitable for systems biology studies with photosynthetic bacteria. Metabolites. 2013;3(4):912–930. doi: 10.3390/metabo3040912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burel C., Boujard T., Corraze G., Kaushik S.J., Boeuf G., Mol K.A., Van Der Geyten S., Kühn E.R. Incorporation of high levels of extruded lupin in diets for rainbow trout (Oncorhynchus mykiss): nutritional value and effect on thyroid status. Aquaculture. 1998;163(3–4):325–345. [Google Scholar]
- Burrells C., Williams P.D., Southgate P.J., Wadsworth S.L. Dietary nucleotides: a novel supplement in fish feeds 2. Effects on vaccination, salt water transfer, growth rates and physiology of Atlantic salmon (Salmo salar L.) Aquaculture. 2001;199(1–2):171–184. [Google Scholar]
- Carver J.D., Walker W.A. The role of nucleotides in human nutrition. JNB (J. Nutr. Biochem.) 1995;6(2):58–72. [Google Scholar]
- Cashion T., Le Manach F., Zeller D., Pauly D. Most fish destined for fishmeal production are food-grade fish. Fish Fish. 2017;18(5):837–844. [Google Scholar]
- Chowdhury A.J.K., Zakaria N.H., Abidin Z.A.Z., Rahman M.M. Phototrophic purple bacteria as feed supplement on the growth, feed utilization and body compositions of Malaysian Mahseer, Tor tambroides juveniles. Sains Malays. 2016;45(1):135–140. [Google Scholar]
- Do H.H., Tabrett S., Hoffman K., Köppel P., Lucas J.S., Barnes A.C. Dietary nucleotides are semi-essential nutrients for optimal growth of black tiger shrimp (Penaeus monodon) Aquaculture. 2012;366–367:115–121. [Google Scholar]
- Eding E.H., Kamstra A., Verreth J.A.J., Huisman E.A., Klapwijk A. Design and operation of nitrifying trickling filters in recirculating aquaculture: a review. Aquacult. Eng. 2006;34(3):234–260. [Google Scholar]
- FAO . FAO; Rome: 2016. The State of World Fisheries and Aquaculture 2016. [Google Scholar]
- FAO . FAO; Rome: 2018. The State of World Fisheries and Aquaculture 2018. [Google Scholar]
- Farhangi M. Growth, physiological and immunological responses of rainbow trout (Oncorhynchus my kiss) to different dietary inclusion levels of dehuded lupin (Lupinus angustifolius) Aquacult. Res. 2001;32(Suppl. 1):329–340. [Google Scholar]
- Gamboa-Delgado J., Márquez-Reyes J.M. Potential of microbial-derived nutrients for aquaculture development. Rev. Aquacult. 2018;10(1):224–246. [Google Scholar]
- Garling D.L., Jr., Wilson R.P. Optimum dietary protein to energy ratio for channel catfish fingerlings, Ictalurus punctatus. J. Nutr. 1976;106(9):1368–1375. doi: 10.1093/jn/106.9.1368. [DOI] [PubMed] [Google Scholar]
- Glencross B. The nutritional management of barramundi, Lates calcarifer - a review. Aquacult. Nutr. 2006;12(4):291–309. [Google Scholar]
- Glencross B., Curnow J., Hawkins W., Kissil G.W.M., Peterson D. Evaluation of the feed value of a transgenic strain of the narrow-leaf lupin (Lupinus angustifolius) in the diet of the marine fish, Pagrus auratus. Aquacult. Nutr. 2003;9(3):197–206. [Google Scholar]
- Glencross B., Hawkins W., Curnow J. Nutritional assessment of Australian canola meals. II. Evaluation of the influence of the canola oil extraction method on the protein value of canola meals fed to the red seabream (Pagrus auratus, Paulin) Aquacult. Res. 2004;35(1):25–34. [Google Scholar]
- Glencross B.D., Booth M., Allan G.L. A feed is only as good as its ingredients - a review of ingredient evaluation strategies for aquaculture feeds. Aquacult. Nutr. 2007;13(1):17–34. [Google Scholar]
- Glencross B., Blyth D., Irvin S., Bourne N., Campet M., Boisot P., Wade N.M. An evaluation of the complete replacement of both fishmeal and fish oil in diets for juvenile Asian seabass, Lates calcarifer. Aquaculture. 2016;451:298–309. [Google Scholar]
- Gong Y., Guterres H.A.D.S., Huntley M., Sorensen M., Kiron V. Digestibility of the defatted microalgae Nannochloropsis sp and Desmodesmus sp when fed to Atlantic salmon, Salmo salar. Aquacult. Nutr. 2018;24(1):56–64. [Google Scholar]
- Hardy R.W. Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal. Aquacult. Res. 2010;41(5):770–776. [Google Scholar]
- Hoang Do H., Tabrett S., Hoffmann K., Koeppel P., Barnes A.C. The purine nucleotides guanine, adenine and inosine are a dietary requirement for optimal growth of black tiger prawn, P. monodon. Aquaculture. 2013;408:100–105. [Google Scholar]
