Abstract
Purpose: To evaluate the prognostic value of diffusion kurtosis imaging (DKI) for survival prediction of patients with high-grade glioma (HGG). Materials and methods: DKI was performed for fifty-eight patients with pathologically proven HGG by using a 3-T scanner. The mean kurtosis (MK), mean diffusivity (MD) and fractional anisotropy (FA) values in the solid part of the tumor were measured and normalized. Univariate Cox regression analysis was used to evaluate the association between overall survival (OS) and sex, age, Karnofsky performance status (KPS), tumor grade, Ki-67 labeling index (LI), extent of resection, use of chemoradiotherapy, MK, MD, and FA. Multivariate Cox regression analysis including sex, age, KPS, extent of resection, use of chemoradiotherapy, MK, MD, and FA was subsequently performed. Spearman’s correlation coefficient for OS and the area under receiver operating characteristic curve (AUC) for predicting 2-year survival were calculated for each DKI parameter and further compared. Results: In univariate Cox regression analyses, OS was significantly associated with the tumor grade, Ki-67 LI, extent of resection, use of chemoradiotherapy, MK, and MD (P < 0.05 for all). Multivariate Cox regression analyses indicated that MK, MD (hazard ratio = 1.582 and 0.828, respectively, for each 0.1 increase in the normalized value), extent of resection and use of chemoradiotherapy were independent predictors of OS. The absolute value of the correlation coefficient for OS and AUC for predicting 2-year survival by MK (rho = -0.565, AUC = 0.841) were higher than those by MD (rho = 0.492, AUC = 0.772), but the difference was not significant. Conclusion: DKI is a promising tool to predict the survival of HGG patients. MK and MD are independent predictors. MK is potentially better associated with OS than MD, which may lead to a more accurate evaluation of HGG patient survival.
Keywords: Diffusion kurtosis imaging, high-grade glioma, overall survival
Introduction
High-grade gliomas (HGGs) are brain tumors associated with high morbidity and mortality [1]. The median survival time after diagnosis is 1 to 2 years despite aggressive surgical treatment, radiotherapy, and chemotherapy [2,3].
The outcomes and treatment response vary due to the heterogeneity in molecular subtypes [4]. Accumulating evidence has shown that some molecular markers are reliable for HGG prognosis, such as Ki-67 and isocitrate dehydrogenase (IDH) [5-10]. However, this information could be obtained only through invasive approaches and could not be dynamically monitored. Therefore, the development of a noninvasive method is crucial to dynamically guide individual therapeutic plans.
Diffusion-weighted imaging (DWI) and diffusion tensor imaging (DTI) have been valuable in evaluating the survival of glioma patients [4,11-15]. However, these methods may have bias in reflecting the tumor microstructure due to their inherent limitations [16].
Diffusion kurtosis imaging (DKI) is an advanced technique for quantifying the non-Gaussianity of water motion in vivo [17], and conventional parameters such as the mean diffusivity (MD) and fractional anisotropy (FA) could also be derived from this method. Previous studies suggested that diffusion kurtosis may be a marker for microstructural complexity and is superior to conventional diffusion parameters in grading gliomas and reflecting tumor proliferation [16,18-23]. However, the value of DKI in predicting the survival of HGG patients has not been investigated. Therefore, this study aimed to evaluate the prognostic value of DKI for HGG patient survival.
Materials and methods
Patient population
This study was approved by the local ethics committee. Informed consent was obtained from every patient before inclusion. Between July 2012 and May 2015, eighty-two patients were enrolled and underwent a series of magnetic resonance imaging (MRI) examinations. The inclusion criteria were as follows: 1) those who were newly diagnosed and were ultimately confirmed to have primary HGG and 2) those with complete preoperative DKI data. The exclusion criteria were as follows: 1) poor image quality, namely, obvious artifacts or head motion, 2) surgery performed more than 4 weeks after the MR data collection, or 3) loss to follow-up. Ultimately, 12 patients were excluded because of a non-HGG diagnosis or because they did not undergo surgery in our institution, and 12 patients were excluded because they were lost to follow-up; a total of 58 patients were included.
Clinical information
The clinical characteristics, including sex, age, Karnofsky performance status (KPS), symptoms and their duration, resection or biopsy, extent of resection, and use of chemoradiotherapy were recorded.
