Abstract
Diabetes-related infections have become challenging and important public health problems in China and around the world. P. aeruginosa plays an important role in diabetic foot infections. As a gram-negative opportunistic pathogen, P. aeruginosa causes recurrent and refractory infections that are characterized by biofilm formation. Previous studies have demonstrated that biofilm-challenged wounds typically take longer to heal than non-biofilm-challenged normal wounds in diabetic mouse models. In the present study, we sought to explore the mechanism via which insulin treatment affects cyclic di-GMP signaling in P. aeruginosa-infected chronic wounds in db/db diabetic mice. We found that the wounds of diabetic mice healed more slowly than those of nondiabetic mice. Moreover, wound healing in diabetic mice treated with insulin exhibited a considerable delay. Peptide nucleic acid-fluorescence in situ hybridization (PNA-FISH) was used to detect biofilms on P. aeruginosa-infected wound tissues. Increased intracellular c-di-GMP levels promoted biofilm formation in wound tissues from nondiabetic mice. Greater biofilm formation was observed in the wounds of insulin-treated diabetic mice than in the wounds of untreated diabetic mice or nondiabetic mice, in both the PAO1/plac-yhjH- and PAO1-infected groups. Quantitative RT-PCR indicated that upon infection with PAO1/Plac-yhjH (the low c-di-GMP expression strain), the expression of IL-4 RNA was significantly higher in diabetic mice treated with insulin than in untreated diabetic mice or nondiabetic mice at each observation time point. Peak expression of IFN-γ occurred earlier in diabetic mice treated with insulin than in untreated diabetic mice with each of the experimental strains. Finally, P. aeruginosa harboring the plasmid pCdrA: gfp s was used as a reporter strain to monitor c-di-GMP levels. We found that insulin could promote biofilm formation by increasing intracellular c-di-GMP levels in vitro. Taken together, these data demonstrate that insulin treatment increases intracellular c-di-GMP levels, promotes biofilm formation and prolongs the inflammation period during the healing of infected wounds, resulting in delayed wound healing.
Keywords: Insulin, pseudomonas aeruginosa, biofilm, cyclic di-GMP, wound infection, inflammation
Introduction
In China, approximately 92.4 million adults (50.2 million men and 42.2 million women), or 9.7% of the adult population, have diabetes, and more than 148.2 million adults (76.1 million men and 72.1 million women) have prediabetes [1]. There are several complications associated with diabetes, ranging from heart disease to blindness. In addition, the most common complications experienced by patients with diabetes are infections, such as skin and lung infections. Foot infections are a common and serious problem among patients with diabetes [2]. Therefore, diabetes-related infectious diseases have become a challenging and important public health problem in China and around the world.
Pseudomonas aeruginosa plays an important role in chronic wound infections and cystic fibrosis-associated pneumonia by forming biofilms. Although a wide range of bacterial species have been cultured from infected chronic wounds, including Staphylococcus aureus, P. aeruginosa, Enterococcus faecalis, coagulase-negative staphylococci, Proteus species, and anaerobic bacteria [3,4], P. aeruginosa is the most common gram-negative rod-shaped bacterium isolated from the soft tissue or ulcerated skin of patients with diabetic foot infections (DFIs) [5,6]. P. aeruginosa is also a major pathogen in patients with chronic lung infections and is the primary cause of morbidity and mortality in cystic fibrosis (CF) [7].
P. aeruginosa is a gram-negative opportunistic pathogen that causes recurrent and refractory infections characterized by biofilm formation. Biofilms are defined as structured communities of a large number of bacteria that adhere to inert or living surfaces; secrete a polysaccharide matrix, fibrinogen, lipoprotein, and DNA; and are enveloped in this self-produced polymeric matrix [8]. Biofilms are significantly different from planktonic bacteria, and biofilm bacteria are protected against antibacterial treatment and phagocytosis and are able to evade the innate and adaptive immune systems [9]. Therefore, P. aeruginosa biofilm infection leads to serious clinical problems.
Multiple mechanisms are involved in the regulation of P. aeruginosa biofilm formation, and these mechanisms mainly involve cyclic diguanosine-5’-monophosphate (c-di-GMP), small RNAs (sRNAs) and quorum sensing (QS) [10]. C-di-GMP was discovered by Benziman et al. [11] as an activator of cellulose synthase in Gluconacetobacter xylinus in 1987 [12] and was recently identified as a novel bacterial global secondary messenger that is considered to be a master regulator of the transition between the biofilm and planktonic lifestyles. Diguanylate cyclases (DGCs) and phosphodiesterases (PDEs) regulate the formation and degradation of c-di-GMP, respectively. DGC activity is associated with the GGDEF domain, while PDE activity is associated with the EAL or HD-GYP domain. High intracellular levels of c-di-GMP promote biofilm formation by increasing the surface adherence of bacterial cells, cell aggregation and the secretion of extracellular polymeric substances. In contrast, low intracellular levels of c-di-GMP inhibit biofilm formation and stimulate bacterial motility [13]. Among the processes associated with biofilm formation, surface attachment, swimming motility, swarming motility, twitching motility, and the production of the extracellular matrix are regulated by c-di-GMP signaling [14].
A previous study demonstrated that biofilm-challenged wounds typically take longer to heal than non-biofilm-challenged normal wounds in a diabetic mouse model [15]. Currently, there is much controversy regarding whether insulin can be used to treat P. aeruginosa infections. One study reported that low-dose insulin treatment can attenuate the sepsis caused by P. aeruginosa infection after severe burn injuries [16]. However, Chase Watters’ group found that insulin treatment could not decrease the P. aeruginosa bacterial load in diabetic wounds and instead enhanced biofilm formation by this bacterium [17,18]. To date, there have been no studies on the role of the c-di-GMP signaling pathway in wound healing in diabetic mouse models, and no pathway studies have confirmed whether intervention with insulin clears bacterial loads or promotes the formation of P. aeruginosa biofilms.
Therefore, the main purpose of this study was to explore the effect of insulin on the healing of wounds infected with P. aeruginosa in diabetic mice. In addition, we examined whether these effects were associated with intracellular c-di-GMP signaling. Finally, in this study, the effect of insulin on P. aeruginosa biofilm formation in the diabetic mouse model was confirmed at the level of the c-di-GMP signaling pathway. Three P. aeruginosa strains with different levels of intracellular c-di-GMP and different biofilm formation abilities were chosen as experimental strains: the control strain PAO1, the low c-di-GMP expression strain PAO1/Plac-yhjH and the high c-di-GMP expression strain PAO1ΔwspF. We assessed the intracellular levels of c-di-GMP in P. aeruginosa by transcriptionally fusing the c-di-GMP-responsive cdrA promoter to the gene encoding green fluorescent protein (GFP), a method that was developed by Rybtke et al. [19]. We found that insulin treatment promoted biofilm formation by increasing intracellular c-di-GMP levels in vitro. In vivo, the wounds of diabetic mice healed more slowly than those of nondiabetic mice, and among these mice, the slowest wound healing was observed for diabetic mice treated with insulin. We observed increased inflammatory cell infiltration (via hematoxylin-eosin (HE) staining), increased levels of released inflammatory factors and enhanced biofilm formation (by peptide nucleic acid-fluorescence in situ hybridization (PNA-FISH)) in diabetic mice treated with insulin. Taken together, these data demonstrated that insulin treatment increased intracellular c-di-GMP levels, promoted biofilm formation, and prolonged the inflammatory period, leading to delayed diabetic wound healing.
Materials and methods
Bacterial strains and growth conditions
This study used the sequenced P. aeruginosa PAO1 wild-type strain, the PAO1ΔwspF strain (a wspF mutant of PAO1 constructed by allelic exchange), the PAO1/Plac-yhjH strain (PAO1 harboring the Plac-yhjH vector), and a PAO1 strain harboring the PcdrA-gfp vector (to provide a fluorescent readout of the intracellular level of c-di-GMP in P. aeruginosa), all of which were generously donated by Dr. Liang Yang (Nanyang Technological University, Singapore). The P. aeruginosa strains were grown at 37°C in Luria-Bertani (LB) medium or ABTGC [20] medium and on LB agar or ABTGC agar at 37°C. Antibiotics were supplied where necessary at the following concentrations: the PAO1/Plac-yhjH strain was grown with 10 µg/ml tetracycline, and the P. aeruginosa strain harboring the PcdrA-gfp vector was grown with 200 µg/ml carbenicillin. Before each experiment, frozen P. aeruginosa cells stored at -80°C were resuscitated and inoculated onto an LB agar plate with or without antibiotics by using a 1-µL inoculation loop. A single colony was picked and subcultured in LB broth in a temperature-regulated shaker set at 37°C, with shaking at 200 rpm for 14~16 hours. Then, the overnight culture was diluted with fresh ABTGC medium to the appropriate bacterial cell density for subsequent experiments.
Animals
Male, 8-week-old, db/db mice were purchased from the Biomedical Research Institute of Nanjing University. Homozygous BKS.Cg-Dock7m+/+Leprdb/Nju mice were deemed diabetic if their blood glucose levels were greater than 20 mmol/L, while wild-type BKS.Cg-Dock7m+/+Leprdb/Nju mice were deemed nondiabetic. All the mice were raised in specific pathogen-free (SPF)-level rooms in the Animal Center Laboratory of Guangxi University (Nanning, People’s Republic of China), fed a standard chow diet, provided free access to tap water and acclimated for 1 week prior to surgery. This protocol was conducted according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication 85-23, revised 1996) and was approved by the Animal Care and Use Committee of Guangxi Medical University, People’s Republic of China.
