Abstract
Studies have suggested trichosanthin (TCS) exerts antitumor activity mainly through direct cytotoxicity toward cancer cells and immune regulation. In this study, we conducted the proliferation and apoptosis assay on A20 cells and endothelial cells (ECs) with different concentrations of TCS and investigated the levels of gene expression linked to angiogenesis. Herein, a new mechanism that TCS inhibits murine B-cell lymphoma growth by anti-angiogenesis was reported. First, TCS inhibit tumor growth and prolonged survival significantly in vivo, and TCS depressed the formation of new blood vessels around the tumor in a dose-dependent manner. Further studies showed that the platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31)-positive endothelial cell numbers, and the serum levels of MMP-2 and MMP-9 were also lower in the study group than controls. However, TCS did neither change the ratio of T cells and NK cells in the spleen of treated mice nor affect the proliferation and apoptosis of A20 cells in vitro. Additionally, the newly formed blood vessels in chorioallantoic membranes treated with TCS were significantly reduced. Last, TCS may suppress the proliferation, induce apoptosis and decrease tube formation and migration of endothelial cells (ECs). And, the mRNA and protein levels of VEGF in ECs treated with TCS were lower than that in the control group. These findings confirm that inhibitory effect of TCS on A20 murine B-cell lymphoma growth is mediated via anti-angiogenesis, and which may be associated with the down-regulation of VEGF and MMPs expression. This is an indication that TCS may represent a natural anti-angiogenic drug for lymphoma therapy.
Keywords: Trichosanthin, lymphoma, angiogenesis, vascular endothelial growth factor, A20 cells, endothelial cells
Introduction
Lymphoma is a systemic disease, requiring systemic treatment, such as, chemotherapy, radiotherapy, biotherapy, immunotherapy, bone marrow transplantation. Although the development of diverse chemotherapies now can achieve a good therapeutic effect, it is incurable in the vast majority of cases. From 1960 to 2006, the relative survival rate increased from 31% to 69%, leading to the increasing of long-term survivors [1]. However, the advances in medical treatment have far outpaced the use of complementary and alternative medicine (CAM) knowledge in this population [2]. The use of CAM among patients with hematological malignancies is prevalent and herbal medicine is one of the leading CAM modalities [3].
Trichosanthin (TCS), extracted from the root tubers of the Chinese medical herb Trichosanthes kirilowi, has been used as an abortifacient for 1,500 years in China for its high toxicity on trophoblasts. Modern pharmacological study indicate that TCS is a type I ribosome-inactivating protein (RIP) comprising 247 amino acids [4]. Over the past 20 years, the antitumor activity of TCS has aroused considerable interest of researchers [5]. Studies have shown that TCS plays an anti-tumor role through different mechanisms, such as direct cytotoxicity [6-8], immune regulation [9,10], demethylation function [11].
It has reported that TCS can also inhibit tumor angiogenesis. TCS could reduce level of vascular endothelial growth factor (VEGF) transcription and secretion in JAR cells, and inhibit the angiogenesis signal induced by tumor cells and mediated by extracellular signal-regulated kinase in endothelial cells (ECs) and blood vessels [12]. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) is a 130-kDa member of the immunoglobulin superfamily that is expressed on ECs, platelets and most leukocytes [13]. Research suggests that CD31 mediates vasculogenesis and angiogenesis [14].
Matrix metalloproteinases (MMPs), a family of at least 24 zinc-dependent endopeptidases, which degrades the basement membrane and almost all protein components of the extracellular matrix (ECM), were believed to play an essential role in angiogenesis [15]. Traditionally, MMP-2 and MMP-9 have been correlated with the invasive stage of carcinomas, because of their ability to degrade type IV collagen that a major constituent of basement membranes [16]. There is evidence to suggest that MMP-2 and MMP-9 functioned as important factors for tumor invasion, metastasis and angiogenesis [17].
There is growing interest in exploiting this traditional herbal for the treatment of hematological cancers, especially lymphomas. Here we report the inhibitory effects of TCS against the murine B-cell lymphoma in vivo. The potential antitumor mechanisms were also unveiled.
Materials and methods
Trichosanthin
TCS Injection (1.2 mg/mL) was purchased from Shanghai Jinshan Pharmacyeutical CO., Ltd (Shanghai, China). In the study, the same batch of TCS was used, its purity is more than 99% as indicated by SDS-PAGE analysis.
Mice
Male BABL/C mices (6 weeks old, 20-22 g) were purchased from College of Veterinary Medicine Yangzhou University and housed in a pathogen-free environment in center of Laboratory Animal, Jiangsu Province Hospital of TCM. All mice were treated humanely in strict accordance with Institutional Animal Care and Use Committee guidelines, and all animal procedures were approved by the Animal Care and Scientific Committee of Jiangsu Province Hospital of TCM.
