Abstract
Background: The S100 gene family encodes low molecular weight proteins implicated in cancer progression. In the present study, we explored the effects and underlying mechanisms of calcium-binding protein A11 (S100A11 protein) in hypopharyngeal squamous cell carcinoma (HSCC). Methods: RT-qPCR and western blot analysis were used to detect the mRNA and protein expression of S100A11, EGFR, MMP2, CD44, and MMP9. CCK-8, colony formation, wound healing and transwell invasion assays were performed to evaluate the effects of S100A11 on HSCC cells. Results: In our study, we observed that the level of S100A11 expression was significantly upregulated in HSCC tissues and cell lines. S100A11 inhibition increased the effects of 5-Fu on FaDu cells proliferation in vitro. In addition, S100A11 inhibition decreased the migration ability of FaDu cells. Additionally, the expression of migration-related proteins including EGFR, MMP2, CD44, and MMP9 were down-regulated when S100A11 was knocked down. Moreover, the expression of phosphorylated-PI3K (p-PI3K), phosphorylated-Akt (p-Akt), phosphorylated-mTOR (p-mTOR) and BCL-2 in FaDu cells were dramatically decreased. Conclusions: Our results suggested that S100A11 could activate the PI3K/Akt/mTOR signaling pathway in HSCC tumorigenesis.
Keywords: S100A11, hypopharyngeal squamous cell carcinoma, PI3K/Akt/mTOR, migration
Introduction
Hypopharyngeal squamous cell carcinoma (HSCC) exhibits rapid proliferation and aggressive invasion, but the pathogenesis has not been thoroughly elucidated. Current management of this cancer includes conventional surgery treatment, radiation therapy, and chemotherapy, but the treatment efficiency is limited, and the 5-year survival rate remains unsatisfied [1,2]. The prognosis for patients with HSCC is among the worst for head and neck carcinoma [3,4]. Therefore, the identification and development of novel strategies for early detection and early treatment of HSCC are highlighted.
The S100 protein is a small, acidic protein of size 10-12 kDa, found only in vertebrates, and is one of the 20 members of the multigene EF-hand calcium-binding protein family [5]. S100A11 is a minor known member of the S100 protein family. The gene encoding S100A11 is also referred to as S100C or Cassegrain and is located on chromosome 1q21 together with the genes of the other 15 S100 family proteins [6]. In 1991, S100A11 was first identified in chicken gizzard smooth muscle [7]. Recently, abnormally expressed S100A11 was found in tumor progression. For example, S100A11 is overexpressed in laryngeal cancer [8], prostate cancer [9], uterine leiomyosarcoma [10], cholangiocarcinoma [11], colorectal carcinoma [12] and non-small cell lung cancer (NSCLC) [13]. In contrast, S100A11 expression is decreased and associated with poor survival in bladder cancer [14], suggesting that the roles of S100A11 in carcinogenesis are more complex. However, the expression and underlying mechanism of S100A11 in HSCC progression remain unclear.
In the present study, we investigated the expression and underlying mechanism of S100A11 in HSCC. Our data showed that S100A1 overexpression might protect the cells from death induced by 5-FU and increased HSCC cells migration. In addition, we suggested that the effects of S100A11 in HSCC tumorigenesis might regulate by PI3K/Akt/mTOR signaling pathway.
Materials and methods
Patients and tissue samples
Primary HSCC tissues and adjacent non-tumor tissues were acquired from 16 cases, who had undergone curative surgeries at Department of Otolaryngology-Head and Neck Surgery, Shanghai Changzheng Hospital. No patients received local or systemic treatment before the operation. All the samples were collected, immediately snap frozen in liquid nitrogen, and stored at -80°C for the following experiments. Written informed consents were obtained from every participant in this present study, and all the manipulates were approved by the ethics committee of the Second Military Medical University. The clinical information was shown in Table 1.
Table 1.