Chronically wounded diabetic mouse model
A diabetic mouse model with chronic wounds was established as described by Watters C and colleagues. Briefly, the mice were anesthetized and shaved, and a dorsal full-thickness surgical excision wound was created (size: 1.5 × 1.5 cm). Then, the wounds were covered with a transparent wound care accessory, i.e., a semipermeable polyurethane dressing (OPSITE, Smith & Nephew, Hull, England). Approximately 104 colony forming units (CFUs) of P. aeruginosa was injected into the gap between the polyurethane dressing and the wound. Some groups of diabetic mice were administered hypodermic injections with 2 units of Humulin® N insulin (Eli Lilly, Indianapolis, IN, USA) daily.
Pathological analysis of infected wound tissue
The infected wound tissue (size range: more than 2 mm of the visible wound margin and as deep as the surface of the mouse crest) was extracted completely from the back of the mouse at each observation time point. The tissue was then fixed in 10% (v/v) formalin solution, embedded in paraffin, sectioned, and stained with HE to visualize the inflammatory reactions of the cells. PNA-FISH was used to identify the bacteria in infected chronic wounds according to the protocol described by Fazli et al. [20]. In brief, paraffin-embedded tissue samples were deparaffinized by treatment with xylene (twice for 5 min), 99.9% ethanol (twice for 3 min), and 96% ethanol (twice for 3 min) and then washed three times in Milli-Q water for 3 min each. One drop of an approximately 300 nmol/L concentration of a PNA-FISH probe that was conjugated to Texas Red and specific for P. aeruginosa 16S rRNA was applied to the tissue section, which was then covered with a cover slip. The samples were incubated at 55°C for 90 min. The cover slip was removed, and the slides were washed in warm washing buffer at 55°C for 30 min and then air-dried in the dark.
Antibiotic resistance assay
The amikacin resistance of P. aeruginosa in the wounds was examined in accordance with Chase Watters’ method. Chronically wounded diabetic mice infected with P. aeruginosa were obtained as described above. A sterile gauze pad was placed over the wound and under the transparent wound care accessory (OPSITE). On days 4, 8, 12, and 16 post surgery, the mice were euthanized, and the gauze pads were removed from the wounds. The gauze pads were sheared in half, weighed, and then immersed in sterile PBS or a 200 mg/mL amikacin solution for 5 hours. The amikacin-treated gauze pad segments were neutralized in PBS for 10 min and then immersed in PBS. Then, the gauze pads were thoroughly homogenized. The obtained suspension was serially diluted and plated to determine the CFU/g of the gauze pad. The amikacin tolerance of P. aeruginosa in the wounds was determined from the CFU/g value obtained after treatment, which was divided by the CFU/g value obtained for the PBS-treated gauze section and multiplied by 100.
Quantitative RT-PCR assay
Infected wound tissue samples were collected as described above. On days 4, 8, 12, and 16 post surgery, the tissue samples were weighed and placed in a precooled 1.5-ml EP tube with RNA Protector and then stored at -80°C. The total RNA was extracted from the above tissue samples by following the manufacturer’s instructions for the MiniBEST Universal RNA Extraction Kit (TaKaRa, Japan). The first-strand complementary DNA (cDNA) was synthesized according to the manufacturer’s recommendations for the PrimeScriptTM RT Reagent Kit and then purified by using gDNA Eraser.
Specific primer sets for genes encoding murine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward: 5’-AAGGTCGGAGTCAACGGATT-3’; reverse: 5’-TTGATGACAAGCTTCCCGTT-3’), interferon-γ (IFN-γ) (forward: 5’-TATAGCTGCCATCGGCTGAC-3’; reverse: 5’-AAGCCAAGATGCAGTGTGTAGC-3’), interleukin-4 (IL-4) (forward: 5’-TACCAGGAGCCATATCCACGGATG-3’; reverse: 5’-TGTGGTGTTCTTCGTTGCTGTGAG-3’), and interleukin-6 (IL-6) (forward: 5’-TCCATCCAGTTGCCTTCTTG-3’; reverse: 5’-AAGCCTCCGACTTGTGAAGTG-3’) were used to amplify DNA templates on a real-time PCR system (ABI 7500, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions for the SYBR Premix Ex Taq II PCR Kit. These samples were used to study the effect of insulin on P. aeruginosa in vitro.
Biofilm formation assay
A microtiter plate-based biofilm assay was used to quantitate the biofilm mass after treatment, as described by Merritt et al. [21]. In brief, an overnight culture of P. aeruginosa was diluted to approximately 106 CFU/ml with ABTGC medium supplemented with denatured insulin or Humulin® N insulin at a final concentration of 200 nU/ml, 200 µU/ml, or 200 mU/ml. The bacteria were inoculated directly into sterile microtiter plates at 200 µl per well, and five wells were used per concentration per strain. The plates were incubated in a temperature-regulated shaker at 37°C and 110 rpm for 12 hours. The planktonic bacteria were removed from each microtiter plate by pipetting gently, and the plates were then washed three times with PBS. The plates were air-dried for 15 min at room temperature, and 220 µl of 0.1% (w/v) crystal violet solution was then added to each well. The wells were stained for 15 min at room temperature. The crystal violet solution was removed by rinsing the wells three times with sterile distilled water. The plates were air-dried, and 33% (v/v) acetic acid was then added to each stained well. The plates were incubated for 15 min at room temperature. The crystal violet/acetic acid solution in each well was mixed by pipetting, and 200 µl of the contents of each well was then transferred to a separate well in a new 96-well plate. The optical density (OD) of the sample in each well was measured spectrophotometrically at a wavelength of 595 nm.
c-di-GMP reporter assay
The fluctuations in intracellular c-di-GMP levels were monitored indirectly by detecting changes in the output of the PcdrA-gfp reporter in P. aeruginosa according to a method previously described by Chua et al. [19] and Christensen et al. [22]. Briefly, an overnight culture of P. aeruginosa was diluted to approximately 106 CFU ml-1 with fresh ABTGC medium supplemented with Humulin® N insulin or denatured insulin (the final amount of insulin was 200 nU, 200 µU, or 200 mU). Next, 200 µl of each culture was transferred into individual wells of a 96-well microplate. Then, the microplate was sealed with Parafilm and incubated at 37°C, with shaking at 185 rpm for 12 hours. The optical density at 600 nm (OD600) and the GFP expression (excitation 490 nm/emission 515 nm) from PcdrA-gfp in P. aeruginosa were measured using a Thermo Fisher spectrophotometer (SN: 3020-128). The OD600 and GFP fluorescence (in relative fluorescence units (RFUs)) were recorded for each well of the 96-well microplate. The RFU values are the fluorescence intensity units corrected for cell density.
Effects of Humulin® N insulin on P. aeruginosa growth in vitro
An overnight P. aeruginosa culture was diluted to approximately 106 CFU ml-1 with fresh ABTGC medium supplemented with Humulin® N insulin or denatured insulin. Humulin® N insulin or denatured insulin was added to a final concentration of 200 nU/ml, 200 µU/ml, or 200 mU/ml. Next, 3 ml of diluted overnight culture was transferred to a sterile 10-ml BD Falcon tube and incubated at 37°C, with shaking at 185 rpm for 24 hours. Growth was monitored by determining the CFU count at 4, 8, 16, and 24 hours.
All the in vitro experiments described above were performed in triplicate and repeated at least three times, and the results are shown as the mean ± SD.
Statistical analysis
The results were analyzed by the SPSS18.0 software package. The mean values of the statistical results for the measured data were presented as the mean ± standard error. Significant differences between groups were determined by analysis of variance (ANOVA) based on the mean values for the groups, which corresponded to a normal distribution. P-values less than 0.05 were considered to be statistically significant.
Results
The db/db mouse model is the most appropriate model for studying diabetic wound-healing interventions, as it is more appropriate than Akita and streptozocin-induced C57BL/6J mice [23]. This model is reproducible and can provide more than 28 days to demonstrate delayed wound healing following biofilm challenge, with low mortality [24]. P. aeruginosa biofilm-infected diabetic wounds in db/db mice are suitable as a chronic wound infection model for studying the role of microbial biofilms in chronic wound infections and the effects of specific biofilm treatments on wound healing. However, there have been no published studies on the role of P. aeruginosa biofilms and the c-di-GMP signaling pathway in wound healing in diabetic mouse models with insulin intervention. Therefore, we compared the factors that affected the healing of wounds infected with the PAO1 wild-type strain, PAO1/Plac-yhjH strain (with low intracellular c-di-GMP expression) and PAO1ΔwspF strain (with high intracellular c-di-GMP expression). Subsequently, for each strain, the mice were divided into three groups: nondiabetic mice (Figure 1A), diabetic mice (Figure 1B) and diabetic mice treated with insulin (Figure 1C). If purulence under the surgical semipermeable polyurethane dressing was observed on the third day post infection, the model was considered to have been successfully generated (Figure 1B, 1C). Based on this criterion, a mouse model with wounds infected with P. aeruginosa strains exhibiting different levels of intracellular c-di-GMP expression was successfully established. The mortality rate of this model was low, with a value of 1.04%, and only 2 deaths occurred among the 196 diabetic mice, one in the group infected with the PAO1 control strain and one in the group infected with the PAO1/Plac-yhjH strain (with low c-di-GMP expression).
