Abstract
NADPH oxidase 4 (NOX4) is one of the main sources of reactive oxygen species, and plays a crucial role in the occurrence and development of tumors. However, there is currently little evidence demonstrating that NOX4 expression is associated with gastric cancer. To establish whether NOX4 plays a role in gastric cancer progression and prognosis, we performed immunohistochemistry on gastric cancer tissues and paired adjacent normal tissues from 90 gastric cancer patients to detect and compare NOX4 expression. Next, we analyzed the association between NOX4 expression and clinicopathological characteristics. Survival analysis was performed to explore the association between NOX4 expression and the prognosis of gastric cancer patients. Furtherly, we investigated the effect of NOX4-knockdown using siRNA on gastric cancer progression in vitro and in vivo. Our results revealed that NOX4 expression in gastric cancer tissues is higher than in paired adjacent normal tissues (P = 0.0009). NOX4 expression is significantly correlated with tumor size (P = 0.0321), lymphatic metastasis (P = 0.0125) and vascular invasion (P = 0.0017) and a poor prognosis (P = 0.0000) in gastric cancer patients. NOX4 depletion could significantly inhibit the invasion, proliferation, EMT and MMP7 expression of gastric cancer cells and suppress the progression of gastric cancer in vivo. In conclusion, NOX4 is related to gastric cancer development and predicts a poor prognosis. NOX4 may play an essential role in the progression of gastric cancer, and is a promising target for the prevention and treatment of gastric cancer.
Keywords: NOX4, gastric cancer, progression, prognosis, reactive oxygen species
Introduction
Gastric cancer is one of the most common malignancies and the second most common cause of cancer-related deaths in China [1]. The incidence of gastric cancer and the mortality rate have increased over the years, with over 679,000 new cases and 498,000 deaths in China in 2015 [1]. Treatment approaches for gastric cancer include surgery, radiotherapy, chemotherapy, gene therapy and immune therapy. Despite these options, the prognosis of patients with gastric cancer remains poor [2]. Lymph node invasion and distant metastasis are the main factors contributing to a poor prognosis [3]. The identification of novel diagnostic and prognostic biomarkers could contribute to improved outcomes in patients with gastric cancer.
The metastasis of cancer is a complex pathological process involving a redox imbalance. In cancer, reactive oxygen species (ROS) lead to genomic instability and trigger several signaling cascades involved in proliferation, neovascularization, migration, invasion and extravasation into distant sites [4-6].
NADPH oxidase 4 (NOX4) is one of the main sources of ROS. In fact, the only confirmed function of NOX4 is generating ROS [7]. NOX4 has been reported to be involved in several diseases, including cancer [7,8]. For example, overexpression of NOX4 promotes cancer progression in colorectal cancer and is associated with a poor prognosis [9]. Additionally, NOX4 promotes lung cancer progression through regulation of glycolysis [10]. NOX4 is involved in the induction of epithelial-mesenchymal transition in breast cancer cells [11]. Furthermore, NOX4 can induce renal tumorigenesis by accumulating hypoxia-inducible factor 2a (HIF2a) in the nucleus [12]. Currently, there is no evidence demonstrating that NOX4 expression is correlated with gastric cancer progression. In this study, we detected and compared NOX4 expression in gastric cancer tissues and paired adjacent normal tissues from 90 patients using immunohistochemistry (IHC) analysis. We also investigated the correlation between NOX4 expression and clinicopathological characteristics and explored the value of NOX4 expression in predicting prognosis. What’s more, we investigated the effect of NOX4-knockdown on biological characteristics of gastric cancer cells. Furtherly, we explore the in vivo effect of NOX4 on tumor progression.
Materials and methods
Clinical tissue samples
This study was approved by the ethics committee of Nanjing Drum Tower Hospital, and all patients provided written informed consent. All gastric cancer tissues and paired adjacent normal tissues were obtained from patients who underwent surgery at the Nanjing Drum Tower Hospital (Nanjing, Jiangsu, China) beginning in 2016. None of the patients received chemotherapy, radiotherapy or any other anti-tumor therapy before surgery. None of the patients had any other cancer diagnosis beyond gastric cancer.
IHC staining
Formalin-fixed and paraffin-embedded samples were cut into sections then de-paraffinized and rehydrated. The primary antibodies used were monoclonal antibodies against NOX4 (1:200 dilution; Abcam, USA). All sections were heated at 98°C in 10 mM citrate buffer for 25 min and then cooled to room temperature for antigen retrieval. Sections were incubated in 0.3% H2O2 for 25 min for the inactivation of endogenous peroxidases. After blocking with 5% goat serum dissolved in phosphate buffered saline for 30-60 min, the sections were incubated with primary antibody against NOX4 overnight at 4°C, then stained with diaminobenzidine solution and counter-stained with hematoxylin. Two senior pathologists independently evaluated and recorded the IHC results in a blinded manner. Scoring was comprehensively conducted according to the percentage of positively stained cells (0 = 0%-5% of cells, 1 = 6%-25% of cells, 2 = 26%-50% of cells, 3 = 51-75% of cells and 4 = 76%-100% of cells) and staining intensity (0 = no staining, 1 = weak staining, 2 = moderate staining and 3 = strong staining). The final NOX4 score was calculated as the product of both grades.
Cell culture
The human normal gastric epithelial cell line GES, GC cell line MKN-45 and AGS were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were all maintained in RPMI 1640 medium (Gibco, Waltham, MA, USA) containing 10% FBS, penicillin (100 μg/mL) and streptomycin (100 μg/mL). All cells were incubated at 37°C in a humidified incubator with 5% CO2. Medium was renewed every 3 days and cells were passaged upon reaching 70-90% confluence.
siRNA transfection assay
Gastric cancer cells were transfected with 100 nM normal control siRNA or NOX4-siRNA from Shanghai Genomeditech Co., Ltd (Shanghai, China) using Lipofectamine 3000 reagent (Thermo Fisher Scientific, Waltham, USA) according to the manufacturer’s instructions. The knockdown efficiency was examined by RT-qPCR and Western blot. Gastric cancer cells were collected after 48 h post-transfection for qRT-PCR, Western blot and detection of ROS.
