Abstract
Cysteine-rich intestinal protein 1 (CRIP1) is overexpressed in colorectal cancer (CRC) tissues and functions as an oncogene in regulating the migration and invasion of CRC cells. However, the underlying mechanism is unclear. CRIP1 has a role in zinc absorption and functions as an intracellular zinc transport protein. Here, we aimed to focus on the function of zinc and its underlying mechanism in CRC and determine whether CRIP1 promotes invasion and CRC metastasis through excessive zinc-induced epithelial-mesenchymal transition (EMT) by affecting the phosphorylated glycogen synthase kinase (GSK)-3beta. The results showed that ZnSO4 (Zn2+) supplementation in medium increased the labile intracellular zinc content. Furthermore, excessive Zn2+ supplementation activated the GSK3/mTOR signaling pathway in both SW620 and LoVo cells, and excessive Zn2+ supplementation promoted migration, invasion, and EMT of SW620 and LoVo cells. This migration promotion was alleviated by the specific mTOR inhibitor rapamycin, indicating that the GSK3/mTOR signaling pathway was involved in this process. CRIP1 silencing increased the labile intracellular zinc content and inhibited EMT and GSK3/mTOR signaling pathway. CRIP1 silencing alleviated the zinc supplementation effects on migration, invasion, EMT, and GSK3/mTOR signaling pathway. In conclusion, excessive Zn2+ promotes migration and invasion capabilities of SW620 and LoVo cells through GSK3/mTOR signaling pathway-induced EMT.
Keywords: Excessive zinc, epithelial-mesenchymal transition, cysteine-rich intestinal protein 1, migration and invasion capabilities
Introduction
Colorectal cancer (CRC), that is, the development of cancer in the colon or rectum, is the second most commonly diagnosed cancer in women, the third most commonly diagnosed cancer in men, and the fourth most common cause of cancer death after lung, stomach, and liver cancers [1]. The morbidity and mortality rates are high in developing countries [2-4] and CRC greatly threatens human life and health. CRC cells have a strong ability to migrate and invade, leading to recurrence, which finally results in death of patients. Therefore, factors that may affect the invasion and CRC metastasis should be evaluated.
Cysteine-rich intestinal protein 1 (CRIP1), a member of the LIM/double zinc finger protein family, was overexpressed in CRC, and CRIP1 silencing suppressed migration and invasive capability of CRC cells [5]. In addition, CRIP1 was overexpressed in the CRC cell line E1, possessing increased motility and invasiveness as compared to the parental HCT-116 cells [6]. Moreover, abnormal expression of CRIP1 has been demonstrated in several tumor types, including thyroid carcinoma, breast, osteosarcoma, cervical, and prostate tumors [7-12]. These results indicate that CRIP1 may be a potential treatment target for malignant tumors, especially for the invasion and metastasis of cancer. As an intracellular zinc transport protein, CRIP1 is involved in zinc absorption [13-15]. Considering CRIP1’s important role in regulating CRC invasion, metastasis, and zinc absorption, we predicted that zinc might play a role in regulating CRC invasion and metastasis. This study aimed to demonstrate the predicted role of zinc in regulating CRC invasion and metastasis and determine its related underlying mechanism.
Zinc has been reported to induce an increase in phosphorylated (p) glycogen synthase kinase (GSK)-3beta level, which could be inhibited by rapamycin, a specific mechanistic target of rapamycin kinase (mTOR) inhibitor in SH-SY5Y neuroblastoma cells [16]. GSK-3beta can bind to and phosphorylate Snail at two consensus motifs to dually regulate the function of Snail, triggering the epithelial-mesenchymal transition (EMT) of epithelial cells by repressing the E-cadherin expression [17,18]. However, whether zinc can affect the EMT of tumor cells remains unclear. At present, we discuss the role of zinc and CRIP1 in regulating the EMT of CRC. In addition, we discuss whether CRIP1 promotes CRC invasion and metastasis through zinc-regulated EMT by affecting the phosphorylation of GSK-3beta.
Materials and methods
Cell culture
The human colon cancer cell lines SW620 and LoVo were purchased from America Type Culture Collection (Manassas, VA, USA) and cultured in the condition according to the instruction of supplier.
siRNA interference
The siRNA oligonucleotides for targeting the CRIP1 gene (siCRIP1, sense: 5’-GCAACAAGGAGGUGUACUUTT-3’) and negative control siRNA (siNC) were obtained from GenePharma (Shanghai, China). Cells were transfected with the 100 nM siCRIP1 or siNC using the LipofectamineTM 2000 (Promega, Madison, WI, USA).