MR protocol
All image acquisitions were performed on a 3-T MR scanner (Discovery MR750, GE Medical Systems, Milwaukee, WI, USA) with a 32-channel head coil.
DKI data were obtained by using a spin-echo echo-planar imaging (SE-EPI) sequence (repetition time (TR) = 6,500 ms, echo time (TE) = 85 ms, number of excitations (NEX) = 1, matrix = 128 × 128, number of sections = 43, slice thickness = 3 mm, gap = 0 mm, field of view (FOV) = 256 × 256 mm2, b = 0, 1,250 and 2,500 s/mm2, twenty-five uniformly distributed directions for each nonzero b-value were applied, and acquisition time = 5 min 45 s). Array Spatial Sensitivity Encoding Technique (ASSET), Real Time Field Adjustment, and Phase Correct were performed to increase the imaging quality.
Routine clinical sequences were applied and served as the references for DKI, including the transverse T1 fluid-attenuated inversion recovery (FLAIR, TR = 2,992 ms, TE = 24 ms, inversion time (TI) = 869 ms) before and after gadolinium administration, transverse T2 fast spin-echo (FSE, TR = 4,599 ms, TE = 102 ms) and T2-FLAIR (TR = 8,000 ms, TE = 160 ms, TI = 2,100 ms).
MR data processing
The DKI data were corrected for eddy current distortions and head motion by using the FMRIB Software Library (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki). Maps of the mean kurtosis (MK), MD, and FA values were calculated with the Diffusional Kurtosis Estimator (version 2.5.1, Medical University of South Carolina). Coregistration was performed to match the diffusion parametric maps with the anatomical images. Two blinded neuroradiologists (with 8 and 5 years of experience, respectively) independently drew regions of interest (ROIs) around the solid part of each tumor (areas with gadolinium enhancement or with the lowest T2 signal intensity) and around the contralateral normal-appearing white matter (cNAWM), avoiding regions with cysts, necrosis, hemorrhage, obvious vessels, edema, and calcifications. The values from the solid tumors were then normalized by dividing by the values from the cNAWM. The two sets of values were used for interobserver variability analysis, and only the normalized values measured by the neuroradiologist with 8 years of experience were further analyzed.
Pathological data acquisition and analysis
The histological type and grade of the tumors were determined according to the WHO classification criteria [24,25]. Immunohistochemical staining for Ki-67 was performed by using the Envision method (Clone No. UMAB107, dilution 1:300). The Ki-67 labeling index (LI) was obtained by calculating the percentage of positively stained nuclei in the hot-spot areas.
Follow-up
The overall survival (OS) was defined as the length of time from the date of DKI to death. Patients still alive or lost to follow-up were censored. The last follow-up was in March 2018.
Statistical analysis
Statistical analyses were performed with SPSS (Version 19.0.0, IBM, Armonk, NY, USA) and R software (version 3.5.2; http://www.r-project.org/). Interobserver variation was assessed by calculating the intraclass correlation coefficient (ICC). Univariate Cox regression analysis was performed to evaluate the association between OS and sex, age, KPS, grade, Ki-67 LI, extent of resection, use of chemoradiotherapy, MK, MD and FA. Multivariate Cox regression analysis was further performed to identify the independent factors predictive of death from tumor-related causes. Spearman correlation analysis was used to evaluate the correlation between OS and each DKI parameter for the deceased HGG patients. The ability of each DKI parameter to predict 2-year survival was evaluated using time-dependent receiver operating characteristic (ROC) analysis. The Spearman’s correlation coefficient and area under the curve (AUC) of each DKI parameter were further compared to obtain the better predictor.
Results
Among the 58 patients who were finally included, 26 had anaplastic astrocytoma, 4 had anaplastic oligodendroglioma, and 28 had glioblastoma. The main symptoms were epilepsy, headache, nausea, limb weakness, and vomiting. Fifty-four patients died from tumor-related causes during follow-up, and their average OS was 567.379 days. The other clinical and pathological features are shown in Table 1.
Table 1.