Figure 1.
A mouse model of chronic wounds infected with P. aeruginosa was established for three groups: nondiabetic mice (A), diabetic mice (B) and diabetic mice treated with insulin (C).
The effect of insulin on the body weights and wound healing rates of diabetic mice with chronic wounds infected with P. aeruginosa
Among the PAO1 wild-type-, PAO1/plac-yhjH- and PAO1ΔwspF-infected mice, the nondiabetic and diabetic mice lost an average of 3.22% to 8.53% of their body weight, while the diabetic mice treated with insulin gained an average of 3.38% to 12.4% of their body weight over the 16 days of the experiment. Among these mice, the percent gain in body weight observed for the diabetic mice treated with insulin was significantly higher than the loss in weight observed for the diabetic mice infected with PAO1/plac-yhjH or PAO1ΔwspF (Figure 2A-C). These results demonstrated that the diabetic mice treated with insulin were healthier than the nontreated diabetic mice.
Figure 2.
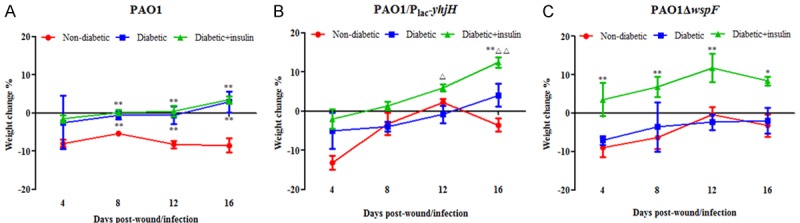
The nondiabetic and diabetic mice exhibited a loss of body weight, while the diabetic mice treated with insulin gained body weight, among mice infected with the (A) PAO1 wild-type strain, (B) PAO1/plac-yhjH strain and (C) PAO1ΔwspF strain over the 16 days of the experiment. The mice were weighed every 4 days, and the percent change in body weight was calculated by the following equation: (Wt-W0)/W0 × 100, where Wt was the weight on the day of observation, and W0 was the weight on the day of surgery. One-way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparison test was used to determine the differences between groups. Note: *P < 0.05 compared with the nondiabetic group, **P < 0.01 compared with the nondiabetic group, ΔP < 0.05 compared with the diabetic group, ΔΔP < 0.01 compared with the diabetic group.
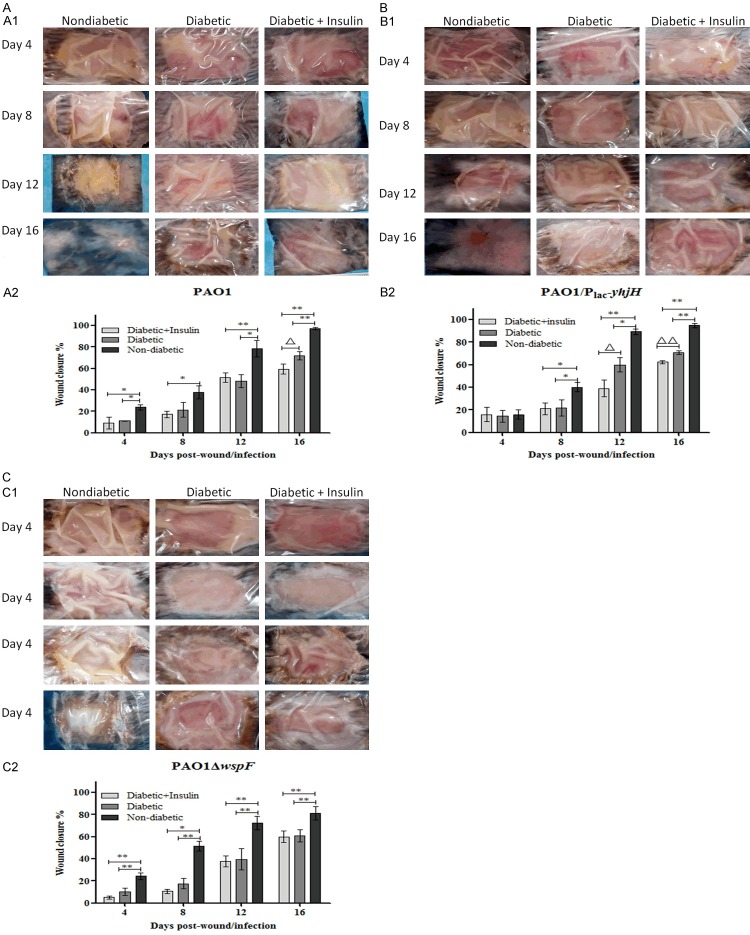
The percent wound closure was calculated by the following equation: (A0-At)/A0 × 100, where A0 was the wound area on the day of surgery, and At was the wound area on the day of observation. The mice were anesthetized, and the area of wound closure was measured by a ruler on the day of surgery and at 4-day intervals post surgery. On day 4 post surgery, only the wounds of the nondiabetic mice infected with PAO1/plac-yhjH were similar to those of diabetic mice and diabetic mice treated with insulin, and the average percent wound closure values were 16.04, 14.5 and 16.01% respectively. The wound-healing rates of the nondiabetic mice infected with PAO1/plac-yhjH were higher than those of the other two groups. Subsequently, on each day of observation post surgery, the percent wound closure for nondiabetic mice was higher than that for diabetic mice or diabetic mice treated with insulin among the mice infected with the PAO1 wild-type, PAO1/plac-yhjH and PAO1ΔwspF strains (Figure 3A-C). Among the mice infected with the PAO1 wild-type, PAO1/plac-yhjH and PAO1ΔwspF strains, by day 16, the wounds of the nondiabetic mice were almost completely closed, with percent wound closure values of 97.11, 94.83 and 81.07%, respectively (Figure 3A-C). Interestingly, by day 16 in mice infected with the PAO1 wild-type strain and by day 12 and day 16 in mice infected with the PAO1/plac-yhjH strain, the percent wound closure in diabetic mice treated with insulin was significantly lower than that in untreated diabetic mice (Figure 3A and 3B). By day 16 in mice infected with the PAO1 wild-type strain, the percent wound closure values for diabetic mice and diabetic mice treated with insulin were 71.8% and 59.46%, respectively. In addition, by day 12, the percent wound closure values for diabetic mice and diabetic mice treated with insulin were 60 and 38.98%, respectively, and by day 16, these values were 70.9 and 62.32% respectively, for mice infected with the PAO1/plac-yhjH strain (Figure 3B).
Figure 3.
(A) Wound closure in the P. aeruginosa infection model was delayed in the PAO1 control group of diabetic mice. All wounds were measured at 4-day intervals to assess wound closure (representative images are shown in A1). The percent wound closure was determined using the following equation: (A0-At)/A0 × 100, where A0 was the wound area on the day of surgery, and At was the wound area on the day of observation (A2). One-way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparison test was used. Note: *P < 0.05 compared with the nondiabetic group, **P < 0.01 compared with the nondiabetic group, ΔP < 0.05 compared with the diabetic group. (B) Wound closure in the P. aeruginosa infection model was delayed in the PAO1/Plac-yhjH-infected group of diabetic mice. All wounds were measured at 4-day intervals to assess wound closure (representative images are shown in B1). The percent wound closure was determined using the following equation: (A0-At)/A0 × 100, where A0 was the wound area on the day of surgery, and At was the area of the wound on the day of observation (B2). One-way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparison test was used. Note: *P < 0.05 compared with the nondiabetic group, **P < 0.01 compared with the nondiabetic group, ΔP < 0.05 compared with the diabetic group, ΔΔP < 0.01 compared with the diabetic group. (C) Wound closure in the P. aeruginosa infection model was delayed in the PAO1ΔwspF-infected group of diabetic mice. All wounds were measured at 4-day intervals to assess wound closure (representative images are shown in C1). The percent wound closure was determined using the following equation: (A0-At)/A0 × 100, where A0 was the wound area on the day of surgery, and At was the area of the wound on the day of observation (C2). One-way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparison test was used. Note: *P < 0.05 compared with the nondiabetic group, **P < 0.01 compared with the nondiabetic group.