Correlation between expression of S100A11 and clinic pathological features in HSCC patients
| Parameters | No. of patients (%) | S100A11 expression | P value | |
|---|---|---|---|---|
|
| ||||
| N=16 | Low (%) | High (%) | ||
| Gender | 0.013 | |||
| Male | 15 (93.75) | 5 (31.25) | 10 (62.5) | |
| Female | 1 (6.25) | 0 (0) | 1 (6.25) | |
| Age (years) | ||||
| Mean | 60.3±8.7 | |||
| Range | 44-76 | |||
| Clinical stage | 0.001 | |||
| I | 1 (6.25) | 0 (0) | 1 (6.25) | |
| II | 1 (6.25) | 1 (6.25) | 0 (0) | |
| III | 1 (6.25) | 0 (0) | 1 (6.25) | |
| IV | 13 (81.25) | 4 (25.00) | 9 (56.25) | |
| Tumor location | 0.004 | |||
| Piriform sinus | 11 (68.75) | 2 (12.5) | 9 (56.25) | |
| Postericoid region | 4 (25.00) | 1 (6.25) | 3 (18.75) | |
| Posterior hypopharynx | 1 (6.25) | 0 (0) | 1 (6.25) | |
Cell culture and treatment
Human hypopharyngeal squamous carcinoma cells (FaDu) were bought from American Type Culture Collection (ATCC, Maryland, USA). Cells were cultured in high glucose Dulbecco’s modified Eagle’s medium (DMEM) (Gibco) supplemented with 10% fetal bovine serum (FBS) (Gibco) at 37°C in a humidified atmosphere with 5% CO2.
Knockdown of S100A11 expression were performed upon the transfection of S100A11 small interfering RNA (siRNA) in FaDu cells. The siRNAs to human S100A11 were chemically synthesized by BioTend (Aibosi, Shanghai) according to the sequence by Howell et al [15]. S100A11 sequences of selected regions to be targeted by the siRNAs were: 5’-CUA CUG UAA UAG UUA GAU AGU TT-3’ (forward) and 5’-UGC CGA AUC AGC AUU GAU GAU TT-3’ (reverse) for S100A11; and 5’-UAC US GA CAU GGC AGU UUT-3’ (forward) and 5’-AUG UUG CUG GAU UGA UCA UUC-3’ (reverse) for the negative control siRNA. S100A11 siRNAs were transfected into FaDu cells using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions. The effect of S100A11 gene knockdown was confirmed by western blot and qRT-PCR as described below.
Western blot analysis
The proteins were extracted from the frozen human tissues and treated cells were lysed using RIPA protein extraction reagent (Beyotime, China) supplemented with PMSF (Roche). Approximately 25 μg of protein extracts were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto nitrocellulose membranes (Sigma), and were probed with primary antibodies (S100A11, EGFR, CD44, MMP2, MMP9, PI3K, Akt, mTOR, p-PI3K, p-Akt, p-mTOR, Bcl-2 and GAPDH) (CST) overnight at 4°C. The blots were then incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody (Abcam) at room temperature for 1 h. The protein bands were revealed by ECL method and imaged using a BioSpectrum Gel Imaging System (Bio-Rad).
RNA isolation and qRT-PCR analysis
Total RNA was isolated from the human tissues and cells using TRIzol reagent (Invitrogen) according to the instructions, cDNA was reversibly transcribed from the isolated mRNA using the RevertAidtm First Strand cDNA Synthesis Kit (ThermoFisher Scientific, USA). The PCR reaction contained 2 μl of cDNA, 0.8 μl of each primer and 10 μl of QPK-201 SYBR Green master mix (ThermoFisher Scientific, USA) in a final volume of 20 μl. Quantitative real-time PCR was performed with the ABI 7300 system from Applied Biosystems. A total of 42 cycles of PCR were performed with 15 seconds at 95°C and 30 seconds at 60°C per cycle. The relative expression of genes was calculated with 2-ΔΔCt. Three independent repetitions were carried out.
Cell proliferation assay
Cell proliferation was measured by the cell proliferation reagent CCK-8 (Roche). After the cells (1 × 103/well) were plating in the 96-well microtiter plates (Corning, NY, USA), 10 μL of CCK-8 was added to each well at the time of harvest. 2 h after adding CCK-8, the absorbance of the converted were recorded at 450 nm to determine the cell viability.
Colony formation assay
Colony formation assays were performed as previously described [16,17]. Transfected cells were seeded into 6-well plates at a density of 500 cells/well and incubated at 37°C in a humidified incubator for 2 weeks. The medium is updated every three days. In the end, the cells were washed with PBS, fixed in 1 ml of methanol for 45 mins, and stained with 0.4% crystal violet for 30 mins at room temperature. The number of colonies containing more than 50 cells was counted and averaged.
Wound healing assay
Transfected cells were inoculated into 6-well plates and incubated with growth medium as described above. 24 h after transfection, confluent monolayers were injured with a yellow sterile pipette tip. The cell debris was removed by washing with PBS and the wound area of the cell monolayer was imaged using an inverted microscope equipped with a digital camera (Olympus).
