Abstract
Background: Glucose metabolism is an essential energy source for mammalian preimplantation embryonic development. Therefore, we aimed to analyze the expression of all 12 known glucose transporters (facilitated solute carrier family 2, Slc2a) during early mouse embryo development. Methods: Gene and protein expression of Slc2a transporters in oocytes and embryos were assessed by the TaqMan gene expression assay and confocal immunofluorescence, respectively. Results: Except for Slc2a2, the other 11 Slc2a transcripts were detected in oocytes. Transcripts of Slc2a1, Slc2a3, Slc2a4, and Slc2a8 were the most enriched and detected in preimplantation embryos. The transcription of other Slc2a isoforms was barely detectable or absent after fertilization; however, they were detected in blastocysts, except for Slc2a10 and Slc2a13. Embryo culture in the simple defined medium caused a reduction in transcription of Slc2a1, Slc2a3, Slc2a4, and Slc2a8 in blastocyst; yet, amino acids partially reversed this impaired transcription of Slc2a1 and Slc2a4. SLC2A1 and SLC2A4 proteins were detected at all embryonic stages with nuclear accumulation in the embryos at the early cleavage stage. SLC2A3 and SLC2A8 were not detected in embryos until the eight-cell stage. The cellular membrane localization of SLC2A1, SLC2A3, and SLC2A8 occurred after compaction and was characterized in blastocysts. SLC2A4 was evenly distributed in the cytoplasm and nuclei without its characteristic membrane localization. Indinavir sulfate (a SLC2A4 inhibitor) decreased the rate of development and prevented glucose utilization in embryos after compaction. These inhibitory activities were partially reversed by exogenous insulin. Conclusion: The results unveil distinct expression patterns of individual Slc2a glucose transporters during early embryo development. Taken together, they provide novel insights into the understanding and management of glucose metabolic infertility in assisted-reproductive technologies (ART).
Keywords: Assisted reproductive technology, SLC2A, gene expression, glucose transporters, preimplantation embryo
Introduction
Glucose is an essential source of energy for the metabolic needs of normal preimplantation embryonic development. Glucose uptake occurs at all stages of development. In the oocytes and embryos during the early preimplantation development, before compaction, glucose consumption is low; therefore pyruvate and lactate act as the major energy sources, utilized via the Krebs cycle [1], while the eight-cell-stage and post-compaction embryos use glucose [2]. Exposure to glucose improves development, prevents apoptosis and is necessary for the transcription of certain genes [3-6]. Glucose is hydrophilic and must be transported into the cell by dedicated transporters that are encoded by genes known collectively as the solute carrier family 2 (SLC2A) (also known as the glucose transporter gene family, GLUT) [7,8]. Currently, 14 isoforms of SLC2A genes have been identified in humans; SLC2A11 and SLC2A14 are not reported in mice. These isoforms exhibit similar structural architecture, consisting of 12 transmembrane-spanning helices and an exofacial N-linked glycosylation site [8,9]. However, they differ in their tissue distribution, cellular and sub-cellular localization, kinetic properties, and affinity for glucose and other hexoses. SLC2A1 and SLC2A4 are the most extensively studied SLC2A members in mammalian tissues. SLC2A4 is the canonical insulin-responsive glucose transporter and is predominantly expressed in adult tissues [9,10]. In reproduction, SLC2A1, SLC2A3, and SLC2A8 are commonly recognized as the crucial glucose transporters and are expressed in the oocytes and preimplantation embryos of mouse [11]. Individual knockout of these genes is known to cause developmental impairments [12-14]. However, the relative expression of individual SLC2A isoforms has been reported to vary among different developmental stages [11], and their roles remain unclear.
The alterations in glucose transport and metabolism during the early stages of development can affect long-term development and an abnormal environment can cause nutrient transport dysfunction, leading to decreased fertility and adverse fetal outcomes [15,16]. Studies in mice have shown that hyperglycemia in preimplantation embryos downregulates SLC2A, resulting in lower intracellular glucose, abnormal metabolism, and increased apoptosis at the blastocyst stage [17]. In ART, the embryos cultured under suboptimal conditions exhibit lowered development rates [18]. Additionally, diabetes is the most common pre-existing medical condition in pregnant mothers, and despite the advances in care, adverse outcomes are still 3-9 times higher in diabetic pregnancies than in non-diabetic pregnancies [16]. However, the detailed expression pattern and functional role of these transporters in regulating glucose uptake in the preimplantation embryos are still largely unexplored.
The present study aimed to analyze the expression pattern of all known mouse SLC2A isoforms in the oocytes and preimplantation embryos of mouse, with particular attention to SLC2A4 and its roles during preimplantation. Studies on the mechanisms of normal glucose transport are critical to understand the distribution and role of glucose transporters during preimplantation under ART conditions in order to improve outcomes in the increasingly metabolic maternal environment.
Material and methods
Animals
The use of animals complied with the guidelines of the Wenzhou Medical University, Zhejiang Province, China and the Shanghai Institute of Planned Parenthood Research for the Care and Use of Animals for Scientific Purposes, and was approved by the Institutional Animal Care and Ethics Committee. Hybrid (DBA/He × C57BL/6) mice were used in the experiments.
Embryo collection and culture
Six-week-old female mice were super ovulated by the intraperitoneal injection of 5 IU pregnant mare serum gonadotropin (Ningbo Second Hormone Factory, Ningbo city, Zhejiang province, China), followed by 5 IU of human chorionic gonadotrophin (hCG) after 48 h (Livzon, Zhuhai, China). The females were either paired with males of proven fertility or left unpaired. Pregnancy was confirmed by the presence of a copulation plug the following morning (day 1). The oocytes or zygotes were recovered 18-20 h post-hCG from unmated and mated females, respectively, and freed from their cumulus cells by brief exposure to 300 IU hyaluronidase (Sigma-Aldrich, St Louis, MO). The two-, four-, and eight-cell embryos and blastocysts were isolated from the oviducts and/or uterus of plug-positive female mice at 40, 60, 68, and 90 h after hCG injection, respectively. The oocytes and embryos were collected in HEPES-buffered modified human tubal fluid medium. All components of the media were of tissue culture grade (Sigma-Aldrich); the media also contained 3 mg/mL bovine serum albumin (Sigma-Aldrich). The one-cell embryos, 20 h post-hCG, were cultured as a cohort of 10 embryos in 10 µL of KSOM (Potassium simplex optimized medium) medium in 60-well plates (LUX 5260; Nunc, Naperville, IL) overlaid by approximately 2 mm of heavy paraffin oil (Sigma-Aldrich) and incubated at 37°C with 5% carbon dioxide. The treatments were performed in KSOM medium with/without the supplementation of (i) amino acids: non-essential amino acids and essential amino acids (Thermo Fisher Scientific); (ii) 10, 50, or 250 µM indinavir sulfate ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-5-[[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]amino]-5-oxopentyl]-N-tert-butyl-4-(pyridin-3-ylmethyl)piperazine-2-carboxamide sulfate) (Abcam, Cambridge UK) and/or (iii) 10 µg/mL bovine insulin (Sigma-Aldrich) for up to 96 h.