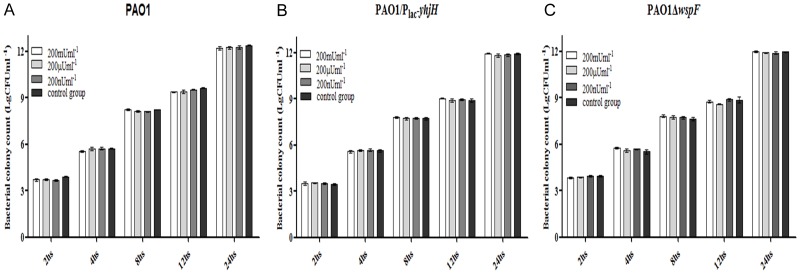
Insulin treatment did not have any effect on the bacterial loads of P. aeruginosa-infected chronic wounds in diabetic mice but increased resistance to amikacin
Every 4 days during observation, one group of mice was euthanized, and the tissue removed from the backs of the mice was weighed. The CFU/g value was determined as a measure of the bacterial loads in the wounds (Figure 4). The CFU count in the wound tissue was determined by a standard colony counting method. The bacterial load increased from an infecting dose of 104 CFU to approximately 106-7 CFU/g of wound tissue by day 4 or day 8 in all the groups of mice. By day 16, the CFU/g value for the nondiabetic mice was lower than that for the diabetic mice infected with PAO1/Plac-yhjH or PAO1. In general, the wounds of the nondiabetic mice closed by approximately 81.07 to 97.11% during the experiment, and it took an additional 12 to 16 days before the wounds of the diabetic mice closed to the same degree. These data indicate that the nondiabetic mice may have been more effective at clearing the infection than the diabetic mice, leading to faster wound healing. The blood, liver and spleen tissues that were extracted from the infected mice were cultured; however, no colonies were obtained in this experiment, suggesting that the P. aeruginosa infection did not spread systemically but was localized to only the wound bed.
Figure 4.
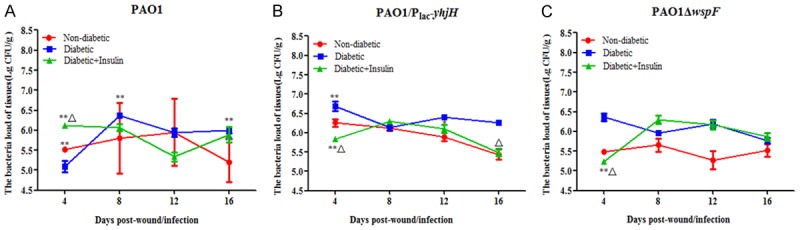
CFU/g values were measured to determine the bacterial loads in the wounds. Insulin treatment did not have any effect on the bacterial loads in chronic wounds of diabetic mice infected with the (A) PAO1 wild-type strain, (B) PAO1/Plac-yhjH strain and (C) PAO1ΔwspF strain. One-way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparison test was used. Note: *P < 0.05 compared with the nondiabetic group, **P < 0.01 compared with the nondiabetic group, ΔP < 0.05 compared with the diabetic group, ΔΔP < 0.01 compared with the diabetic group.
Next, we determined the resistance of the bacteria in the chronic wounds to amikacin, which is commonly used as an antipseudomonal agent. We did not observe any changes among the nondiabetic mice, diabetic mice and diabetic mice treated with insulin in the groups infected with either the PAO1 wild-type strain or the PAO1ΔwspF strain (Figure 5A and 5C). However, in the group infected with the PAO1/plac-yhjH strain (Figure 5B), we observed that the resistance of P. aeruginosa to amikacin was significantly higher for the diabetic mice treated with insulin than for the nondiabetic mice or the untreated diabetic mice at days 4 and 16, with values of 2.79, 3.44, and 7.09%, respectively, on day 4 and 4.47, 9.97, and 14.2%, respectively, on day 16 (Figure 5B).
Figure 5.
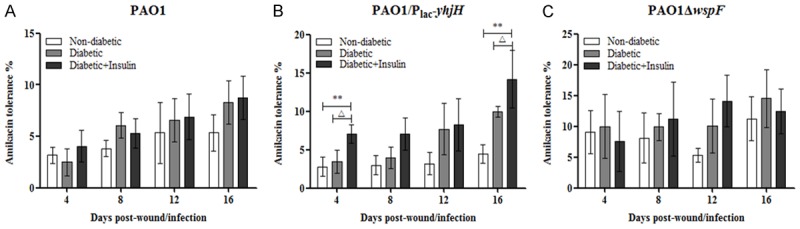
The resistance of P. aeruginosa to amikacin in diabetic mice treated with insulin was higher than that in nondiabetic mice and untreated diabetic mice. In particular, in the PAO1/Plac-yhjH-infected group, the resistance of diabetic mice treated with insulin was significantly higher than that of nondiabetic mice or untreated diabetic mice at days 4 and 16. The mice were anesthetized, and the gauze pads were removed from the wounds and treated with amikacin or PBS on the day of surgery and at 4-day intervals post surgery. The amikacin resistance of P. aeruginosa in the wounds was calculated by the following equation: (Ra CFU/g)/(Rp CFU/g) × 100, where Ra CFU/g refers to gauze pads treated with amikacin, and Rp CFU/g refers to gauze pads treated with PBS. One-way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparison test was used. Note: *P < 0.05 compared with the nondiabetic group, **P < 0.01 compared with the nondiabetic group, ΔP < 0.05 compared with the diabetic group, ΔΔP < 0.01 compared with the diabetic group.
P. aeruginosa aggregates were most prevalent in the wounds of diabetic mice treated with insulin
HE staining was used to observe the inflammatory reactions of the cells in the infected sections (Figure 6). There were more polymorphonuclear leucocytes (PMNs) in the wounds of the diabetic mice treated with insulin than in those of untreated diabetic mice and nondiabetic mice. P. aeruginosa aggregates were observed by PNA-FISH with a fluorescence microscope. More P. aeruginosa aggregates were observed with the PAO1ΔwspF strain (with high intracellular levels of c-di-GMP) than with the other strains. Some scattered P. aeruginosa aggregates were observed with the PAO1/Plac-yhjH strain (with low intracellular levels of c-di-GMP) (Figure 7). A high level of P. aeruginosa aggregation was observed in diabetic mice treated with insulin and infected with either the PAO1 wild-type strain or the PAO1/Plac-yhjH strain (Figure 8).
Figure 6.
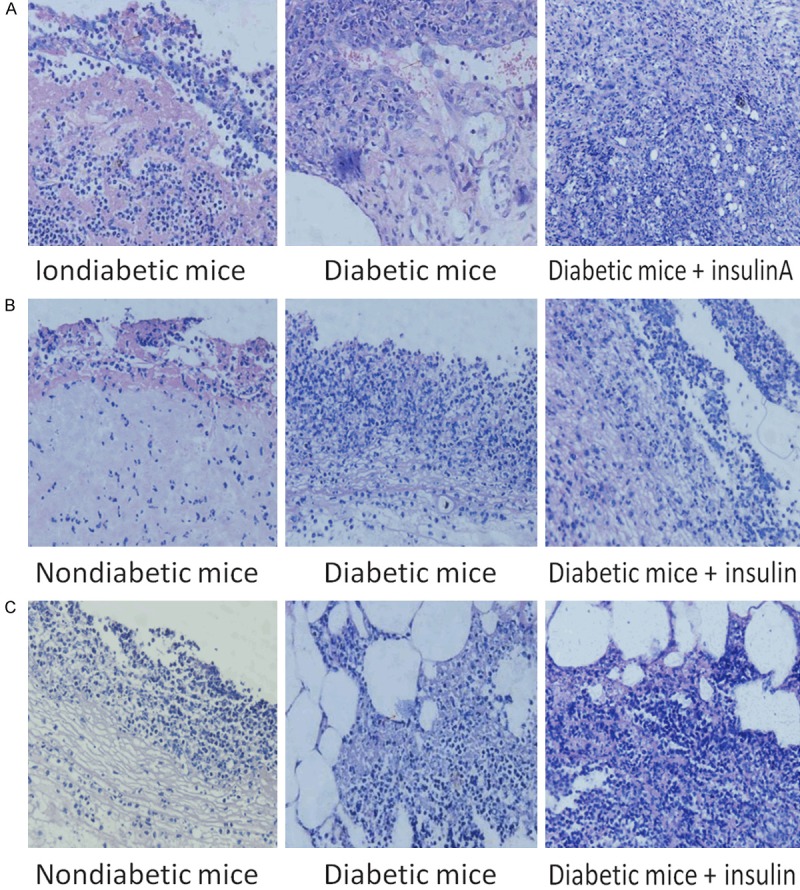
Deparaffinized pathological tissue samples stained with HE to observe the inflammatory reactions of the cells in the infected sections. Arrows indicate P. aeruginosa cells observed by microscopy. Tissues infected with the (A) PAO1 wild-type strain, (B) PAO1/Plac-yhjH strain and (C) PAO1ΔwspF strain.
Figure 7.