Cell lines
The mouse B-cell lymphoma A20 cells were obtained from molecular biology laboratory, Jiangsu Province Hospital of TCM. Endothelial cells (ECs) were prepared as described [18]. The cells were cultured at 37°C under 5% CO2 in RPMI 1640 medium (Biowest, Nuaillé, France) containing 10% heat-inactivated fetal bovine serum (Biowest, Nuaillé, France), 100 IU/mL penicillin and 100 mg/mL streptomycin sulfate.
Animal experiments
In vivo assay, approximately 1.0 × 106 A20 cells in 100 μL PBS were injected hypodermically into the right forelimbs of male BABL/c mice. After the onset of visible solid tumor, mice were randomized to four groups and were given a daily intraperitoneal injection of TCS (0.2, 0.4 and 0.8 mg/kg) or vehicle (200 µL PBS) for 7 days. The tumor dimensions were measured every two days using a digital caliper, and tumor volume was calculated following the formula: V = 0.52 × length × width2. Three weeks after tumor implantation, the mice were sacrificed and the tumors were excised and weighed. To further investigate the anti-lymphoma activity of TCS in subcutaneous murine A20 tumor model, the survival times of mice were recorded (5 mice per group).
For tumor peritumorous angiogenesis experiment, solid tumor models were induced and grouped as outlined above. The therapeutic period was 7 days. After the onset of visible solid tumor, mice were sacrificed. About 0.5 mL blood was taken by removalling eyeball for ELISA assay of Matrix metalloproteinases (MMPs). Tumors and peritumorous blood vessels were photographed. The tumor tissue along with spleen were also collected and analyzed.
Proliferation assay in vitro
The inhibitory effect of TCS on growth of A20 and ECs in 96-well plates was evaluated by Cell Counting Kit-8 (CCK-8) assay (Dojindo, Kumamoto, Japan). Briefly, cells were treated with different concentrations of TCS (0, 2.5, 5, 10, 20 and 40 μg/mL) for 24, 48 and 72 h. After treatment, cell proliferation was detected following the manufacturer’s protocol.
Apoptosis assay in vitro
The apoptosis was assessed using Annexin V-FITC/propidium iodide (PI) method with flow cytometry (FCM). Briefly, A20 and ECs were incubated in 6-well plates with different concentrations of TCS (treatment concentration depends on proliferation assay) for 48 h. Then, Annexin V-FITC/PI staining of the cells was performed according to the manufacturer’s instructions (Biouniquer, China). The stained cells were analyzed within 1 h by FCM (Beckman, Epic Altra, USA).
Immune function analysis
Splenocytes from tumor-bearing BABL/c mice were harvested by pressing through a nylon mesh. Red blood cells were lysed using Red Blood Cell Lysis Buffer (Beyotime, Nantong, China) and then PBS was used to wash cells. Lymphocyte populations were identified using CD4-TITC, CD8-FITC, CD3-PE and NK-1.1-PE, and the stained samples were analyzed on a Beckman Coulter FCM500.
Peritumorous angiogenesis and hematoxylin-eosin (HE) staining for micro vessel density (MVD)
Morphological characteristics of microvasculature on the surface of mice subcutaneous tumor were photographed, then the tumor was processed for HE staining for measurement of MVD using Carl Zeiss microscope as reported earlier [19].
Fluorescence immunostaining
Tumors were fixed in 4% paraformaldehyde overnight, and then kept in 30% sucrose at 4°C for 48 h. The 3 μm slices were prepared with frozen section machine. Slices were blocked with PBS containg 10% normal rat serum for 1 h at room temperature. Immunostaining of the cells was performed using the anti-CD31-FITC mAb (eBioscience) for 60 min at 4°C. Slides were observed using a confocal laser scanning microscope (Ziess, LSM510).
Chick embryo chorioallantoic membrane assay
An 8-day embryo was used. A 1-2 cm2 window in the shell concealing the air sac was punctured carefully. Then the shell membrane was removed to expose the chorioallantoic membrane. A sterilized filter paper (5 mm diameter) soaked in TCS (2.5, 10, 40 μg/mL) or normal saline (NS) was implanted on the chorioallantoic membrane. After incubated for another 3 days, the chorioallantoic membrane microvessels were photographed and quantified using Image Pro Plus.
Transwell migration assay
The transwell plate (Becton, Dickinson) was coated with 0.1% gelatin for 30 min and rinsed three times with PBS. Then extracellular matrix (ECM) containing 4 ng/mL VEGF was added to the lower chamber and 4 × 104 ECs were seeded into each well in the top chamber. After that, cells were incubated with TCS (6 μg/mL) for 4 h. Non-migrated cells were gently wiped off the upper surfaces of the membrane and then the membrane containing migrated cells was fixed with 4% paraformaldehyde for 20 min. Images were recorded and migrated cells were counted manually.
Tube formation assay
Matrigel was thawed overnight at 4°C. A pre-chilled 96-well plates was coated with 50 μL Matrigel and then incubated at 37°C for 30 min. ECs (2 × 104/well) were seeded onto Matrigel and incubated with 6 μg/mL TCS for 18 h at 37°C, 5% CO2. EC tubular structure was examined under an inverted microscope. Tube formation at different concentrations of TCS was normalized to control (with NS added).