Taq mangene expression assay
The RNA extraction and reverse transcription were performed as described previously [19]. Briefly, the mouse brain RNA was extracted using an RNeasy Mini kit (Qiagen) according to the manufacturer’s instructions. A cohort of 20 oocytes and embryos collected from the reproductive tracts and culture media were washed in cold Dulbecco’s phosphate-buffered saline to remove media and transferred at a minimal volume to the RNA lysis buffer. This was followed by three cycles of freezing in liquid nitrogen and thawing by vortexing. The RNA was purified using the TURBO DNA-free kit according to the instruction of the manufacturer (Applied Biosystems/Ambion).
Reverse transcription was performed with 2.5 µM random decamers (Applied Biosystems/Ambion). The cDNA equivalent to 1.5 oocytes or embryos was amplified with TaqMan probes by polymerase chain reaction (PCR) according to the manufacturer’s instructions: 10 min at 95°C, 40 cycles of denaturation for 15 s at 95°C, and extension/annealing for 1 min at 60°C in a LightCycler 480 (Roche Applied Science). Positive controls consisted of the genes of interest in the mouse brain (cDNA equivalent to 20 ng brain tissue) and the internal housekeeper gene H2afz in the embryos. Negative controls for all reactions included those without reverse transcriptase or without the RNA sample (to test for any RNA or DNA contamination, respectively). The cDNA was amplified by the TaqMan gene expression assay. The assessed genes are listed in Table 1. The Ct values were calculated by the system software. The delta Ct values were a measure of changes in the transcript of the tested gene relative to that of the housekeeping gene H2afz in the embryo. A plot of 2-(normalized Delta CT) is shown.
Table 1.
TaqMan® Gene Expression Assay of solute carrier family 2, facilitated glucose transporter and reference gene
| Gene name | Gene Bank number | Assay number | Catalog number | Amplicon length | Exon boundary | Assay location |
|---|---|---|---|---|---|---|
| Slc2a1 | NM_011400.3 | Mm00441480_m1 | 4331182 | 73 | 8-9 | 1278 |
| Slc2a2 | NM_031197.2 | Mm00446229_m1 | 4331182 | 61 | 9-10 | 1287 |
| Slc2a3 | NM_011401.4 | Mm00441483_m1 | 4331182 | 87 | 3-4 | 626 |
| Slc2a4 | NM_009204.2 | Mm00436609_g1 | 4331182 | 92 | 3-4 | 532 |
| Slc2a5 | NM_019741.3 | Mm00600311_m1 | 4331182 | 95 | 7-8 | 1216 |
| Slc2a6 | NM_001177627.1 | Mm00554217_m1 | 4331182 | 64 | 2-3 | 343 |
| Slc2a7 | NM_001085529.1 | Mm01260619_m1 | 4351372 | 66 | 8-9 | 1057 |
| Slc2a8 | NM_019488.4 | Mm00444634_m1 | 4331182 | 70 | 4-5 | 570 |
| Slc2a9 | NM_001012363.2 | Mm00455122_m1 | 4331182 | 66 | 8-9 | 1031 |
| Slc2a10 | NM_130451.3 | Mm01249519_m1 | 4331182 | 78 | 3-4 | 1547 |
| Slc2a11 | Not in mouse | |||||
| Slc2a12 | NM_178934.4 | Mm00619244_m1 | 4331182 | 65 | 1-2 | 304 |
| Slc2a13 | NM_001033633.3 | Mm01306489_m1 | 4331182 | 62 | 2-3 | 843 |
| H2afz | NM_016750.3 | Mm02018760_g1 | 4331182 | 94 | 1-1 | 167 |
Epi- and confocal immunofluorescence
Immunofluorescence was performed as previously described [20]. After fixation and blocking, the embryos were incubated overnight at 4°C with primary antibodies: 2 μg/mL rabbit anti-SLC2A1, rabbit anti-SLC2A3, anti-SLC2A4, and rabbit anti-SLC2A8 polyclonal IgG (AbCaM) or an equivalent concentration of isotype control immunoglobulin (negative control). The primary antibodies were detected by incubating with secondary antibodies coupled to FITC (goat anti-rabbit FITC conjugated IgG) (Sigma) for 1 h at room temperature. The nuclear DNA was stained with 10 µg/mL Hoechst 33342 (Sigma). The slides were mounted with Vecta Mount Solution (Vector Labs, CA, USA), and photographed using a Nikon A1+ Confocal Microscope System (Nikon, Tokyo, Japan).
Western blot
Fresh embryos were collected and washed three times in cold PBS and then transferred to extraction buffer containing Triton X-100 (Bio-Rad), 24 mM deoxycholic acid, 0.2% (w/v) sodium dodecyl sulfate (SDS), 20 mM NaF, 20 mM Na4P2O7, 2 mM phenylmethanesulphonylfluoride, 3.08 mM aprotinin, 42 mM leupeptin, and 2.91 mM pepstatin A (all from Sigma) in PBS. Embryos were lyzed by three cycles of freezing in liquid nitrogen and thawing (with vortexing) and then were immediately subjected to Western blot or stored at -80°C for future use. The samples were diluted with Laemmli loading buffer and separated on 4-20% Mini-PROTEAN® TGX™ Gel (BioRad) using Bio apparatus (Bio-Rad, CA, USA). The proteins were transferred on Trans-Blot® Turbo™ Mini PVDF with Trans-Blot Turbo Transfer System (Bio-Rad). The membrane was incubated in 10 mL of blocking buffer containing 2.5% (w/v) skim milk powder (Diploma, New Zealand) and then stained with 0.2 μg/mL rabbit anti-SLC2A4 IgG at 4°C overnight on shaker. Primary antibody was detected with 1:5000 Horse Radish Peroxidase (HRP) conjugated secondary antibody (Sigma) and detected using by Tanon 4200 Chemiluminescent Imaging System (Tanon, China). The membrane was incubated in Super Signal@ West Femto (Pierce, Rockford, IL). The membrane was stripped by incubation in 200 mM NaOH (Sigma) for 30 min at room temperature and reprobed with 0.1 µg/mL anti-rabbit OCT4 polyclonal IgG (Abcam) or 0.1 µg/mL anti-rabbit ACTIN IgG (Sigma).