ZnSO4 supplementation and experimental group
To detect the effect of ZnSO4 supplementation on the cell survival rate, SW620 and LoVo cells were seeded on a 96-well plate and treated with 0.5, 5, 10, 50, 100, or 200 µM ZnSO4 for 24 h. To determine the effects of ZnSO4 supplementation on the levels of p-mTOR and p-GSK-3beta, SW620 and LoVo cells were seeded on a 6-well plate and treated with 5, 10, or 50 µM ZnSO4 for 24 h, or with 5 µM ZnSO4 for 12, 24, or 48 h. To determine the effects of 50 µM ZnSO4 and/or 50 nM rapamycin on migration and invasion capabilities, SW620 and LoVo cells were seeded on a 6-well plate, treated with 50 µM ZnSO4, 50 µM ZnSO4 plus 50 nM rapamycin, or 50 nM rapamycin for 24 h, and harvested for Transwell assays and western blotting. To determine the effects of CRIP1 silencing on the level of p-mTOR and p-GSK-3beta, SW620 and LoVo cells were seeded on a 6-well plate and transfected with siCRIP1 and siNC for 48 h. To determine the effects of CRIP1 silencing on the function of ZnSO4, SW620 and LoVo cells were seeded on a 6-well plate, pre-transfected with siCRIP1 and siNC for 24 h, and subsequently treated with 5 µM ZnSO4 for 24 h.
Cell proliferation assay
After being treated with 0.5, 5, 10, 50, 100, or 200 µM ZnSO4 for 24 h, cell proliferation was monitored using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega) following the manufacturer’s protocol.
Labile intracellular zinc content
At the end of different treatment, labile intracellular zinc in SW620 and LoVo cells treated as described above was monitored using Zinquin ethyl ester (Abcam, Cambridge, MA, USA). Briefly, cells were harvested and washed three times with phosphate-buffered saline (PBS) to remove extracellular Zn prior to labelling with Zinquin. Then, cells were suspended in PBS and 5 × 106 cells/ml were seeded in black-bottom 96-well plates, incubated with 25-uM Zinquin at 37°C for 30 min. The fluorescence intensity was determined using a multiplate reader SuPerMax 3000FA (Shanghai Flash spectrum biological technology co. LTD) at 368 and 490 nm, respectively. The results in relative light units were obtained from raw data minus the reagent blank and expressed as the percentage of fluorescence-intensity controls.
Western blotting
The total protein isolation, protein concentration measurement, and western blot were carried out according the method described previously [5]. The primary antibodies, anti-CRIP1, anti-mTOR, anti-mTOR (phospho S2448), anti-GSK-3beta, anti-GSK-3beta (phospho Y216), were purchased from Abcam. The primary antibody of internal reference, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Transwell assay
After treated with different condition, transwell assays for cell migration or invasion were carried out according the method described previously [5]. Finally, the number of migrated or invasive cell was counted in five randomly selected fields under a phase contrast microscope (magnification: 200 ×; IX71, Olympus, Tokyo, Japan). Assays were performed in triplicate. The number of invasive or migrated cells was adjusted by dividing with the survival rate.
Statistical analysis
All results are presented as the mean ± standard deviation and were analyzed using the Statistical Package for the Social Science (SPSS) version 19.0 software (IBM SPSS, Armonk, NY, USA). Intergroup comparisons of more than two groups were conducted using one-way analysis of variance (ANOVA), followed by post hoc tests of the least significant difference for multiple pairwise comparisons. Intergroup comparisons of two groups were conducted using Student’s t-test. A P-value of < 0.05 was considered to indicate a statistically significant difference.
Results
Zn2+ supplementation increased the labile zinc levels in the CRC cells
The CRC cell lines SW620 and LoVo were treated with different concentrations of ZnSO4 (Zn2+) for 24 h, and the cell survival rate was calculated using cell proliferation assay. The results showed that 0.5 µM Zn2+ supplementation did not decrease the survival rate of both SW620 and LoVo cells (Figure 1A). The inhibition rate of 5-, 10-, and 50-µM Zn2+ on SW620 and LoVo cell proliferation was < 5% (Figure 1A). The cell survival rate was clearly inhibited after 100-, 125-, 150-, or 200-µM Zn2+ supplementation (Figure 1A). Although 50-µM Zn2+ can significantly inhibit the survival rate of SW620 and LoVo, the survival rate remains to be > 95%. Because we investigated the effects of Zn2+ supplementation on migration and invasion capabilities, 5-, 10-, and 50-µM Zn2+ concentrations that cannot obviously suppress cell proliferation were selected for the following assays.
Figure 1.