- Hülsen T., Batstone D.J., Keller J. Phototrophic bacteria for nutrient recovery from domestic wastewater. Water Res. 2014;50(0):18–26. doi: 10.1016/j.watres.2013.10.051. [DOI] [PubMed] [Google Scholar]
- Hülsen T., Hsieh K., Lu Y., Tait S., Batstone D.J. Simultaneous treatment and single cell protein production from agri-industrial wastewaters using purple phototrophic bacteria or microalgae – a comparison. Bioresour. Technol. 2018;254:214–223. doi: 10.1016/j.biortech.2018.01.032. [DOI] [PubMed] [Google Scholar]
- Johnson M.B., Wen Z. Development of an attached microalgal growth system for biofuel production. Appl. Microbiol. Biotechnol. 2010;85(3):525–534. doi: 10.1007/s00253-009-2133-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Kobayashi M. Anoxygenic Photosynthetic Bacteria. 1995. Waste remediation and treatment using anoxygenic phototrophic bacteria; pp. 1269–1282. [Google Scholar]
- Kobayashi M., Tchan Y.T. Treatment of industrial waste solutions and production of useful by-products using a photosynthetic bacterial method. Water Res. 1973;7(8):1219–1224. [Google Scholar]
- Langeland M., Vidakovic A., Vielma J., Lindberg J.E., Kiessling A., Lundh T. Digestibility of microbial and mussel meal for Arctic charr (Salvelinus alpinus) and Eurasian perch (Perca fluviatilis) Aquacult. Nutr. 2016;22(2):485–495. [Google Scholar]
- Li P., Gatlin D.M. Nucleotide nutrition in fish: current knowledge and future applications. Aquaculture. 2006;251(2–4):141–152. [Google Scholar]
- Loo P.L., Chong V.C., Ibrahim S., Sabaratnam V. Manipulating culture conditions and feed quality to increase the survival of larval marble goby Oxyeleotris marmorata. N. Am. J. Aquacult. 2015;77(2):149–159. [Google Scholar]
- Matassa S., Batstone D.J., Hülsen T., Schnoor J., Verstraete W. Can direct conversion of used nitrogen to new feed and protein help feed the world? Environ. Sci. Technol. 2015;49(9):5247–5254. doi: 10.1021/es505432w. [DOI] [PubMed] [Google Scholar]
- Matassa S., Boon N., Pikaar I., Verstraete W. Microbial protein: future sustainable food supply route with low environmental footprint. Microb. Biotech. 2016;9(5):568–575. doi: 10.1111/1751-7915.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung K.K., Choi K.M., Yin C.R., Lee K.Y., Im W.T., Ju H.L., Lee S.T. Odorous swine wastewater treatment by purple non-sulfur bacteria, Rhodopseudomonas palustris, isolated from eutrophicated ponds. Biotechnol. Lett. 2004;26(10):819–822. doi: 10.1023/b:bile.0000025884.50198.67. [DOI] [PubMed] [Google Scholar]
- Naylor R.L., Hardy R.W., Bureau D.P., Chiu A., Elliott M., Farrell A.P., Forster I., Gatlin D.M., Goldburg R.J., Hua K., Nichols P.D. Feeding aquaculture in an era of finite resources. Proc. Natl. Acad. Sci. U. S. A. 2009;106(36):15103–15110. doi: 10.1073/pnas.0905235106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norsker N.-H., Barbosa M.J., Vermuë M.H., Wijffels R.H. Microalgal production — a close look at the economics. Biotechnol. Adv. 2011;29(1):24–27. doi: 10.1016/j.biotechadv.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Ormerod J.G., Ormerod K.S., Gest H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch. Biochem. Biophys. 1961;94(3):449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Øverland M., Skrede A. Yeast derived from lignocellulosic biomass as a sustainable feed resource for use in aquaculture. J. Sci. Food Agric. 2017;97(3):733–742. doi: 10.1002/jsfa.8007. [DOI] [PubMed] [Google Scholar]
- Ozkan A., Kinney K., Katz L., Berberoglu H. Reduction of water and energy requirement of algae cultivation using an algae biofilm photobioreactor. Bioresour. Technol. 2012;114(Suppl. C):542–548. doi: 10.1016/j.biortech.2012.03.055. [DOI] [PubMed] [Google Scholar]
- Pikaar I., Matassa S., Rabaey K., Bodirsky B.L., Popp A., Herrero M., Verstraete W. Microbes and the next nitrogen revolution. Environ. Sci. Technol. 2017;51(13):7297–7303. doi: 10.1021/acs.est.7b00916. [DOI] [PubMed] [Google Scholar]
- Robaina L., Izquierdo M.S., Moyano F.J., Socorro J., Vergara J.M., Montero D., Fernández-Palacios H. Soybean and lupin seed meals as protein sources in diets for gilthead seabream (Sparus aurata): nutritional and histological implications. Aquaculture. 1995;130(2–3):219–233. [Google Scholar]