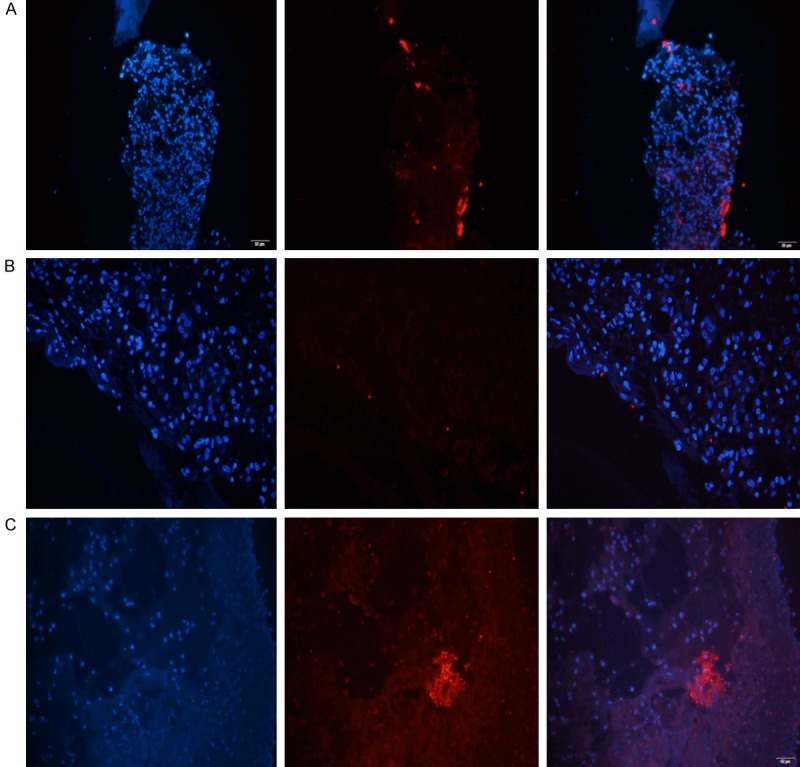
P. aeruginosa aggregates were observed by PNA-FISH with a Texas Red-labeled P. aeruginosa-specific probe (red). The (A) PAO1 wild-type strain, (B) PAO1/Plac-yhjH strain and (C) PAO1ΔwspF strain observed with a fluorescence microscope. A high level of P. aeruginosa aggregation was observed with the PAO1ΔwspF strain (with high intracellular levels of c-di-GMP). Some scattered P. aeruginosa aggregates were observed with the PAO1/Plac-yhjH strain (with low intracellular levels of c-di-GMP).
Figure 8.
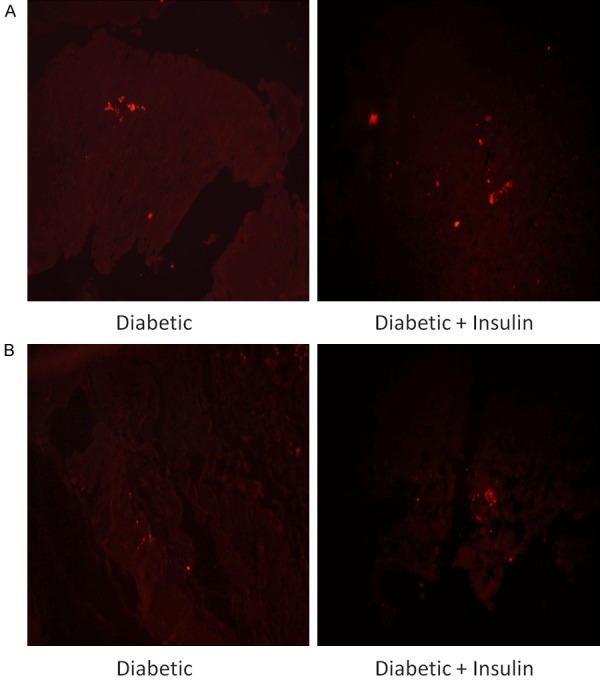
Effect of insulin treatment on the biofilms in wound tissue infected with the (A) PAO1 wild-type strain or (B) PAO1/Plac-yhjH strain. A high level of P. aeruginosa aggregation was observed in diabetic mice treated with insulin and infected with either the PAO1 wild-type strain or the PAO1/Plac-yhjH strain.
Insulin treatment promoted the expression of IL-4 and IFN-γ RNA in P. aeruginosa-infected wounds in diabetic mice
To identify the mechanism by which insulin participates in the immune response, RT-qPCR was used to detect the expression levels of IL-4 and IFN-γ RNA in P. aeruginosa-infected wounds in diabetic mice. We found that the expression of IL-4 RNA in each group increased with time post infection (Figure 9). For the PAO1/Plac-yhjH strain (with low c-di-GMP expression levels), at each observation time point, the expression of IL-4 in diabetic mice treated with insulin was significantly higher than that in the untreated diabetic group or the nondiabetic group (P < 0.05). The peak expression of IFN-γ in diabetic mice treated with insulin occurred earlier than it did in the untreated diabetic group with each of the strains tested (Figure 10).
Figure 9.
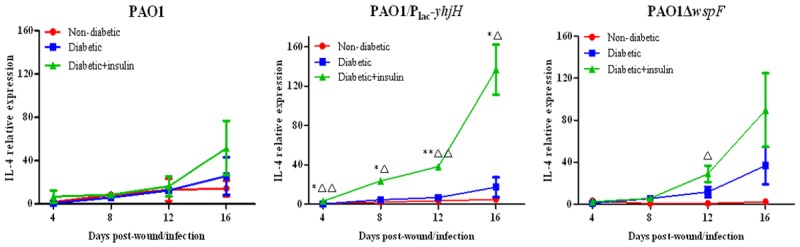
Effect of insulin treatment on the expression of IL-4 RNA in infected chronic wound tissue from diabetic mice. Note: *P < 0.05 compared with the nondiabetic group, **P < 0.01 compared with the nondiabetic group, ΔP < 0.05 compared with the diabetic group, ΔΔP < 0.01 compared with the diabetic group.
Figure 10.
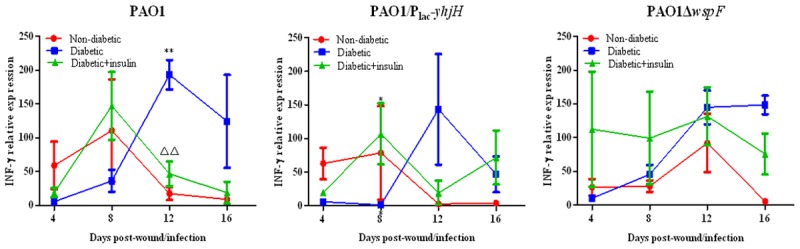
Effect of insulin treatment on the expression of IFN-γ RNA in infected chronic wound tissue from diabetic mice. Note: *P < 0.05 compared with the nondiabetic group, **P < 0.01 compared with the nondiabetic group, ΔP < 0.05 compared with the diabetic group, ΔΔP < 0.01 compared with the diabetic group.
Insulin affected biofilm formation via the c-di-GMP signaling pathway in vitro
Chase Watters’ group found that insulin treatment promoted biofilm formation activity in the P. aeruginosa wild-type PAO1 strain. Therefore, we tested whether insulin treatment enhanced biofilm formation by increasing the intracellular c-di-GMP levels of P. aeruginosa in a microtiter plate-based biofilm assay. First, we determined whether insulin or denatured insulin could affect the growth of P. aeruginosa strains with different intracellular c-di-GMP levels. Among the mice infected with the PAO1, PAO1ΔwspF or PAO1/Plac-yhjH strain, no changes were observed in the growth of the P. aeruginosa strains with different intracellular c-di-GMP levels in response to Humulin® N insulin, at amounts ranging from 200 nU to 200 mU, or denatured Humulin® N insulin (Figure 11). We next tested whether Humulin® N insulin affected the ability of P. aeruginosa to form biofilms by a microtiter plate-based biofilm assay. We observed that Humulin® N insulin but not denatured insulin could promote the formation of biofilms in the PAO1 and PAO1/Plac-yhjH strains in a manner that was independent of insulin concentration (Figure 12A and 12B). However, we did not observe any effect on the biofilm formation of the PAO1ΔwspF strain (Figure 12C). Finally, we examined whether insulin could increase intracellular c-di-GMP levels in P. aeruginosa under the conditions described above. We found that Humulin® N insulin significantly promoted the expression of the intracellular c-di-GMP biosensor PcdrA-gfp in the PAO1 strain treated with 200 mU of insulin and in the PAO1/Plac-yhjH strains treated with 200 nU to 200 mU of insulin (Figure 13B). However, in contrast, Humulin® N insulin did not promote, and instead suppressed, the expression of the intracellular c-di-GMP biosensor PcdrA-gfp with 200 mU of insulin in the PAO1ΔwspF group (Figure 13B). These data demonstrate that Humulin® N insulin promoted P. aeruginosa biofilm formation by increasing intracellular c-di-GMP levels in P. aeruginosa strains with intermediate or low intracellular c-di-GMP levels, while the opposite trend was observed for the strain with high c-di-GMP levels.
Figure 11.
Effect of insulin on the growth of the (A) PAO1 strain, (B) PAO1/Plac-yhjH strain and (C) PAO1ΔwspF strain. We found that there were no changes in the growth of P. aeruginosa strains with different intracellular c-di-GMP levels in response to any concentration of insulin or denatured insulin.
Figure 12.
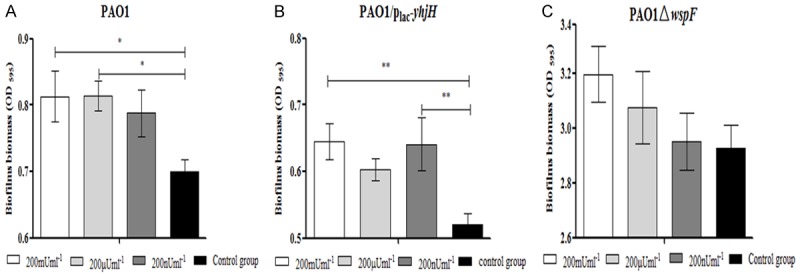
Effect of insulin on biofilm formation of the (A) PAO1 strain, (B) PAO1/Plac-yhjH strain and (C) PAO1ΔwspF strain in microplates. An overnight culture was diluted with ABTGC medium supplemented with Humulin® N insulin or denatured insulin with a final amount of 200 nU, 200 µU, 200 mU or 200 mU. Biofilm formation was monitored after 12 hours of continuous cultivation. The mean and SD from at least triplicate experiments are shown. Note: *P < 0.05 compared with the control group, **P < 0.01 compared with the control group.