D-glucose consumption assay in culture media
The concentration of D-glucose in the media was determined using a glucose assay kit (Sigma-Aldrich) according to the manufacturer’s instructions. This enzymatic method is based on the consequent increase in nicotinamide adenine dinucleotide (NAD) content, measured by the absorbance at 340 nm, which is directly proportional to glucose concentration. A reduction in glucose concentration in the treatment media compared with that in the media without the embryo represented glucose consumption in the media; the medium was collected from each treatment at 24, 48, 72, and 96 h after zygote culture.
Statistical analyses
The statistical analyses were performed with the SPSS version 22.0 software for Windows (SPSS Inc., Chicago, IL, USA). The cell number, normalized transcript number, and glucose consumption rate were quantitatively analyzed by the univariate analysis of variance. The dependent variable, test treatments as the independent variables, and experimental replicates were incorporated in the model as a covariate. The tests for main factor effects and interaction effects were performed. The differences between individual treatments were determined by multiple comparisons with the least significance difference test. The development rate of blastocysts and hatching blastocysts was assessed by the binary logistic regression analysis.
Results
Transcription of Slc2a genes in the oocyte and preimplantation embryos of mouse
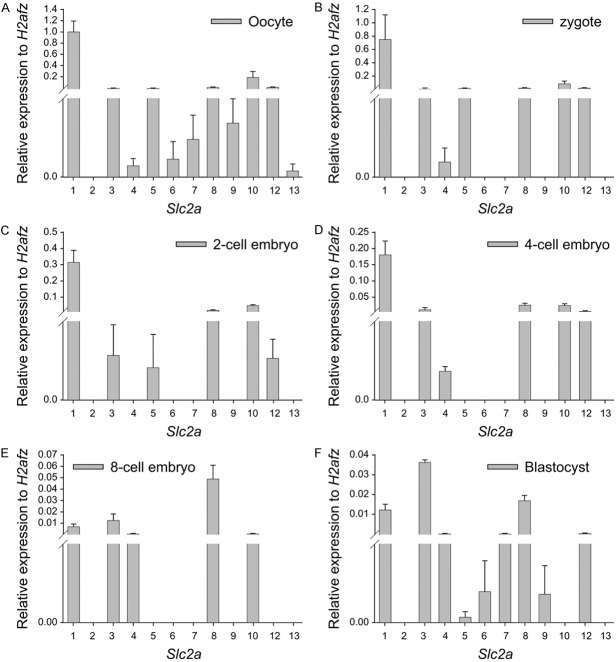
TaqMan gene expression assay was used to quantitatively assess the level of transcripts of 12 members of SLC2A glucose transporter family relative to the expression of the housekeeping gene H2afz at different developmental stages. The expression of the housekeeping gene H2afz was relatively stable throughout preimplantation and was employed as an internal reference [23]. All SLC2A isoforms, except SLC2A2, were detected in the oocytes (Figure 1A). SLC2A2 and SLC2A13 were not identified in the embryos throughout preimplantation, whereas SLC2A1, SLC2A3, and SLC2A8 transcripts were detected in the embryos throughout preimplantation (Figure 1B-F). SLC2A1 was the most expressed SLC2A in the oocytes, zygotes, and two- and four-cell embryos (Figure 1A-D); SLC2A8 was the most represented in the eight-cell embryos; and SLC2A3 in the blastocysts (Figure 1E, 1F). SLC2A5 and SLC2A12 were not observed in the embryos at the eight-cell stage but were redetected in the blastocysts (Figure 1B-E). SLC2A10 was detected in the embryos during the early development stages, except at the blastocyst stage (Figure 1B-E). SLC2A4 was not detected at the two-cell stage but redetected from the four-cell to blastocyst stages (Figure 1B-E). The transcripts of SLC2A6, SLC2A7, and SLC2A9 were not detected in the early preimplantation embryos until the blastocyst stage (Figure 1B-E). A comparison of expression of each SLC2A member at different developmental stages revealed that SLC2A1, SLC2A3, SLC2A4, and SLC2A8 were the main SLC2A members expressed during the early mouse embryo development. The transcription of SLC2A1 gradually decreased, whereas, that of SLC2A3 increased with development. In contrast, SLC2A8 transcription was relatively low in the embryos at the early cleavage stage, and significantly high in the eight-cell stage embryos and blastocysts (Figure 1B-E).
Figure 1.
Expression of Slc2a genes in the oocytes and preimplantation embryos of the mouse by the TaqMan gene expression assay. The mean ± S.E.M of the Slc2a members normalized to the relative transcript number of H2afz in the oocytes (A), zygotes (B), two-cell embryos (C), four-cell embryos (D), eight-cell embryos (E), and blastocysts (F) from four independent replicates. Each reaction is representative of RNA equivalent to 1.5 oocytes or embryos. The expression of Slc2a in the brain tissue as a positive control was demonstrated in 11 isoforms of Slc2a genes, except Slc2a7.
Embryo culture caused the reduction of Slc2a transcription
Further study showed that the transcription of Slc2a1, Slc2a3, Slc2a4, and Slc2a8 was lower in the blastocysts cultured in KSOM medium compared with the blastocysts collected from reproductive tract (fresh) (P < 0.001) (Figure 2). This reduced expression was partially reversed by the addition of both non-essential and essential amino acids into KSOM medium for Slc2a1 and Slc2a4 (P < 0.01), but not for Slc2a3 and Slc2a8 (Figure 2).
Figure 2.
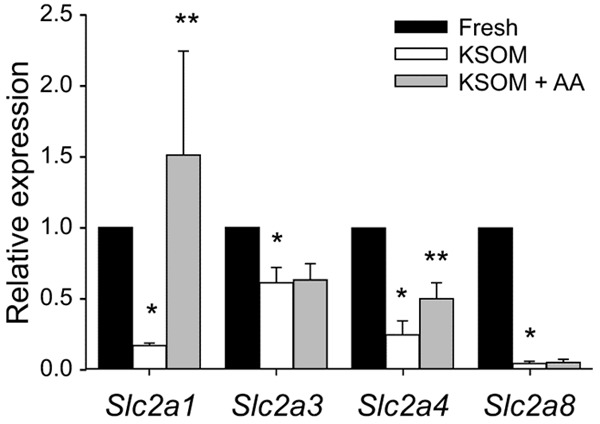
The effect of embryo culture on Slc2a expression in the mouse blastocyst. The mean ± S.E.M of normalized expression of SLC2A in the blastocysts derived from zygote stage in vitro culture in KSOM with or without amino acids, compared to the blastocyst collected from reproductive tracts (fresh blastocyst), was analyzed by TaqMan gene expression assay. The data were representative of five independent replicates, each with 10 blastocysts per treatment. *P < 0.001, compared to corresponding fresh blastocyst; **P < 0.01, compared to the corresponding treatments without amino acids.
