Abstract
The prognosis of patients with advanced hepatocellular carcinoma (HCC) remains obscure. From a clinical point of view, the ERK MAPK pathway and the PI3K/AKT pathway are activated in the majority of liver cancer. In addition, long term used to single agent treatment of HCC, frequently results in reverse activation of the ERK MAPK pathway or the PI3K/AKT pathway, leading to drug resistance. Thus, it is important to research the mechanism of combination agents that could suppress different pathways to treat HCC. Here, we found that combination natural product magnolin with BRAF inhibitor SB590885 synergistically suppressed the proliferation, promoted cell cycle arrest and apoptosis in hepatocellular carcinoma cells Bel-7402 and SK-Hep1. Furthermore, we demonstrated that the magnolin and the SB590885 combination led to increased impaired proliferation via inhibition of the ERK MAPK pathway and the PI3K/AKT pathway. These findings highlight the important role of agent combination and provided the approaches of therapeutic improvement for patients with advanced HCC.
Keywords: Magnolin, SB590885, hepatocellular carcinoma cells, PI3K-AKT/mTOR pathway, ERK MAPK pathway
Introduction
As the increasing incidence and mortality, cancer is the key cause of death in the world and is a worldwide problem. Among cancers, hepatocellular carcinoma is identified for the fifth most common cancer and the second major cause of cancer related mortality worldwide [1-6]. Several studies have reported the molecular characterization of hepatocellular carcinoma (HCC) [7-9]. However, HCC that is diagnosed at an advanced stage remains very poor prognosis.
As the development of drug resistance, the efficacy of the single therapeutic agents is limited [10]. Thus, new approaches that inhibit the tumor growth have important role in clinical impact. Combined treatments could be more effective for suppressing cancer and could be more effective for achieving treatment of cancer patients. Magnolin is an active ingredient of volatile oil from Magnoliafargesii [11,12], which has been traditionally used as an oriental medicine to treat nasal congestion associated with headaches, sinusitis, inflammation, and allergic rhinitis [13-15]. Previous studies have shown that magnolin suppresses cell migration and invasion through targeting the ERKs/RSK2 signaling pathway [14-16]. Recent study has demonstrated that magnolin inhibits prostate cancer cell growth in vitro and in vivo through the PI3K/AKT pathway [12]. Furthermore, magnolin also promotes the autophagy and cell cycle arrest in colorectal cancers through suppressing the LIF/Stat3/Mcl-1 dependent manner [17]. SB590885 is a serine/threonine-protein kinase B-Raf (B-RAF) inhibitor. Previous studies have shown that the combination of SB590885 and AKT inhibitor ZSTK474 impacts on proliferation of papillary thyroid cancer cell lines via inhibition of the ERK MAPK and PI3K/AKT signaling pathway [18,19]. However, whether the combination of magnolin and SB590885 inhibits the hepatocellular carcinoma (HCC) growth is very poor.
Therefore, in this study we combined magnolin with SB590885 to test whether this therapeutic strategy could provide an improvement in treatment of advanced HCC, without increased toxicity. Our results demonstrated that the magnolin and the SB590885 combination resulted in impacting on proliferation via inhibiting the ERK MAPK pathway and the PI3K/AKT pathway in HCC cells.
Materials and methods
Cell culture
The HCC cell lines Bel-7402 and SK-Hep1 were obtained from the Shanghai Genechem Co (Shanghai, China). The cell lines were cultured in RPMI-1640 medium supplied with 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) and 10% fetal bovine serum (Gibco, Carlsbad, CA, USA) were maintained at 37°C in a humidified incubator containing 5% CO2.
Cell viability assay
Cell viability was tested using the CCK-8 assay according to the manufacturer’s instructions. Cells (7×103)/well were seeded into a 96-well plate and cultured in regular growth medium containing 10% fetal bovine serum. After 24 h, cells were exposed to serial dilutions of magnolin (25 μM, 50 μM, 75 μM, 100 μM, 125 μM) SB590885 (3 μM, 6 μM, 9 μM, 12 μM, 15 μM). After the cells were incubated at 37°C for 48 h, the medium was washed and 100 μL of RPMI-1640 and 10 μL of CCK-8 reagent were added. The cells were incubated for 2 h at 37°C. Finally, the absorbance was measured at 450 nm using Spectra Max i3X (Molecular Devices, Silicon Valley, CA, USA). All cell viability assays were performed at least three times. The combination index (CI), which was calculated by the Chou-Talalay equation, demonstrates synergistic effects with CI <1, additive effects with CI =1, and antagonism with CI >1 [20].