- Sasaki K., Noparatnaraporn N., Nagai S. Bioconversion of Waste Materials to Industrial Products. 1991. Use of photosynthetic bacteria for the production of SCP and chemicals from agroindustrial wastes; pp. 223–262. [Google Scholar]
- Shapawi R., Ting T.E., Al-Azad S. Inclusion of purple non-sulfur bacterial biomass in formulated feed to promote growth, feed conversion ratio and survival of Asian seabass lates calcarifer juveniles. J. Fish. Aquat. Sci. 2012;7(6):475–480. [Google Scholar]
- Shepherd C.J., Jackson A.J. Global fishmeal and fish-oil supply: inputs, outputs and markets. J. Fish Biol. 2013;83(4):1046–1066. doi: 10.1111/jfb.12224. [DOI] [PubMed] [Google Scholar]
- Sorensen M., Berge G.M., Reitan K.I., Ruyter B. Microalga Phaeodactylum tricornutum in feed for Atlantic salmon (Salmo salar) - effect on nutrient digestibility, growth and utilization of feed. Aquaculture. 2016;460:116–123. [Google Scholar]
- Sorensen M., Gong Y.Y., Bjarnason F., Vasanth G.K., Dahle D., Huntley M., Kiron V. Nannochloropsis oceania-derived defatted meal as an alternative to fishmeal in Atlantic salmon feeds. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0179907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer A. Insect proteins-a new source for animal feed: the use of insect larvae to recycle food waste in high-quality protein for livestock and aquaculture feeds is held back largely owing to regulatory hurdles. EMBO Rep. 2015;16(6):676–680. doi: 10.15252/embr.201540528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadesse I., Isoaho S.A., Green F.B., Puhakka J.A. 2003. Removal of Organics and Nutrients from Tannery Effluent by Advanced Integrated Wastewater Pond Systems® Technology; pp. 307–314. [PubMed] [Google Scholar]
- Tangendjaja B. In: Feed and Feeding Practices in Aquaculture. Davis A., editor. Elsevier; Amsterdam: 2015. pp. 141–169. [Google Scholar]
- Torrissen O.J., Christiansen R. Requirements for carotenoids in fish diets. Zeitschrift Fur Angewandte IchthyologieJ. Appl. Ichthyol. 1995;11(3–4):225–230. [Google Scholar]
- Troell M., Naylor R.L., Metian M., Beveridge M., Tyedmers P.H., Folke C., Arrow K.J., Barrett S., Crépin A.-S., Ehrlich P.R., Gren Å., Kautsky N., Levin S.A., Nyborg K., Österblom H., Polasky S., Scheffer M., Walker B.H., Xepapadeas T., de Zeeuw A. Does aquaculture add resilience to the global food system? Proc. Natl. Acad. Sci. Unit. States Am. 2014;111(37):13257–13263. doi: 10.1073/pnas.1404067111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tveteras S., Asche F. International fish trade and exchange rates: an application to the trade with salmon and fishmeal. Appl. Econ. 2008;40(13):1745–1755. [Google Scholar]
- Ubilava D. El Nino Southern Oscillation and the fishmeal-soya bean meal price ratio: regime-dependent dynamics revisited. Eur. Rev. Agric. Econ. 2014;41(4):583–604. [Google Scholar]
- Van der Rest M., Gingras G. The pigment complement of the photosynthetic reaction center isolated from Rhodospirillum rubrum. J. Biol. Chem. 1974;249(20):6446–6453. [PubMed] [Google Scholar]
- Vidakovic A., Langeland M., Sundh H., Sundell K., Olstorpe M., Vielma J., Kiessling A., Lundh T. Evaluation of growth performance and intestinal barrier function in Arctic Charr (Salvelinus alpinus) fed yeast (Saccharomyces cerevisiae), fungi (Rhizopus oryzae) and blue mussel (Mytilus edulis) Aquacult. Nutr. 2016;22(6):1348–1360. [Google Scholar]
- Wade N.M., Cheers S., Bourne N., Irvin S., Blyth D., Glencross B.D. Dietary astaxanthin levels affect colour, growth, carotenoid digestibility and the accumulation of specific carotenoid esters in the Giant Tiger Shrimp, Penaeus monodon. Aquacult. Res. 2017;48(2):395–406. [Google Scholar]
- Yaakob Z., Ali E., Zainal A., Mohamad M., Takriff M.S. An overview: biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res. 2014;21(1) doi: 10.1186/2241-5793-21-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Yang H., Zhang W., Huang Z., Liu S.J. Rhodocista pekingensis sp. nov., a cyst-forming phototrophic bacterium from a municipal wastewater treatment plant. Int. J. Syst. Evol. Microbiol. 2003;53(4):1111–1114. doi: 10.1099/ijs.0.02500-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.