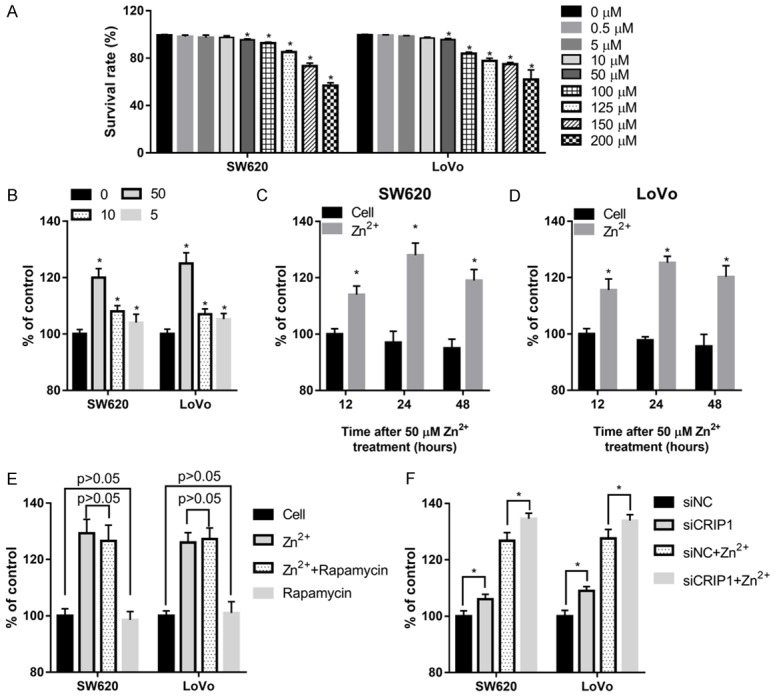
The effects of zinc supplementation in medium on the cell survival rate and labile zinc content in SW620 and LoVo cells. (A) The effects of different ZnSO4 (Zn2+) concentrations on the cell survival rate after being treated for 24 h, as calculated using cell proliferation assay. (B-D) Changes of the labile zinc content in SW620 and LoVo cells treated with 5-, 10-, and 50-µM Zn2+ for 24 h (B), or treated with 50-µM Zn2+ for 12, 24, and 48 h (C, D). (E, F) Changes of the labile zinc content in SW620 and LoVo cells treated with 50-µM Zn2+ and/or 50-nM rapamycin (E), or transfected with CRIP1 siRNA (siCRIP1) or negative control siRNA (siNC) with or without 50-µM Zn2+ supplemented in the medium (F). *P < 0.05, when compared to the 0-µM Zn2+ treated group or to the cell group at 12, 24, and 48 h.
After treating with 5-, 10-, and 50-µM Zn2+ supplementation in the medium for 24 h, the labile intracellular zinc content was increased in both the SW620 and LoVo cells, with 50-µM Zn2+ having the most obvious promotion effect (Figure 1B). Following this, SW620 and LoVo cells were treated with 50-µM Zn2+ supplementation in the medium for 12, 24, and 48 h. Our findings indicate that 50-µM Zn2+ supplementation can increase the labile intracellular zinc content from 12 to 48 h, reaching a peak at 24 h (Figure 1C and 1D). These results all indicate that zinc supplementation can increase the labile intracellular zinc content in CRC cells. The specific mTOR inhibitor rapamycin cannot affect the labile intracellular zinc content with or without 50-µM Zn2+ supplementation in the medium (Figure 1E). However, the labile intracellular zinc content was increased in siCRIP1 transfected cells with or without 50-µM Zn2+ supplementation in medium (Figure 1F).
Zinc supplementation activated the GSK3/mTOR signaling pathway in the CRC cells
After the treatment with 5-, 10-, and 50-µM Zn2+ supplementation in medium for 24 h, the phosphorylation levels of GSK-3beta and mTOR were increased in both the SW620 and LoVo cells, with 50-µM Zn2+ having the most obvious promotion effect (Figure 2A). Following this, SW620 and LoVo cells were treated with 50-µM Zn2+ supplementation in medium for 12, 24, and 48 h. Our findings indicate that 50-µM Zn2+ supplementation can increase the phosphorylation levels of GSK-3beta and mTOR in a time-dependent manner (Figure 2B and 2C). These results indicate that zinc supplementation can activate the GSK3/mTOR signaling pathway in the CRC cells.
Figure 2.
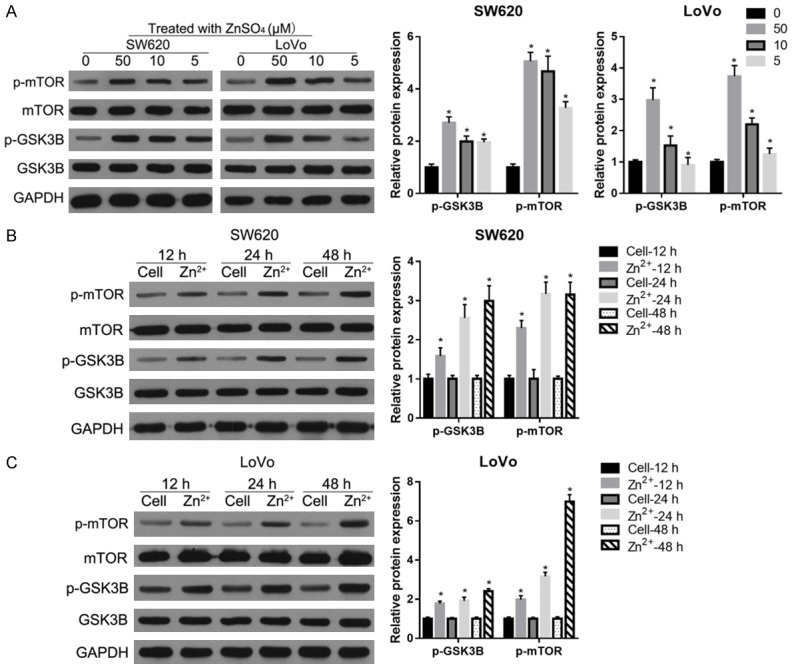
Zinc supplementation activated the GSK3/mTOR signaling pathway in the CRC cells. The phosphorylation levels of GSK-3beta (p-GSK3B) and mTOR (p-mTOR) after being treated with 5-, 10-, and 50-µM Zn2+ for 24 h (A) or with 50-µM Zn2+ for 12, 24, and 48 h (B, C). On the left are the representative graphs of the western blot. On the right are the statistical results of relative p-GSK3B and p-mTOR expression levels referenced to both GAPDH and total protein levels. *P < 0.05, when compared to the 0-µM Zn2+ treated group or to the cell group at 12 h, 24, and 48 h.
