Abstract
Mesenchymal stem cells (MSCs) are multipotent, non-hematopoietic stem cells capable of differentiating into varieties of mature cell types such as osteoblasts, chondrocytes, adipocytes, and myoblasts. MSCs can be isolated from different kinds of tissues and cultivated in vitro for amplification and passage easily. These cells have drawn researcher’s attention lately due to their ability of tissue repair, properties of hematopoiesis support and function of immunoregulation through the secretion of a variety of cytokines and growth factors that have both paracrine and autocrine activities. MSCs can regulate the proliferation of T cells, the antibodies secretion of B cells, maturation of DC, polarization of macrophages and also have many effects on neutrophils such as the suppression of NO secretion, inhibition of apoptosis, reduction of their infiltration, decreasing of N-Formy l-L-Methionine-L-leucy l-L-phenylalanine, induction of respiratory bursts and promotion of survivals. In some conditions, MSCs exert their function of treatment through immunoregulation. We reviewed the multifaceted roles of MSCs in communicating with immune cells mainly neutrophils in both in vivo and in vitro experiments. MSCs may provide promising trends for cell therapy in future.
Keywords: MSCs, neutrophils, immunoregulation
Introduction
Different types of stromal cells, including endothelial cells, smooth muscle cells, reticular cells, and osteoblasts, are found within the bone marrow cavity, where they form a niche that plays an indispensable role in supporting the survival, growth, and differentiation of hematopoietic progenitor cells [1-3]. A subset of mesodermal progenitor cells named multipotent mesenchymal stromal cells or more commonly, mesenchymal stem cells (MSCs) are primarily found in the stromal component of the bone marrow and many other tissues such as umbilical cord, and adipose [4-6]. MSCs are fibroblast-like and plastic-adherent having the potency to self-renew and differentiate into distinct types of cell lineages such as osteoblasts, chondrocytes, adipocytes, tenocytes, myotubes, neural cells, and hematopoietic-supporting stroma, either in vitro or in vivo [7,8]. In the early 2000s, the research group of Bartholomew A and Di Nicola M reported that MSCs have an immunoregulatory effects on lymphocytes [9,10]. From then on, more researches about the immunoregulatory properties of MSCs have been reported. MSCs can regulate the proliferation, differentiation, maturation and immune functions of myeloid cells like monocytes, dendritic cells, macrophages, myeloid-derived suppressor cells and granulocytes [11-15].
Neutrophils are one kind of granulocytes that originate from hematopoietic stem cells and mature in the bone marrow and are subsequently released into the blood of peripheral vasculature [16]. They are the largest quantity of leukocytes in the circulatory system of peripheral blood, reaching up to 70% of total leukocytes in the human body [17]. Neutrophils make up the first line of host defense and protect hosts from pathogenic attacks via mechanisms such as phagocytosis, release of cytokines and granules and formation of extracellular trap [18]. Neutrophils are also major phagocytes which have a primary of clearing extracellular pathogens [16]. Furthermore, they participate in the modulation of adaptive immunity by interacting with various adaptive immune cells [19]. In addition to the host defense and immunomodulation, neutrophils are also involved in the pathogenesis and progression of many diseases, including inflammation, autoimmune disorders and cancer [17]. Interestingly, effects of MSCs have been tested in a variety of immune diseases such as collagen-induced arthritis (CIA) [20], graft-versus-host disease (GVHD) [21] and inflammatory bowel disease (IBD) [22]. It is evident that MSCs may interact with neutrophils with or without the conditions of diseases. In this review, we will discuss research advances in the interaction between MSCs and some immune cells including neutrophils and also consider their evolving clinical potential in diseases.