Expression of SLC2A proteins during preimplantation
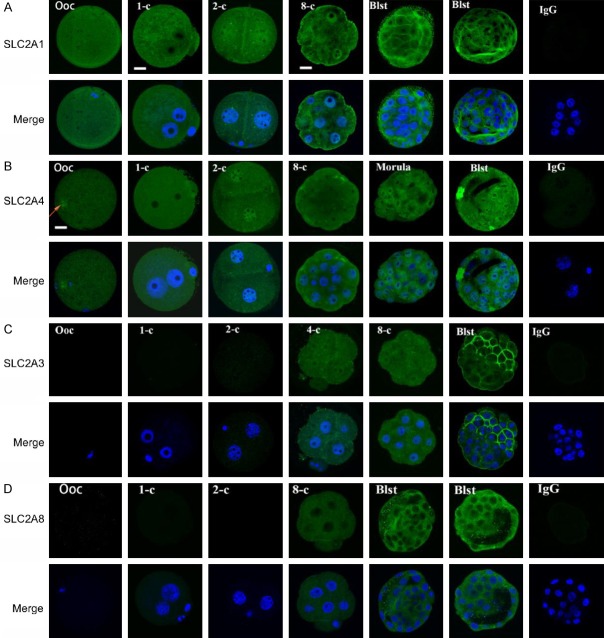
Confocal immunofluorescence detected SLC2A1 and SLC2A4 in the oocytes and embryos at all stages of preimplantation (Figure 3A, 3B). SLC2A1 was evenly distributed in the oocytes and zygotes. In two-cell embryos, more staining was observed in the nuclei than in the cytoplasm. This nuclear expression was evident until the eight-cell stage with a gradual decrease in the cytoplasm. The translocation of the protein to the cellular membrane was characteristic of the blastocyst (Figure 3A). SLC2A4 was of maternal origin with more staining in the MII chromosome (as indicated by an arrow, (Figure 3B). It was evenly expressed in the zygotes, excluding the nucleoli, and accumulated in the nuclei of two-cell stage embryos (Figure 3B). The protein was not translocated to the membrane until the blastocyst stage (Figure 3B). SLC2A3 and SLC28 were not detected until the four-eight cell stage of embryos. SLC2A3 was evenly expressed in the cytoplasm and nuclei in the four- and eight-cell embryos and a specific membrane staining was observed in the blastocysts (Figure 3C). SLC2A8 cellular membrane localization was detected in the blastocyst (Figure 3D). There was no difference in expression of SLC2A1, SLC2A4, SLC2A3 and SLC2A8 between inner cell mass and trophectoderm cells (Figure 3A-D).
Figure 3.
Expression of SLC2A in the oocytes and preimplantation embryos from the reproductive tract of mouse analyzed by confocal microscopy. The images of SLC2A1, SLC2A3, SLC2A4, and SLC2A8 obtained by Hoechst 33342 staining of DNA are representative of 20 oocytes or embryos at different stages. Non-immune IgG equivalent was used as a negative control for the primary antibody (four-cell embryos and blastocysts as examples). Scale bar = 10 µm for all images.
Roles of SLC2A4 during preimplantation
Indinavir is an HIV protease inhibitor that can act as a potent inhibitor of SLC2A by binding to the cytosolic domains of SLC2A, and SLC2A4-mediated glucose transport is sensitive to this compound with an IC50 of 50-100 µM [22-24]. The culture of zygotes in KSOM medium containing 10-250 µM indinavir sulfate caused a dose-dependent reduction in the rate of blastocyst formation (P < 0.001) and hatching blastocysts at 96 h (P < 0.001) (Figure 4A, 4B). A higher dose of indinavir sulfate prevented 60% of zygotes from developing into blastocysts in the indinavir sulfate-supplemented medium, and none of those derived blastocysts hatched (Figure 4A). The treatment with indinavir sulfate also decreased the number of cells accumulated in the blastocysts (P < 0.001) and increased nucleus fragmentation in the derived blastocysts (P < 0.01) (Figure 4B). The analysis of developmental landmarks at 24 h intervals of culture showed that indinavir sulfate did not affect the rate of formation of two-, four-, and eight-cell embryos (or morula) at 24, 48, and 72 h, compared with the corresponding rates in control (Figure 4C). However, the total number of cells accumulated in these embryos was lower (P < 0.001) at 48, 72, and 96 h in a dose-dependent manner (P < 0.05, P < 0.001, and P < 0.001, respectively) (Figure 4C).
Figure 4.
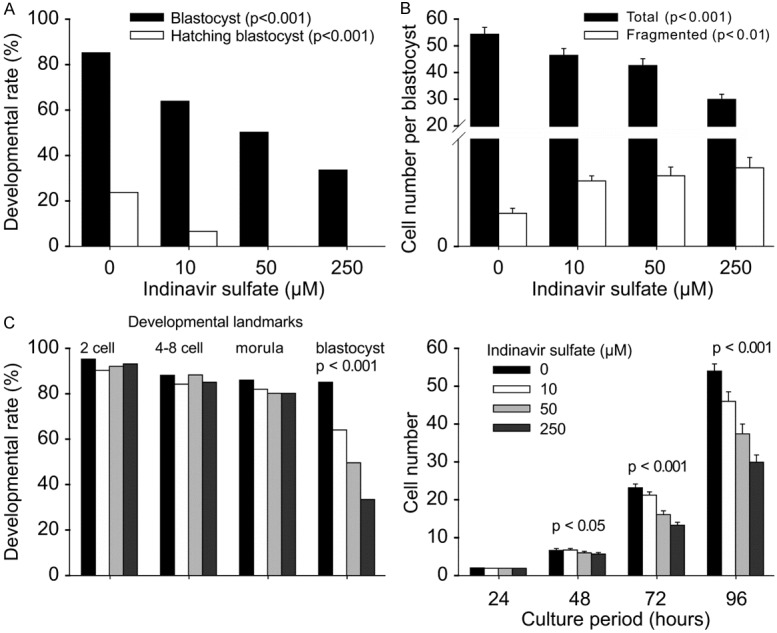
Role of SLC2A4 during mouse preimplantation development. The developmental outcomes of hybrid zygotes cultured in media containing a range of indinavir sulfate concentrations for 96 h, including the rate of blastocyst formation and hatching blastocysts (A), mean ± S.E.M of the number of cells accumulated in each blastocyst and fragmented cells (B), the rate and mean ± S.E.M of the number of total cells for each developmental landmark at 24 h intervals (C). The results are representative of three independent replicates with at least 60 embryos in each treatment. *P < 0.001 compared with that of the control.