Lentiviral transduction
The control lentiviral vector encoding scrambled shRNA and a lentiviral vector containing gene-specific shRNAs against NOX4 were purchased from Shanghai Genomeditech (Shanghai, China). Human GC cells were transduced with the lentiviral particles along with polybrene and were selected by puromycin (2 μg/μL) (Thermo Fisher Scientific, Waltham, USA) for 10 days. The knockdown efficiency was verified by Western blot.
Cell proliferation
For edu assays, all performances were according to manufacturer’s instructions. Edu kit (KGA331-100) were purchased from Key-GENE BioTECH (Nanjing, China).
Cells were seeded in 96-well plates, 5×103 per well and cultured for 48 h. Then, cells were incubated in 50 μM EDU solutions for 8-10 h at 37°C and cells were fixed using 4% paraformaldehyde at 37°C for 30 min. After washed, cells were permeabilized using PBS containing 0.5% Triton X-100 for 15 min. Then cells were incubated with Apollo staining solution for 20 min. At last, the cells were washed using NaCl/Pi for 3 times, and then incubated with DAPI (KeyGEN BioTECH, Nanjing, Jiangsu, China).
Cell invasion assay
The invasive ability of GC cells was evaluated by 24-well transwell system (8-μm pore; BD Biosciences). GC cells were transfected with siRNA-control or siRNA-NOX4. Transwell chambers with a polycarbonate membrane insert were pre-covered with 0.2% Matrigel. The lower chamber was filled with 600 μL RPMI 1640 medium contained 5% FBS as a chemoattractant. A 200 μL suspension of GC cells (5×104 cells) were seeded in the upper chamber and incubated at 37°C in 5% CO2 for 24 h. Non-migrating cells were gently removed with cotton swabs. Next, the wells were fixed in methanol for 20 min and stained with crystal violet (KeyGEN BioTECH, Nanjing, Jiangsu, China) for 30 min. The number of invaded cells was counted in five different fields with a microscope.
Colony-forming assay
The human GC cells were plated at a low density (5000 cells/100-mm plate) and incubated at 37°C in 5% CO2 for 10 days until cells in control-well formed colonies with suitable size (≥ 50 cells per colony). Remove medium and wash cells using PBS, followed by fixation using 4% paraformaldehyde at 37°C for 10 min. Then cells were stained with 0.5% crystal violet (KeyGEN BioTECH, Nanjing, Jiangsu, China) at 37°C for 15 min. Remove crystal violet and wash the wells using PBS for several times. The morphology of colonies was analyzed after air-dry. The results were showed as the number of scattered or compact colonies.
Detection of ROS
For measurements of intracellular ROS, gastric cancer cell lines and normal gastric epithelial cell line were incubated with 30 mM of H2DCFDA (Sigma, St. Louis, USA) for 20-30 min at 37°C, collected, and analyzed by FCM on a flow cytometer (Becton Dickinson Bioscience, CA, USA).
Western blot analysis
Cells were lysed in lysis buffer on ice and lysates were centrifuged by centrifugation (10 min, 12000×g, 4°C). Then, total protein was resolved by 8%-12% SDS-PAGE and samples were electro-transferred onto an Amersham Hybond™-P (PVDF) membrane (GE Healthcare, Pittsburgh, USA) in a transfer buffer. PVDF membrane was blocked with 5% skimmed milk at 37°C for 2 h and target bands were incubated at 4°C overnight with anti-NOX4 (1:2000, Abcam), anti-MMP-2 (1:1000, Abcam), anti-MMP-7 (1:2000, Abcam), anti-MMP-9 (1:1000, CST), anti-MMP-13 (1:2000, Abcam), anti-Nanog (1:1000, Abcam), anti-Bmi-1 (1:10000, Abcam), anti-Notch3 (1:1000, Abcam), anti-Vimentin (1:2000, Abcam), anti-ZO-1 (1:1000, Abcam), anti-N-Cadherin (1:1000, Abcam), anti-E-Cadherin (1:1000, Santa Cruz) and anti-GAPDH (1:2000, Abcam) antibodies, respectively. Then, the bands were incubated with HRP conjugated goat anti-rabbit IgG (Sigma, A6154) at 37°C for 2 h. The Bands were visualized using the Amersham™ ECL Detection System (GE Healthcare, Pittsburgh, USA).
Reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR)
Cells were harvested and total RNA was extracted using the RNA extraction kit (Tiangen, Beijing, China) following the manufacturer’s instructions. cDNA was synthesized using the Reverse Transcriptase System (Takara, Kyoto, Japan). All the primers were designed and synthesized by Genomeditech (Shanghai, China). The sequences were NOX4 forward, AGGAGAACCAGGAGATTGTTG and reverse, GGGATGACTTATGACCGAAAT; GAPDH forward, AGCCACATCGCTCAGACAC and reverse, GCCCAATACGACCAAATC. Next, the target cDNAs were amplified via quantitative real-time RCR with SYBR on a RT-PCR instrument (ABI 7500). Cycling conditions were as follow: 95°C for 30 s (1 cycle); 95°C for 10 s, 60°C for 30 s (40 cycles); 95°C for 15 s, 60°C for 60 s, and 95°C for 15 s (1 cycle). Human GAPDH served as the internal standard. Relative mRNA expression was calculated using the 2-ΔΔCt method.
In vivo model
Human GC cells MKN-45 and MKN-45 with NOX4 knockdown (dissolved in PBS, 5×106/100 μl, 100 μM/per nude mouse) were injected subcutaneously in the right flank of BALB/c nude mice. 12 tumor-bearing mice were divided into two groups (control group, NOX4-KD group). Seven days later, Tumor volumes (length × width2/2) were measured every three days. On 28th day, Mice were sacrificed and tumors were harvested. The animal care and experimental protocols were carried out according to the guidelines established by National Institutes of Health. The protocol was approved by the Animal Use and Care Committee of the Nanjing Drum Tower Hospital.