The characteristics of MSC
Friedenstein and his colleagues were the first to point out that MSCs were fibroblast-like cells [23] characterized morphologically by a small cell body which is long and thin. The cell body contains a large, round nucleus with a prominent nucleolus which is surrounded by finely dispersed chromatin particles, giving the nucleus a clear appearance. The remainder of the cell body contains a small amount of Golgi apparatus, rough endoplasmic reticulum, mitochondria and poly ribosomes. MSCs are widely dispersed and the adjacent extracellular matrix is populated by a few reticular fibrils but is devoid of the other types of collagen fibrils [24]. MSCs can be isolated from a variety of adult or neonatal tissues, primarily bone marrow, fat tissue, dental pulp, placenta, umbilical cord and amniotic fluid [25-27].
Although the terms mesenchymal stem cell and marrow stromal cell have been used interchangeably for many years, neither term is sufficiently descriptive. Mesenchyme is embryonic connective tissue that is derived from the mesoderm and can differentiate into connective tissue but does not differentiate into hematopoietic cells [28]. On the other hand, stromal cell is connective tissue cell that forms the supportive structure in which the functional cells of the tissue reside, which is an accurate description for one function of MSC but fails to convey the relatively recently discovered roles of MSC in the repair of tissue [29]. The term encompasses multipotent cell derived from other non-marrow tissues, such as placenta, umbilical cord blood, adipose tissue, adult muscle, corneal stroma or the dental pulp of deciduous baby teeth [30,31]. The cell does not have the capacity to reconstitute an entire organ. Until today, the contributions of many laboratories validated Caplan’s concept of a mesenchymal stem cell [32] and made it possible to realize that these osteogenic cells were indeed capable of differentiating into several types of clonal connective cells such as osteoblasts, chondrocytes, myocytes, adipocytes, neurons and oligodendrocytes [33-36].
Immunoregulatory function of MSC
MSCs are characterized by immunophenotypes such as CD11b-, CD14-, CD34-, CD45-, HLA-DR-, CD73+, CD90+, CD105+ and have been shown to possess a broad spectrum of immunoregulatory capabilities, affecting both adaptive and innate immunity [37]. They can also produce chemokines and proteases that are likely to play a role either in their immunomodulatory or migratory function [10,38,39]. MSCs inhibit the proliferation and maturation of immune cells and suppress the immune reaction both in vitro and in vivo in a non-MHC restricted manner [40,41]. Therefore, MSCs are considered to be hypo-immunogenic which display low expression levels of HLA class I and no expression of HLA class II and costimulatory molecules such as CD40, CD80, and CD86 [41]. Basically, MSCs could exert their widespread immunomodulatory effects on the innate and adaptive immune system. Ex-vivo amplified MSCs have also been shown to suppress the activity of a broad range of immune cells, including T cells, B cells, dendritic cells (DCs), natural killer T (NKT) cells, macrophages and neutrophils (Figure 1).
Figure 1.
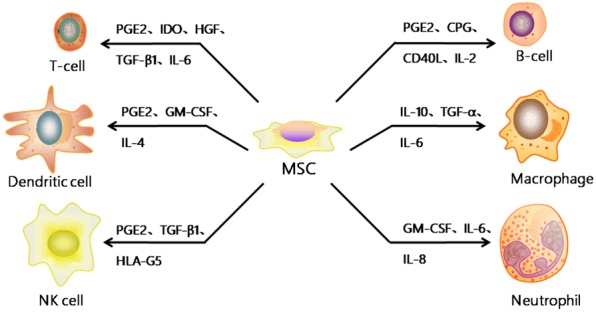
The effects of MSC on immune cells. The secretion of IL-6, IL-8 and GM-CSF by MSC increases neutrophil migration to the site of infection/injury, enhancing their activation and phagocytosis whilst promoting their survival. Furthermore, MSC can secrete other cytokines like PGE2, IDO, HGF, CPG, IL-2, IL-4, IL-10, TGF-β1 and so on to affect the proliferation, differentiation, maturation and function of other immune cells such as T cell, B cell, Dendritic cell, Macrophage and NK cell.