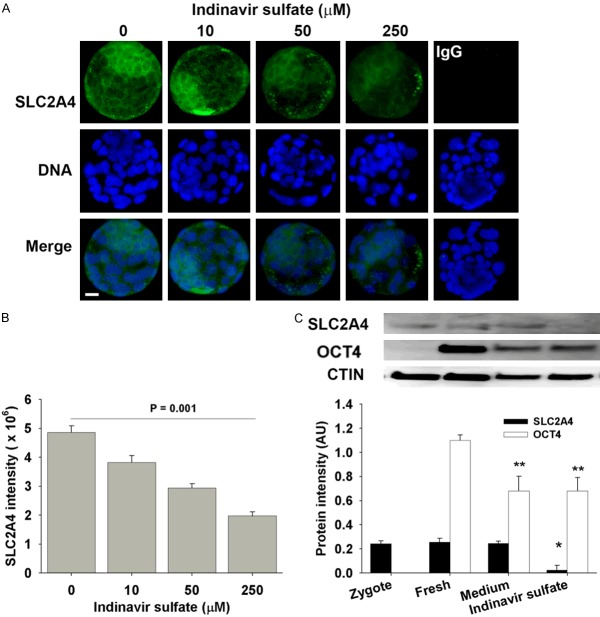
The effect of indinavir sulfate on SLC2A4 expression in blastocyst
Epi-immunofluorescence showed that indinavir sulfate reduced the staining of SLC2A4 protein in the blastocyst in a dose-dependent manner (P < 0.001) (Figure 5A, 5B). It reduced overall level of the staining within ICM and trophoblast cells of whole embryo rather than particular cellular compartments, suggesting its role may not be involved in membrane translocalization of SLC2A4. Western blot detected SLC2A4 in zygotes and blastocysts (Figure 5C). There was similar level of SLC2A4 in the in vivo-derived blastocysts to the blastocysts derived from zygote stage in KSOM medium, but markedly reduced by indinavir sulfate (P < 0.001). Indinavir sulfate did not alter the expression of embryonic pluripotent biomarker (OCT4), although in vitro derived blastocysts had lower level of OCT4 compared to the fresh blastocysts (P < 0.01). OCT4 was a little detected in the zygote stage.
Figure 5.
The effect of indinavir sulfate on SLC2A4 in blastocyst. The whole-session epi-immunofluorescent images of SLC2A4 staining (A) and intensity (B) in the blastocysts derived from hybrid zygotes in KSMO medium supplemented with or without indinavir sulfate. The results were representative of three independent replicates with at least 30 embryos in each treatment. Negative control was the staining of non-immune IgG in blastocyst (medium only as example). Scalar bar = 10 for all images. (C) Western blot analyzed the expression of SLC2A4 and OCT4 in zygotes, fresh blastocyst (collected from reproductive tracts), and the blastocysts derived in KSOM medium or supplemented with 250 µM indinavir sulfate from zygote stage. The results were representative of three independent replicates with 70 embryos loaded in each lane. *P = 0.001, compared to fresh or medium-derived blastocysts. **P < 0.01, compared to fresh blastocysts.
Exogenous insulin partially reversed indinavir sulfate-mediated inhibition of SLC2A4 activity
We further hypothesized that the inhibitory effect of indinavir sulfate on embryo development occurred through the insulin-responsive SLC2A members. Exogenous insulin alone did not affect the rate of blastocyst formation and the number of cells in the blastocyst formed (Figure 6A). The addition of insulin in the media together with indinavir sulfate might have partially reversed the indinavir sulfate-mediated inhibition of blastocyst formation (P < 0.001) and cell number (Figure 6A). To analyze glucose uptake of the embryos, the glucose concentration in the culture media was measured by the glucose consumption assay, and the proportion of residual glucose was reported at several time points (Figure 6B). The results showed negligible changes in glucose concentration prior to 48 h in the control and treatment media. A significant reduction in the glucose concentration occurred after 72 h of culture. In the control media, approximately one-third of the glucose was utilized by the zygotes after 72 h of culture and only one third remained after 96 h of culture. Indinavir sulfate prevented the embryo glucose uptake; this effect was clear after 72 h of treatment, and the residual glucose at 96 h of culture was almost two-fold higher in the 250 µM indinavir sulfate-supplemented media compared with that in control (P < 0.01). This effect was also partially reversed by exogenous insulin treatment (P < 0.05).
Figure 6.
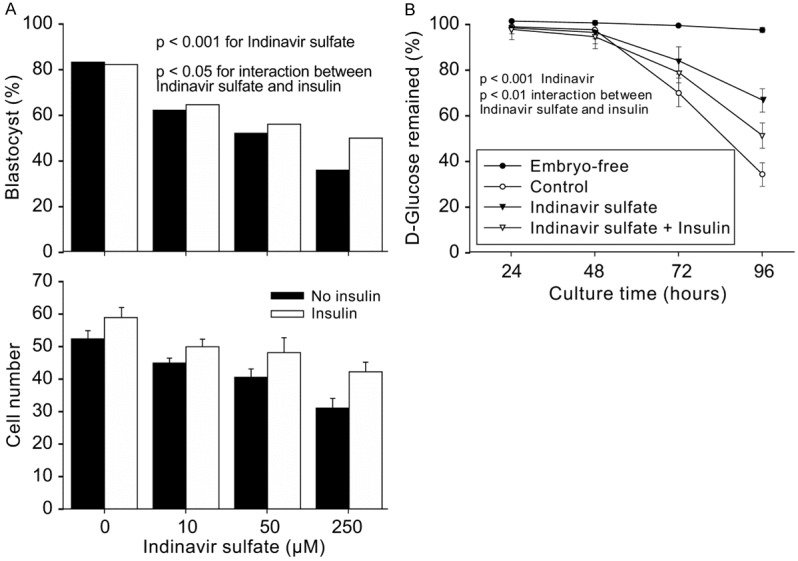
Effect of insulin on SLC2A4-dependent development and glucose consumption. A. The outcomes of blastocyst formation and cell accumulation in blastocysts derived after zygote culture for 96 h in KSOM medium with treatments including (1) with or without 10 µg/mL insulin and (2) with or without indinavir sulfate. The results are representative of three independent replicates with at least 70 embryos for each treatment. P > 0.05 for the effect of insulin on blastocyst formation rate and cell number; P < 0.001 for indinavir sulfate; P < 0.05 for interaction between indinavir sulfate and insulin. B. The proportion of glucose remaining in the culture media collected at four developmental time points. The results are representative of six independent replicates. In each replicate, 30 µL of media from five groups of same treatment were collected and stored at -20°C. The glucose assay was performed after 96 h of culture.