In silico analysis
To investigate the relationship between NOX4 expression and survival rate in gastric cancer, we generated Kaplan-Meier plots (https://http://kmplot.com/analysis/).
Statistical analysis
Data was analyzed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 7 (San Diego, CA, USA) software. A paired t-test was used to compare NOX4 expression in gastric cancer tissue and the paired adjacent normal tissue. The chi squared test, chi squared test with continuity correction and Fisher’s exact probability method were used to analyze the connection between NOX4 expression and clinicopathological characteristics in the 90 patients with gastric cancer. All tests were two-tailed, and the differences were statistically significant when P < 0.05.
Results
Baseline patient characteristics
Detailed clinicopathological characteristics of the 90 gastric cancer patients are presented in Table 1. The median age at surgery was 57.8 years (range: 39-82 years), and the ratio of male to female was 2.75:1. A total of 12 cases were well differentiated, 54 were moderately differentiated, and 24 were poorly differentiated. On the basis of the seventh edition of the AJCC staging criteria, the number of phase I, II, III and IV cases were 18, 21, 36 and 15 respectively. According to the TNM staging criteria, 56 cases exhibited N2 or N3 lymph node metastasis, while 34 cases exhibited N0 or N1 lymph node metastasis. Three cases exhibited distant metastasis, while the remaining 87 cases did not.
Table 1.
Summary of NOX4 expression and clinicopathologic features of gastric cancer patients
| Variable | Cases (n) | NOX4 expression | p value | |
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Age | ||||
| ≤ 60 | 28 | 12 | 16 | 0.4128 |
| > 60 | 62 | 21 | 41 | |
| Gender | ||||
| Male | 66 | 24 | 42 | 0.9212 |
| Female | 24 | 9 | 15 | |
| Tumor size (cm) | ||||
| < 3.5 | 36 | 18 | 18 | 0.0321* |
| ≥ 3.5 | 54 | 15 | 39 | |
| AJCC Stage | ||||
| I + II | 39 | 17 | 22 | 0.2333 |
| III + IV | 51 | 16 | 35 | |
| Depth of invasion | ||||
| Mucosa/Submucosa | 15 | 7 | 8 | 0.3786 |
| Muscularis/Serosa | 75 | 26 | 49 | |
| T Stage | ||||
| T1 + T2 | 28 | 12 | 16 | 0.306 |
| T3 + T4 | 62 | 19 | 41 | |
| N Stage | ||||
| N0 + N1 | 34 | 18 | 16 | 0.0125* |
| N2 + N3 | 56 | 15 | 41 | |
| M Stage | ||||
| Negative | 87 | 33 | 54 | 0.2955 |
| Positive | 3 | 0 | 3 | |
| Venous invasion | ||||
| Absence | 33 | 19 | 14 | 0.0017** |
| Presence | 57 | 14 | 43 | |
| Nerve invasion | ||||
| Absence | 31 | 14 | 17 | 0.2254 |
| Presence | 59 | 19 | 40 | |
| Lauren Classification | ||||
| Intestinal | 36 | 16 | 20 | 0.8445 |
| Diffuse | 32 | 12 | 20 | |
| Mixed | 12 | 5 | 7 | |
| Histologic grade | ||||
| WD | 12 | 5 | 7 | 0.9106 |
| MD | 54 | 19 | 35 | |
| PD | 24 | 9 | 15 | |
P < 0.05;
P < 0.01.
NOX4 expression in gastric cancer tissues and normal peritumoral tissues
We detected and graded NOX4 expression in gastric cancer tissues and paired normal peritumoral tissues. Representative IHC staining of two groups is shown in Figure 1A and 1B. High NOX4 expression was detected in gastric cancer tissues in 63.3% (57/90) of cases and in normal peritumoral tissues in only 23.3% (21/90) of cases. Compared with paired normal peritumoral tissues, 71.1% (64/90) of gastric cancer cases had higher NOX4 expression scoring, 13.3% (12/90) of cases had equal NOX4 expression scoring, and 15.5% cases had lower NOX4 expression scoring (Table 1). Therefore, NOX4 expression in gastric cancer tissues was higher than in paired normal peritumoral tissues (P < 0.001; Figure 1C).
Figure 1.
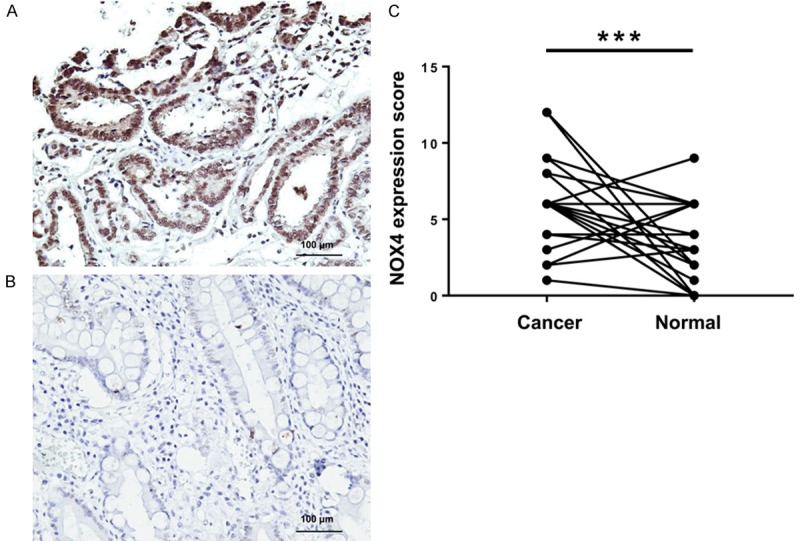
NOX4 expression in gastric cancer tissues and normal peritumoral tissues. A. Representative expression of NOX4 in gastric cancer tissues (magnification ×200). B. Representative expression of NOX4 in adjacent normal tissues (magnification ×200). C. Comparison of NOX4 expression in gastric cancer tissues and paired adjacent normal tissues. ***P < 0.001.