Immunoregulatory effects of MSC on T cell
The main features of the T cell response are cell proliferation and cytokines secretion. Inhibition of the proliferation of T cell is the most significant effect of MSCs on T cells. In vitro, MSCs are capable of suppressing the proliferation of T cell induced by mitogen and alloantigen [42,43], as well as activation of T cells by CD3 and CD28 antibodies [42]. Suppression of T cell proliferation by MSCs has no immunological restriction; similar suppressive effects being observed with cells that were autologous or allogeneic to the responder cells [42,43]. MSCs also modulate immune responses through the induction of regulatory T cells which are important in maintaining immune homeostasis and self-tolerance. However, depletion of regulatory T cells has no effect on the suppression of T cell proliferation by MSCs, and MSCs physically hinder T cells from contact with antigen presenting cells (APCs) [44]. Human adipocyte-MSCs exert an anti-proliferative effect on mouse splenic T cells in vitro, primarily through COX-2 expression [45]. Human adipose-AT-MSCs obtained from kidney donors induced a 2.1-fold increase in the percentage of CD25+ CD127- FoxP3+ cells within the population of CD4+ T cell from all stimulated CD25- cells. The majority of cells within the fraction of induced Treg had a methylated FoxP3 gene Treg-specific demethylated region indicating that they were not of natural regulatory T cells origin [46].
Although the exact mechanism underlying the immunosuppressive effects of MSCs is still not clear, most evidences support that soluble factors are involved. These factors include PGE2, IDO, HGF and TGF-β1 [47]. Additionally, it is well-established that IFN-γ plays an important role in the enhancement of MSCs suppressive activity. Furthermore, increased evidences support that MSCs inhibit the proliferation and/or functions of CD4+ Th1, Th17, CD8+ T cell, and NK cell predominantly via the secretion of soluble factors including TGF-β1 and HGF [48,49]. Again, MSCs play a key role in cytotoxic CD8+ T cells against intracellular pathogens. Schurch and colleagues reported that IFN-γ can promote the release of hematopoietic cytokines, including IL-6 from MSCs, which in turn reduces the expression of the transcription factors Runx-1 and Cebpα in early hematopoietic progenitor cells, increases myeloid differentiation, triggers the temporary activation of emergency myelopoiesis and promote clearance of the infection [50].
Accumulated in vitro data have demonstrated that the proliferation of T cells stimulated with either polyclonal mitogens, allogeneic cells or specific antigens are inhibited by MSCs [9,51-55]. This inhibition is considered to be mediated through arrest of the lymphocytes in the G0/G1 phase of the cell cycle. In addition, MSCs have also been reported to influence the cytokine secretion profile of the different T-cell subsets, as their addition to an in vitro activated T-cell culture resulted in decreased production of the pro-inflammatory cytokines: IFN-γ, TNF-α, IL-6 and IL-17 and increased levels of anti-inflammatory cytokines such as IL-4 and IL-1 [54,56,57]. Taken together, these results could indicate a possible MSC-mediated alternation in Th1/Th2 balance. Several research groups have demonstrated that MSCs are also capable of inhibiting the in vitro induction of CTL-mediated cytotoxicity [54,58,59], though how these stem cells exert an inhibitory effect on an already activated effector cytotoxic T cell is yet to be clarified.
Immunoregulatory effects of MSC on B cell
B cells are a type of lymphocyte in the humoral immunity of the adaptive immune system specialized in antigen presentation and antibody production. Recent studies have shown that MSCs can inhibit several functions of B cells. Corcione and colleagues showed that the proliferation of B cell was inhibited by MSCs through an arrest in the G0/G1 phase of the cell cycle. Possible interactions between MSCs and B cells have received relatively little scrutiny [60]. Most published results to date indicate that MSCs inhibit in vitro activation of the proliferation of B cell as well as immunoglobulin production [60,61]. MSCs inhibited the secretion of B cell resulting in a significant impairment of the production of IgM, IgG, and IgA. The expression of CXCR4, CXCR5, CCR7, CXCL12 and CXCL13 in B-cell was significantly down-regulated by MSCs, suggesting that MSCs affected the chemotactic properties of B cells. In addition, MSCs also affected the migration of B cells to inflammatory regions under the guidance of cell adhesion molecules and receptors of inflammatory chemokines [62].