Zinc supplementation promotes migration, invasion, and EMT activation through the GSK3/mTOR signaling pathway
After being treated with 50-µM Zn2+ for 24 h, the migration and invasion capabilities of SW620 and LoVo cells were examined using the Transwell assays. The results showed that a 50-µM Zn2+ treatment could increase the number of migratory and invasive cells (Figure 3A). In addition, we found that a 50-µM Zn2+ supplementation significantly decreased the protein level of the epithelial marker, E-cadherin, and correspondingly increased the levels of mesenchymal marker, vimentin (Figure 3B). In contrast, the specific mTOR inhibitor rapamycin alleviated the effects of Zn2+ supplementation on the migration and invasion capabilities and EMT markers (Figure 3A and 3B). Compared to the cell group, rapamycin can suppress migration and invasion capabilities and the expression of EMT markers (Figure 3A and 3B). These results indicate that a 50-µM Zn2+ supplementation promotes migration, invasion, and EMT of SW620 and LoVo cells and that the GSK3/mTOR signaling pathway was involved in this process.
Figure 3.
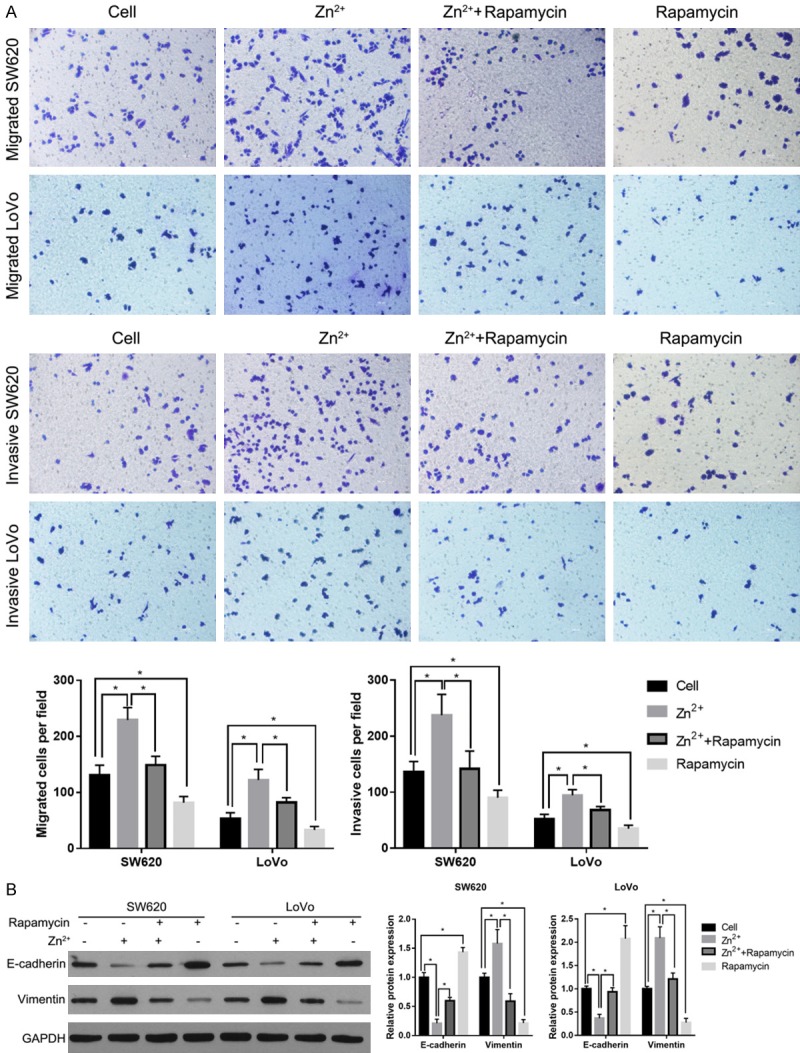
Zinc supplementary in medium promoted migration, invasion, and EMT, and the specific mTOR inhibitor rapamycin alleviated the effects of Zn2+ supplementation. A: The effects of 50-µM Zn2+ supplementation on the migration and invasion capabilities of SW620 and LoVo cells. B: The effects of 50-µM Zn2+ supplementation on the expression levels of EMT markers. The representative graphs are shown on the left. The statistical results are shown on the right. *P < 0.05.
CRIP1 silencing inhibits EMT and GSK3/mTOR signaling pathway
Our previous study indicated that CRIP1 silencing could effectively inhibit migration and invasion of CRC cells [5]. To further determine whether CRIP1 silencing suppressed invasion and CRC metastasis by inhibiting the EMT, we detected the expression levels of EMT markers and the phosphorylation levels of p-GSK-3beta and p-mTOR after transfection with siCRIP1 or siNC. Compared to the siNC transfection group, siCRIP1 transfection significantly increased the protein level of the epithelial marker, E-cadherin, and correspondingly decreased the levels of mesenchymal marker, vimentin (Figure 4A). In addition, the phosphorylation levels of GSK-3beta and mTOR decreased in both SW620 and LoVo cells after being transfected with siCRIP1 compared to those transfected with siNC (Figure 4B). These results all indicate that CRIP1 silencing inhibits EMT and GSK3/mTOR signaling pathway.