Detailed information and characteristics of the patients with HGGs
| Characteristics | Value, n (%) | Mean ± SD |
|---|---|---|
| Total | 58 | |
| Age | 45.830±12.775 (years) | |
| Sex (Male) | 32 (55.172%) | |
| KPS | 60.900±9.589 (%) | |
| Duration of symptoms | 134.550±321.900 (days) | |
| Diagnosed with epilepsy | 11 (18.966%) | |
| Surgery | 58 (100.00%) | |
| Extent of resection | ||
| GTR | 33 (56.897%) | |
| < GTR | 25 (43.103%) | |
| Ki-67 LI | 24.550±19.095 (%) | |
| Grade | ||
| Grade III | 30 (51.724%) | |
| Grade IV | 28 (48.276%) | |
| Use of a standardized chemoradiotherapy protocol | 30 (51.724%) | |
| Deceased from tumor-related causes | 54 (93.103%) | |
| OS (for deceased patients) | 54 (93.103%) | 567.379±65.489 (days) |
KPS: Karnofsky performance status; GTR: gross total resection; Ki-67 LI: Ki-67 labeling index; OS: overall survival.
Table 2 shows the interobserver variability of the measurements. The ICCs were between 0.960 and 0.987, indicating excellent reproducibility.
Table 2.
Interobserver variability of the measurements of HGG patients performed by two observers
| Region | Parameters | Interobserver variability as the ICC (95% CI) |
|---|---|---|
| Solid region of the tumor | MK | 0.960 (0.933-0.976) |
| MD | 0.970 (0.950-0.982) | |
| FA | 0.972 (0.953-0.983) | |
| cNAWM | MK | 0.975 (0.959-0.985) |
| MD | 0.970 (0.950-0.982) | |
| FA | 0.987 (0.978-0.992) |
MK: mean kurtosis; MD: mean diffusivity; FA: fractional anisotropy; cNAWM: contralateral normal-appearing white matter; MK: mean kurtosis; MD: mean diffusivity; FA: fractional anisotropy.
Table 3 shows the hazard ratio (HR) of each factor in the univariate Cox regression analyses. Reduced OS was significantly associated with a high tumor grade (HR = 2.371, P = 0.003), high Ki-67 LI (HR = 1.021 per 1% increase, P = 0.004) and high MK value (HR = 1.516 per 0.1 increase, P < 0.001), while treatment with gross tumor resection (GTR) (HR = 0.533, P = 0.024), use of a standardized chemoradiotherapy protocol (HR = 0.442, P = 0.004), and a high MD value (HR = 0.853 per 0.1 increase, P < 0.001) were significantly associated with a long OS. In contrast, sex, age, KPS, and FA were not significantly associated with OS (P > 0.05 for all).
Table 3.
Univariate Cox regression analyses demonstrating the associations between OS and sex, age, KPS, tumor grade, Ki-67 LI, extent of resection, MK, MD and FA in patients with HGGs
| Regressor | HR (95% CI) | P Value |
|---|---|---|
| Sex (Male) | 0.917 (0.533-1.577) | 0.754 |
| Age | 1.002 (0.979-1.027) | 0.842 |
| KPS | 1.000 (0.969-1.033) | 0.978 |
| Tumor grade (Grade IV) | 2.371 (1.343-4.187) | 0.003* |
| Ki-67 LI | 1.021 (1.007-1.036)# | 0.004* |
| Extent of resection (GTR) | 0.533 (0.308-0.922) | 0.024* |
| Use of a standardized chemoradiotherapy protocol | 0.442 (0.255-0.766) | 0.004* |
| MK | 1.516 (1.292-1.779)# | < 0.001* |
| MD | 0.853 (0.782-0.929)# | < 0.001* |
| FA | 1.080 (0.900-1.297)# | 0.407 |
indicates P < 0.05;
indicates the hazard ratio and 95% confidence interval for each 1% (for Ki-67 LI) or 0.1 (for MK, MD and FA) increase in the parameter value.
HR: hazard ratio; CI: confidence interval; KPS: Karnofsky performance status; Ki-67 LI: Ki-67 labeling index; GTR: gross total resection; MK: mean kurtosis; MD: mean diffusivity; FA: fractional anisotropy.