Transwell invasion assay
The invasive ability of treated HSCC cells was analyzed by transwell assay using 8-µm pores chambers (Corning, USA) with Matrigel matrix (BD Biosciences, USA). Treated cells were collected and re-suspended in DMEM medium, making the final concentration 1 × 105 cells/ml. The upper chamber was filled with 200 µl cells suspension, and the lower chamber was filled with 500 µl medium containing 20% FBS. After cultured for 24 h, the invaded cells were fixed and stained, and then counted using a microscope.
Immunofluorescence staining
Treated FaDu cells (1 × 106 cells/well) were fixed and permeabilized using 4% formaldehyde for 15 min at room temperature. Non-specific sites were blocked by treatment with 1% bovine serum albumin (BSA) in PBS for 1 h at room temperature. Next, the cells were incubated overnight at 4°C with primary antibodies, and washed 3 times with PBS. Next, cells were incubated with secondary antibody for 1 h at room temperature. After washing twice with PBS, the cells were stained with 4’,6-diamidino-2-phenylindole (DAPI; Sigma) for 30 s at room temperature. Fluorescence images were obtained using upright fluorescence microscope (Olympus) equipped with a 40 × objective lens.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 software from at least three independent experiments. All of the data were expressed as the mean ± standard deviation (SD). Multiple groups comparisons were carried out using one-way analysis of variance (ANOVA). Statistical significance was determined as P<0.05.
Results
S100A11 is increased in HSCC
S100A11 expression levels in 16 pairs of HSCC tissues and adjacent non-tumor tissues were detected by using western blot and qRT-PCR. Western blot showed that S100A11 expression was significantly increased in HSCC tissues compared with adjacent non-tumor tissues (Figure 1A and 1C, P<0.05). QRT-PCR showed that S100A11 mRNA expression levels in 16 pairs of HSCC tissues was also upregulated compared to non-tumor tissues (Figure 1D, P<0.05). In addition, we explored S100A11 expression in HSCC cells, results showed that S100A11 mRNA expression was significantly increased in HSCC cells (Figure 1B, P<0.05). These data indicate that up-regulation of S100A11 might contribute to HSCC carcinogenesis.
Figure 1.
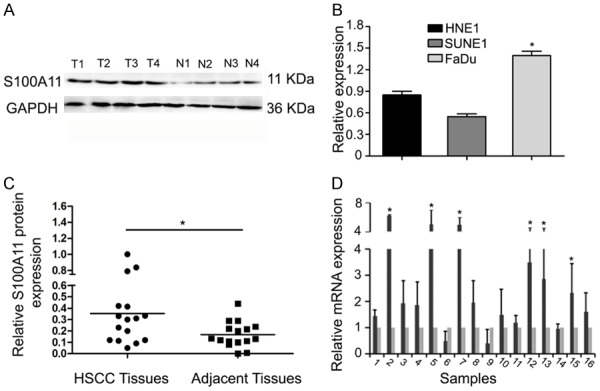
S100A11 is upregulated in HSCC. A, C. S100A11 expression in HSCC tissues and adjacent non-tumor tissues were detected by Western blot. B. S100A11 mRNA expression levels in HSCC cells was explored by qRT-PCR. D. S100A11 expression levels in HSCC tissues and adjacent non-tumor tissues were detected by using qRT-PCR. *P<0.05.
S100A11 influences the sensitivity of FaDu cells to 5-FU
To understand the potential roles of S100A11 in HSCC, FaDu cells were first transfected with siRNA to specifically target S100A11 or a negative control siRNA. The transfection efficiency was confirmed by western blotting and qRT-PCR (Figure 2A and 2B, P<0.05). Then, the effects of S100A11 on FaDu cells was determined by CCK-8 and colony formation assays. CCK-8 results showed that there is no significant difference among the S100A11 siRNA group, the negative control siRNA group and the untreated control group at the three time points (24, 48 and 72 h) (Figure 2C, P>0.05). Furthermore, similar results were obtained in colony formation assays (Figure 2D and 2E, P>0.05). Moreover, FaDu cells were treated with a combination of 5-FU and S100A11 siRNA or with a combination of 5-FU and negative control siRNA. Results showed that S100A1 inhibition might promote FaDu cells death induced by 5-FU (Figure 2F, P<0.05).
Figure 2.