A recent study by Lee and colleagues showed that the conditioned medium of MSCs infected with a mycoplasma strain, Mycoplasma arginini, had marked inhibitory effects on Ig production by lipopolysaccharide/interleukin-4-induced B cells compared with mycoplasma-free MSC-CM [63]. Yan and colleagues found that BAFF in MSCs was expressed at a higher level after TLR4-priming, indicating that TLR4 and a downstream pathway play a role in BAFF secretion and thus exert an important function in B lymphocyte-related immune regulation [64]. Another recent study highlighted galectin-9 (Gal-9) strongly up-regulated upon activation of the cells by IFN-γ. Their results showed that Gal-9 is a major mediator of the anti-proliferative and functional effects of MSCs not only on T cells but also on B cells. Moreover, Gal-9 and activated MSCs contribute to the suppression of antigen triggered immunoglobulin release [65]. On the contrary, Rosado and colleagues reported that bone marrow-MSCs were able to promote in vitro proliferation and differentiation of transitional and naive B cells isolated from both healthy donors and pediatric patients with SLE upon stimulation with CpG, soluble CD40L, anti-Ig antibodies and IL-2 [66]. Meanwhile, B cells treated with UC-MSCs resulted in an increase of proliferation, differentiation into plasma cells and production of antibodies in vitro [67]. These conflicting results hint that it is important to distinguish the direct action of MSCs on B cells from indirect effects mediated by other cell types contained in the different culture conditions.
Immunoregulatory effects of MSC on DC
DCs are the main antigen presenting cells (APCs) in the immune system. Their main function is to present antigenic material on the cell surface to T cells. APCs significantly affect the balance between helper and regulatory T cells and establish self-antigen tolerance [13]. MSCs have been shown to inhibit the in vitro maturation of monocytes and hematopoietic progenitor cells into DCs [68,69] as well as down-regulate the expression of MHC class II, CD11c, CD83 and co-stimulatory molecules on mature DCs. These effects, along with the MSCs ability to decrease the production of the pro-inflammatory cytokines: IL-12 [70] and TNF-α and to up-regulate the production of the anti- inflammatory cytokine IL-10 [71] in monocytes, suggests that MSCs possess the potency to impair both the antigen presentation function of the DCs as well as their pro-inflammatory potential. The co-culture of MSCs with DCs resulted in a reduction of CCR7 chemokine expression by DCs following stimulation. The maturation of DCs in the presence of MSCs showed a significantly lower migration of DCs towards CCL19 [72]. Zhang and colleagues have shown that MSCs inhibit the up-regulation of CD1a, CD40, CD80, CD86 and HLA-DR during the differentiation of DCs and prevent an increase in CD40, CD86 and CD83 expression during the maturation of DCs [73]. In addition, Jiang and colleagues have also shown that the co-culture of MSCs can strongly inhibit the initial differentiation of monocytes into DCs. Subsequently, CD83 expression was significantly reduced in the maturation of DCs treated with MSCs, suggesting that their status was immature. At the same time, a decrease in the expression of presentation molecules (HLA-DR and CD1a) and co-stimulation molecules (CD80 and CD86) and the down-regulation of the secretion of IL-12 have also been observed [74]. Furthermore, the differentiation of bone marrow progenitors into DCs cultured with conditioned supernatants from MSCs was partially inhibited by IL-6 secretion. Also, MSC-secreted PGE2 plays a major role in inhibiting the maturation of DCs, as well as its inhibitory effect on DC maturation by acting early [75].