Figure 4.
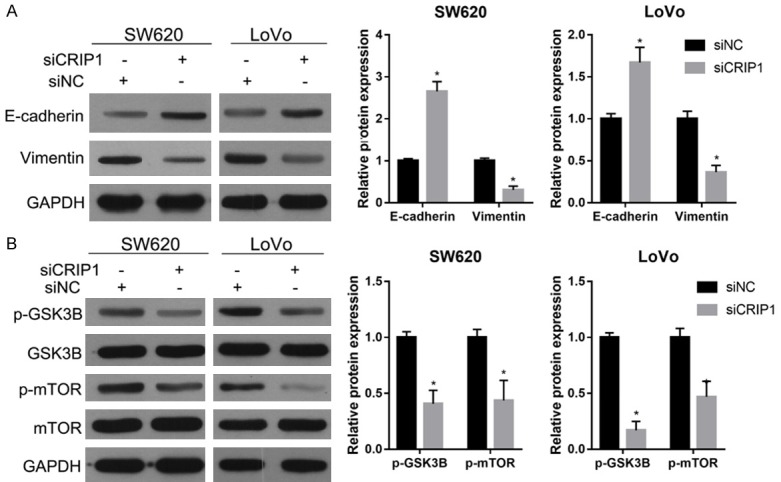
CRIP1 silencing inhibited the expression levels of EMT markers (A) and the phosphorylation levels of p-GSK3B and p-mTOR (B). On the left are the representative graphs of the western blot. On the right are the statistical results of relative protein expression levels. *P < 0.05, when compared to the siNC group.
CRIP1 silencing alleviated the effects of zinc supplementation on the migration, invasion, EMT, and GSK3/mTOR signaling pathway
To further investigate the relationship between CRIP1 and zinc supplementation in regulating migration, invasion, EMT, and GSK3/mTOR signaling pathway, SW620 and LoVo cells were treated with 50-µM Zn2+ after being transfected with siNC or siCRIP1 for 24 h. The results of Transwell assays showed that the number of migratory and invasive cells in the siCRIP1 transfection and zinc supplementation group (siCRIP1+Zn2+) was less than that in the siNC transfection and zinc supplementation group (siNC+Zn2+) (Figure 5A and 5B). The results of western blotting showed that the protein level of the epithelial marker, E-cadherin, was increased in the siCRIP1+Zn2+ group compared to that in the siNC+Zn2+ group (Figure 5C). In addition, the level of mesenchymal marker vimentin and the phosphorylation levels of GSK-3beta and mTOR were decreased in the siCRIP1+Zn2+ group compared to those in the siNC+Zn2+ group (Figure 5C and 5D). These results indicated that CRIP1 silencing alleviated the effects of zinc supplementation on migration, invasion, EMT, and GSK3/mTOR signaling pathway.
Figure 5.
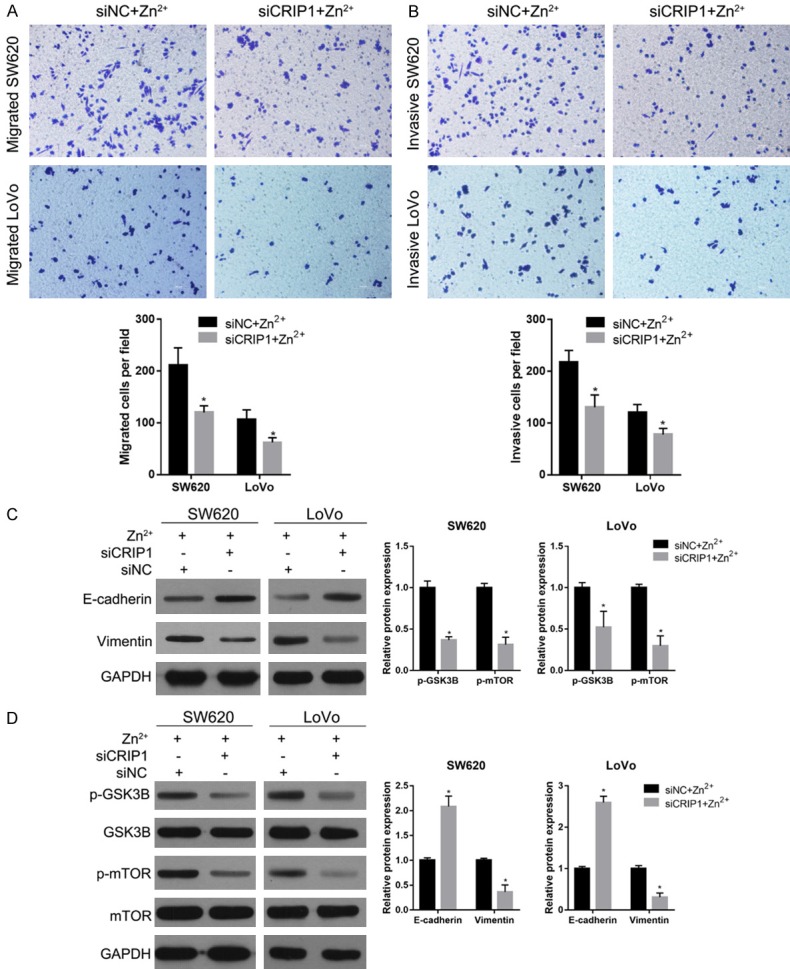
CRIP1 silencing alleviated the effects of zinc supplementation in medium. (A, B) The migration and invasion capabilities of SW620 and LoVo cells treated with 50-µM Zn2+ after transfected with siNC or siCRIP1 for 24 h detected using Transwell assays. The upper graphs provide the representative graph results, whereas the lower graphs provide the statistical results. (C, D) The expression levels of EMT markers (C) and the phosphorylation levels of p-GSK3B and p-mTOR (D) in SW620 and LoVo cells treated with 50 µM Zn2+ after being transfected with siNC or siCRIP1 for 24 h. On the left are the representative graphs of the western blot. On the right are the statistical results of the relative protein expression levels. *P < 0.05, when compared to the siNC+Zn2+ group.