Discussion
In the present study, the TaqMan gene expression assay was carried out to analyze the expression of all 12 known isoforms of the mouse SLC2A glucose transporter in the oocytes and embryos at each stage of preimplantation in the mouse. The most-expressed SLC2A isoforms were Slc2a1, Slc2a3, Slc2a4, and Slc2a8 during mouse preimplantation development. To the best of our knowledge, the present study is the first to report the presence of SLC2A1 and SLC2A4 proteins in the embryos at all stages of preimplantation. We also observed nuclear localization of these glucose transporters in the embryos at the cleavage stage. SLC2A3 and SLC2A8 were not detected until the four- to eight-cell embryo stages. The characteristic cellular membrane staining was initially visualized after compaction.
The deficiency of SLC2A1 results in glucose transporter type 1 syndrome, causing embryonic malformations due to hyperglycemia in maternal diabetes [14] and homozygous GLUT1-null mice that do not survive beyond embryonic day 14 [25]. SLC2A1 plays an important role in the survival of pre-implantation embryos [14,25]. The SLC2A1 gene was the highest expressed SLC2A isoform in the oocytes, and its transcription was similar to that of the housekeeper gene H2afz. This gene can, therefore, be a potential biomarker to assess oocyte quality [26]. SLC2A1 expression was found throughout the preimplantation development and translocated to the cellular membrane after compaction, suggesting that this isoform is responsible for constitutive or basal glucose uptake [6,26,27]. The presence of nuclear Slc2a1 in the embryos at the cleavage stage is interesting for future research.
The transcription of Slc2a3 and Slc2a8 increased during embryo development and was significantly higher during compaction and blastocyst formation. This was consistent with their protein expression and localization during development, suggesting that they are the main glucose transporters participating in glucose utilization from compaction to complete embryo development. Slc2a3 mediates the uptake of glucose, 2-deoxyglucose, galactose, mannose, xylose, and fucose, and probably dehydroascorbate [28]. This protein is essential for post-implantation [29] and is lethal for embryos [12]. In blastocysts, Slc2a3 is the most abundant SLC2A gene, suggesting that SLC2A3 may be involved in determining the metabolic priorities of the embryo [6]. Besides the fact that Slc2a8 deficiency in mice results in reproductive and growth impairments, Slc2a8 is suggested to have a constitutive and significant role in glucose uptake [13,30].
The Slc2a4 gene is predominantly expressed in adult tissues responsible for the bulk of whole-body glucose disposal [31,32] and is a principal transporter isoform mediating insulin-stimulated glucose uptake [33]. Slc2a4 has not been reported in embryos at early stages of preimplantation, except in the blastocysts of mouse and rabbit [31]. To the best of our knowledge, the present study is the first to report the dynamic expression pattern of SLC2A4 mRNA and SLC2A4 protein in the oocyte and embryos at all stages of preimplantation. Maternal Slc2a4 mRNA was obviously degraded abruptly after fertilization whereas the protein remained. This reciprocal pattern of expression of transcripts and protein is not paradoxical and is particularly significant during the period of maternal-zygotic transition [19]. Similar to that of SLC2A1, SLC2A4 accumulation in the nuclei of embryos at the cleavage stage indicates a novel function of SLC2A4 that should be studied. Unlike the other SLC2A members, SLC2A4 did not exhibit the characteristic membrane localization in the embryos, but it was primarily localized intracellularly in the unstimulated state. This indicates that it is redistributed in the plasma membrane in response to acute insulin and other stimuli [34]. A previous pharmacological study with indinavir sulfate [38] unveiled the roles of SLC2A4 during preimplantation. Indinavir sulfate acts by binding to the cytoplasmic glucose binding site of SLC2A4 to prevent glucose transport [23], rather than affecting SLC2A4 translocation. Indinavir, similar to all first-generation HIV protease inhibitors, contains a core peptidomimetic structure flanked by hydrophobic moieties and acts as a potent non-competitive inhibitor of zero-trans SLC2A4-mediated glucose transport. However, it has a negligible effect on the activity of SLC2A1 transporter [24]. The results of the present study revealed that its inhibition resulted in ~60% cell loss without affecting the developmental rate, suggesting the activity of other SLC2A glucose transporters. However, the developmental delay by indinavir was observed from relatively early cleavage stages (i.e., from the second to third embryonic cell cycle (48 h of culture)), during which nuclear SLC2A4 accumulation occurred. It should be noted that as a protease inhibitor, the pharmacological effects of indinavir on SLC2A4 activities may not be selective, particularly at the higher dose. Therefore, the activities of SLC2A4 during the preimplantation development of embryo warrants to be defined in the future with powerful research tools.
Continued development of an embryo and the establishment of implantation require glucose as an energy source. SLC2A1 and SLC2A3 were not expected to be insulin-stimulated glucose transporters [35]. Their simultaneous presence, together with insulin-stimulated SLC2A4 and SLC2A8 [31,33,35] during preimplantation corresponds to the critical time of embryo development when the embryonic fuel requirement switches from the oxidation of lactate and pyruvate via the Krebs cycle to anaerobic metabolism of glucose via glycolysis [1]. The embryos can grow to the blastocyst stage in simple media, at least partially, by autocrine and paracrine mechanisms, as several autocrine factors (such as insulin-like growth factor (IGF)-1 and IGF-2) are secreted by the embryos, and their receptors are expressed in the preimplantation embryos [36]. The embryos alone do not produce insulin, but exogenous insulin and IGF-1 can increase the developmental rate by improving blastocyst hatching and growing the cell number per blastocyst when the mouse embryos are cultured in simple media supplemented with glucose [37]. Insulin stimulates glucose uptake into embryos by enriching the concentration of SLC2A4 proteins in the plasma membrane [34]. Indinavir preferably binds to the cytosolic domain of SLC2A4 over SLC2A1, thus decreasing glucose consumption by the embryo. Given the presence of other SLC2A transporters that are not sensitive to indinavir, the influence of indinavir is relatively limited.
Moreover, exogenous insulin might not directly reverse the action of indinavir against SLC2A4, but rather induce a change in the cellular localization of other insulin-responsive SLC2As, possibly including SLC2A8. Therefore, the presence of two main insulin-responsive (SLC2A4 and SLC2A8) and two main insulin-non-responsive (SLC2A1 and SLC2A3) glucose transporters might help maintain normal glucose consumption in the embryos. However, whether indinavir affects other insulin-responsive or non-responsive SLC2A glucose transporters remains unclear. The dynamic expression pattern of SLC2A glucose transporters and their regulatory mechanisms coinciding with embryonic development might allow flexibility during embryo development in order to adapt to a changing environment.