Colony formation assay
The HCC cell lines Bel-7402 and SK-Hep1 were incubated in a six-well plate with 100 μM magnolin and 12 μM SB590885 for 10 days as previously described [12,19]. Colonies were stained with crystal violet and then counted under microscope.
Cell cycle and apoptosis analysis
The HCC cell lines Bel-7402 and SK-Hep1 were incubated in a complete cell culture medium with 100 μM magnolin and 12 μM SB590885 for 48 h. The cells were collected in cold PBS and next incubated with 150 μL RNase A (10 μg/mL) for 30 min at 37°C in the dark, stained with 400 μL propidium iodide (PI) (50 μg/mL)and placed at 4°C in the dark for 30 min [12,21]. The stained cells were analyzed using a flow cytometer (BD Bioscience, CA, US) [21]. For apoptosis analyses, high-affinity Annexin-V (AV) and PI (BD Biosciences, CA, USA) were used as previously described [22].
Western blot analysis
SK-Hep1 and Bel-7402 cells were plated into 6-well plates for 24 h, and then treated with 100 µM magnolin, 12 µM SB590885 and the combination for 48 h. The total protein from the cells was extracted using RIPA buffer with proteinase inhibitors (Beyotime, China). Protein samples were subjected to SDS-PAGE and then transferred to PVDF membranes (Millipore, US). After blocking with 5% BSA for 1 hour, the membranes were incubated with primary antibodies such as Bcl-2, Bax, cleaved PARP, cleaved caspase-9, P21, P27, CyclinD1, p-MEK, MEK, p-ERK, ERK, AKT, p-AKT, P70S6K, p-P70S6K, S6, p-S6, β-actin (Cell Signaling Technology, Inc. (Danvers, MA, USA)) and GAPDH (Qian Chen Biotech, Shanghai) at 4°C overnight. After washing thrice, the membranes were incubated with the secondary anti-rabbit or anti-mouse antibody for 1.5 h at room temperature. Protein bands were visualized by a BIO-RAD ChemiDoc Imaging System.
Statistical analysis
Statistical analyses were performed using SPSS 17.0 (IBM, Armonk, New York, USA). Graphs were completed with GraphPad Prism 7.0 software (GraphPad software, San Diego, CA, USA). All experiments mentioned above were performed at three independent experiments. Statistical significance was determined by Student’s t-test, and a two-sided P-value <0.05 was considered statistically significant.
Results
Combined treatment of magnolin with SB590885 influences viability and clonogenic potential of hepatocellular carcinoma cells
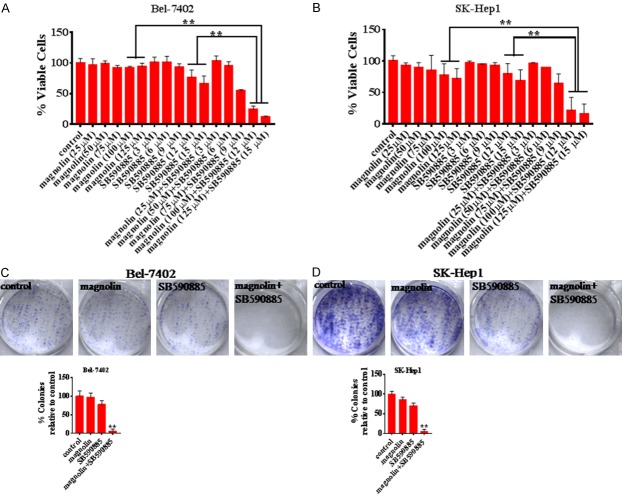
To evaluate the effect of the combination treatment on hepatocellular carcinoma, we tested the effects on morphology, viability of Bel-7402 and SK-Hep1 cells. We found that magnolin treatment for 48 hours did not inhibit the viability of Bel-7402 and SK-Hep1 cells, meanwhile SB590885 single treatment suppressed the viability of Bel-7402 and SK-Hep1 cells. Intriguingly, combination significantly decreased the cell viability than any other single treated groups (Figure 1A and 1B). The CI values measuring the degree of drug interaction suggested that there is a synergistic interaction between magnolin and SB590885 (Table 1). Furthermore, we performed the clonogenic assay, over a 10-day treatment, although SB590885 single treated group exhibited the suppressed single cell colony progression, the combination almost eliminated colony formation (Figure 1C and 1D). Taken together, these results indicate that the combination of magnolin and SB590885 synergistically inhibited the growth of hepatocellular carcinoma cells and induced the cell death.