Multivariate Cox proportional hazards analysis was further performed for sex, age, KPS, extent of resection, use of chemoradiotherapy, MK, MD and FA with respect to OS. Due to the colinearity that exists among MK, MD and FA, these three parameters were not inputted together into one Cox model; instead, three Cox models were performed for each DKI parameter. After adjusting by sex, age and KPS, MK (HR = 1.582 per 0.1 increase, P < 0.001) was a significant predictor of reduced OS, and it was a risk factor; MD (HR = 0.828 per 0.1 increase, P < 0.001) was also a significant predictor of OS and was a protective factor; however, FA (HR = 0.996 per 0.1 increase, P = 0.971) was not a significant predictor of OS.
In addition, both treatment with GTR (Cox model 1: HR = 0.299, P < 0.001; Cox model 2: HR = 0.362, P = 0.002; Cox model 3: HR = 0.420, P = 0.008) and use of a standardized chemoradiotherapy protocol (Cox model 1: HR = 0.482, P = 0.024; Cox model 2: HR = 0.303, P < 0.001; Cox model 3: HR = 0.369, P = 0.002) were significant predictors of OS, and they were both protective factors. In contrast, the other factors were not significant predictors of OS, and the corresponding P values and HRs (95% CIs) are shown in Table 4.
Table 4.
Comparison of the ability of DKI parameters to evaluate HGG patient survival using multivariate Cox regression analyses
| Regressor | Cox model 1 | Cox model 2 | Cox model 3 | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Sex (Female) | 0.806 (0.435-1.495) | 0.494 | 0.970 (0.523-1.800) | 0.924 | 1.129 (0.601-2.121) | 0.705 |
| Age | 0.995 (0.971-1.020) | 0.698 | 1.001 (0.976-1.026) | 0.959 | 0.993 (0.969-1.017) | 0.547 |
| KPS | 1.021 (0.986-1.057) | 0.250 | 1.016 (0.982-1.053) | 0.359 | 1.012 (0.976-1.051) | 0.516 |
| Extent of resection (GTR) | 0.299 (0.154-0.582) | < 0.001* | 0.362 (0.190-0.692) | 0.002* | 0.420 (0.222-0.795) | 0.008* |
| Use of a standardized chemoradiotherapy protocol | 0.482 (0.255-0.909) | 0.024* | 0.303 (0.156-0.590) | < 0.001* | 0.369 (0.197-0.690) | 0.002* |
| MK | 1.582 (1.316-1.901)# | < 0.001* | - | - | - | - |
| MD | - | - | 0.828 (0.756-0.907)# | < 0.001* | - | - |
| FA | - | - | - | - | 0.996 (0.816-1.216)# | 0.971 |
indicates P < 0.05;
indicates the hazard ratio and 95% confidence interval for each 0.1 increase of the parameter value.
HR: hazard ratio; CI: confidence interval; KPS: Karnofsky performance status; GTR: gross total resection; MK: mean kurtosis; MD: mean diffusivity; FA: fractional anisotropy.
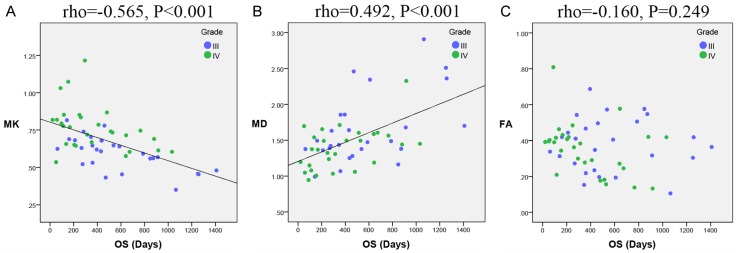
To compare the ability of the three DKI parameters to predict HGG patient survival, Spearman correlation analysis was performed to evaluate the correlation between the survival time and each DKI parameter for the 54 deceased HGG patients. OS was significantly correlated with MK (rho = -0.565, P < 0.001) and MD (rho = 0.492, P < 0.001). In contrast, FA was not significantly correlated with survival time (rho = -0.160, P = 0.249). The scatter diagrams demonstrating the correlations between OS and MK, MD and FA are shown in Figure 1. The correlation coefficient of MK was higher than that of MD, although the difference between these two correlation coefficients was not significant (Z = 0.512, P = 0.608). The correlation coefficient of MK was significantly higher than that of FA (Z = 2.418, P = 0.016). Additionally, time-dependent ROC curves were also created, as shown in Figure 2. The 2-year survival period was evaluated because the average OS of the HGG patients was 567.379 days, which is nearly 2 years. The curves indicated that MK (AUC = 0.841) had a better performance than MD (AUC = 0.772) and FA (AUC = 0.506) in predicting the 2-year survival of HGG patients. The AUC of MK was higher than that of MD (P = 0.283), but this difference was not significant, and the AUC of MK was significantly higher than that of FA (P = 0.002).