The present study demonstrated that the transcription of SLC2A5, SLC2A6, SLC2A7, SLC2A9, and SLC2A12 was relatively low or silent during the early stages of preimplantation but resumed in the blastocyst stage. This indicates a particular requirement for different types of glucose transporters by the embryos in the blastocyst stage, where the embryonic cell lines are formed, and cell differentiation has begun. To the best of our knowledge, the present study is the first to report the transcription of SLC2A5, SLC2A6, and SLC2A7 during mouse preimplantation [11]. However, we observed that the expression of several Slc2a isoforms, such as SLC2A2 and SLC2A9, differed from those reported previously [17].
Slc2a9 was not detectable until blastocyst stage [38]. Although these differences could be partially due to limitation of research technology, the biological nature of these novelty warrants future study.
Present study showed that embryo culture, though supplemented with amino acids, was insufficient to maintain the Slc2a transcription. Along with the clinical findings that metabolic disorders (such as hyperglycemia) [17,39] affect fertility, ART outcomes suggest that the creation of a novel culture system focusing on the maintenance of glucose metabolism during preimplantation development will be particularly beneficial for metabolic-related infertility in ART.
Conclusion
The present study summarized the expression pattern of all known SLC2A glucose transporter genes in the embryos at different developmental stages and mainly analyzed the role and regulation of SLC2A4 during preimplantation development in the mouse. It is understandable that the requirement of glucose metabolism for the preimplantation development is tightly regulated by the overall activities of multiple SLC2As, whereas the physiology of individual SLC2A warrants further studies, particularly, during the early cleavage stages where the embryonic genome activation occurs. This study provides an insight into the mechanism of glucose utilization and may help optimize ART conditions during preimplantation development in vitro.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China to XJ (81471458) and Natural Science Foundation of Zhejiang Province to JL (LY16C120001). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This study protocol conformed to clinical research guidelines and was approved by the research ethics committee of Wenzhou Medical University, Second Affiliated Hospital. The experimental work was carried out at the Wenzhou Medical University, Second Affiliated Hospital.
Disclosure of conflict of interest
None.
Abbreviations
- ART
assisted reproductive technology
- GLUT
glucose transporter gene family
- hCG
human chorionic gonadotrophin
- IGF
insulin-like growth factor
- PCR
polymerase chain reaction
- SLC2A
solute carrier family 2
References
- 1.Martin KL, Leese HJ. Role of developmental factors in the switch from pyruvate to glucose as the major exogenous energy substrate in the preimplantation mouse embryo. Reprod Fertil Dev. 1999;11:425–433. doi: 10.1071/rd97071. [DOI] [PubMed] [Google Scholar]
- 2.Leese HJ, Conaghan J, Martin KL, Hardy K. Early human embryo metabolism. Bioessays. 1993;15:259–264. doi: 10.1002/bies.950150406. [DOI] [PubMed] [Google Scholar]
- 3.Jansen S, Pantaleon M, Kaye PL. Characterization and regulation of monocarboxylate cotransporters Slc16a7 and Slc16a3 in preimplantation mouse embryos. Biol Reprod. 2008;79:84–92. doi: 10.1095/biolreprod.107.066811. [DOI] [PubMed] [Google Scholar]
- 4.Pantaleon M, Scott J, Kaye PL. Nutrient sensing by the early mouse embryo: hexosamine biosynthesis and glucose signaling during preimplantation development. Biol Reprod. 2008;78:595–600. doi: 10.1095/biolreprod.107.062877. [DOI] [PubMed] [Google Scholar]
- 5.Gardner DK, Pool TB, Lane M. Embryo nutrition and energy metabolism and its relationship to embryo growth, differentiation, and viability. Semin Reprod Med. 2000;18:205–218. doi: 10.1055/s-2000-12559. [DOI] [PubMed] [Google Scholar]
- 6.Pantaleon M, Kaye PL. Glucose transporters in preimplantation development. Rev Reprod. 1998;3:77–81. doi: 10.1530/ror.0.0030077. [DOI] [PubMed] [Google Scholar]
- 7.Joost HG, Bell GI, Best JD, Birnbaum MJ, Charron MJ, Chen YT, Doege H, James DE, Lodish HF, Moley KH, Moley JF, Mueckler M, Rogers S, Schürmann A, Seino S, Thorens B. Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am J Physiol Endocrinol Metab. 2002;282:E974–976. doi: 10.1152/ajpendo.00407.2001. [DOI] [PubMed] [Google Scholar]
- 8.Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review) Mol Membr Biol. 2001;18:247–256. doi: 10.1080/09687680110090456. [DOI] [PubMed] [Google Scholar]
- 9.Wood IS, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr. 2003;89:3–9. doi: 10.1079/BJN2002763. [DOI] [PubMed] [Google Scholar]
- 10.Watson RT, Kanzaki M, Pessin JE. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr Rev. 2004;25:177–204. doi: 10.1210/er.2003-0011. [DOI] [PubMed] [Google Scholar]
- 11.Purcell SH, Moley KH. Glucose transporters in gametes and preimplantation embryos. Trends Endocrinol Metab. 2009;20:483–489. doi: 10.1016/j.tem.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt S, Hommel A, Gawlik V, Augustin R, Junicke N, Florian S, Richter M, Walther DJ, Montag D, Joost HG, Schürmann A. Essential role of glucose transporter GLUT3 for post-implantation embryonic development. J Endocrinol. 2009;200:23–33. doi: 10.1677/JOE-08-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adastra KL, Frolova AI, Chi MM, Cusumano D, Bade M, Carayannopoulos MO, Moley KH. Slc2a8 deficiency in mice results in reproductive and growth impairments. Biol Reprod. 2012;87:49. doi: 10.1095/biolreprod.111.097675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heilig CW, Saunders T, Brosius FC 3rd, Moley K, Heilig K, Baggs R, Guo L, Conner D. Glucose transporter-1-deficient mice exhibit impaired development and deformities that are similar to diabetic embryopathy. Proc Natl Acad Sci U S A. 2003;100:15613–15618. doi: 10.1073/pnas.2536196100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, Cummings EA, O’Connell C, Jangaard K. Fetal and neonatal outcomes of diabetic pregnancies. Obstet Gynecol. 2006;108:644–650. doi: 10.1097/01.AOG.0000231688.08263.47. [DOI] [PubMed] [Google Scholar]