Figure 1.
Anti-proliferation effects of combination treatments in HCC cells. After treatment with magnolin (25 μM, 50 μM, 75 μM, 100 μM, 125 μM) and SB590885 (3 μM, 6 μM, 9 μM, 12 μM, 15 μM) in combination or single treatment for 48 h, the CCK-8 assay was used to determine cell viability (A) Bel-7402 cell line, (B) SK-Hep1 cell line. These results are mean ± SD of three independent experiments performed in triplicate. **P<0.001 versus single treatments (one-way ANOVA followed by a Student-Newman-Keuls test). The combination of 100 μM magnolin and 12 μM SB590885 significantly inhibited colony formation in cells (C) Bel-7402 cell line (D) SK-Hep1 cell line. These results are mean ± SD of three independent experiments performed in triplicate. **P<0.001 versus single treatments (one-way ANOVA followed by a Student-Newman-Keuls test).
Table 1.
Synergistic indexes of combination treatment with Magnolin and SB590885 in Bel-7402 and Sk-Hep1 hepatocellular carcinoma cell lines
| Bel-7402 | Sk-Hep1 | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Magnolin (μM) | SB590885 (μM) | CI | Magnolin (μM) | SB590885 (μM) | CI |
| 25 | 3 | 1.04 | 25 | 3 | 1.01 |
| 50 | 6 | 1.61 | 50 | 6 | 0.81 |
| 75 | 9 | 0.39 | 75 | 9 | 0.51 |
| 100 | 12 | 0.28 | 100 | 12 | 0.21 |
| 125 | 15 | 0.19 | 125 | 15 | 0.20 |
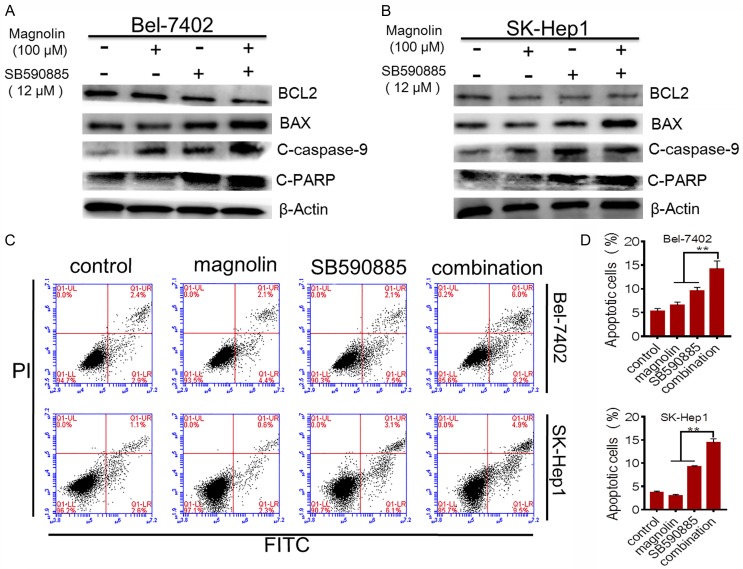
Combination treatment induced the cell apoptosis in hepatocellular carcinoma cells
Apoptosis is identified as an underlying antitumor mechanism. Thus we tested the effects of the combined treatment on apoptosis in Bel-7402 and SK-Hep1 cells. We found that combination of magnolin and SB590885 significantly promoted the apoptosis in Bel-7402 and SK-Hep1 cells compared to single agent treatment via the apoptosis assay (FITC/PI staining) (Figure 2). Furthermore, to confirm these results, using western blot analysis, we found that the expression of BAX, cleaved caspase-3 and cleaved PARP increased in combination treated group compared to single treated group (Figure 2). However, the level of anti-apoptosis protein BCL-2 decreased in combination treated group compared to single treated group (Figure 2). These data suggested that magnolin facilitated SB590885 promoted apoptosis in HCC cells.