MSCs inhibit the effector properties of DCs in vitro, including antigen processing and presentation to T cells by inhibiting the activation of mitogen activated protein kinase occurring during TLR4 stimulation. Again, in vitro exposure of DCs to MSCs as well as intravenous MSCs in vivo resulted in significant down-regulation of CCR7 and CD49d, two molecules involved in the original DCs in lymphoid organs. This event leads to inhibition of migration to draining lymph nodes and subsequent alteration of priming of naive T cells specific for the antigen [76].
Immunoregulatory effects of MSC on NKT cell
NKT cells are part of the innate immune system and link the adaptive immune system to the innate immune system. Once activated, these cells can perform the function assigned to Th and Tc cells by enhancing the cell-mediated immune response [77]. In addition, NKT cells play a key role in eliminating virus-infected cells and tumor cells. MSCs can influence innate immunity through their inhibition to the cytotoxicity of NK cells by down-regulating the expression of NKp30, NKp44 and NKG2D activating receptors and suppressing the production of IFN-γ [78,79]. MSCs alter the phenotype of NKT cells and suppress the proliferation, cytokine secretion, and cytotoxicity towards HLA class I-expressing targets. Some of these effects require cell-to-cell contact, while others are influenced by soluble factors, notably TGF-β1 and PGE2, suggesting the existence of various mechanisms of NKT cells suppression mediated by cell supplement medium (CSM) [80]. Spaggiari and colleagues also showed that MSCs could inhibit IL-2-induced proliferation of non-activated NKT cells [71]. MSCs, via HLA-G5, affect innate immunity by inhibiting both NKT cell-mediated cytolysis and IFN-γ secretion [81]. Therefore, MSCs manage to inhibit NKT cell proliferation and immune regulatory function by involving multiple cytokines and the signal pathway.
Immunoregulatory effects of MSC on macrophage
Macrophages are differentiated from monocytes that reside in tissues. They play a key role in the innate immune system and are particularly specialized in the removal of dead cells and cell debris. Macrophage is also an important immune effector cell involving a cell-mediated immune response. Increasing evidences have shown that the regulation of macrophages by MSCs is essential for the inflammatory response and repair of tissue lesions. Researchers have reported that MSCs injection can modulate the response of the immune system by interacting with monocytes and macrophages to reprogram. Treated monocytes and macrophages produce large amounts of IL-10 and the treatment decreases circulating amounts of TNF-α and IL-6 [82]. A recent study has shown that mouse MSCs tilt macrophages to an M2 phenotype independently of cell contact. MSCs exerted this effect by inhibiting NF-KB p65 and activating STAT3 pathways [83]. In addition, the preferential shift of the macrophage phenotype from M1 to M2 may be related to the immune modulation characteristics of MSCs [84]. The discovery of M1 monocytes and macrophages transforming into M2 macrophages in the presence of MSCs could be very important for the development of inflammatory biology and the treatment of inflammatory diseases.
Immunoregulatory effects of MSC on neutrophil
The bone marrow is not only the organ where hematopoiesis occurs but also the site where a large amount of non-proliferating neutrophils is retained in the storage pool of bone marrow sinusoids [72,76]. The bone marrow reserve with an estimated mass between 25-30 times larger than the circulating mass of granulocytes, represents in conditions of increased demand a readily available source of neutrophils that possess the same functional properties as their peripheral counterparts [76,85]. Interestingly, MSCs, which exert their homeostatic functions through both paracrine mechanisms involving the release of soluble factors and contact-dependent mechanisms, would line the bone marrow extravascular space, forming a network that interpolates with the sinusoids, where neutrophils of the bone marrow reserve reside. MSCs also suppress the in vitro production of hydrogen peroxide in activated neutrophils, thus suggesting that these stem cells can potentially limit the intensity of a respiratory burst upon inflammatory stimulation [86].