Enzyme-linked immunosorbent assay (ELISA)
The serum levels of MMP-2 and MMP-9 in A20 inoculated mice were detected using the ELISA kit (BOSTER, Wuhan, China) following the manufacturer’s protocol.
ECs were untreated or given 3, 6 μg/mL TCS (IC50 = 5.9 ± 0.2 μg/mL) for 72 h respectively. And the concentrations of vascular endothelial growth factor (VEGF) were determined using ELISA kit (BOSTER, Wuhan, China).
Real-time PCR
ECs were treated with NS or given 3 μg/mL and 6 μg/mL TCS for 72 h. cDNA was made with the SuperScript III First Strand kit (Invitrogen, Carlsbad, California, USA) according to the instructions of manufacturer using random hexamers. Real-time PCR was performed using SybrGreen PCR master mix (Applied Biosystems). The primer pair for VEGF was: forward primer 5’-TGCACCCACGACAGAAGG-3’, reverse primer 5’-GCACACAGGACGGCTTGA-3’ (154 bp) [20].
Statistical analysis
The SPSS 17.0 software was used for statistical analysis. Statistical analysis was performed applying unpaired Student t tests. Survival data were calculated according to the Kaplan-Meier method. All values were expressed as mean values ± standard deviations (means ± SD) and the significance was set at P < 0.05.
Results
TCS inhibit mouse A20 lymphoma growth in vivo
To investigate whether the anti-tumor activity of TCS could also be seen on lymphoma, we analyzed the effect of TCS on mouse B-cell lymphoma A20 cells in vivo experiments. TCS inhibits the growth of A20 tumor cells in mice in a dose-dependent manner. Tumors treated with middle (0.4 mg/kg) and high dosage (0.8 mg/kg) of TCS were significantly smaller than those in the control or low dosage (0.2 mg/kg) groups (P < 0.05) (Figure 1A). Consistent with this finding, the tumor size and weight of the middle and high dosage groups were lighter than that of other two groups (P < 0.05) (Figure 1B, 1C). In addition, the survival time of mice was obviously longer among those in the middle and high dosage groups, compared to those in other two groups (P < 0.05), and no statistical significance between the control and low dosage groups (P > 0.05) (Figure 1D).
Figure 1.
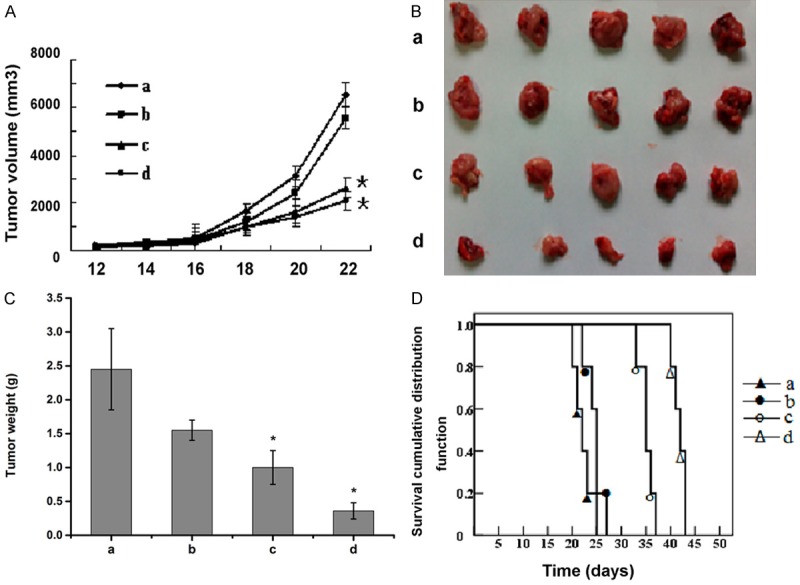
Effect of TCS on murine B-cell lymphoma growth in vivo. A. Mean tumor volume measured by caliper during the experiment. B. Photograph of tumors isolated from mouse homograft after three weeks. C. Quantitative analysis of tumor weights three weeks after implantation. D. Survival curve of tumor-bearing mice after tumor implantation. Data are represented by the mean ± SD (n = 5, *P < 0.05 vs. group a). a: saline control group; b: 0.2 mg/kg TCS group; c: 0.4 mg/kg TCS group; d: 0.8 mg/kg TCS group. Male BALB/c mice were subcutaneously grafted with 1 × 106 A20 cells. Starting from day 14 after tumor cell grafting, animals were treated intraperitoneal injection daily with different dosage of TCS or PBS.