Figure 2.
Effect of combination treatment on HCC cell apoptosis. BCL2, BAX, cleaved caspase-9 and cleaved PARP were tested using western blot analysis (A) Bel-7402 cell line, (B) SK-Hep1 cell line. Additive effects of combination treatment of magnolin and SB590885 on apoptosis in HCC cell lines after 48 h of exposure (C) Bel-7402 cell line (D) SK-Hep1 cell line. These results are mean ± SD of three independent experiments performed in triplicate. **P<0.001 versus single treatments (one-way ANOVA followed by a Student-Newman-Keuls test).
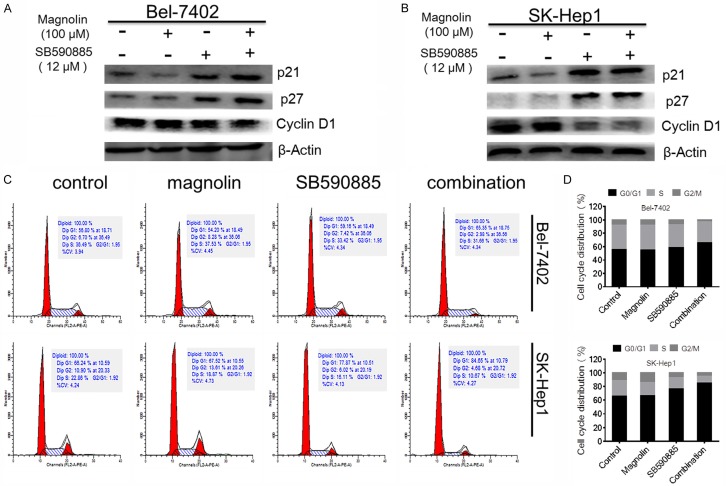
Combination treatment induced the cell cycle arrest in hepatocellular carcinoma cells
Because of the potent inhibitory effects of the combination of magnolin and SB590885 on HCC cell proliferation and viability, we next determined the cell cycle progression after drug treatment. Flow cytometry analyses demonstrated that combination treatment promoted cell cycle arrest in the G0/G1 phase (Figure 3). To confirm these results, we performed western blot analysis and found that the expression of p21 and p27 increased in combination treated group compared to single treated group (Figure 3). In contrast, the level of cyclinD1 decreased in combination treated group compared to single treated group (Figure 3). These data suggested that combination magnolin and SB590885 promoted cell cycle arrest in HCC cells.
Figure 3.
Effect of combination treatments on HCC cell cycle arrest. The p21, p27, and cyclinD1 were tested using western blot analysis (A) Bel-7402 cell line, (B) SK-Hep1 cell line. Additive effects of combination treatment of magnolin and SB590885 on cell cycle arrest in HCC cell lines after 48 h of exposure (C) Bel-7402 cell line (D) SK-Hep1 cell line. These results are performed with three independent experiments in triplicate.
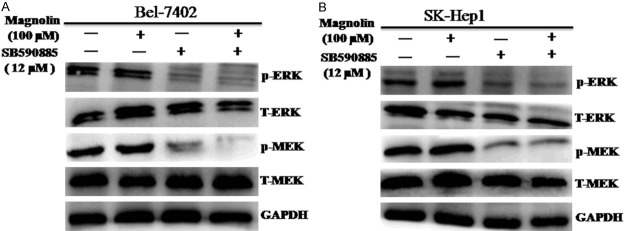
Combined treatment of magnolin with SB590885 regulates the ERK MAPK pathway in hepatocellular carcinoma cells
To determine the underlying molecular mechanism, we investigated the effects of magnolin and SB590885 on the ERK MAPK pathways in HCC cells. We found that combination treatment magnolin and SB590885 significantly suppressed the phosphorylation levels of MEK and ERK in Bel-7402 and SK-Hep1 cells (Figure 4A and 4B). These results suggested that combination magnolin and SB590885 suppressed the growth of hepatocellular carcinoma cells via inhibited the ERK MAPK signaling pathway.
Figure 4.
Effect of combination treatment on the ERK MAPK pathway in HCC cell lines. The p-ERK, total-ERK, p-MEK and total-MEK were tested via using western blot analysis (A) Bel-7402 cell line, (B) SK-Hep1 cell line. These results are performed with three independent experiments in triplicate.