Figure 1.
Correlations between OS and the DKI parameters. Scatter diagrams demonstrating the correlations between OS and (A) MK, (B) MD, and (C) FA. MK and MD were found to be significantly correlated with OS. In contrast, FA was not significantly correlated with OS. OS: overall survival; MK: mean kurtosis; MD: mean diffusivity; FA: fractional anisotropy.
Figure 2.
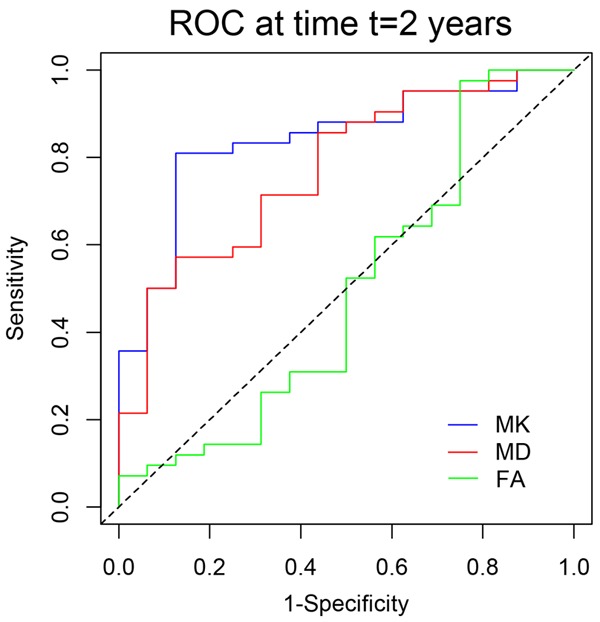
Time-dependent ROC curves of each DKI parameter for predicting the 2-year survival of HGG patients. The AUCs of MK, MD and FA were 0.841, 0.772 and 0.506, respectively. The AUC of MK was higher than that of MD and was significantly higher than that of FA. ROC: receiver operating characteristic curve; DKI: diffusion kurtosis imaging; HGG: high-grade glioma; AUC: area under curve; MK: mean kurtosis; MD: mean diffusivity; FA: fractional anisotropy.
Discussion
Our results showed that MK and MD are both significantly associated with the survival time of patients who were newly diagnosed with HGG. Patients with tumors that display high MK values or low MD values are prone to having short lifespans after diagnosis.
To the best of our knowledge, no study correlating MK with survival time for patients with gliomas or HGGs has been published. In this study, MK was found to be an independent predictor of reduced OS, and MK was a risk factor. A higher MK indicates higher complexity of the microstructures within the tumor, which can present as tumors with greater nuclear atypia, higher cellular pleomorphism, more necrosis and more microvascular proliferation [26]. This increased complexity usually indicates tumors to be more aggressive and therefore may result in reduced OS.
Several studies have shown that a low apparent diffusion coefficient (ADC) was associated with a decrease in survival for patients with gliomas [27-31]. Saksena et al. demonstrated that DTI parameters can be used to evaluate progression-free survival in patients with glioblastomas. The authors believe that these parameters could be useful for treatment planning as HGGs with low minimal ADC values may be treated more aggressively than those with high ADC values [4]. In this study, the MD value calculated from the DKI data was used instead of the conventional ADC because like the ADC, MD also reflects the water diffusivity but may define water diffusivity in the brain more precisely than ADC. Similarly, a low MD was also found to be an independent predictor of reduced OS, and MD was a protective factor, which is in accordance with previously published studies. An inverse relationship between the ADC and glioma grade has been demonstrated, and this relationship is associated with tumor cellularity [32]. A low MD is indicative of high cellularity and hence a large tumor burden [33]; therefore, a low MD is associated with a short survival period.