Inhibition of A20 lymphoma growth by TCS is mediated via anti-angiogenesis and not related to direct cytotoxicity and immune response
In order to reveal the underlying mechanism of TCS anti-tumor effect in mouse A20 lymphoma homograft model, we examined the effect of TCS on the viability and the apoptosis of A20 cells. A20 cells were incubated in medium containing 2.5-40 µg/mL of TCS for 24 h, 48 h and 72 h, respectively. CCK-8 cell viability assay (Table S1) and flow cytometry apoptosis analysis (Figure S1) showed that TCS did not have cytotoxicity to A20 cells under the conditions mentioned above.
To investigate the effect of TCS on immunomodulatory in mice inoculated with A20 lymphoma, we analyzed the changes of spleen lymphocyte subsets in tumor-bearing mice by FCM. Compared with the control group, the percentages of immune cells, such as CD4+/CD8+ T cells and natural killer (NK) cells in the spleen of the TCS-treated tumor-bearing mice, did not change significantly (Table S2). These data indicated that the immunomodulatory function of TCS might not be directly involved in its anti-lymphoma activity.
Tumor growth also depends on angiogenesis. In a follow-up experiment, we verified the inhibitory effect of TCS on tumor angiogenesis. Surprisingly, peripheral angiogenesis was reduced in mice treated with TCS (Figure 2A). Further, there was a dramatic decrease in MVD of tumor section in animals treated with middle and high dosage TCS (Figure 2B). And the Vessels/High Power Fields (V/HPF) of these two groups were (12 ± 4) and (9 ± 3), whereas the V/HPFs of control and low dosage groups were (31 ± 7) and (24 ± 5) respectively (Figure 2C). The immunofluorescence staining of CD31-FITC also showed that the number of vascular ECs in treatment groups was attenuated compared to the control (Figure 2D). The results of ELISA indicated that the serum levels of MMP-2 and MMP-9 were higher in control and low dose TCS group, did not differ significantly between the groups. Compared with the other two groups, the levels of MMP-2 and MMP-9 were significantly decreased in the middle and high dose groups, with statistical significance (Figure 2E, 2F). These results validated that MMP-2 and MMP-9 has played an important role in TCS mediated anti-angiogenesis.
Figure 2.
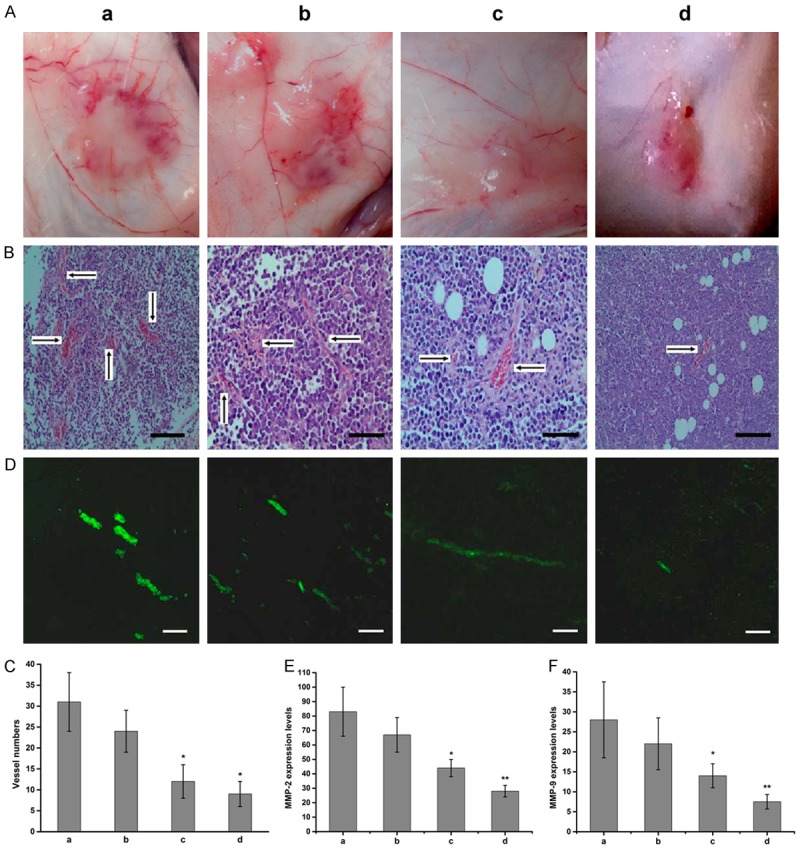
TCS inhibits tumor angiogenesis and the expression of MMPs. A. Photograph of peritumorous angiogenesis in each group. B. Measurement of tumor micro vessel density (MVD) by Hematoxylin and Eosin (HE) staining in control and TCS treated mice. Bar, 20 μm. C. Quantitative analysis of tumor MVD (n = 5, magnification 200). D. Representative immunofluorescence images of blood vessel formation in subcutaneous tumors stained with platelet endothelial cell adhesion molecule-1 (CD31-FITC) (green) (200). Bar, 10 μm. E. The expression levels of MMP-2 in serum of A20 inoculated mice (n = 5, P < 0.05, P < 0.01 vs. group a). F. The expression levels of MMP-9 in serum of A20 inoculated mice (n = 5, P < 0.05, P < 0.01 vs. group a). Data are represented by the mean ± SD. a: saline control group; b: 0.2 mg/kg TCS group; c: 0.4 mg/kg TCS group; d: 0.8 mg/kg TCS group.