Association of clinicopathological characteristics and NOX4 expression in gastric cancer
NOX4 expression levels were associated with tumor size, lymph node status and nerve invasion (P < 0.05; Table 1). No significant association between NOX4 expression levels and other clinicopathological characteristics was demonstrated (P > 0.05; Table 1). The percentage of samples with high NOX4 expression was increased in patients with N2 or N3 lymph node metastasis (73.2%, 41/56) compared to those with N0 or N1 lymph node metastasis (47.1%, 16/34; P < 0.05). The percentage of tissue samples showing high NOX4 expression was also increased in patients with venous invasion (75.4%, 43/57) compared to those without (42.42%, 14/33; P < 0.01) and in patients with large tumor sizes (72.2%, 39/54) compared with those with small tumors (50%, 18/36; P < 0.05). Although not significant, high NOX4 expression was also observed patients with advanced AJCC stages (68.6%, 35/51), advanced T stages (66.1%, 41/62), distant metastasis (100%, 3/3), nerve invasion (67.8%, 40/59), and deeper invasion (65.3%, 49/75) compared with patients with primary AJCC stages (56.4%, 22/39; P > 0.05), primary T stages (57.1%, 16/28; P > 0.05), no distant metastasis (62.1%, 54/87; P > 0.05), no nerve invasion (54.8%, 17/31; P > 0.05), and shallower invasion (53.3%, 8/15; P > 0.05), respectively.
In silico analysis of survival
To evaluate the association between NOX4 expression levels and prognosis in human gastric cancer, we performed survival analysis using Kaplan-Meier curves. The total number of gastric cancer patients included in the analysis was 876. The median overall survival time in high NOX4 expressing patients (n = 401, 23.2 months; Table 2) was significantly decreased as compared to low NOX4 expressing patients (n = 475, 39.7 months; Table 2). Overall survival was significantly decreased in high NOX4 expressing patients compared with low NOX4 expression cases (P = 0.000; Figure 2A). Analysis of 641 gastric cancer patients for progression-free survival showed significantly decreased median progression-free survival in high NOX4 expressing patients (n = 466, 14.5 months; Table 2) compared with low NOX4 expressing patients (n = 175, 93.2 months; Table 2). Progression-free survival was also significantly decreased in high NOX4 expressing patients compared with low NOX4 expression cases (P = 0; Figure 2B). Overall, high NOX4 expression was positively correlated with a poor prognosis in gastric cancer.
Table 2.
Summary of overall survival and progression-free survival analyses
| Analysis category | NOX4 Expression | Median survival (months) | p value | HR | 95% CI for HR |
|---|---|---|---|---|---|
| Overall survival | High (n = 401) | 23.2 | 0.000 | 1.59 | 1.35-1.89 |
| Low (n = 475) | 39.7 | ||||
| Progression-free survival | High (n = 466) | 14.5 | 0.000 | 1.98 | 1.54-2.55 |
CI, confidence interval; HR, hazard ratio.
Figure 2.
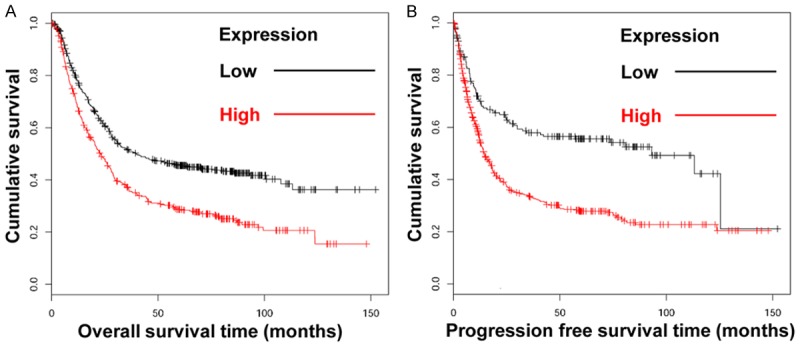
In silico analysis of overall survival and progression-free survival. A. Kaplan-Meier analysis of the overall survival rate of patients expressing NOX4 (P = 0.000). B. Kaplan-Meier analysis of the progression-free survival rate of patients expressing NOX4 (P = 0.000).
NOX4 promotes the invasion and proliferation of gastric cancer cells
To investigate the role that NOX4 played in the regulation of biological characteristics of gastric cancer cells, NOX4 was depleted by siRNA transfection. The knockdown efficiency was verified at the levels of transcription and translation (Figure 3A, 3B). Knockdown of NOX4 significantly inhibited the invasion of gastric cancer cells (Figure 3C, 3D). What’s more, Knockdown of NOX4 effectively reduce the percentage of EDU positive gastric cancer cells (Figure 3E, 3F) and obviously suppress the colony formation of gastric cancer cells (Figure 3G), indicating that NOX4 could promote proliferation of gastric cancer cells. In conclusion, NOX4 promotes the invasion and proliferation of gastric cancer cells.
Figure 3.