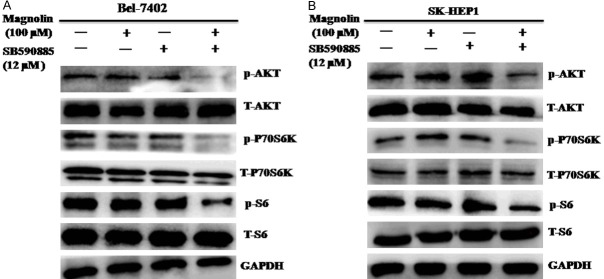
Combined treatment of magnolin with SB590885 regulates the PI3K/AKT/mTOR pathway in hepatocellular carcinoma cells
Single agent treatment inhibited the ERK MAPK pathway often resulted in reversing activation the PI3K/AKT/mTOR pathway. Thus we also investigated the effects of magnolin and SB590885 on the PI3K/AKT/mTOR pathways in HCC cells. We found that combination treatment magnolin and SB590885 significantly suppressed the phosphorylation levels of AKT, P70S6K and S6 in Bel-7402 and SK-Hep1 cells (Figure 5A and 5B). These results suggested that combination magnolin and SB590885 suppressed the growth of hepatocellular carcinoma cells via inhibited the PI3K/AKT/mTOR signaling pathway.
Figure 5.
Effect of combination treatment on the PI3K/AKT/mTOR pathway in HCC cell lines. The p-AKT, total-AKT, p-P70S6K, total-P70S6K, p-S6 and total-S6 were tested via using western blot analysis (A) Bel-7402 cell line, (B) SK-Hep1 cell line. These results are performed with three independent experiments in triplicate.
Discussion
HCC is one of the most prevalent and lethal cancers with multiple genetic aberrations in worldwide. Although the new therapeutic agents have been identified for treatment of cancer patients in recent years, the optimal and effective use of targeted therapies of treatment the hepatocellular carcinoma (HCC) remain unclear. Thus agent combination based chemotherapy could be more effectively for treatment the cancer patients. In this study, we provide evidence that combined treatments have important role in the chemoprevention of HCC.
Sorafenib is a small-molecule inhibitor that acts on VEGFR, platelet-derived growth factor receptor, and Raf kinases, known as extended the survival of advanced hepatocellular carcinoma (HCC) patients [10,23]. However, the efficacy of sorafenib is limited by the development of drug resistance. Our results indicate that the combination of magnolin and (BRAF) inhibitors SB590885 significantly inhibits the proliferation and viability in hepatocellular carcinoma (HCC). Consistent with recent observations [12,17], we found that the combination of magnolin and SB590885 induces apoptosis along with G0/G1 phase cell cycle arrest in cancer cells. In addition, our results indicated that the combined treatment groups significantly inhibited the clonogenic potential of both Bel-7402 and SK-Hep1 cells. These data suggested that the combination of magnolin and SB590885 suppressed the growth of hepatocellular carcinoma cells by decree sing cell proliferation rate.
Several studies have shown that the phosphatidylinositol-3 kinase PI3K/AKT/mTOR pathway is activated in approximately 50% of patients with HCC [24,25]. Long term use of the ERK MAPK inhibitor in treatment of HCC, frequently results in reverse activation of the PI3K/AKT pathway, leading to drug resistance. For example, sorafenib has been demonstrated to activate the PI3K/AKT pathway in HCC cells [26] and this over-activation is identified to be one of the mechanisms of resistance to sorafenib treatment [27]. Previous studies have shown that magnolin suppresses cell migration and invasion through targeting the ERKs/RSK2 signaling pathway [14-16]. Recent study has demonstrated that magnolin inhibits prostate cancer cell growth in vitro and in vivo through the PI3K/AKT pathway [12]. Furthermore, magnolin also promotes the autophagy and cell cycle arrest in colorectal cancers through suppressing the LIF/Stat3/Mcl-1 dependent manner [17]. SB590885 is a serine/threonine-protein kinase B-Raf (B-RAF) inhibitor. Previous studies have shown that the combination of SB590885 and AKT inhibitor ZSTK474 impact on proliferation of papillary thyroid cancer cell lines via inhibition of the ERK MAPK and PI3K/AKT signaling pathway [18,19]. Our results suggested that the combination magnolin and SB590885 suppressed the growth of hepatocellular carcinoma cells via inhibited the ERK MAPK and PI3K/AKT/mTOR signaling pathway. Our findings highlight the key role of the ERK MAPK and PI3K/AKT/mTOR pathway in the resistance to single agent therapies and provide a potential drug regimen of magnolin in combination with SB590885 for treating patients with HCC in the clinical treatment.