Inhibitory effects of TCS on angiogenesis in chorioallantoic membrane
The inhibitive effect of TCS against angiogenesis was further assessed by the chorioallantoic membrane assay. Compared to control, the newly formed vessels in chorioallantoic membranes treated with TCS were significantly reduced (Figure 3A). Quantitative analysis shows TCS could inhibit angiogenesis and the effect was dose-dependent (Figure 3B).
Figure 3.
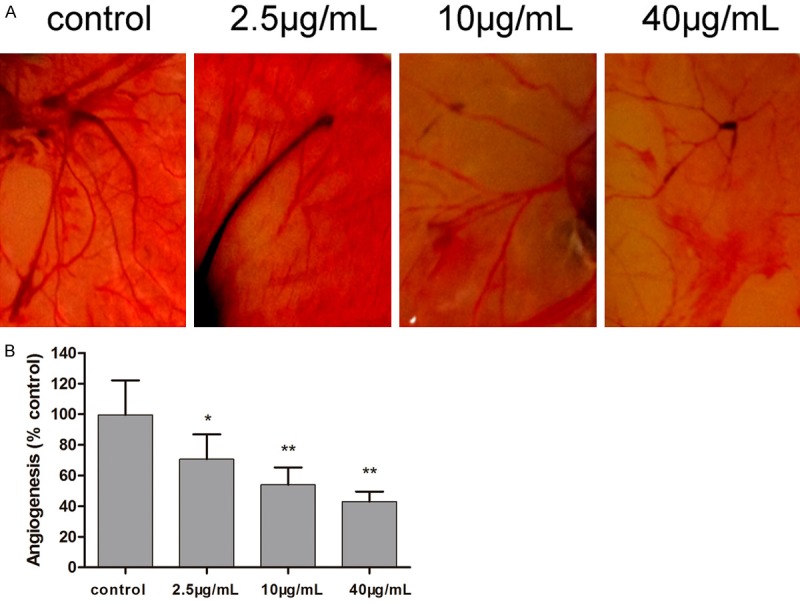
Inhibitory effects of TCS on angiogenesis by chorioallantoic membrane assay. A. Representative images of control and TCS-treated chorioallantoic membranes. B. Quantitative analysis of new vessel formation in the chorioallantoic membrane assay. The 8-day-old chorioallantoic membrane was treated with different concentrations of TCS for 72 h and then patterns of angiogenesis were photographed. Values are mean ± SD (n = 5, *P < 0.05, **P < 0.01. vs. control); control: normal saline group.
Effect of TCS on ECs
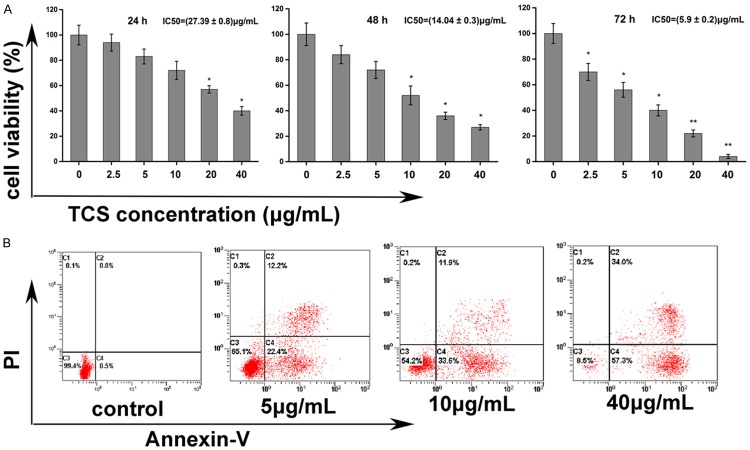
Since cell proliferation is an essential step in angiogenesis, the effect of TCS on ECs proliferation was assessed. The results of CCK-8 assay have shown that TCS treatment significantly reduces the viability of ECs (Figure 4A). After 24, 48 and 72 h of treatment, TCS exerted a significant cytotoxic effect on ECs, showing IC50 values of (27.39 ± 0.8) μg/mL, (14.04 ± 0.3) μg/mL and (5.9 ± 0.2) μg/mL TCS, respectively.
Figure 4.
Effect of TCS on ECs viability and apoptosis. A. Effect of TCS on cell viability determined by CCK-8 assay. ECs were treated for 24, 48 and 72 h in 96-well plate in the presence of different doses of TCS (0, 2.5, 5, 10, 20 and 40 μg/mL). CCK-8 was added to the wells at corresponding time points and then detected at an optical density (OD) of 450 nm. Data are represented by the mean ± SD. *P < 0.05, **P < 0.01 vs. control (0 μg/mL TCS). B. The detection of TCS induced apoptosis with Annexin V-Fluorescein and PI staining. ECs were cultured in the absence or presence of 5 μg/mL, 10 μg/mL, 15 μg/mL TCS. After 48 h, cells were harvested, stained with PI/Annexin V and then analyzed by FCM.