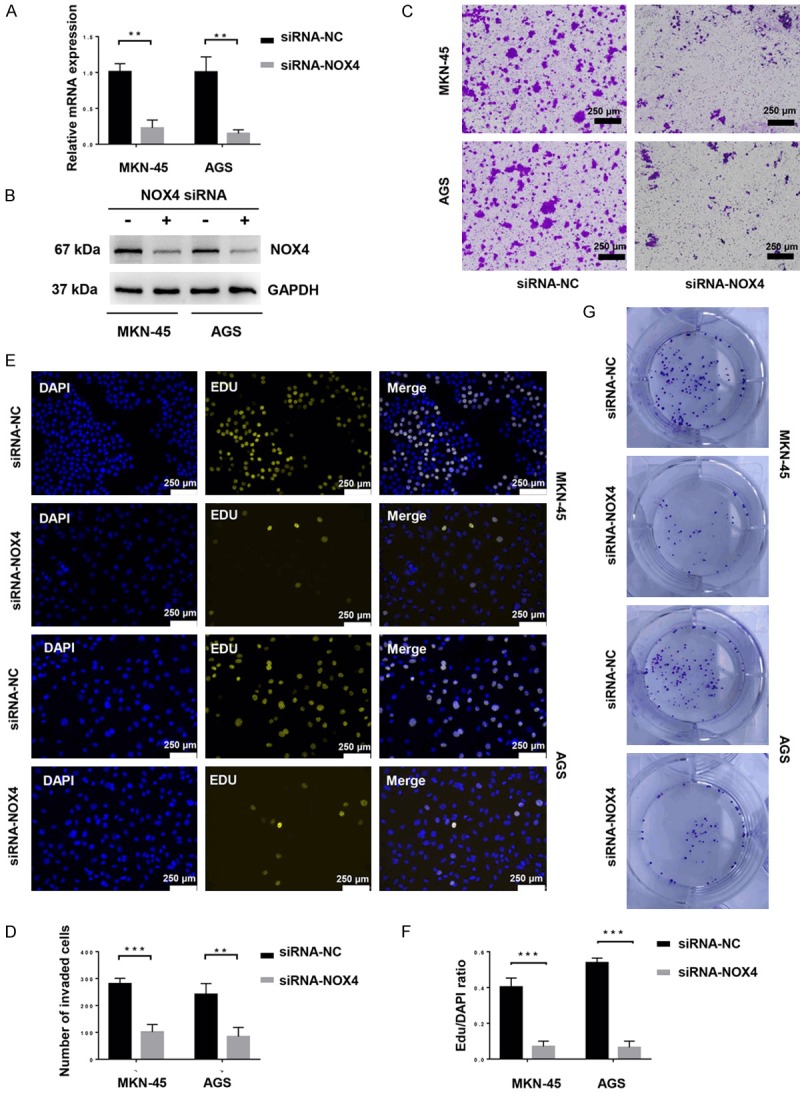
NOX4 promotes the invasion and proliferation of gastric cancer cells. MKN-45 and AGS cells were transfected with siRNA-Negative Control or siRNA-NOX4 for 24 h. A. Relative mRNA expression of NOX4 were measured by RT-qPCR. **P < 0.01. B. The expression of NOX4 was detected by western blot with the indicated antibodies. C. Invasion of gastric cancer cells and gastric cancer cells with NOX4 knockdown were assessed by a Matrigel invasion assay system (magnification ×100). D. The average number of invaded cells in each field was measured. **P < 0.01, ***P < 0.001. E. Proliferation of gastric cancer cells and gastric cancer cells with NOX4 knockdown were assessed by EDU incorporation (magnification ×100). F. The average EDU/DAPI ratio of cells in each field was measured. ***P < 0.001. G. Colony-formation of gastric cancer cells and gastric cancer cells with NOX4 knockdown were assessed by colony-forming assay. All experiments were performed three independent times.
Knockdown of NOX4 inhibits ROS generation, epithelial mesenchymal transition and MMP7 expression of gastric cancer cells
In cancer, reactive oxygen species (ROS) lead to genomic instability and trigger several signaling cascades involved in proliferation, neovascularization, migration, invasion and extravasation into distant sites [4-6]. And NADPH oxidase 4 (NOX4) is one of the main sources of ROS [7]. ROS levels in gastric cancer cells increased compared with that in the normal gastric epithelial cell line, GES-1 (Figure 4A). What’s more, knockdown of NOX4 significantly decreased ROS generation of gastric cancer cells (Figure 4B).
Figure 4.
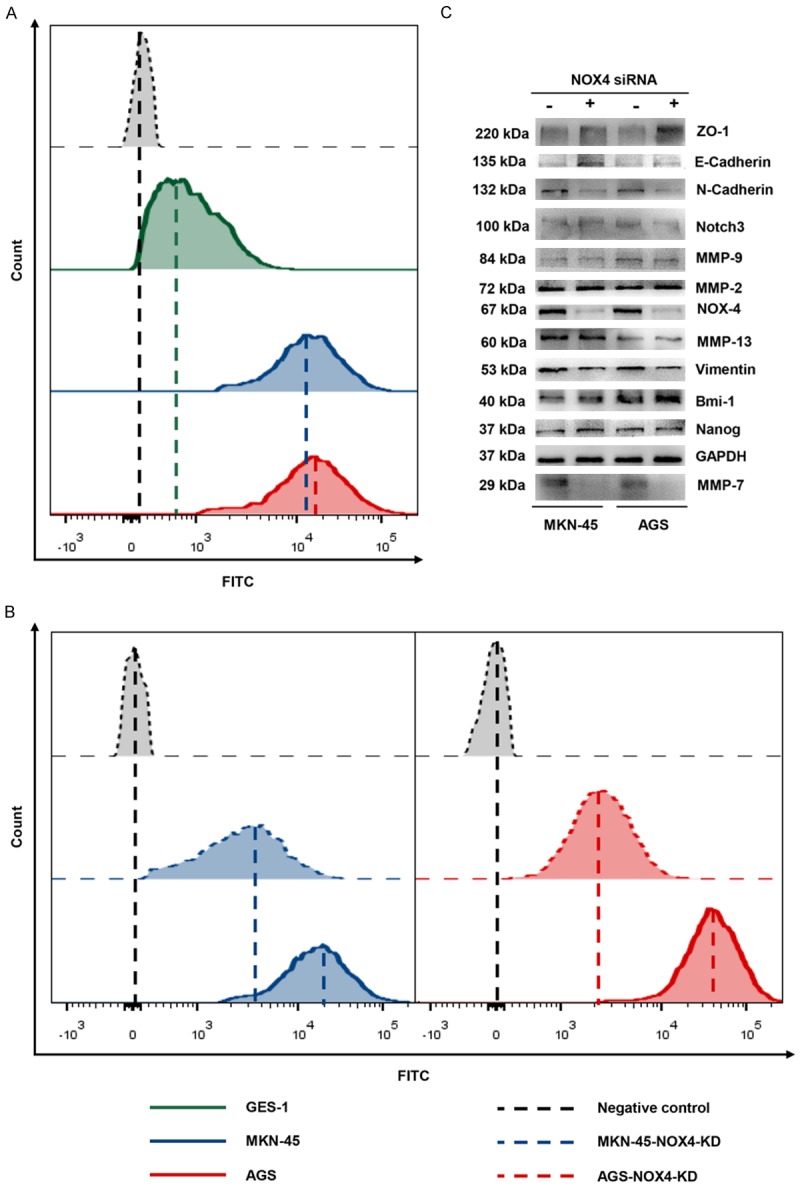
Knockdown of NOX4 inhibits ROS generation, epithelial mesenchymal transition and MMP7 expression of gastric cancer cells. MKN-45 and AGS cells were transfected with siRNA-Negative Control or siRNA-NOX4 for 24 h. A. ROS generation by normal gastric epithelial cell line, GES-1, gastric cancer cell line, MKN-45 and AGS cells as measured by flow cytometry using H2DCFDA. B. ROS generation by gastric cancer cells and gastric cancer cells with NOX4 knockdown as measured by flow cytometry using H2DCFDA. C. The expression of NOX4, MMP-2, MMP-7, MMP-9, MMP-13, Nanog, Bmi-1, Notch3, Vimentin, ZO-1, N-Cadherin and E-Cadherin in GC cells and GC cells with NOX4 knockdown was measured by immunoblotting with the indicated antibodies. GAPDH served as a loading control.