Discussion
Zinc, an essential micronutrient, is required for the activity of > 300 enzymes, including key kinases, proteases, and phosphatases. The benefits of zinc supplementation have been widely accepted. In addition, its tumor-suppressing role and the association between zinc deficiency and cancer have been widely reported [19-22]. Our previous study indicated that CRIP1 is overexpressed in the CRC tissues and cell lines, and CRIP1 functioned as an oncogene in regulating migration and invasion of CRC cells [5]. CRIP1 has a role in zinc absorption and functions as an intracellular zinc transport protein [13-15]. These results suggested that an overactivity of intracellular zinc transport may also be involved in cancer development. CRC cells have a notable capability to migrate and invade; therefore, we focused on the function and underlying mechanism of excessive zinc supplementation on CRC cell migration and invasion capabilities.
First, 50-µM Zn2+ supplementation in medium increased the labile intracellular zinc content and enhanced the migratory and invasive capabilities of SW620 and HT29 cells, indicating that 50-µM Zn2+ supplementation elicited the effects on CRC cell migration and invasion. However, our present study showed that the survival rate of SW620 and LoVo cells was clearly inhibited after 100-, 125-, 150-, or 200-µM Zn2+ supplementation, indicating that excessive Zn2+ supplementation inhibits the effects on CRC cell growth. These inhibiting effects of Zn2+ supplementation have also been reported previously [23]. Although 50-µM Zn2+ can significantly inhibit the survival rate of SW620, the survival rate remains > 95% in our study. Thus, the effects of 50-µM Zn2+ supplementation on the cell survival rate are limited. In other words, 50-µM Zn2+ supplementation cannot obviously suppress the cell growth. This result is consistent with the previous study [23]. In addition, the number of invasive or migrated cells was adjusted by dividing with the survival rate. Therefore, the conclusion that 50-µM Zn2+ supplementation promotes CRC cell migration and invasion cannot be mediated by its limited inhibiting effect on CRC cell growth. Zinc is an essential trace element with cofactor functions in a large number of proteins, such as zinc finger proteins [24]. However, some zinc finger proteins functioned as oncogene [25,26], but some as tumor suppressor [27]. Therefore, we predicted that different concentrations of labile intracellular zinc may activate various proteins, resulting in different changes in the cells. These may explain the double-edged role in the development of CRC. Zinc concentration in the cell culture medium can simulate normal physiological concentrations of zinc. As such, 50-µM Zn2+ supplementation in a cell culture medium can stimulate a zinc excess condition, which suggests that an excess of zinc at some concentrations may play a cancer-promoting role in the development of CRC. This conclusion can be supported by the reports in Hep-2 tumor cells [28]. External zinc has also been reported to stimulate proliferation of tumor Hep-2 cells [28]. These results indicated that zinc does not always play a beneficial role. Clinicians and patients may need to reconsider the practice of using zinc supplements. However, further in vivo studies should be conducted to confirm this finding. In addition, only one concentration of Zn2+ was investigated in this study. The function of excessive zinc supplementation on CRC cell migration and invasion should be further confirmed by more concentration of Zn2+ in other CRC cells.