FA was not significantly correlated with survival time, which is slightly different from the results of a previous study [4]. The reported relationship between FA and tumor cellularity is controversial [34]. Therefore, the relationship between FA and survival time may require further investigation.
Both the absolute value of the correlation coefficient for OS and AUC for predicting 2-year survival by MK were higher than those by MD, but the difference was not significant, indicating that MK is potentially better associated with HGG patient survival than MD. The lack of a noticeable difference in AUCs between MK and MD may be due to the limited patient cohort in this study. In contrast, both the correlation coefficient and AUC of MK were significantly higher than those of FA, indicating that MK performs better than FA in predicting the survival of HGG patients. Therefore, as a better imaging predictor than the other DKI parameters, MK has great potential to more accurately evaluate the survival of HGG patients and better manage individual treatment plans.
This study still has several limitations. The results of our analysis are mainly limited by the relatively small patient cohort of our study. Therefore, this study did not further analyze the association of DKI parameters with OS when stratified by HGG grade (grades III and IV). However, we believe that the conclusions drawn from this study are still valuable for clinics. Another limitation is that 7 patients did not undergo Ki-67 detection, but we believe that this had only a slight effect on the statistical analyses.
In conclusion, DKI is a promising tool to predict HGG patient survival. MK and MD are independent imaging predictors of survival time; MK is potentially better associated with OS than MD, and MK may lead to a more accurate evaluation of HGG patient survival. As a potentially better imaging predictor than the other DKI parameters, MK may be more helpful in guiding treatment strategies to prolong OS for patients with HGGs.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81570462 and No. 30870702), the Natural Science Foundation of Fujian Province (No. 2018J05135) and Joint Funds for the Innovation of Science and Technology, Fujian province (Grant number: 2017Y9024). The authors thank Hao Zhang, Haijun Sang and Siquan Wang for their assistance with following up with the patients and Bangwei Zeng and Chanchan Liu for their assistance with the statistical analyses.
Disclosure of conflict of interest
None.
References
- 1.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 2.Oh J, Henry RG, Pirzkall A, Lu Y, Li X, Catalaa I, Chang S, Dillon WP, Nelson SJ. Survival analysis in patients with glioblastoma multiforme: predictive value of choline-to-N-acetylaspartate index, apparent diffusion coefficient, and relative cerebral blood volume. J Magn Reson Imaging. 2004;19:546–554. doi: 10.1002/jmri.20039. [DOI] [PubMed] [Google Scholar]
- 3.Marijnen CA, van den Berg SM, van Duinen SG, Voormolen JH, Noordijk EM. Radiotherapy is effective in patients with glioblastoma multiforme with a limited prognosis and in patients above 70 years of age: a retrospective single institution analysis. Radiother Oncol. 2005;75:210–216. doi: 10.1016/j.radonc.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Saksena S, Jain R, Narang J, Scarpace L, Schultz LR, Lehman NL, Hearshen D, Patel SC, Mikkelsen T. Predicting survival in glioblastomas using diffusion tensor imaging metrics. J Magn Reson Imaging. 2010;32:788–795. doi: 10.1002/jmri.22304. [DOI] [PubMed] [Google Scholar]
- 5.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, Feuerstein BG, Aldape K. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Colman H, Zhang L, Sulman EP, McDonald JM, Shooshtari NL, Rivera A, Popoff S, Nutt CL, Louis DN, Cairncross JG, Gilbert MR, Phillips HS, Mehta MP, Chakravarti A, Pelloski CE, Bhat K, Feuerstein BG, Jenkins RB, Aldape K. A multigene predictor of outcome in glioblastoma. Neuro Oncol. 2010;12:49–57. doi: 10.1093/neuonc/nop007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kayaselcuk F, Zorludemir S, Gumurduhu D, Zeren H, Erman T. PCNA and Ki-67 in central nervous system tumors: correlation with the histological type and grade. J Neurooncol. 2002;57:115–121. doi: 10.1023/a:1015739130208. [DOI] [PubMed] [Google Scholar]