Neutrophils are the most prevalent innate immune cells, responding to microbial challenge by accumulating at the wound site within minutes of injury. These non-proliferative, phagocytic cells respond to microbial challenge by releasing bactericidal molecules, reactive oxygen species and producing neutrophil extracellular traps (webs of chromatin derived from the neutrophil nucleus containing proteases) [87]. Neutrophils, also known as polymorphonuclear (PMN) leucocytes are the major cell type that constitutes innate immune system. They comprise approximately 50-70% of leucocytes and predominate in eliminating pathogens that induce acute inflammation. Elimination of pathogens by neutrophils involves a series of physiological sequences that comprise chemotaxis, phagocytosis and microbial killing.
It has been demonstrated within mice that MSCs residing in tissue are central to the recruitment of neutrophils, exhibiting a pro-inflammatory phenotype and secreting chemotactic cytokines such as IL-6, IL-8, GM-CSF and macrophage inhibitory factor [88]. These findings are further evidenced by results from a murine sepsis model where infusion of MSCs was shown to aid bacterial clearance through enhancing neutrophil phagocytic activity [89]. Neutrophils are notoriously short-lived immune cells and their survival is central to the elimination of infection and in facilitating tissue repair [90].
Effects of MSC on the oxidative metabolism of neutrophil
Neutrophils undergo a spontaneous oxidative response that can be increased by different stimuli, such as the bacterial-derived peptide formy l-l-methionine-l-leucy l-l-phenylalanine (f-MLP) [91]. Several studies indicate a close relationship between neutrophil apoptosis and oxidant production [92]. The effects of MSCs on the oxidative metabolism of neutrophil were investigated by assessing the oxidative status of neutrophils incubated with or without MSCs for 1 hour and then exposed to f-MLP or medium for 30 minutes. Intracellular hydrogen peroxide levels were detected by flow cytometry. The results showed that MSCs significantly down-regulated the production of intracellular hydrogen peroxide in both unstimulated and f-MLP-stimulated neutrophils [91].
MSC inhibits apoptosis and preserves the viability of neutrophil
The microenvironment of bone marrow establishes close interactions between MSCs and bone marrow sinusoids in a putative perivascular niche. These vessels contain a large storage pool of mature non-proliferating neutrophils [72]. Researchers have investigated the effects of human bone marrow MSC on the survival and effector functions of neutrophil. MSCs from healthy donors, at very low ratios (up to 1:500) of MSC: neutrophil, significantly inhibited the apoptosis of resting and IL-8-activated neutrophils and dampened N-f-MLP-induced respiratory burst. The anti-apoptotic activity of MSCs did not require cell-to-cell contact, as shown by trans-well experiments. Antibody neutralization experiments demonstrated that the key MSC-derived soluble factor responsible for neutrophil protection from apoptosis was IL-6, which is signaled by activating STAT-3 transcription factor. Furthermore, the expression of IL-6 was detected in MSCs by real-time PCR and ELISA. Finally, recombinant IL-6 was found to protect neutrophils from apoptosis in a dose-dependent manner. However, MSC had no effects on neutrophil phagocytosis, expression of adhesion molecules, and chemotaxis in response to IL-8, f-MLP, or C5a [93].
It is shown that human MSCs inhibit the proliferation of most of the immune cells. However, there are innate immune cells such as neutrophils and other polymorphonuclear cells that do not require an extensive proliferation prior to their effector function. The effect of MSCs on neutrophils in the presence of complete and serum-deprived culture media was investigated. The viability of neutrophils increases in the presence of MSCs. Annexin V and propidium iodide (PI) have been utilized to confirm whether the enhancement of neutrophil’s viability is due to a reduction in programmed cell death (PCD). MSCs significantly rescue neutrophils from apoptosis at 1, 5 and 10% of FBS supplementation. The fractions of viable and dead cells were increased and decreased respectively in the presence of MSCs. These results indicate that MSCs rescue neutrophils from nutrient or serum-deprived cell death. However, whether this effect is exerted through a specific signaling pathway or confining neutrophils in resting state by MSCs requires further investigation [93].