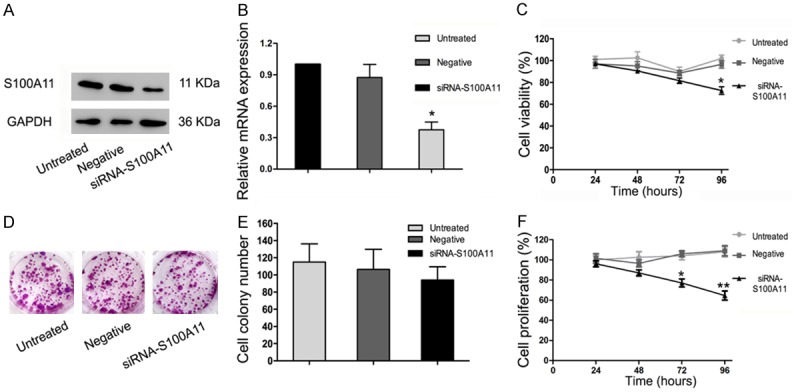
S100A11 influences the sensitivity of FaDu cells to 5-FU. A, B. Relative expression of S100A11 at both protein and mRNA levels in FaDu cells transfected with siRNA. C. The cell survival rate of siRNA-transfected FaDu cells was determined by CCK-8 assays. D, E. Colony formation showed that the colony numbers were not significantly different between the S100A11 siRNA, negative control siRNA and untreated control groups. F. S100A1 inhibition promoted FaDu cells death induced by 5-FU. **P<0.01, *P<0.05.
S100A11 inhibition reduces FaDu cells migration ability
Transwell assay showed that the number of cells penetrating the polycarbonate membrane in the S100A11 siRNA group, the negative control siRNA group and the untreated control group was 20.4±2.88/5 fields, 47.2±5.72/5 fields, and 47±3.81/5 fields, respectively, which showed a significant difference among groups (Figure 3A and 3B, P<0.05). Wound healing assay showed a similar result as transwell assay. The relative migratory distance of the cells in the S100A11 siRNA, negative control siRNA and untreated control groups was 9.25±0.5, 14±1.41, and 15.5±1.29, respectively (Figure 3C and 3D, P<0.05). These results showed that S100A11 inhibition does not affect proliferation but decreases the ability of FaDu cells to migrate. Thus, we demonstrated that S100A11 expression is correlate with HSCC cells migration ability.
Figure 3.
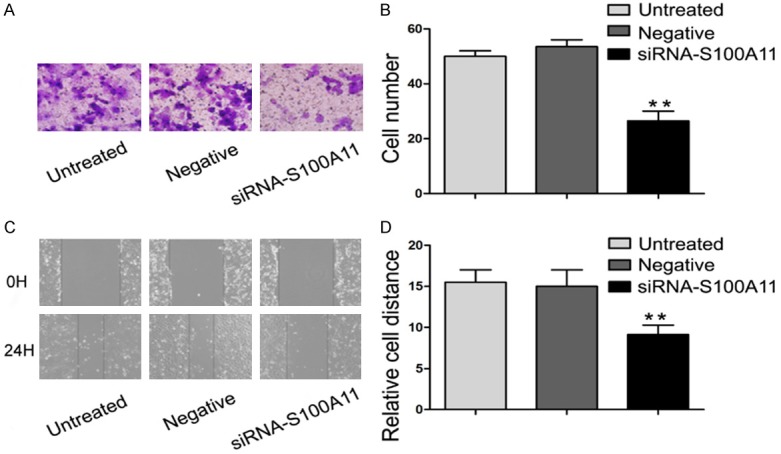
S100A11 inhibition reduced FaDu cells migration. A, B. Effects of S100A11 inhibition on FaDu cells invasion were detected by transwell assay. C, D. Effects of S100A11 suppression on FaDu cells migration were detected by wound healing assay. **P<0.01.