- 8.Neder L, Colli BO, Machado HR, Carlotti CG Jr, Santos AC, Chimelli L. MIB-1 labeling index in astrocytic tumors--a clinicopathologic study. Clin Neuropathol. 2004;23:262–270. [PubMed] [Google Scholar]
- 9.Molenaar RJ, Radivoyevitch T, Maciejewski JP, van Noorden CJ, Bleeker FE. The driver and passenger effects of isocitrate dehydrogenase 1 and 2 mutations in oncogenesis and survival prolongation. Biochim Biophys Acta. 2014;1846:326–341. doi: 10.1016/j.bbcan.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Molenaar RJ, Verbaan D, Lamba S, Zanon C, Jeuken JW, Boots-Sprenger SH, Wesseling P, Hulsebos TJ, Troost D, van Tilborg AA, Leenstra S, Vandertop WP, Bardelli A, van Noorden CJ, Bleeker FE. The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro Oncol. 2014;16:1263–1273. doi: 10.1093/neuonc/nou005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang W, Pope WB, Harris RJ, Hardy AJ, Leu K, Mody RR, Nghiemphu PL, Lai A, Cloughesy TF, Ellingson BM. Diffusion MR characteristics following concurrent radiochemotherapy predicts progression-free and overall survival in newly diagnosed glioblastoma. Tomography. 2015;1:37–43. doi: 10.18383/j.tom.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elson A, Paulson E, Bovi J, Siker M, Schultz C, Laviolette PS. Evaluation of pre-radiotherapy apparent diffusion coefficient (ADC): patterns of recurrence and survival outcomes analysis in patients treated for glioblastoma multiforme. J Neurooncol. 2015;123:179–188. doi: 10.1007/s11060-015-1782-5. [DOI] [PubMed] [Google Scholar]
- 13.Poussaint TY, Vajapeyam S, Ricci KI, Panigrahy A, Kocak M, Kun LE, Boyett JM, Pollack IF, Fouladi M. Apparent diffusion coefficient histogram metrics correlate with survival in diffuse intrinsic pontine glioma: a report from the Pediatric Brain Tumor Consortium. Neuro Oncol. 2016;18:725–734. doi: 10.1093/neuonc/nov256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaw TM, Pope WB, Cloughesy TF, Lai A, Nghiemphu PL, Ellingson BM. Short-interval estimation of proliferation rate using serial diffusion MRI predicts progression-free survival in newly diagnosed glioblastoma treated with radiochemotherapy. J Neurooncol. 2014;116:601–608. doi: 10.1007/s11060-013-1344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zolal A, Juratli TA, Linn J, Podlesek D, Sitoci Ficici KH, Kitzler HH, Schackert G, Sobottka SB, Rieger B, Krex D. Enhancing tumor apparent diffusion coefficient histogram skewness stratifies the postoperative survival in recurrent glioblastoma multiforme patients undergoing salvage surgery. J Neurooncol. 2016;127:551–557. doi: 10.1007/s11060-016-2063-7. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Chen X, Chen D, Wang Z, Li S, Zhu W. Grading and proliferation assessment of diffuse astrocytic tumors with monoexponential, biexponential, and stretched-exponential diffusion-weighted imaging and diffusion kurtosis imaging. Eur J Radiol. 2018;109:188–195. doi: 10.1016/j.ejrad.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53:1432–40. doi: 10.1002/mrm.20508. [DOI] [PubMed] [Google Scholar]