Apoptotic ratio was evaluated by FCM. After incubation with different concentrations (5, 10, 15 μg/mL) of TCS for 48 h (IC50 was about 14 ± 0.3 μg/mL), the percentage of early apoptosis cells (Annexin V+/PI-) increased to 22.4%, 33.6%, and 57.3%, respectively, as compared to 0.5% in the control group. The percentage of late apoptosis cells (Annexin V+/PI+) increased to 12.2%, 11.9%, and 34%, respectively, as compared to negative in the control (Figure 4B).
Tube formation assay on Matrigel was adopted to demonstrate the effect of TCS on angiogenesis. As a result, the formation of capillary-like, branched vessel networks apparently in the control group. Compared with the control, TCS can restrain significantly the tubular formation and the branching number of ECs (Figure 5A, 5B).
Figure 5.
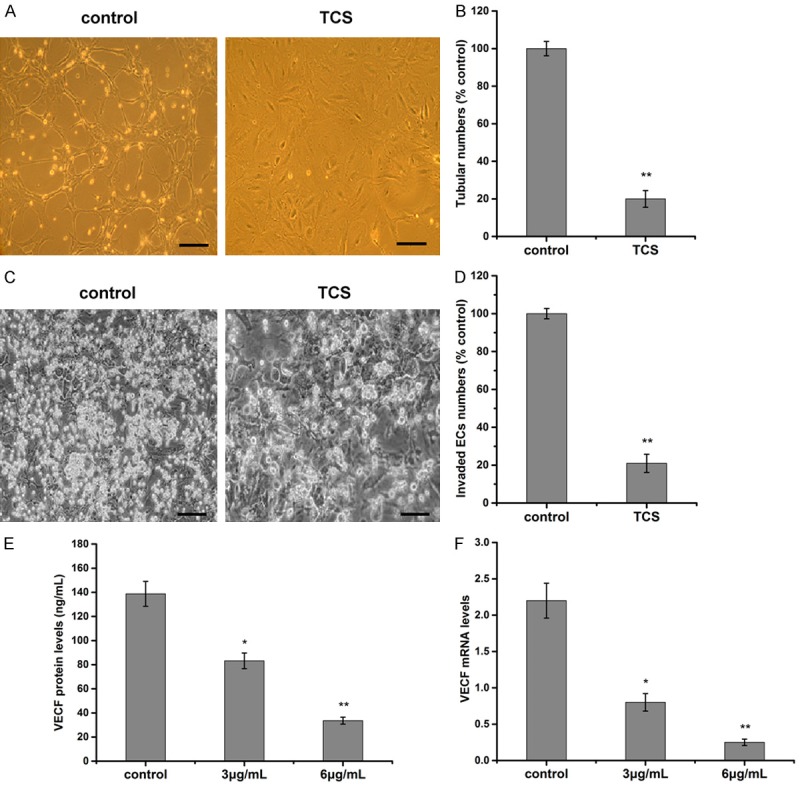
Effect of TCS on ECs. A. The image of tube formation of ECs. ECs (2 × 104 cells) and TCS were added on Matrigel layers. After 18 h of incubation with 6 μg/mL TCS or normal saline, ECs tube-like formation was assessed with an inverted photomicroscope (200 ×), Bar, 20 μm. B. The statistical results of vessel number of ECs. Control as 100%. Tubular structures were quantified by manual counting of low power fields (200 ×). C. Graphical representative image of transwell invasion assay. ECs (4 × 104 cells/well) treated with 6 μg/mL TCS were seeded in the upper chamber, and the bottom chamber was filled with ECM medium containing 4 ng/mL VEGF. Cells with an irregular shape in images are cells that migrated into the lower chamber (200 ×). Bar, 20 μm. D. The statistical results of invaded cell numbers. Control wells as 100%. E. Effect of TCS on VEGF protein secretion in ECs. The levels of VEGF protein were analyzed by ELISA. F. Effect of TCS on VEGF mRNA levels in ECs. Levels of VEGF mRNA were analyzed by real-time PCR. Data are represented by the mean ± SD (n = 3). *P < 0.05, **P < 0.01 vs. control; control: normal saline group.
Cell invasion plays an important role in angiogenesis and is necessary for tumor growth and metastasis. A transwell migration assay was carried out to examine the effect of TCS on ECs invasion. The number of invaded ECs drastically reduced when the cells were treated with 6 μg/mL TCS (Figure 5C). Quantitative analysis showed the inhibitory activities could reach 80% (Figure 5D).