Down-regulation of S100A11 influences the migration-related proteins expression
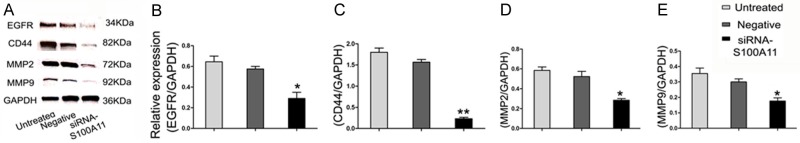
In order to more accurately locate the S100A11 protein expression, we performed immunofluorescence staining on FaDu cells. The results showed that S100A11 protein was highest in the untreated group, and reached the lowest level in the siRNA group, suggesting that the knockdown effects of the siRNA-S100A11 in FaDu cells. Moreover, we found that the protein of S100A11 is localization in cytoplasm of HSCC cells (Figure 4). Previous studies showed that EGFR, CD44, MMP2 and MMP9 proteins play important roles in the invasion and metastasis of cancer cells [18-20]. Hence, we analyzed the EGFR, CD44, MMP2 and MMP9 protein expression in FaDu cells. Results showed that the relative protein expression levels of EGFR, CD44, MMP2 and MMP9 was significantly decreased in the S100A11 siRNA group (Figure 5A-E, P<0.05). Overall, S100A11 inhibition could reduce these migration-related proteins expression in HSCC cells.
Figure 4.
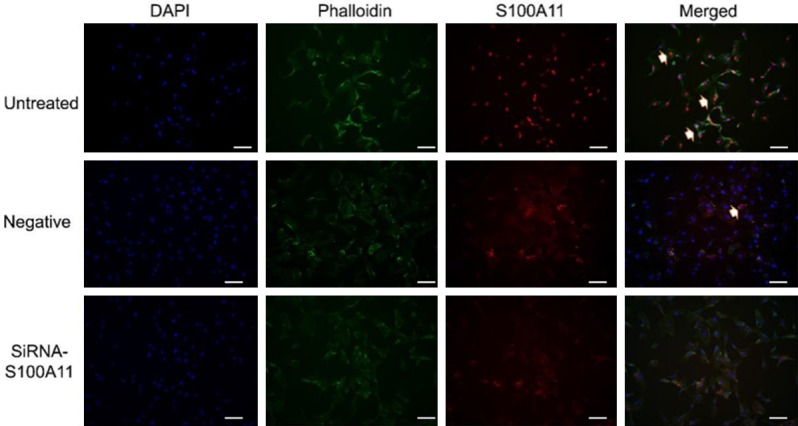
Immunofluorescence staining for marker S100A11. S100A11 protein was highest in the untreated group, and reached the lowest level in the siRNA group. The arrow showed the expression aggregation area of S100A11 protein. (Blue: DAPI, green: Phalloidin, red: S100A11).
Figure 5.
S100A11 inhibition influences migration-related gene expression. (A) Migration-related proteins expression was determined by Western blot. (B-E) The relative protein expression of EGFR (B), CD44 (C), MMP2 (D) and MMP9 (E) in FaDu cells transfected with S100A11 siRNA and negative control siRNA group. **P<0.01, *P<0.05.
S100A11 activates PI3K/Akt/mTOR signaling pathway of FaDu cells
Furthermore, RT-qPCR and western blot assays were used to detect the effect of S100A11 on PI3K/Akt/mTOR signaling pathway. we found that the mRNA expressions of PI3K, Akt, mTOR and bcl-2 were significantly decreased in the S100A11 siRNA group (Figure 6A, P<0.05). Western blot assay showed that the protein expressions of p-PI3K, p-Akt, p-mTOR and Bcl-2 in the S100A11 siRNA group were dramatically decreased (Figure 6B and 6C, P<0.05). However, the total-PI3K (t-PI3K), total-Akt (t-Akt) and total-mTOR (t-mTOR) protein expression was not changed (Figure 6B and 6C, P>0.05). These results indicated that S100A11 is positively correlated with PI3K/Akt/mTOR signaling pathway in HSCC carcinogenesis. However, whether S100A11 promotes migration in HSCC carcinogenesis is directly by activating PI3K/Akt/mTOR signaling pathway remain to be explored.
Figure 6.
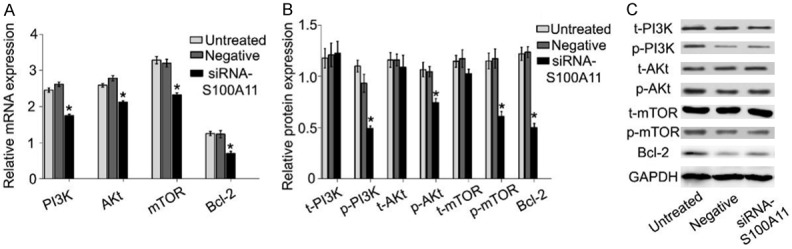
S100A11 inhibition inactivate PI3K/Akt/mTOR signaling pathway. A. Relative PI3K/Akt/mTOR g pathway related gene expression in FaDu cells was determined by qRT-PCR. B, C. Western blot was used to detect the effect of S100A11 on PI3K/Akt/mTOR pathway related gene expression. *P<0.05.