Figure 13.
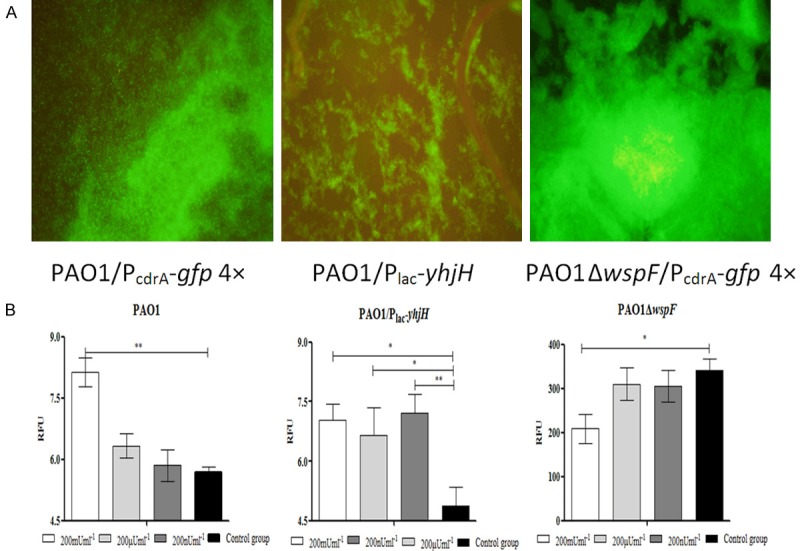
Effect of insulin on PcdrA-gfp expression in the PAO1 strain, PAO1/Plac-yhjH strain and PAO1ΔwspF strain in microplates. The PAO1 strain containing the PcdrA-gfp vector, which can provide a fluorescent readout of the intracellular level of c-di-GMP in P. aeruginosa, was resuscitated and grown at 37°C in Luria-Bertani (LB) medium. After 24 hours of cultivation, the microplates were observed by fluorescence microscopy (A). PcdrA-gfp fluorescence and OD600 values were measured and recorded as relative fluorescence unit (RFU) values after 12 hours of incubation. The mean and SD of RFUs from at least triplicate experiments are shown (B). Note: *P < 0.05 compared with the control group, **P < 0.01 compared with the control group.
Discussion
The presence of biofilms has been confirmed in infected chronic wounds via PNA-FISH, scanning electron microscopy (SEM), and detection of the extracellular polymeric substance (EPS) matrix by illumination of the alginate surrounding P. aeruginosa; these specimens originated from patients with DFIs or from chronic wound models of diabetes [2,25-28]. However, whether biofilms in wounds hinder wound healing is a subject of debate. Increasing evidence has shown that the presence of biofilms in wounds leads to delayed healing [2,28,29]. It is well known that the presence of biofilms in wounds delays wound healing in a variety of ways, including by activating the host response to the bacteria, which is characterized by persistent inflammation, continuous oxidative damage, fibroblast senescence and depletion of beneficial growth factors needed for tissue resolution in a chronic wound [30]. However, Trøstrup H reported that P. aeruginosa biofilms hamper murine central wound healing by suppressing the vascular epithelial growth factor [31]. Whether insulin treatment can be used to treat P. aeruginosa infections is currently a controversial subject. Low-dose insulin treatment has been reported to decrease the mortality rate of sepsis caused by P. aeruginosa infection after severe burn injury. However, as reported by Watters et al. [16], in chronic wound models of diabetes, biofilms are highly prevalent in STZ-induced wounds in diabetic mice, and most importantly, high biofilm formation was observed in the wounds of insulin-treated diabetic mice [18]. Subsequently, studies have shown that high levels of lysed neutrophils in the wounds of diabetic mice treated with insulin, combined with decreased levels of macrophages that clear the cellular detritus, contribute to enhanced eDNA levels, thereby enhancing P. aeruginosa biofilm formation [17].
In our study, we observed that the increase in the body weights of diabetic mice treated with insulin was significantly greater than that observed for untreated diabetic mice between the PAO1/plac-yhjH- and PAO1ΔwspF-infected groups, which indicated that insulin treatment indeed improved the overall condition of diabetic mice. For example, in the PAO1-wild-type-infected group, only the diabetic mice treated with insulin gained body weight. As weight loss is a sign of disease progression or infection, these data showed that insulin treatment can improve the overall health of diabetic mice with P. aeruginosa-infected chronic wounds [32]. Brown et al. showed that wound closure was impaired, with significantly greater severity, in diabetic mice infected with P. aeruginosa than in nondiabetic mice infected with P. aeruginosa [33]. Our result was similar to the results reported by Brown et al. [33]. However, we found dramatic differences in wound closure between nondiabetic and diabetic mice among the groups infected with the PAO1 wild-type, PAO1/plac-yhjH or PAO1ΔwspF strain. The slowest wound healing was observed with the PAO1ΔwspF strain (with high intracellular c-di-GMP levels) among the nondiabetic mice, diabetic mice and diabetic mice treated with insulin. Most importantly, we observed that the wound closure in diabetic mice treated with insulin was significantly slower than that in untreated diabetic mice in the PAO1 wild-type-infected group (day 16) and the PAO1/plac-yhjH-infected group (day 12 and day 16). P. aeruginosa produces biofilms that increase the resistance of this species to antibiotics [34,35]. C-di-GMP is involved in the regulation of cellular functions, especially biofilm formation and dispersion; high intracellular levels of c-di-GMP promote biofilm formation, and low c-di-GMP levels cause biofilm dispersal. We also observed that the PAO1/plac-yhjH strain in the wounds of the diabetic mice was significantly more tolerant to amikacin (day 4 and day 16) than that same strain in the wounds of nondiabetic mice, and the highest antibiotic tolerance was observed in diabetic mice treated with insulin. However, the changes in wound healing and amikacin resistance were not related to the changes in the colony counts of the wound tissues. Therefore, these data suggest that one of the primary factors underlying the changes in wound healing and amikacin resistance was increased biofilm formation.
We used PNA-FISH to determine whether the wounds were infected with P. aeruginosa, and ConA-FITC was then used to stain the exopolysaccharide to confirm biofilm formation. Notably (and unexpectedly), more biofilm was observed in the wounds of insulin-treated diabetic mice infected with PAO1/plac-yhjH or PAO1 than in the wounds of untreated diabetic mice or nondiabetic mice. These in vivo data indicate that the formation of biofilms in the wounds of diabetic mice treated with insulin was promoted at intermediate or low levels of intracellular c-di-GMP, which was associated with c-di-GMP signaling in P. aeruginosa. In other words, insulin treatment likely promoted P. aeruginosa biofilm formation by affecting the c-di-GMP signaling pathway, resulting in delayed wound healing and increased antibiotic resistance. In vitro studies showed that there were no significant changes in the growth of P. aeruginosa strains with different intracellular c-di-GMP levels in response to insulin or denatured insulin. We also found that insulin could significantly promote the expression of the intracellular c-di-GMP biosensor PcdrA-gfp in the PAO1 wild-type strain with 200 mU of insulin and in the PAO1/plac-yhjH strain with 200 nU to 200 mU of insulin. However, in the PAO1ΔwspF-infected group, in vitro studies showed that insulin and denatured insulin did not affect biofilm formation. Similarly, we found that insulin treatment did not significantly prolong wound healing in diabetic mice or lead to increased rates of drug resistance in vivo. We speculate that there is already a very high concentration of intracellular c-di-GMP in the PAO1ΔwspF strain, leaving no room to improve biofilm formation. The intracellular c-di-GMP levels in the PAO1ΔwspF strain were not enhanced by drugs or other means. Rybtke et al. [19] showed that reporter constructs provided a fluorescent readout of the intracellular levels of c-di-GMP in P. aeruginosa strains with different levels of c-di-GMP and could detect the increased turnover of c-di-GMP mediated by the treatment of P. aeruginosa with the phosphodiesterase-inducer nitric oxide. These data proved that insulin did not promote the growth of P. aeruginosa but promoted the formation of biofilms by increasing intracellular c-di-GMP synthesis in P. aeruginosa strains with intermediate and low levels of c-di-GMP.