Second, we found that excessive zinc supplementation activated the GSK3/mTOR signaling pathway in SW620 and LoVo cells. This regulatory mechanism has also been reported in SH-SY5Y neuroblastoma cells [16]. GSK-3beta can bind to and phosphorylate Snail at two consensus motifs to dually regulate the function of Snail, which triggers the EMT of epithelial cells through repressing E-cadherin expression [17,18]. Due to a 50-µM Zn2+ supplementation that enhanced the EMT of SW620 and LoVo cells, we predicted that zinc enhanced EMT of SW620 and LoVo cells through the activation of GSK3/mTOR signaling pathway, which was confirmed by our results. We found that the effects of zinc supplementation on the EMT were blocked by rapamycin, a specific mTOR inhibitor. Moreover, rapamycin alleviated the effects of a 50-µM Zn2+ supplementation on the migratory and invasive capabilities of SW620 and LoVo cells. Cancer-associated EMT is known to contribute to increased invasiveness and metastasis [29]. Based on these results, we predicted that excessive Zn2+ would promote the migratory and invasive capabilities of SW620 and LoVo cells through GSK3/mTOR signaling pathway-induced EMT. Our previous study demonstrated that CRIP1 silencing inhibited the migratory and invasive capabilities of SW620 and LoVo cells [5] and that CRIP1 silencing inhibited the EMT and GSK3/mTOR signaling pathway. In addition, CRIP1 silencing alleviated the effects of zinc supplementation on migration, invasion, EMT, and GSK3/mTOR signaling pathway. Furthermore, the labile intracellular zinc content was increased in siCRIP1 transfected cells with 50-µM Zn2+ supplementation in medium. As the intracellular zinc transport role of CRIP1, we predicted that CRIP1 affect GSK3/mTOR signaling pathway by increasing the zinc transport to the target protein. However, other zinc transporters also existed in the CRC cells, such as G protein-coupled receptor 39 [30]. Moreover, many enzymes or signaling pathways could also be regulated by zinc [20,28,31,32]; thus, the underlying mechanism in which excessive Zn2+ promotes the migratory and invasive capabilities of SW620 and LoVo cells may be more complex than the results indicated in our report.
In conclusion, our results indicated that excessive zinc supplementation enhanced the migratory and invasive capabilities of SW620 and LoVo cells, and GSK3/mTOR signaling pathway-induced EMT was involved in this regulation. In addition, CRIP1 silencing alleviated the effects of excessive zinc supplementation on migration, invasion, EMT, and GSK3/mTOR signaling pathway. Based on these results, we predicted that CRIP1 promoted EMT through zinc-induced p-GSK-3beta in the CRC. Although further studies in vivo are needed to confirm the function and underlying mechanisms of zinc on CRC cell migratory and invasive capabilities, our data reveal a novel molecular basis for the understanding and clinical treatment of CRC.
Acknowledgements
This study was supported by the Scientific Research Fund of Xinxiang Medical University (No. 505124), National Natural Science Foundation of China (No. 81772524, 81702850, 31800658) and the Educational Commission of Henan Province, China (No. 18A310023).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59:366–378. doi: 10.3322/caac.20038. [DOI] [PubMed] [Google Scholar]
- 3.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, Ries LA. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung JJ, Lau JY, Goh KL, Leung WK. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol. 2005;6:871–876. doi: 10.1016/S1470-2045(05)70422-8. [DOI] [PubMed] [Google Scholar]
- 5.He G, Zou L, Zhou L, Gao P, Qian X, Cui J. Cysteine-rich intestinal protein 1 silencing inhibits migration and invasion in human colorectal cancer. Cell Physiol Biochem. 2017;44:897–906. doi: 10.1159/000485357. [DOI] [PubMed] [Google Scholar]
- 6.Lin Q, Tan HT, Lim TK, Khoo A, Lim KH, Chung MC. iTRAQ analysis of colorectal cancer cell lines suggests Drebrin (DBN1) is overexpressed during liver metastasis. Proteomics. 2014;14:1434–1443. doi: 10.1002/pmic.201300462. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Miller C, Mosher R, Zhao X, Deeds J, Morrissey M, Bryant B, Yang D, Meyer R, Cronin F, Gostout BS, Smith-McCune K, Schlegel R. Identification of cervical cancer markers by cDNA and tissue microarrays. Cancer Res. 2003;63:1927–1935. [PubMed] [Google Scholar]
- 8.Gomez del Pulgar T, Bandres E, Espina C, Valdes-Mora F, Perez-Palacios R, Garcia-Amigot F, Garcia-Foncillas J, Lacal JC. Differential expression of Rac1 identifies its target genes and its contribution to progression of colorectal cancer. Int J Biochem Cell Biol. 2007;39:2289–2302. doi: 10.1016/j.biocel.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Ludyga N, Englert S, Pflieger K, Rauser S, Braselmann H, Walch A, Auer G, Hofler H, Aubele M. The impact of cysteine-rich intestinal protein 1 (CRIP1) in human breast cancer. Mol Cancer. 2013;12:28. doi: 10.1186/1476-4598-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma XJ, Salunga R, Tuggle JT, Gaudet J, Enright E, McQuary P, Payette T, Pistone M, Stecker K, Zhang BM, Zhou YX, Varnholt H, Smith B, Gadd M, Chatfield E, Kessler J, Baer TM, Erlander MG, Sgroi DC. Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci U S A. 2003;100:5974–5979. doi: 10.1073/pnas.0931261100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Williamson M, Bott S, Brookman-Amissah N, Freeman A, Nariculam J, Hubank MJ, Ahmed A, Masters JR. Hypomethylation of WNT5A, CRIP1 and S100P in prostate cancer. Oncogene. 2007;26:6560–6565. doi: 10.1038/sj.onc.1210472. [DOI] [PubMed] [Google Scholar]