In order to explore the molecular mechanisms of TCS on ECs, we analyzed the expression of one angiogenesis-related growth factor, VEGF. ELISA results showed that, in TCS 3 μg/mL group and TCS 6 μg/mL group, the secretion amount of VEGF were (83.2 ± 6.5) ng/mL and (33.6 ± 2.9) ng/mL respectively, lower than that in the control group (138.7 ± 10.3) ng/mL (P < 0.05; P < 0.01) (Figure 5E). Additionally, real-time PCR demonstrated TCS inhibited the expression of VEGF mRNA in ECs at 72 h (Figure 5F).
Discussion
In this manuscript, we report the therapeutic properties and the mechanism of TCS toward murine B lymphoma. First, TCS exhibits anti-tumor activity against A20 lymphoma in tumor-bearing mice. Since its anti-tumor action was believed to be due to its cytotoxicity [21], we anticipate that the main mechanism of TCS on A20 cells may be related to direct cytotoxicity. In contrast, in our study, TCS did not show any effects on A20 cells viability and apoptosis rate in vitro, so the anti-lymphoma activity was not attribute to the cytotoxicity effect of TCS. In addition, several studies indicated that TCS manifest immunoregulatory effects, which are involved in its antitumor activities [9,10]. TCS also did not affect the immune function of mice with A20 lymphoma in our research. These results suggest that the anti-tumor activity of TCS may not be result from its cytotoxicity or immunoregulatory effect. Surprisingly, the formation of new blood vessels around the tumor in vivo was inhibited, which is found to be in line with the experimental results in MVD of tumor and chorioallantoic membrane assay. Immunofluorescence staining of CD31 demonstrated that the proliferation of tumor-derived vascular ECs was depressed in A20 lymphoma section, which effectively inhibited the occurrence of tumor. This was easily verifiable via the experiment in vitro with ECs.
The process of angiogenesis is regulated by a series of positive and negative factors. VEGF, a well-studied initial factor of angiogenesis, promotes many of the events needed for angiogenesis, such as the proliferation and migration of ECs [22]. MMPs have been regarded as major critical molecules in the process of tumor-induced angiogenesis, tumor invasion [23]. Our study showed that the formation of new blood vessels was inhibited and the number of invaded endothelial cells decreased in TCS treated mice; besides, the low expression and secretion levels of VEGF in ECs were observed. This indicates that TCS causes repression of VEGF expression plays an essential role in the regulation of anti-angiogenesis. Furthermore, TCS suppressed the expression of serum MMPs in A20 tumor-bearing mice. Several studies have shown that MMPs played an important role in the processes of embryonic development and reproduction [24], and the expression of MMP-2 and MMP-9 significantly increased in placenta and endometrium of the pregnant guinea-pig [25]. This shows that the course of tumor metastasis and gestation actually shared the same characteristics in aspect of MMPs. Therefore, TCS inhibits the expression of MMPs caused not only antitumor effect, but also is the major factor inducing abortion. If it is the case, it just supports the conclusion of our study. Previous study also suggested that there is a direct relationship between VEGF and the expressions of MMPs, their expressions being enhanced by VEGF [26]. However, no experimental verification has been carried out in our research.
Taken together, our date has indicated that inhibited tumor angiogenesis ability could be important mechanisms of TCS in anti-tumor anti-metastasis. However, we know little about the molecular mechanisms underlying the condition. Further investigations are required to clarify the mechanisms of its inhibition effect on tumor angiogenesis.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.American Cancer Society. Cancer treatment and survivorship facts & figureures 2012-2013. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 2.Rausch Osian S, Leal AD, Allmer C, Maurer MJ, Nowakowski G, Inwards DJ, Macon WR, Ehlers SL, Weiner GJ, Habermann TM, Cerhan JR, Thompson CA. Widespread use of complementary and alternative medicine among non-Hodgkin lymphoma survivors. Leuk Lymphoma. 2015;56:434–439. doi: 10.3109/10428194.2014.916803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Arye E, Attias S, Tadmor T, Schiff E. Herbs in hemato-oncological care: an evidence-based review of data on efficacy, safety, and drug interactions. Leuk Lymphoma. 2010;51:1414–1423. doi: 10.3109/10428194.2010.487622. [DOI] [PubMed] [Google Scholar]
- 4.Shaw PC, Yung MH, Zhu RH, Ho WK, Ng TB, Yeung HW. Cloning of trichosanthin cDNA and its expression in escherichia coli. Gene. 1991;97:267–272. doi: 10.1016/0378-1119(91)90061-f. [DOI] [PubMed] [Google Scholar]