Discussion
Surgical removal of tumors is the most straightforward and effective treatment for cancer, however, surgical resection, including laryngectomy, results in the deterioration of communication skills and quality of life due to tracheostoma [1]. Though a number of novel treatments, including targeted molecular therapy, gene therapy, and immunological therapy, have been investigated to inhibit the growth and metastasis of hypopharyngeal carcinoma, no significant progress has been made [4]. In this study, we observed that a reduction in S100A11 might inhibit the migration of FaDu cells and increase the effect of 5-Fu on the growth inhibition of FaDu cells. These findings provide a basis for the development of novel strategies for the early detection and treatment of hypopharyngeal carcinoma.
5-FU is widely used to treat squamous cell carcinoma, however, cancer cells frequently evade drug-induced death signals [21]. As a result, chemoresistance is the major impediment of 5-FU-based therapies. Therefore, the identification of new molecular targets of 5-FU treatment will provide new approaches to increase 5-FU efficacy for HSCC. Recent studies showed that Wnt pathway play important roles in chemotherapy resistance of various malignancies [22]. In the present study, we observed that S100A11 inhibition increases the chemosensitivity of FaDu cells to 5-Fu in vitro. Thus, concurrently treating patients with 5-FU and S100A11 inhibitors may be a viable option for HSCC chemotherapies in the future.
At present, no insight exists concerning the role of S100A11 in HSCC, and the exact mechanism of S100A11 in promoting metastasis is poorly understood. In this study, the analysis of the relationship between S100A11 expression and migration ability in FaDu cells implies that the overexpression of S100A11 might play a role in the metastasis of HSCC. The abnormal expression of S100A11 has been found in many types of cancers. However, the molecular mechanism responsible for the regulation of S100A11 gene expression is still unknown. Previous research showed that DNA methylation might have a role in the control of S100A11 expression, in certain cancer systems, methylation at critical CpG sites within the first intron of the gene regulates transcription [23,24]. Recent study reported that S100A11 promoted the viability and proliferation of human pancreatic cancer PANC-1 cells through the upregulation of the PI3K/AKT signaling pathway [25].
PI3K/Akt/mTOR signaling pathway is involved in growth, differentiation, proliferation, survival of cancer cells [17]. AKT as a key protein in the pathway play an important role in regulating cell viability, growth and proliferation, while PI3K as an upstream effector of the PI3K/AKT signaling pathway, may induce the activation of AKT through phosphorylating threonine and/or serine residues [26-28]. However, the role of PI3K/Akt/mTOR signaling pathway in HSCC progression remains unclear. In the present study, we found that the S100A11 could activate the PI3K/Akt/mTOR signaling pathway, suggesting inactivation of S100A11 or PI3K/Akt/mTOR signaling pathway might inhibit HSCC processes.
Although our experimental data are significantly different, S100A11 must be further tested as a marker associated with metastasis of hypopharyngeal cancer in more patient samples.
In conclusion, our study suggested that S100A11 might play important regulatory roles in the promotion of HSCC migration and the protection of cancer cell death induced by 5-Fu. Additionally, we revealed that S100A11 might regulate PI3K/Akt/mTOR signaling pathway in HSCC tumorigenesis. It was suggested that S100A11 might act as an effective therapeutic candidate for HSCC treatment.
Acknowledgements
This research was supported by Natural Science Foundation of Shanghai (17ZR1438900) and the funds of Shanghai Health and Family Planning Commission (20174Y0176 and 201444).
Disclosure of conflict of interest
None.