Moreover, It was demonstrated that epithelial mesenchymal transition (EMT), enhanced stemness and overexpression of matrix metalloproteinase (MMP) are associated with various tumor functions, such as tumor initiation, tumor cell migration and metastasis [13-15]. To explore the mechanism behind which NOX4 promotes the invasion and proliferation of gastric cancer cells, we detect expression of EMT phenotypes, stemness associated protein and MMPs in ordinary GC cells and GC cells with NOX4 knockdown. The knockdown efficiency was verified at the levels of transcription and translation (Figure 4C). Knockdown of NOX4 had little effects on the stemness associated protein levels including Bmi-1, Notch3 and Nanog as well as MMP2/9/13 expression (Figure 4C). However, Knockdown of NOX4 could decrease MMP7 expression, which is involved in malignant biological characteristics of several cancers [16-18] (Figure 4A). Intriguingly, knockdown of NOX4 enhanced epithelial phenotype associated protein expression including ZO-1 and E-cadherin, but decreased mesenchymal phenotype-associated protein expression, indicating that knockdown of NOX4 inhibited epithelial mesenchymal transition (Figure 4C). Hence, NOX4 might be involed in the EMT of gastric cancer cells, which is related to carcinogenic and metastatic features of cancer [19].
NOX4 promotes progression of gastric cancer cells in vivo
To investigate the in vivo effect of NOX4 on tumor progression, a xenograft model was established. MKN-45 cells were infected with lentivirus expressing shRNA-NC or shRNA-NOX4. The infection efficiency was validated by fluorescence (Figure 5A). The Efficiency of knockdown was verified at the level of translation (Figure 5B). A total of 5×106 MKN-45 or MKN-45NOX4-KD cells were subcutaneously injected in the BALB/C nude mice. Volumes of tumor were measured every three days. 28 days after subcutaneous injection, all mice were sacrificed and the volumes and weight of tumors were measured. The volume, weight and growth rate of tumors in the MKN-45NOX4-KD group were much lower than the MKN-45 group (Figure 5C-E). In conclusion, NOX4 promotes progression of gastric cancer cells in vivo.
Figure 5.
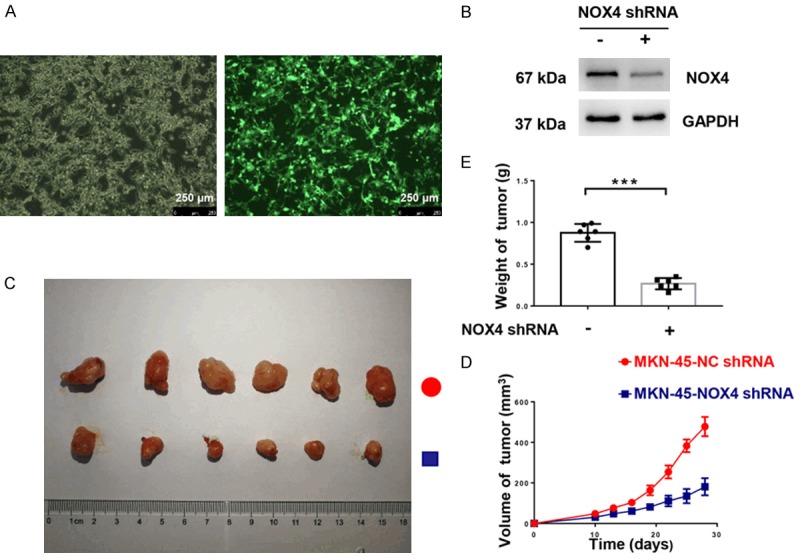
NOX4 promotes progression of gastric cancer cells in vivo. Human GC cells MKN-45 and MKN-45 with NOX4 knockdown (dissolved in PBS, 5×106/100 μl, 100 μM/per nude mouse) were injected subcutaneously in the right flank of BALB/c nude mice. A. Verifying of infection efficiency by fluorescence (magnification ×100). B. Validation of knockdown efficiency of shRNA-NOX4 using Western blot. C. Image of tumors of mice in group-MKN-45-NC shRNA and group-MKN-45-NOX4 shRNA. D. Volume of tumor of mice in group-MKN-45-NC shRNA and group-MKN-45-NOX4 shRNA at indicated time. E. Weight of tumor of mice in group-MKN-45-NC shRNA and group-MKN-45-NOX4 shRNA on 28th days. ***P < 0.001.