- 5.Sha O, Niu J, Ng TB, Cho EY, Fu X, Jiang W. Anti-tumor action of trichosanthin, a type 1 ribosome-inactivating protein, employed in traditional chinese medicine: a mini review. Cancer Chemother Pharmacol. 2013;71:1387–1393. doi: 10.1007/s00280-013-2096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang EF, Zhang CZ, Zhang L, Wong JH, Chan YS, Pan WL, Dan XL, Yin CM, Cho CH, Ng TB. Trichosanthin inhibits breast cancer cell proliferation in both cell lines and nude mice by promotion of apoptosis. PLoS One. 2012;7:e41592. doi: 10.1371/journal.pone.0041592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He DX, Yau K, He XH, Shi HJ, Zheng YT, Tam S. Conversion of trichosanthin-induced CD95 (fas) type I into type II apoptotic signaling during Herpes simplex virus infection. Mol Immunol. 2011;48:2000–2008. doi: 10.1016/j.molimm.2011.06.217. [DOI] [PubMed] [Google Scholar]
- 8.Zheng YT, Zhang WF, Ben KL, Wang JH. In vitro immunotoxicity and cytotoxicity of trichosanthin against human normal immunocytes and leukemia-lymphoma cells. Immunopharmacol Immunotoxicol. 1995;17:69–79. doi: 10.3109/08923979509052721. [DOI] [PubMed] [Google Scholar]
- 9.Li CT, Lin CH, Kao TY, Wu MF, Yeh CS, Yeh KT, Ko JL. The mechanisms of action of tianhua (TM) on antitumor activity in lung cancer cells. Pharm Biol. 2010;48:1302–1309. doi: 10.3109/13880201003789432. [DOI] [PubMed] [Google Scholar]
- 10.Cai YC, Xiong SD, Zheng YJ, Luo FF, Jiang P, Chu YW. Trichosanthin enhances anti-tumor immune response in a murine lewis lung cancer model by boosting the interaction between TSLC1 and CRTAM. Cell Mol Immunol. 2011;8:359–367. doi: 10.1038/cmi.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang YL, Song HM, Hu HJ, Cui L, You CC, Huang LM. Trichosanthin inhibits DNA methyltransferase and restores methylation-silenced gene expression in human cervical cancer cells. Mol Med Rep. 2012;6:872–878. doi: 10.3892/mmr.2012.994. [DOI] [PubMed] [Google Scholar]
- 12.He DX, Jin J, Zheng YT, Bruce IC, Tam S, Ma X. Anti-angiogenesis effect of trichosanthin and the underlying mechanism. Biochem Biophys Res Commum. 2013;430:735–740. doi: 10.1016/j.bbrc.2012.11.080. [DOI] [PubMed] [Google Scholar]
- 13.Newman PJ. The biology of PECAM-1. J Clin Invest. 1997;100:S25–S29. [PubMed] [Google Scholar]
- 14.DeLisser HM, Christofidou-Solomidou M, Strieter RM, Burdick MD, Robinson CS, Wexler RS, Kerr JS, Garlanda C, Merwin JR, Madri JA, Albelda SM. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol. 1997;151:671–677. [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson C. Matrix metalloproteinases and angiogenesis. Curr Opin Nephrol Hypertens. 2002;11:295–299. doi: 10.1097/00041552-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Stetler-Stevenson WG. Type IV collagenases in tumor invasion and metastasis. Cancer Metastasis Rev. 1990;9:289–303. doi: 10.1007/BF00049520. [DOI] [PubMed] [Google Scholar]
- 17.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 18.Zhang MH, Tang H, Guo ZH, An HZ, Zhu XJ, Song WG, Guo J, Huang X, Chen TY, Wang JL, Cao XT. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol. 2004;5:1124–1133. doi: 10.1038/ni1130. [DOI] [PubMed] [Google Scholar]
- 19.Prabhakar BT, Khanum SA, Shashikanth S, Salimath BP. Antiangiogenic effect of 2-benzoyl-phenoxy acetamide in EAT cell is mediated by HIF-1α and down regulation of VEGF of in-vivo. Invest New Drugs. 2006;24:471–478. doi: 10.1007/s10637-006-6587-0. [DOI] [PubMed] [Google Scholar]
- 20.Asano T, Kaneko E, Shinokazi S, Imai Y, Shibayama M, Chiba T, Ai M, Kawakami A, Asaoka H, Nakayama T, Mano Y, Shimokado K. Hyperbaric oxygen induces basic fibroblast growth factor and hepatocyte growth factor expression, and enhances blood perfusion and muscle regeneration in mouse ischemic hind limbs. Circ J. 2007;71:405–411. doi: 10.1253/circj.71.405. [DOI] [PubMed] [Google Scholar]
- 21.Shaw PC, Lee KM, Wong KB. Recent advances in trichosanthin, a ribosome-inactivating protein with multiple pharmacological properties. Toxicon. 2005;45:683–689. doi: 10.1016/j.toxicon.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 22.Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat. 1995;36:127–137. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- 23.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 24.Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 25.Corthorn J, Rey S, Chacón C, Valdés G. Spatio-temporal expression of MMP-2, MMP-9 and tissue kallikrein in uteroplacental units of the pregnant guinea-pig (cavia porcellus) Reprod Biol Endocrinol. 2007;5:27. doi: 10.1186/1477-7827-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem. 2002;277:36288–36295. doi: 10.1074/jbc.M201674200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.