Acknowledgements
This work was supported by Natural Science Foundation of China [81660611], Science & Technology Foundation of Health and Family Planning Commission of Guizhou Province [gzwjkj2017-1-049], Science & Technology Plan of Zunyi [(2018)16], The start-up Foundation of Zunyi Medical University [F-903], The Xinmiao Foundation of Zunyi Medical University [(2017)5733-025].
Disclosure of conflict of interest
None.
References
- 1.Singh AR, Joshi S, Burgoyne AM, Sicklick JK, Ikeda S, Kono Y, Garlich JR, Morales GA, Durden DL. Single agent and synergistic activity of the “first-in-class” dual PI3K/BRD4 inhibitor SF1126 with sorafenib in hepatocellular carcinoma. Mol Cancer Ther. 2016;15:2553–2562. doi: 10.1158/1535-7163.MCT-15-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Sartorius K, Sartorius B, Aldous C, Govender PS, Madiba TE. Global and country underestimation of hepatocellular carcinoma (HCC) in 2012 and its implications. Cancer Epidemiol. 2015;39:284–290. doi: 10.1016/j.canep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Nasr M, Nafee N, Saad H, Kazem A. Improved antitumor activity and reduced cardiotoxicity of epirubicin using hepatocyte-targeted nanoparticles combined with tocotrienols against hepatocellular carcinoma in mice. Eur J Pharm Biopharm. 2014;88:216–225. doi: 10.1016/j.ejpb.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Yamane B, Weber S. Liver-directed treatment modalities for primary and secondary hepatic tumors. Surg Clin North Am. 2009;89:97–113. ix. doi: 10.1016/j.suc.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 7.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 8.Laurent-Puig P, Legoix P, Bluteau O, Belghiti J, Franco D, Binot F, Monges G, Thomas G, Bioulac-Sage P, Zucman-Rossi J. Genetic alterations associated with hepatocellular carcinomas define distinct pathways of hepatocarcinogenesis. Gastroenterology. 2001;120:1763–1773. doi: 10.1053/gast.2001.24798. [DOI] [PubMed] [Google Scholar]
- 9.Chiang DY, Villanueva A, Hoshida Y, Peix J, Newell P, Minguez B, LeBlanc AC, Donovan DJ, Thung SN, Sole M, Tovar V, Alsinet C, Ramos AH, Barretina J, Roayaie S, Schwartz M, Waxman S, Bruix J, Mazzaferro V, Ligon AH, Najfeld V, Friedman SL, Sellers WR, Meyerson M, Llovet JM. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008;68:6779–6788. doi: 10.1158/0008-5472.CAN-08-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeannot V, Busser B, Vanwonterghem L, Michallet S, Ferroudj S, Cokol M, Coll JL, Ozturk M, Hurbin A. Synergistic activity of vorinostat combined with gefitinib but not with sorafenib in mutant KRAS human non-small cell lung cancers and hepatocarcinoma. Onco Targets Ther. 2016;9:6843–6855. doi: 10.2147/OTT.S117743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahn KS, Jung KY, Kim JH, Oh SR, Lee HK. Inhibitory activity of lignan components from the flower buds of Magnoliae fargesii on the expression of cell adhesion molecules. Biol Pharm Bull. 2001;24:1085–1087. doi: 10.1248/bpb.24.1085. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Zou X, Zhang X, Wang F, Zhu W, Zhang G, Xiao J, Chen M. Magnolin inhibits prostate cancer cell growth in vitro and in vivo. Biomed Pharmacother. 2017;87:714–720. doi: 10.1016/j.biopha.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Kim JS, Kim JY, Lee HJ, Lim HJ, Lee DY, Kim DH, Ryu JH. Suppression of inducible nitric oxide synthase expression by furfuran lignans from flower buds of Magnolia fargesii in BV-2 microglial cells. Phytother Res. 2010;24:748–753. doi: 10.1002/ptr.3028. [DOI] [PubMed] [Google Scholar]