- 16.Moley KH. Hyperglycemia and apoptosis: mechanisms for congenital malformations and pregnancy loss in diabetic women. Trends Endocrinol Metab. 2001;12:78–82. doi: 10.1016/s1043-2760(00)00341-6. [DOI] [PubMed] [Google Scholar]
- 17.Moley KH, Chi MM, Mueckler MM. Maternal hyperglycemia alters glucose transport and utilization in mouse preimplantation embryos. Am J Physiol. 1998;275:E38–47. doi: 10.1152/ajpendo.1998.275.1.E38. [DOI] [PubMed] [Google Scholar]
- 18.Jin XL, O’Neill C. Systematic analysis of the factors that adversely affect the rate of cell accumulation in mouse embryos during their culture in vitro. Reprod Biol Endocrinol. 2014;12:35. doi: 10.1186/1477-7827-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin XL, O’Neill C. Regulation of the expression of proto-oncogenes by autocrine embryotropins in the early mouse embryo. Biol Reprod. 2011;84:1216–1224. doi: 10.1095/biolreprod.110.087007. [DOI] [PubMed] [Google Scholar]
- 20.Jin XL, O’Neill C. The regulation of the expression and activation of the essential ATF1 transcription factor in the mouse preimplantation embryo. Reproduction. 2014;148:147–157. doi: 10.1530/REP-13-0535. [DOI] [PubMed] [Google Scholar]
- 21.Mamo S, Gal AB, Bodo S, Dinnyes A. Quantitative evaluation and selection of reference genes in mouse oocytes and embryos cultured in vivo and in vitro. BMC Dev Biol. 2007;7:14. doi: 10.1186/1471-213X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murata H, Hruz PW, Mueckler M. Indinavir inhibits the glucose transporter isoform Glut4 at physiologic concentrations. AIDS. 2002;16:859–863. doi: 10.1097/00002030-200204120-00005. [DOI] [PubMed] [Google Scholar]
- 23.Hresko RC, Hruz PW. HIV protease inhibitors act as competitive inhibitors of the cytoplasmic glucose binding site of GLUTs with differing affinities for GLUT1 and GLUT4. PLoS One. 2011;6:e25237. doi: 10.1371/journal.pone.0025237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hresko RC, Kraft TE, Tzekov A, Wildman SA, Hruz PW. Isoform-selective inhibition of facilitative glucose transporters: elucidation of the molecular mechanism of HIV protease inhibitor binding. J Biol Chem. 2014;289:16100–16113. doi: 10.1074/jbc.M113.528430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Pascual JM, Yang H, Engelstad K, Mao X, Cheng J, Yoo J, Noebels JL, De Vivo DC. A mouse model for Glut-1 haploinsufficiency. Hum Mol Genet. 2006;15:1169–1179. doi: 10.1093/hmg/ddl032. [DOI] [PubMed] [Google Scholar]
- 26.Paczkowski M, Schoolcraft WB, Krisher RL. Fatty acid metabolism during maturation affects glucose uptake and is essential to oocyte competence. Reproduction. 2014;148:429–439. doi: 10.1530/REP-14-0015. [DOI] [PubMed] [Google Scholar]
- 27.Leppens-Luisier G, Urner F, Sakkas D. Facilitated glucose transporters play a crucial role throughout mouse preimplantation embryo development. Hum Reprod. 2001;16:1229–1236. doi: 10.1093/humrep/16.6.1229. [DOI] [PubMed] [Google Scholar]
- 28.Seatter MJ, De la Rue SA, Porter LM, Gould GW. QLS motif in transmembrane helix VII of the glucose transporter family interacts with the C-1 position of D-glucose and is involved in substrate selection at the exofacial binding site. Biochemistry. 1998;37:1322–1326. doi: 10.1021/bi972322u. [DOI] [PubMed] [Google Scholar]
- 29.Ganguly A, Collis L, Devaskar SU. Placental glucose and amino acid transport in calorie-restricted wild-type and Glut3 null heterozygous mice. Endocrinology. 2012;153:3995–4007. doi: 10.1210/en.2011-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carayannopoulos MO, Chi MM, Cui Y, Pingsterhaus JM, McKnight RA, Mueckler M, Devaskar SU, Moley KH. GLUT8 is a glucose transporter responsible for insulin-stimulated glucose uptake in the blastocyst. Proc Natl Acad Sci U S A. 2000;97:7313–7318. doi: 10.1073/pnas.97.13.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tonack S, Fischer B, Navarrete Santos A. Expression of the insulin-responsive glucose transporter isoform 4 in blastocysts of C57/BL6 mice. Anat Embryol (Berl) 2004;208:225–230. doi: 10.1007/s00429-004-0388-z. [DOI] [PubMed] [Google Scholar]
- 32.Tonack S, Ramin N, Garimella S, Rao R, Seshagiri PB, Fischer B, Navarrete Santos A. Expression of glucose transporter isoforms and the insulin receptor during hamster preimplantation embryo development. Ann Anat. 2009;191:485–495. doi: 10.1016/j.aanat.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Navarrete Santos A, Tonack S, Kirstein M, Kietz S, Fischer B. Two insulin-responsive glucose transporter isoforms and the insulin receptor are developmentally expressed in rabbit preimplantation embryos. Reproduction. 2004;128:503–516. doi: 10.1530/rep.1.00203. [DOI] [PubMed] [Google Scholar]
- 34.Furtado LM, Somwar R, Sweeney G, Niu W, Klip A. Activation of the glucose transporter GLUT4 by insulin. Biochem Cell Biol. 2002;80:569–578. doi: 10.1139/o02-156. [DOI] [PubMed] [Google Scholar]
- 35.Czech MP, Corvera S. Signaling mechanisms that regulate glucose transport. J Biol Chem. 1999;274:1865–1868. doi: 10.1074/jbc.274.4.1865. [DOI] [PubMed] [Google Scholar]
- 36.Rappolee DA, Sturm KS, Behrendtsen O, Schultz GA, Pedersen RA, Werb Z. Insulin-like growth factor II acts through an endogenous growth pathway regulated by imprinting in early mouse embryos. Genes Dev. 1992;6:939–952. doi: 10.1101/gad.6.6.939. [DOI] [PubMed] [Google Scholar]
- 37.Mihalik J, Rehak P, Koppel J. The influence of insulin on the in vitro development of mouse and bovine embryos. Physiol Res. 2000;49:347–354. [PubMed] [Google Scholar]
- 38.Carayannopoulos MO, Schlein A, Wyman A, Chi M, Keembiyehetty C, Moley KH. GLUT9 is differentially expressed and targeted in the preimplantation embryo. Endocrinology. 2004;145:1435–1443. doi: 10.1210/en.2003-1264. [DOI] [PubMed] [Google Scholar]
- 39.Long NM, Rule DC, Zhu MJ, Nathanielsz PW, Ford SP. Maternal obesity upregulates fatty acid and glucose transporters and increases expression of enzymes mediating fatty acid biosynthesis in fetal adipose tissue depots. J Anim Sci. 2012;90:2201–2210. doi: 10.2527/jas.2011-4343. [DOI] [PubMed] [Google Scholar]