- 18.Bai Y, Lin Y, Tian J, Shi D, Cheng J, Haacke EM, Hong X, Ma B, Zhou J, Wang M. Grading of gliomas by using monoexponential, biexponential, and stretched exponential diffusion-weighted MR imaging and diffusion kurtosis MR imaging. Radiology. 2016;278:496–504. doi: 10.1148/radiol.2015142173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexiou GA, Tsiouris S, Kyritsis AP, Argyropoulou MI, Voulgaris S, Fotopoulos AD. Assessment of glioma proliferation using imaging modalities. J Clin Neurosci. 2010;17:1233–1238. doi: 10.1016/j.jocn.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Van Cauter S, De Keyzer F, Sima DM, Sava AC, D’Arco F, Veraart J, Peeters RR, Leemans A, Van Gool S, Wilms G, Demaerel P, Van Huffel S, Sunaert S, Himmelreich U. Integrating diffusion kurtosis imaging, dynamic susceptibility-weighted contrast-enhanced MRI, and short echo time chemical shift imaging for grading gliomas. Neuro Oncol. 2014;16:1010–1021. doi: 10.1093/neuonc/not304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cha S. Update on brain tumor imaging: from anatomy to physiology. AJNR Am J Neuroradiol. 2006;27:475–487. [PMC free article] [PubMed] [Google Scholar]
- 22.Blow JJ, Tanaka TU. The chromosome cycle: coordinating replication and segregation. Second in the cycles review series. EMBO Rep. 2005;6:1028–1034. doi: 10.1038/sj.embor.7400557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer EJ, Ikmi A, Gibson MC. Interkinetic nuclear migration is a broadly conserved feature of cell division in pseudostratified epithelia. Curr Biol. 2011;21:485–491. doi: 10.1016/j.cub.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 25.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tietze A, Hansen MB, Ostergaard L, Jespersen SN, Sangill R, Lund TE, Geneser M, Hjelm M, Hansen B. Mean diffusional kurtosis in patients with glioma: initial results with a fast imaging method in a clinical setting. AJNR Am J Neuroradiol. 2015;36:1472–1478. doi: 10.3174/ajnr.A4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui Y, Ma L, Chen X, Zhang Z, Jiang H, Lin S. Lower apparent diffusion coefficients indicate distinct prognosis in low-grade and high-grade glioma. J Neurooncol. 2014;119:377–385. doi: 10.1007/s11060-014-1490-6. [DOI] [PubMed] [Google Scholar]
- 28.Higano S, Yun X, Kumabe T, Watanabe M, Mugikura S, Umetsu A, Sato A, Yamada T, Takahashi S. Malignant astrocytic tumors: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology. 2006;241:839–846. doi: 10.1148/radiol.2413051276. [DOI] [PubMed] [Google Scholar]
- 29.Murakami R, Sugahara T, Nakamura H, Hirai T, Kitajima M, Hayashida Y, Baba Y, Oya N, Kuratsu J, Yamashita Y. Malignant supratentorial astrocytoma treated with postoperative radiation therapy: prognostic value of pretreatment quantitative diffusion-weighted MR imaging. Radiology. 2007;243:493–499. doi: 10.1148/radiol.2432060450. [DOI] [PubMed] [Google Scholar]
- 30.Yamasaki F, Sugiyama K, Ohtaki M, Takeshima Y, Abe N, Akiyama Y, Takaba J, Amatya VJ, Saito T, Kajiwara Y, Hanaya R, Kurisu K. Glioblastoma treated with postoperative radio-chemotherapy: prognostic value of apparent diffusion coefficient at MR imaging. Eur J Radiol. 2010;73:532–537. doi: 10.1016/j.ejrad.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Zulfiqar M, Yousem DM, Lai H. ADC values and prognosis of malignant astrocytomas: does lower ADC predict a worse prognosis independent of grade of tumor?--a meta-analysis. AJR Am J Roentgenol. 2013;200:624–629. doi: 10.2214/AJR.12.8679. [DOI] [PubMed] [Google Scholar]
- 32.Sugahara T, Korogi Y, Kochi M, Ikushima I, Shigematu Y, Hirai T, Okuda T, Liang L, Ge Y, Komohara Y, Ushio Y, Takahashi M. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999;9:53–60. doi: 10.1002/(sici)1522-2586(199901)9:1<53::aid-jmri7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 33.Crawford FW, Khayal IS, McGue C, Saraswathy S, Pirzkall A, Cha S, Lamborn KR, Chang SM, Berger MS, Nelson SJ. Relationship of pre-surgery metabolic and physiological MR imaging parameters to survival for patients with untreated GBM. J Neurooncol. 2009;91:337–351. doi: 10.1007/s11060-008-9719-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang R, Jiang J, Zhao L, Zhang J, Zhang S, Yao Y, Yang S, Shi J, Shen N, Su C, Zhu W. Diffusion kurtosis imaging can efficiently assess the glioma grade and cellular proliferation. Oncotarget. 2015;6:42380–42393. doi: 10.18632/oncotarget.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]