- 14.Lee CJ, Lee HS, Ryu HW, Lee MH, Lee JY, Li Y, Dong Z, Lee HK, Oh SR, Cho YY. Targeting of magnolin on ERKs inhibits Ras/ERKs/RSK2-signaling-mediated neoplastic cell transformation. Carcinogenesis. 2014;35:432–441. doi: 10.1093/carcin/bgt306. [DOI] [PubMed] [Google Scholar]
- 15.Lee CJ, Lee MH, Yoo SM, Choi KI, Song JH, Jang JH, Oh SR, Ryu HW, Lee HS, Surh YJ, Cho YY. Magnolin inhibits cell migration and invasion by targeting the ERKs/RSK2 signaling pathway. BMC Cancer. 2015;15:576. doi: 10.1186/s12885-015-1580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song JH, Lee CJ, An HJ, Yoo SM, Kang HC, Lee JY, Kim KD, Kim DJ, Lee HS, Cho YY. Magnolin targeting of ERK1/2 inhibits cell proliferation and colony growth by induction of cellular senescence in ovarian cancer cells. Mol Carcinog. 2019;58:88–101. doi: 10.1002/mc.22909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu H, Yin S, Zhou S, Shao Y, Sun J, Pang X, Han L, Zhang Y, Gao X, Jin C, Qiu Y, Wang T. Magnolin promotes autophagy and cell cycle arrest via blocking LIF/Stat3/Mcl-1 axis in human colorectal cancers. Cell Death Dis. 2018;9:702. doi: 10.1038/s41419-018-0660-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barollo S, Bertazza L, Baldini E, Ulisse S, Cavedon E, Boscaro M, Pezzani R, Mian C. The combination of RAF265, SB590885, ZSTK474 on thyroid cancer cell lines deeply impact on proliferation and MAPK and PI3K/Akt signaling pathways. Invest New Drugs. 2014;32:626–635. doi: 10.1007/s10637-014-0108-3. [DOI] [PubMed] [Google Scholar]
- 19.Bertazza L, Barollo S, Radu CM, Cavedon E, Simioni P, Faggian D, Plebani M, Pelizzo MR, Rubin B, Boscaro M, Pezzani R, Mian C. Synergistic antitumour activity of RAF265 and ZSTK474 on human TT medullary thyroid cancer cells. J Cell Mol Med. 2015;19:2244–2252. doi: 10.1111/jcmm.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 21.Yin R, Bao W, Xing Y, Xi T, Gou S. MiR-19b-1 inhibits angiogenesis by blocking cell cycle progression of endothelial cells. Biochem Biophys Res Commun. 2012;417:771–776. doi: 10.1016/j.bbrc.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 22.Xu B, Huang Y, Niu X, Tao T, Jiang L, Tong N, Chen S, Liu N, Zhu W, Chen M. Hsa-miR-146a-5p modulates androgen-independent prostate cancer cells apoptosis by targeting ROCK1. Prostate. 2015;75:1896–1903. doi: 10.1002/pros.23068. [DOI] [PubMed] [Google Scholar]
- 23.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 24.Matter MS, Decaens T, Andersen JB, Thorgeirsson SS. Targeting the mTOR pathway in hepatocellular carcinoma: current state and future trends. J Hepatol. 2014;60:855–865. doi: 10.1016/j.jhep.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunter I, Erdal E, Nart D, Yilmaz F, Karademir S, Sagol O, Atabey N. Active form of AKT controls cell proliferation and response to apoptosis in hepatocellular carcinoma. Oncol Rep. 2014;31:573–580. doi: 10.3892/or.2013.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen KF, Chen HL, Tai WT, Feng WC, Hsu CH, Chen PJ, Cheng AL. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J Pharmacol Exp Ther. 2011;337:155–161. doi: 10.1124/jpet.110.175786. [DOI] [PubMed] [Google Scholar]
- 27.Zhai B, Hu F, Yan H, Zhao D, Jin X, Fang T, Pan S, Sun X, Xu L. Bufalin reverses resistance to sorafenib by inhibiting akt activation in hepatocellular carcinoma: the role of endoplasmic reticulum stress. PLoS One. 2015;10:e0138485. doi: 10.1371/journal.pone.0138485. [DOI] [PMC free article] [PubMed] [Google Scholar]