In the clinic, chronic wounds are also frequently infected by multispecies biofilms [4], but only one pathogen, P. aeruginosa, which is a ubiquitous, clinically important, opportunistic pathogen, was used in this study; therefore, the results obtained in this study did not accurately simulate an infected human chronic wound. In this study, we observed the effect of insulin treatment on only the amount of biofilm in the P. aeruginosa-infected chronic wounds, but determining whether insulin treatment can influence the production of biofilm components such as rhamnolipids requires further research. In our in vivo study, we observed that insulin amounts from 200 nU to 200 mU promoted biofilm formation, but it remains unclear whether these insulin amounts correspond to the levels in the infected wounds of diabetic mice in vivo. Information regarding the levels of insulin that reach the peripheral tissue under the mouse skin is scarce; however, it has been reported that 40~70 µU/mL insulin was detected in the periphery 90 s after injection of 400 ng of insulin into the vena cava in rats [36]. Assuming that this result was true in our mice, when the mice were treated with approximately 69 µg of insulin (equal to 2 U), the peripheral insulin concentration would have been 7~12 mU/mL, which clearly promoted the biofilm formation of P. aeruginosa directly in vitro.
The presence and persistence of P. aeruginosa biofilms in chronic wounds can affect the functions of cells such as leukocytes, keratinocytes, endothelial cells, and fibroblasts, and the affected functions include the inflammatory cellular response, the cutaneous innate immune response, and the repair phase of wound healing [37]. In the course of normal wound healing, once the wound tissue is removed, the end of the inflammatory reaction period follows, which is followed by the beginning of the proliferative phase. A chronic wound is characterized by a prolonged duration of the inflammatory reaction, which interferes with wound healing. It was reported that insulin had anti-inflammatory and antioxidant effects in both clinical trials and animal experiments [38,39]. Insulin promotes the polarization of T cells to the Th2 cell type by promoting the phosphorylation of the Th2 extracellular signal-regulated kinase, which plays an anti-inflammatory role not only in septicemia but also in chronic inflammation associated with obesity or type 2 diabetes [40]. Animal experiments and clinical studies have indicated that the immune response to P. aeruginosa infection is a Th2-type response, as observed mainly in patients with pulmonary cystic fibrosis infected with P. aeruginosa [41-43]. According to the latest European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines [44,45], the inflammatory cells in P. aeruginosa-infected wound tissues are mainly PMNs or macrophages, which will determine whether the predominant immune response to the microbes present in P. aeruginosa biofilms consist of either Th2 (antibody) or Th1 (cell-mediated) polarization. Our study found that the RNA expression of IL-4, a characteristic inflammatory factor that is secreted by Th2 cells, increased with time. In particular, in the PAO1/Plac-yhjH strain (with low c-di-GMP expression), the expression of IL-4 RNA in diabetic mice treated with insulin was significantly higher than that in untreated diabetic and nondiabetic mice at each observation time point. In terms of the expression of IFN-γ RNA, a characteristic inflammatory factor secreted by Th1 cells, the peak local expression of this inflammatory factor observed in the P. aeruginosa-infected wounds of diabetic mice treated with insulin was lower and occurred earlier than that in diabetic mice, with expression levels gradually decreasing thereafter. These data demonstrate the dual effects of insulin; on one hand, the clearance of P. aeruginosa decreased via inhibition of the Th1-type immune response, but on the other hand, biofilm formation was promoted by enhanced Th2-type polarization, leading to promotion of the Th2-type immune response, which resulted in a prolonged period of inflammation and the healing of infected wounds.
In conclusion, the delayed healing of infected chronic wounds is affected by a variety of factors, which are complex and variable. In our study, for the first time, insulin was shown to promote P. aeruginosa biofilm formation by increasing intracellular c-di-GMP levels in vitro and in vivo, resulting in delayed wound healing in diabetic mice. In addition, for the first time, insulin was shown to decrease the clearance of P. aeruginosa by inhibiting the Th1-type immune response and promoting biofilm formation by enhancing Th2-type polarization, thereby promoting the Th2-type immune response, which resulted in a prolonged period of inflammation and the healing of infected wounds. However, there have been no clinical studies linking the chronic DFIs caused by P. aeruginosa with c-di-GMP signaling. It has been reported that [46] the naturally induced dispersal agent nitric oxide can reduce intracellular c-di-GMP levels and decrease cell permeability. Compared with other c-di-GMP synthase inhibitors, nitric oxide can induce P. aeruginosa biofilm dispersal [47,48] at relatively low concentrations. Further clinical studies are required to determine whether a nitric oxide donor, such as sodium nitroprusside solution, can be used locally to induce the dispersal of P. aeruginosa biofilms in strains with high intracellular c-di-GMP levels.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 81460003, no. 81760024 and no. 81760743), the Innovation Project of Guangxi Graduate Education (no. 201010 LX039), the Key Research and Development Program of Guangxi (no. AB16380152), the Basic Ability Improvement Project for Young and Middle-aged Teachers in Colleges of Guangxi (no. 2019KY0125) and the Medical Excellence Award Funded by the Creative Research Development Grant from the First Affiliated Hospital of Guangxi Medical University.
Disclosure of conflict of interest
None.
References
- 1.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gjodsbol K, Christensen JJ, Karlsmark T, Jorgensen B, Klein BM, Krogfelt KA. Multiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J. 2006;3:225–231. doi: 10.1111/j.1742-481X.2006.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirketerp-Møller K, Jensen PØ, Fazli M, Madsen KG, Pedersen J, Moser C, Tolker-Nielsen T, Høiby N, Givskov M, Bjarnsholt T. Distribution, organization, and ecology of bacteria in chronic wounds. J Clin Microbiol. 2008;46:2717–2722. doi: 10.1128/JCM.00501-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rastogi A, Sukumar S, Hajela A, Mukherjee S, Dutta P, Bhadada SK, Bhansali A. The microbiology of diabetic foot infections in patients recently treated with antibiotic therapy: a prospective study from India. J Diabetes Complications. 2017;31:407–412. doi: 10.1016/j.jdiacomp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Wu WX, Liu D, Wang YW, Wang C, Yang C, Liu XZ, Mai LF, Ren M, Yan L. Empirical antibiotic treatment in diabetic foot infection: a study focusing on the culture and antibiotic sensitivity in a population from southern China. Int J Low Extrem Wounds. 2017;16:173–182. doi: 10.1177/1534734617725410. [DOI] [PubMed] [Google Scholar]
- 6.Hoiby N, Frederiksen B, Pressler T. Eradication of early pseudomonas aeruginosa infection. J Cyst Fibros. 2005;4(Suppl 2):49–54. doi: 10.1016/j.jcf.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 8.Hoiby N, Ciofu O, Bjarnsholt T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 2010;5:1663–1674. doi: 10.2217/fmb.10.125. [DOI] [PubMed] [Google Scholar]
- 9.Fazli M, Almblad H, Rybtke ML, Givskov M, Eberl L, Tolker-Nielsen T. Regulation of biofilm formation in pseudomonas and burkholderia species. Environ Microbiol. 2014;16:1961–1981. doi: 10.1111/1462-2920.12448. [DOI] [PubMed] [Google Scholar]
- 10.Luo J, Dong B, Wang K, Cai S, Liu T, Cheng X, Lei D, Chen Y, Li Y, Kong J, Chen Y. Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS One. 2017;12:e0176883. doi: 10.1371/journal.pone.0176883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross P, Weinhouse H, Aloni Y, Michaeli D, Weinberger-Ohana P, Mayer R, Braun S, de Vroom E, van der Marel GA, van Boom JH, Benziman M. Regulation of cellulose synthesis in acetobacter xylinum by cyclic diguanylic acid. Nature. 1987;325:279. doi: 10.1038/325279a0. [DOI] [PubMed] [Google Scholar]