- 12.Li HG, Zhao LH, Zhang ZH, Liu JZ, Ren K, Li SY, Su ZJ. The impact of cysteine-rich intestinal protein 1 (CRIP1) on thyroid carcinoma. Cell Physiol Biochem. 2017;43:2037–2046. doi: 10.1159/000484184. [DOI] [PubMed] [Google Scholar]
- 13.Hempe JM, Cousins RJ. Cysteine-rich intestinal protein binds zinc during transmucosal zinc transport. Proc Natl Acad Sci U S A. 1991;88:9671–9674. doi: 10.1073/pnas.88.21.9671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hempe JM, Cousins RJ. Cysteine-rich intestinal protein and intestinal metallothionein: an inverse relationship as a conceptual model for zinc absorption in rats. J Nutr. 1992;122:89–95. doi: 10.1093/jn/122.1.89. [DOI] [PubMed] [Google Scholar]
- 15.Escobar O, Sandoval M, Vargas A, Hempe JM. Role of metallothionein and cysteine-rich intestinal protein in the regulation of zinc absorption by diabetic rats. Pediatr Res. 1995;37:321–327. doi: 10.1203/00006450-199503000-00012. [DOI] [PubMed] [Google Scholar]
- 16.An WL, Bjorkdahl C, Liu R, Cowburn RF, Winblad B, Pei JJ. Mechanism of zinc-induced phosphorylation of p70 S6 kinase and glycogen synthase kinase 3beta in SH-SY5Y neuroblastoma cells. J Neurochem. 2005;92:1104–1115. doi: 10.1111/j.1471-4159.2004.02948.x. [DOI] [PubMed] [Google Scholar]
- 17.Bachelder RE, Yoon SO, Franci C, de Herreros AG, Mercurio AM. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition. J Cell Biol. 2005;168:29–33. doi: 10.1083/jcb.200409067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 19.Pan Z, Choi S, Ouadid-Ahidouch H, Yang JM, Beattie JH, Korichneva I. Zinc transporters and dysregulated channels in cancers. Front Biosci. 2017;22:623–643. doi: 10.2741/4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grattan BJ, Freake HC. Zinc and cancer: implications for LIV-1 in breast cancer. Nutrients. 2012;4:648–675. doi: 10.3390/nu4070648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alder H, Taccioli C, Chen H, Jiang Y, Smalley KJ, Fadda P, Ozer HG, Huebner K, Farber JL, Croce CM, Fong LY. Dysregulation of miR-31 and miR-21 induced by zinc deficiency promotes esophageal cancer. Carcinogenesis. 2012;33:1736–1744. doi: 10.1093/carcin/bgs204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taccioli C, Chen H, Jiang Y, Liu XP, Huang K, Smalley KJ, Farber JL, Croce CM, Fong LY. Dietary zinc deficiency fuels esophageal cancer development by inducing a distinct inflammatory signature. Oncogene. 2012;31:4550–4558. doi: 10.1038/onc.2011.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.John S, Briatka T, Rudolf E. Diverse sensitivity of cells representing various stages of colon carcinogenesis to increased extracellular zinc: implications for zinc chemoprevention. Oncol Rep. 2011;25:769–780. doi: 10.3892/or.2010.1124. [DOI] [PubMed] [Google Scholar]
- 24.Vallee BL, Auld DS. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990;29:5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Shao Y, He Y, Ning K, Cui X, Liu F, Wang Z, Li F. MORC2 promotes development of an aggressive colorectal cancer phenotype through inhibition of NDRG1. Cancer Sci. 2019;110:135–146. doi: 10.1111/cas.13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu B, Xing X, Li X, Guo Q, Xu T, Xu K. ZNF259 promotes breast cancer cells invasion and migration via ERK/GSK3beta/snail signaling. Cancer Manag Res. 2018;10:3159–3168. doi: 10.2147/CMAR.S174745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Yang Q, Guan H, Shi B, Ji M, Hou P. ZNF677 suppresses Akt phosphorylation and tumorigenesis in thyroid cancer. Cancer Res. 2018;78:5216–5228. doi: 10.1158/0008-5472.CAN-18-0003. [DOI] [PubMed] [Google Scholar]
- 28.Rudolf E, Cervinka M. External zinc stimulates proliferation of tumor Hep-2 cells by active modulation of key signaling pathways. J Trace Elem Med Biol. 2008;22:149–161. doi: 10.1016/j.jtemb.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Prieto-Garcia E, Diaz-Garcia CV, Garcia-Ruiz I, Agullo-Ortuno MT. Epithelial-to-mesenchymal transition in tumor progression. Med Oncol. 2017;34:122. doi: 10.1007/s12032-017-0980-8. [DOI] [PubMed] [Google Scholar]
- 30.Cohen L, Sekler I, Hershfinkel M. The zinc sensing receptor, ZnR/GPR39, controls proliferation and differentiation of colonocytes and thereby tight junction formation in the colon. Cell Death Dis. 2014;5:e1307. doi: 10.1038/cddis.2014.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prasad AS. Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr. 2013;4:176–190. doi: 10.3945/an.112.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor KM, Nicholson RI. The LZT proteins; the LIV-1 subfamily of zinc transporters. Biochim Biophys Acta. 2003;1611:16–30. doi: 10.1016/s0005-2736(03)00048-8. [DOI] [PubMed] [Google Scholar]