Abbreviations
- S100A11
Calcium-binding protein A11
- DMEM
Dulbecco Modified Eagle’s Medium
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate-dehydrogenase
- miRNAs
MicroRNAs
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- OD
optical density
- RT-qPCR
reverse transcription quantitative real-time polymerase chain reaction
- SDS-PAGE
sodium dodecyl sulphate polyacrylamide gel electrophoresis
- HSCC
hypopharygeal squamous cell carcinoma
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Qian Y, Liu D, Cao S, Tao Y, Wei D, Li W, Li G, Pan X, Lei D. Upregulation of the long noncoding RNA UCA1 affects the proliferation, invasion, and survival of hypopharyngeal carcinoma. Mol Cancer. 2017;16:68. doi: 10.1186/s12943-017-0635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muto M, Nakane M, Katada C, Sano Y, Ohtsu A, Esumi H, Ebihara S, Yoshida S. Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Cancer. 2004;101:1375–81. doi: 10.1002/cncr.20482. [DOI] [PubMed] [Google Scholar]
- 4.Taguchi T, Nishimura G, Takahashi M, Komatsu M, Sano D, Sakuma N, Yabuki K, Arai Y, Takahashi H, Hata M, Koike I, Oridate N. Treatment results and prognostic factors for advanced squamous cell carcinoma of the hypopharynx treated with concurrent chemoradiotherapy. Cancer Chemother Pharmacol. 2014;73:1147–1154. doi: 10.1007/s00280-014-2448-2. [DOI] [PubMed] [Google Scholar]
- 5.Salama I, Malone PS, Mihaimeed F, Jones JL. A review of the S100 proteins in cancer. Eur J Surg Oncol. 2008;34:357–364. doi: 10.1016/j.ejso.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature) Biochem Biophys Res Commun. 2004;322:1111–1122. doi: 10.1016/j.bbrc.2004.07.096. [DOI] [PubMed] [Google Scholar]
- 7.Todoroki H, Kobayashi R, Watanabe M, Minami H, Hidaka H. Purification, characterization, and partial sequence analysis of a newly identified EF-hand type 13-kDa Ca(2+)-binding protein from smooth muscle and non-muscle tissues. J Biol Chem. 1991;266:18668–18673. [PubMed] [Google Scholar]
- 8.Wang C, Zhang Z, Li L, Zhang J, Wang J, Fan J, Jiao B, Zhao S. S100A11 is a migration-related protein in laryngeal squamous cell carcinoma. Int J Med Sci. 2013;10:1552–9. doi: 10.7150/ijms.5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rehman I, Azzouzi AR, Cross SS, Deloulme JC, Catto JW, Wylde N, Larre S, Champigneuille J, Hamdy FC. Dysregulated expression of S100A11 (calgizzarin) in prostate cancer and precursor lesions. Hum Pathol. 2004;35:1385–1391. doi: 10.1016/j.humpath.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Kanamori T, Takakura K, Mandai M, Kariya M, Fukuhara K, Sakaguchi M, Huh NH, Saito K, Sakurai T, Fujita J, Fujii S. Increased expression of calcium-binding protein S100 in human uterine smooth muscle tumours. Mol Hum Reprod. 2004;10:735–742. doi: 10.1093/molehr/gah100. [DOI] [PubMed] [Google Scholar]
- 11.Svasti J, Srisomsap C, Subhasitanont P, Keeratichamroen S, Chokchaichamnankit D, Ngiwsara L, Chimnoi N, Pisutjaroenpong S, Techasakul S, Chen ST. Proteomic profiling of cholangiocarcinoma cell line treated with pomiferin from derris malaccensis. Proteomics. 2005;5:4504–4509. doi: 10.1002/pmic.200401315. [DOI] [PubMed] [Google Scholar]
- 12.Melle C, Ernst G, Schimmel B, Bleul A, Mothes H, Kaufmann R, Settmacher U, Von Eggeling F. Different expression of calgizzarin (S100A11) in normal colonic epithelium, adenoma and colorectal carcinoma. Int J Oncol. 2006;28:195–200. [PubMed] [Google Scholar]
- 13.Hao J, Wang K, Yue Y, Tian T, Xu A, Hao J, Xiao X, He D. Selective expression of S100A11 in lung cancer and its role in regulating proliferation of adenocarcinomas cells. Mol Cell Biochem. 2012;359:323–332. doi: 10.1007/s11010-011-1026-8. [DOI] [PubMed] [Google Scholar]
- 14.Memon AA, Sorensen BS, Meldgaard P, Fokdal L, Thykjaer T, Nexo E. Down-regulation of S100C is associated with bladder cancer progression and poor survival. Clin Cancer Res. 2005;11:606–11. [PubMed] [Google Scholar]