Discussion
Tumor progression is a complicated process, involving multiple factors and steps. ROS play a crucial role in cancer cell proliferation, resistance to anoikis, neovascularization, migration, invasion, and extravasation to distant sites [20,21]. As one of the main sources of ROS, NOX4 is overexpressed in various malignant tumors, promoting the malignant behavior of tumor cells through various pathways and mechanisms [22,23]. Currently, there is little evidence indicating a correlation between NOX4 expression and gastric cancer. Our study found that NOX4 expression in gastric cancer tissues is clearly higher than in paired adjacent normal tissues and is significantly correlated with lymph node metastasis, tumor size, venous invasion and poor survival. NOX4 expression is, therefore, a promising prognostic biomarker for gastric cancer.
NOX4 is a member of the NOX family, whose only validated function is generating ROS [7]. NOX family-derived ROS can triggered oxidative stress, leading to tumor initiation and progression [24]. Unlike other NOX members, NOX4 interacts with p22phox without the assistance of other proteins; therefore, it is constitutively active and is regulated at the transcriptional level [25-27]. NOX4-derived ROS can induce mitochondrial DNA damage, mitochondrial dysfunction and genomic instability, potentially leading to tumorigenesis [28,29]. Moreover, NOX4-derived-ROS induced by Bcr-Abl promote the transduction of survival signals through inhibiting PP1 in chronic myeloid leukemia [30]. In renal cell carcinoma, NOX4-mediated production of interleukin-6 and -8 is related to inflammation-induced metastasis [31]. P53-induced NOX4 activation promotes cell migration and epithelial-mesenchymal transition in lung epithelial cells [11,32]. NOX4-derived ROS decrease the generation of monocyte chemoattractant protein-1, which is important for tumor formation [33]. Furthermore, in ovarian cancer cells, NOX4-derived ROS are essential for regulation of VEGF and tumor-induced angiogenesis through HIF-1α induction [34]. In this study, NOX4 is involved in regulation of malignant biological characteristics of gastric cancer cells. NOX4 could significantly promote the proliferation and invasion of gastric cancer cells. Also, ROS levels in gastric cancer cells increased compared with that in the normal gastric epithelial cell line, GES-1 and knockdown of NOX4 significantly decreased ROS generation of gastric cancer cells. What’s more, NOX4 is associated with EMT and MMP7 expression which are involved in carcinogenesis and metastasis of gastric cancer [17,35]. Interestingly, ROS derived by NOX could mediate epithelial-mesenchymal transition and metastasis of hepatoma cells [36]. ROS that derived by NOX could induce epithelial-to-mesenchymal transition in glioma [37]. Also, TGF-β-SMAD3-induced NOX4 activation promotes epithelial-mesenchymal transition in breast epithelial cells [11]. Moreover, it was demonstrated that oxidative stress could induce MMP7 expression [38,39]. H2O2 could promote SW620 cell invasion via JNK/C-Jun and ERK/C-Fos activation-mediated MMP-7 expression [38]. ROS-ERK pathway could regulate MMP7 and 9 and uPA levels in colon epithelial cells [39]. Based on these findings and our current study, we conjecture that NOX4 is a key factor that generates ROS involved in the malignant biology of gastric cancer cells. What’s more, NOX4 was highly expressed in gastric cancer tissues compared with paired adjacent normal tissues and high NOX4 expression in gastric cancer tissues was correlated with tumor size, lymphatic metastasis, vascular invasion and a poor prognosis. Interestingly, although not statistically significant, all patients with distant metastasis displayed high NOX4 expression. Survival analysis showed that high NOX4 expression was associated with a poor prognosis. In vivo, NOX4 depletion dramatically inhibit gastric cancer progression. Together, these results indicate that NOX4 may be a crucial regulatory factor in gastric cancer and may be closely associated with the occurrence and development of gastric cancer. NOX4 may become a promising prognostic biomarker for gastric cancer. Targeting of NOX4 activity is a promising strategy for gastric cancer therapy to prolong the survival of gastric cancer patients.
Acknowledgements
This work was supported by This work was sponsored by the National Science Foundation for Young Scholars of China [Grant number 81501380], the National Science Foundation of China [Grant number 81372364], The key research plan and social development project of Jiangsu Province, China [Grant number BE2016603], the National ministry of science and technology projects [Grant number 2016YFC0104105] and the Natural Science Foundation for Young Scholars of Jiangsu Province, China [Grant number BK20150110].
Disclosure of conflict of interest
None.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Cidon EU, Ellis SG, Inam Y, Adeleke S, Zarif S, Geldart T. Molecular targeted agents for gastric cancer: a step forward towards personalized therapy. Cancers (Basel) 2013;5:64–91. doi: 10.3390/cancers5010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steeg PS. Metastasis suppressors alter the signal transduction of cancer cells. Nat Rev Cancer. 2003;3:55–63. doi: 10.1038/nrc967. [DOI] [PubMed] [Google Scholar]
- 4.Peiris-Pagès M, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Metastasis and oxidative stress: are antioxidants a metabolic driver of progression? Cell Metab. 2015;22:956–958. doi: 10.1016/j.cmet.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Schumacker PT. Reactive oxygen species in cancer: a dance with the devil. Cancer Cell. 2015;27:156–157. doi: 10.1016/j.ccell.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med. 2004;37:768–784. doi: 10.1016/j.freeradbiomed.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 8.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin XL, Yang L, Fu SW, Lin WF, Gao YJ, Chen HY, Ge ZZ. Overexpression of NOX4 predicts poor prognosis and promotes tumor progression in human colorectal cancer. Oncotarget. 2017;8:33586–33600. doi: 10.18632/oncotarget.16829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng C, Wu Q, Wang J, Yao B, Ma L, Yang Z, Li J, Liu B. NOX4 supports glycolysis and promotes glutamine metabolism in non-small cell lung cancer cells. Free Radic Biol Med. 2016;101:236–248. doi: 10.1016/j.freeradbiomed.2016.10.500. [DOI] [PubMed] [Google Scholar]