- 12.Hengge R. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol. 2009;7:263–273. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- 13.Ha DG, O’Toole GA. c-di-GMP and its effects on biofilm formation and dispersion: a pseudomonas aeruginosa review. Microbiol Spectr. 2015;3 doi: 10.1128/microbiolspec.MB-0003-2014. MB-0003-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao G, Usui ML, Underwood RA, Singh PK, James GA, Stewart PS, Fleckman P, Olerud JE. Time course study of delayed wound healing in a biofilm-challenged diabetic mouse model. Wound Repair Regen. 2012;20:342–352. doi: 10.1111/j.1524-475X.2012.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gauglitz GG, Toliver-Kinsky TE, Williams FN, Song J, Cui W, Herndon DN, Jeschke MG. Insulin increases resistance to burn wound infection-associated sepsis. Crit Care Med. 2010;38:202–208. doi: 10.1097/CCM.0b013e3181b43236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watters C, Everett JA, Haley C, Clinton A, Rumbaugh KP. Insulin treatment modulates the host immune system to enhance pseudomonas aeruginosa wound biofilms. Infect Immun. 2014;82:92–100. doi: 10.1128/IAI.00651-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watters C, DeLeon K, Trivedi U, Griswold JA, Lyte M, Hampel KJ, Wargo MJ, Rumbaugh KP. Pseudomonas aeruginosa biofilms perturb wound resolution and antibiotic tolerance in diabetic mice. Med Microbiol Immunol. 2013;202:131–141. doi: 10.1007/s00430-012-0277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rybtke MT, Borlee BR, Murakami K, Irie Y, Hentzer M, Nielsen TE, Givskov M, Parsek MR, Tolker-Nielsen T. Fluorescence-based reporter for gauging cyclic di-GMP levels in pseudomonas aeruginosa. Appl Environ Microbiol. 2012;78:5060–5069. doi: 10.1128/AEM.00414-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chua SL, Tan SY, Rybtke MT, Chen Y, Rice SA, Kjelleberg S, Tolker-Nielsen T, Yang L, Givskov M. Bis-(3’-5’)-cyclic dimeric GMP regulates antimicrobial peptide resistance in pseudomonas aeruginosa. Antimicrob Agents Chemother. 2013;57:2066–2075. doi: 10.1128/AAC.02499-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fazli M, Bjarnsholt T, Hoiby N, Givskov M, Tolker-Nielsen T. PNA-based fluorescence in situ hybridization for identification of bacteria in clinical samples. Methods Mol Biol. 2014;1211:261–271. doi: 10.1007/978-1-4939-1459-3_21. [DOI] [PubMed] [Google Scholar]
- 21.Merritt JH, Kadouri DE, O’Toole GA. Growing and analyzing static biofilms. Curr Protoc Microbiol. 2005 doi: 10.1002/9780471729259.mc01b01s00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christensen LD, van Gennip M, Rybtke MT, Wu H, Chiang WC, Alhede M, Hoiby N, Nielsen TE, Givskov M, Tolker-Nielsen T. Clearance of pseudomonas aeruginosa foreign-body biofilm infections through reduction of the cyclic Di-GMP level in the bacteria. Infect Immun. 2013;81:2705–2713. doi: 10.1128/IAI.00332-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao G, Hochwalt PC, Usui ML, Underwood RA, Singh PK, James GA, Stewart PS, Fleckman P, Olerud JE. Delayed wound healing in diabetic (db/db) mice with pseudomonas aeruginosa biofilm challenge: a model for the study of chronic wounds. Wound Repair Regen. 2010;18:467–477. doi: 10.1111/j.1524-475X.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michaels J th, Churgin SS, Blechman KM, Greives MR, Aarabi S, Galiano RD, Gurtner GC. db/db mice exhibit severe wound-healing impairments compared with other murine diabetic strains in a silicone-splinted excisional wound model. Wound Repair Regen. 2007;15:665–670. doi: 10.1111/j.1524-475X.2007.00273.x. [DOI] [PubMed] [Google Scholar]
- 25.James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilms in chronic wounds. Wound Repair Regen. 2008;16:37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 26.Fazli M, Bjarnsholt T, Kirketerp-Moller K, Jorgensen B, Andersen AS, Krogfelt KA, Givskov M, Tolker-Nielsen T. Nonrandom distribution of pseudomonas aeruginosa and staphylococcus aureus in chronic wounds. J Clin Microbiol. 2009;47:4084–4089. doi: 10.1128/JCM.01395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanno E, Toriyabe S, Zhang L, Imai Y, Tachi M. Biofilm formation on rat skin wounds by pseudomonas aeruginosa carrying the green fluorescent protein gene. Exp Dermatol. 2010;19:154–156. doi: 10.1111/j.1600-0625.2009.00931.x. [DOI] [PubMed] [Google Scholar]
- 28.Bjarnsholt T. The role of bacterial biofilms in chronic infections. APMIS Suppl. 2013;121:1–58. doi: 10.1111/apm.12099. [DOI] [PubMed] [Google Scholar]
- 29.Hogsberg T, Bjarnsholt T, Thomsen JS, Kirketerp-Moller K. Success rate of split-thickness skin grafting of chronic venous leg ulcers depends on the presence of pseudomonas aeruginosa: a retrospective study. PLoS One. 2011;6:e20492. doi: 10.1371/journal.pone.0020492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moser C, Pedersen HT, Lerche CJ, Kolpen M, Line L, Thomsen K, Hoiby N, Jensen PO. Biofilms and host response-helpful or harmful. APMIS. 2017;125:320–338. doi: 10.1111/apm.12674. [DOI] [PubMed] [Google Scholar]
- 31.Trostrup H, Lerche CJ, Christophersen LJ, Thomsen K, Jensen PO, Hougen HP, Hoiby N, Moser C. Pseudomonas aeruginosa biofilm hampers murine central wound healing by suppression of vascular epithelial growth factor. Int Wound J. 2018;15:123–132. doi: 10.1111/iwj.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Percival SL, Hill KE, Williams DW, Hooper SJ, Thomas DW, Costerton JW. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen. 2012;20:647–657. doi: 10.1111/j.1524-475X.2012.00836.x. [DOI] [PubMed] [Google Scholar]
- 33.Brown RL, Greenhalgh DG. Mouse models to study wound closure and topical treatment of infected wounds in healing-impaired and normal healing hosts. Wound Repair Regen. 1997;5:198–204. doi: 10.1046/j.1524-475X.1997.50213.x. [DOI] [PubMed] [Google Scholar]
- 34.Teitzel GM, Parsek MR. Heavy metal resistance of biofilm and planktonic pseudomonas aeruginosa. Appl Environ Microbiol. 2003;69:2313–2320. doi: 10.1128/AEM.69.4.2313-2320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson GG, O’Toole GA. Innate and induced resistance mechanisms of bacterial biofilms. Curr Top Microbiol Immunol. 2008;322:85–105. doi: 10.1007/978-3-540-75418-3_5. [DOI] [PubMed] [Google Scholar]
- 36.Pelegrinelli FF, Thirone AC, Gasparetti AL, Araujo EP, Velloso LA, Saad MJ. Early steps of insulin action in the skin of intact rats. J Invest Dermatol. 2001;117:971–976. doi: 10.1046/j.0022-202x.2001.01473.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhao G, Usui ML, Lippman SI, James GA, Stewart PS, Fleckman P, Olerud JE. Biofilms and inflammation in chronic wounds. Adv Wound Care (New Rochelle) 2013;2:389–399. doi: 10.1089/wound.2012.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langouche L, Vanhorebeek I, Vlasselaers D, Vander Perre S, Wouters PJ, Skogstrand K, Hansen TK, Van den Berghe G. Intensive insulin therapy protects the endothelium of critically ill patients. J Clin Invest. 2005;115:2277–2286. doi: 10.1172/JCI25385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeschke MG, Klein D, Bolder U, Einspanier R. Insulin attenuates the systemic inflammatory response in endotoxemic rats. Endocrinology. 2004;145:4084–4093. doi: 10.1210/en.2004-0592. [DOI] [PubMed] [Google Scholar]
- 40.Viardot A, Grey ST, Mackay F, Chisholm D. Potential antiinflammatory role of insulin via the preferential polarization of effector T cells toward a T helper 2 phenotype. Endocrinology. 2007;148:346–353. doi: 10.1210/en.2006-0686. [DOI] [PubMed] [Google Scholar]
- 41.Moser C, Kjaergaard S, Pressler T, Kharazmi A, Koch C, Hoiby N. The immune response to chronic pseudomonas aeruginosa lung infection in cystic fibrosis patients is predominantly of the Th2 type. APMIS. 2000;108:329–335. doi: 10.1034/j.1600-0463.2000.d01-64.x. [DOI] [PubMed] [Google Scholar]
- 42.Moser C, Johansen HK, Song Z, Hougen HP, Rygaard J, Hoiby N. Chronic pseudomonas aeruginosa lung infection is more severe in Th2 responding BALB/c mice compared to Th1 responding C3H/HeN mice. APMIS. 1997;105:838–842. [PubMed] [Google Scholar]
- 43.Hartl D, Griese M, Kappler M, Zissel G, Reinhardt D, Rebhan C, Schendel DJ, Krauss-Etschmann S. Pulmonary T(H)2 response in pseudomonas aeruginosa-infected patients with cystic fibrosis. J Allergy Clin Immunol. 2006;117:204–211. doi: 10.1016/j.jaci.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 44.Hoiby N, Bjarnsholt T, Moser C, Bassi GL, Coenye T, Donelli G, Hall-Stoodley L, Hola V, Imbert C, Kirketerp-Moller K, Lebeaux D, Oliver A, Ullmann AJ, Williams C. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 2015;21(Suppl 1):S1–S25. doi: 10.1016/j.cmi.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 45.Van Alst NE, Picardo KF, Iglewski BH, Haidaris CG. Nitrate sensing and metabolism modulate motility, biofilm formation, and virulence in Pseudomonas aeruginosa. Infect Immun. 2007;75:3780–3790. doi: 10.1128/IAI.00201-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poh WH, Barraud N, Guglielmo S, Lazzarato L, Rolando B, Fruttero R, Rice SA. Furoxan nitric oxide donors disperse pseudomonas aeruginosa biofilms, accelerate growth, and repress pyoverdine production. ACS Chem Biol. 2017;12:2097–2106. doi: 10.1021/acschembio.7b00256. [DOI] [PubMed] [Google Scholar]
- 47.Barnes RJ, Bandi RR, Wong WS, Barraud N, McDougald D, Fane A, Kjelleberg S, Rice SA. Optimal dosing regimen of nitric oxide donor compounds for the reduction of pseudomonas aeruginosa biofilm and isolates from wastewater membranes. Biofouling. 2013;29:203–212. doi: 10.1080/08927014.2012.760069. [DOI] [PubMed] [Google Scholar]
- 48.Amari DT, Marques CN, Davies DG. The putative enoyl-coenzyme a hydratase dspI is required for production of the pseudomonas aeruginosa biofilm dispersion autoinducer cis-2-decenoic acid. J Bacteriol. 2013;195:4600–4610. doi: 10.1128/JB.00707-13. [DOI] [PMC free article] [PubMed] [Google Scholar]