- 15.Howell MD, Fairchild HR, Kim BE, Bin L, Boguniewicz M, Redzic JS, Hansen KC, Leung DY. Th2 cytokines act on S100/A11 to downregulate keratinocyte differentiation. J Invest Dermatol. 2008;128:2248–2258. doi: 10.1038/jid.2008.74. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Zhang J, Miao L, Liu K, Yang S, Pan C, Jiao B. Interleukin-11 promotes the progress of gastric carcinoma via abnormally expressed versican. Int J Biol Sci. 2012;8:383–393. doi: 10.7150/ijbs.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Miao L, Sun W, Jiao B, Wang B, Yao L, Huang C. Wentilactone B from aspergillus wentii induces apoptosis and inhibits proliferation and migration of human hepatoma SMMC-7721 cells. Biol Pharm Bull. 2012;35:1964–1971. doi: 10.1248/bpb.b12-00368. [DOI] [PubMed] [Google Scholar]
- 18.Wobus M, Rangwala R, Sheyn I, Hennigan R, Coila B, Lower EE, Yassin RS, Sherman LS. CD44 associates with EGFR and erbB2 in metastasizing mammary carcinoma cells. Appl Immunohistochem Mol Morphol. 2002;10:34–39. doi: 10.1097/00129039-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Wang SJ, Wreesmann VB, Bourguignon LY. Association of CD44 V3-containing isoforms with tumor cell growth, migration, matrix metalloproteinase expression, and lymph node metastasis in head and neck cancer. Head Neck. 2007;29:550–558. doi: 10.1002/hed.20544. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Liu M, Yang B, Li B, Lu J. Role of siRNA silencing of MMP-2 gene on invasion and growth of laryngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2008;265:1385–91. doi: 10.1007/s00405-008-0684-y. [DOI] [PubMed] [Google Scholar]
- 21.Komatsu M, Shiono O, Taguchi T, Sakuma Y, Nishimura G, Sano D, Sakuma N, Yabuki K, Arai Y, Takahashi M, Isitoya J, Oridate N. Concurrent chemoradiotherapy with docetaxel, cisplatin and 5-fluorouracil (TPF) in patients with locally advanced squamous cell carcinoma of the head and neck. Jpn J Clin Oncol. 2014;44:416–421. doi: 10.1093/jjco/hyu026. [DOI] [PubMed] [Google Scholar]
- 22.Noda T, Nagano H, Takemasa I, Yoshioka S, Murakami M, Wada H, Kobayashi S, Marubashi S, Takeda Y, Dono K, Umeshita K, Matsuura N, Matsubara K, Doki Y, Mori M, Monden M. Activation of Wnt beta-catenin signalling pathway induces chemoresistance to interferon-alpha 5-fluorouracil combination therapy for hepatocellular carcinoma. Br J Cancer. 2009;100:1647–1658. doi: 10.1038/sj.bjc.6605064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, Palanisamy N, Schmidt H, Teruya-Feldstein J, Jhanwar SC, Zelenetz AD, Houldsworth J, Chaganti RS. Deregulation of FCGR2B expression by 1q21 rearrangements in follicular lymphomas. Oncogene. 2001;20:7686–7693. doi: 10.1038/sj.onc.1204989. [DOI] [PubMed] [Google Scholar]
- 24.Zheng F, Hasim A, Anwer J, Niyaz M, Sheyhidin I. LMP gene promoter hypermethylation is a mechanism for its down regulation in Kazak’s esophageal squamous cell carcinomas. Mol Biol Rep. 2013;40:2069–75. doi: 10.1007/s11033-012-2138-2. [DOI] [PubMed] [Google Scholar]
- 25.Xiao M, Li T, Ji Y, Jiang F, Ni W, Zhu J, Bao B, Lu C, Ni R. S100A11 promotes human pancreatic cancer PANC1 cell proliferation and is involved in the PI3K/AKT signaling pathway. Oncol Lett. 2018;15:175–182. doi: 10.3892/ol.2017.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto S, Tomita Y, Hoshida Y, Morooka T, Nagano H, Dono K, Umeshita K, Sakon M, Ishikawa O, Ohigashi H. Prognostic significance of activated Akt expression in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:2846–50. doi: 10.1158/1078-0432.ccr-02-1441. [DOI] [PubMed] [Google Scholar]
- 27.Jiang H, Fan D, Zhou G, Li X, Deng H. Phosphatidylinositol 3-kinase inhibitor (LY294002) induces apoptosis of human nasopharyngeal carcinoma in vitro and in vivo. J Exp Clin Cancer Res. 2010;29:34. doi: 10.1186/1756-9966-29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin Y, Qiu S, Shao N, Zheng J. Fucoxanthin and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) synergistically promotes apoptosis of human cervical cancer cells by targeting PI3K/Akt NF-κB signaling pathway. Med Sci Monit. 2018;24:11–18. doi: 10.12659/MSM.905360. [DOI] [PMC free article] [PubMed] [Google Scholar]