- 11.Boudreau HE, Casterline BW, Rada B, Korzeniowska A, Leto TL. Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radic Biol Med. 2012;53:1489–1499. doi: 10.1016/j.freeradbiomed.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregg JL, Turner RM 2nd, Chang G, Joshi D, Zhan Y, Chen L, Maranchie JK. NADPH oxidase NOX4 supports renal tumorigenesis by promoting the expression and nuclear accumulation of HIF2alpha. Cancer Res. 2014;74:3501–3511. doi: 10.1158/0008-5472.CAN-13-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 14.Saygin C, Matei D, Majeti R, Reizes O, Lathia JD. Targeting cancer stemness in the clinic: from hype to hope. Cell Stem Cell. 2019;24:25–40. doi: 10.1016/j.stem.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Vandenbroucke RE, Libert C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat Rev Drug Discov. 2014;13:904–927. doi: 10.1038/nrd4390. [DOI] [PubMed] [Google Scholar]
- 16.Resovi A, Bani MR, Porcu L, Anastasia A, Minoli L, Allavena P, Cappello P, Novelli F, Scarpa A, Morandi E, Falanga A, Torri V, Taraboletti G, Belotti D, Giavazzi R. Soluble stroma-related biomarkers of pancreatic cancer. EMBO Mol Med. 2018;10:771–779. doi: 10.15252/emmm.201708741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin Y, Grabowska AM, Clarke PA, Whelband E, Robinson K, Argent RH, Tobias A, Kumari R, Atherton JC, Watson SA. Helicobacter pylori potentiates epithelial:mesenchymal transition in gastric cancer: links to soluble HB-EGF, gastrin and matrix metalloproteinase-7. Gut. 2010;59:1037–1045. doi: 10.1136/gut.2009.199794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherf DB, Dally H, Muller P, Werle-Schneider G, Jager B, Edler L, Tuengerthal S, Fischer JR, Drings P, Bartsch H, Risch A. Single nucleotide polymorphisms in matrix metalloproteinase genes and lung cancer chemotherapy response and prognosis. Eur Respir J. 2010;35:381–390. doi: 10.1183/09031936.00125608. [DOI] [PubMed] [Google Scholar]
- 19.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 20.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanley CJ, Mellone M, Ford K, Thirdborough SM, Mellows T, Frampton SJ, Smith DM, Harden E, Szyndralewiez C, Bullock M, Noble F, Moutasim KA, King EV, Vijayanand P, Mirnezami AH, Underwood TJ, Ottensmeier CH, Thomas GJ. Targeting the myofibroblastic cancer-associated fibroblast phenotype through inhibition of NOX4. J Natl Cancer Inst. 2018;110:234–245. doi: 10.1093/jnci/djx121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shanmugasundaram K, Nayak BK, Friedrichs WE, Kaushik D. NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance. Nat Commun. 2017;8:997. doi: 10.1038/s41467-017-01106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 25.Brandes RP, Weissmann N, Schroder K. Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic Biol Med. 2014;76:208–226. doi: 10.1016/j.freeradbiomed.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 26.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 27.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 28.Graham KA, Kulawiec M, Owens KM, Li X, Desouki MM, Chandra D, Singh KK. NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol Ther. 2010;10:223–231. doi: 10.4161/cbt.10.3.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naughton R, Quiney C, Turner SD, Cotter TG. Bcr-Abl-mediated redox regulation of the PI3K/AKT pathway. Leukemia. 2009;23:1432–1440. doi: 10.1038/leu.2009.49. [DOI] [PubMed] [Google Scholar]
- 31.Fitzgerald JP, Nayak B, Shanmugasundaram K, Friedrichs W, Sudarshan S, Eid AA, DeNapoli T, Parekh DJ, Gorin Y, Block K. Nox4 mediates renal cell carcinoma cell invasion through hypoxia-induced interleukin 6- and 8-production. PLoS One. 2012;7:e30712. doi: 10.1371/journal.pone.0030712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boudreau HE, Casterline BW, Burke DJ, Leto TL. Wild-type and mutant p53 differentially regulate NADPH oxidase 4 in TGF-beta-mediated migration of human lung and breast epithelial cells. Br J Cancer. 2014;110:2569–2582. doi: 10.1038/bjc.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordillo G, Fang H, Park H, Roy S. Nox-4-dependent nuclear H2O2 drives DNA oxidation resulting in 8-OHdG as urinary biomarker and hemangioendothelioma formation. Antioxid Redox Signal. 2010;12:933–943. doi: 10.1089/ars.2009.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 35.Li T, Huang H, Shi G, Zhao L, Li T, Zhang Z, Liu R, Hu Y, Liu H, Yu J, Li G. TGF-beta1-SOX9 axis-inducible COL10A1 promotes invasion and metastasis in gastric cancer via epithelial-to-mesenchymal transition. Cell Death Dis. 2018;9:849. doi: 10.1038/s41419-018-0877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong Y, Wu Z, He M, Chen Y, Chen Y, Shen X, Zhao X, Zhang L, Yuan B, Zeng Z. ADAM9 mediates the interleukin-6-induced epithelial-mesenchymal transition and metastasis through ROS production in hepatoma cells. Cancer Lett. 2018;421:1–14. doi: 10.1016/j.canlet.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Kesanakurti D, Maddirela D, Banasavadi-Siddegowda YK, Lai TH, Qamri Z, Jacob NK, Sampath D, Mohanam S, Kaur B, Puduvalli VK. A novel interaction of PAK4 with PPARgamma to regulate Nox1 and radiation-induced epithelial-to-mesenchymal transition in glioma. Oncogene. 2017;36:5309–5320. doi: 10.1038/onc.2016.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho BY, Wu YM, Chang KJ, Pan TM. Dimerumic acid inhibits SW620 cell invasion by attenuating H(2)O(2)-mediated MMP-7 expression via JNK/C-Jun and ERK/C-Fos activation in an AP-1-dependent manner. Int J Biol Sci. 2011;7:869–880. doi: 10.7150/ijbs.7.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steury MD, Lucas PC, McCabe LR, Parameswaran N. G-protein-coupled receptor kinase-2 is a critical regulator of TNFalpha signaling in colon epithelial cells. Biochem J. 2017;474:2301–2313. doi: 10.1042/BCJ20170093. [DOI] [PMC free article] [PubMed] [Google Scholar]