During the beginning phase of inflammation, neutrophils are one of the first-respondent inflammatory cells to migrate towards the site of inflammation. MSCs inhibit in vitro apoptosis of resting and IL-8-activated neutrophils and reduce N-f-MLP-induced respiratory burst while not affecting phagocytosis, expression of adhesion molecules, or the migration capability of neutrophils in response to classic stimuli. Further researches have shown that MSCs rescued neutrophils from apoptosis by constitutive release of IL-6 [93]. Cassatella and colleagues also reported that MSCs, upon TLR activation, may sustain and amplify the functions of neutrophils. Their results showed that TLR3-activated MSCs are powerful in preserving neutrophils viability and function, and a concerted action of endogenously produced IL-6, IFN-β, and GM-CSF determines most of the modulatory effects exerted on PMN by TLR3-activated MSC [94]. Results documented by Maqbool and colleagues also indicated that MSCs rescue neutrophils from nutrient- or serum-deprived cell death [95].
Effects of MSC on bax and MCL-1 expression in neutrophil
Bax, a member of the Bcl-2 family, is a pro-apoptotic mitochondrial protein modulated during spontaneous neutrophil apoptosis [96]. The expression of Bax was evaluated in some reviews by immunocytochemistry and digital image analysis in neutrophils cultured in the absence or presence of MSCs. In these studies, Bax was expressed in neutrophils cultured for 18 hours. However, upon incubation with MSCs for the same time interval, neutrophils displayed significantly decreased Bax expression. In contrast, they found that MCL-1, a well-known mitochondrial anti-apoptotic protein, was significantly up-regulated in neutrophils after 18-hour culture with MSCs compared with neutrophils cultured in medium alone for the same time interval [97] (Table 1).
Table 1.
Cytokines secreted by MSCs and their effects on neutrophils
| Cytokines secreted by MSCs | Functions on Neutrophils | Relevant mechanism | References |
|---|---|---|---|
| IL-2 | Reduces neutrophil infiltration | [96] | |
| IL-4 | Reduces neutrophil infiltration | [96] | |
| IL-6 | Reduces neutrophil infiltration, inhibits neutrophil apoptosis and decreases N-Formyl-L-Methionine-L-leucyl-L-phenylalanine-inducedrespiratory bursts | STAT3 | [85,92] |
| IL-8 | Inhibits neutrophil apoptosis | Modulation of two mitochondrial proteins of the Bcl-2 family, Bax and MCL-1 | [92,93] |
| Down-regulation of Bax and up-regulation of MCL-1 | |||
| IL-10 | Suppresses the functions of neutrophils | Expression of PD-L1 and FasL molecules | [93] |
| IL-17 | Enhances neutrophil phagocytic activity, increases neutrophils maturation and number | Induces G-CSF | [92] |
| CXCL2 | Reduces neutrophil infiltration | [96] | |
| CXCR2 | Attenuates neutrophil recruitment | Enhanced intracellular activation of p38 MAPK phosphorylation | [96] |
| GM-CSF | Promotes neutrophils survival | [85] |
MSC suppresses the nitric oxide (NO) secretion by neutrophil
An assay was conducted to assess NO production of neutrophils. Neutrophils were co-cultured with or without MSCs at the ratio of neutrophil: MSC (1:100) for 24 hours supplemented with or without 5 µg/ml LPS and PMA. The amount of NO released from the cells was derived from the standard curve. The results revealed that PMA served as potent stimulator of neutrophils compared to LPS. Although MSCs were allogenic to respondent neutrophils, MSCs did not stimulate the resting PMN. In the presence of MSCs and PMA, NO production of neutrophils was dramatically reduced more than three folds [93]. Therefore, neutrophils may be one of important immune cells which mediate the forward immunomodulation of MSCs (Figure 2).
Figure 2.
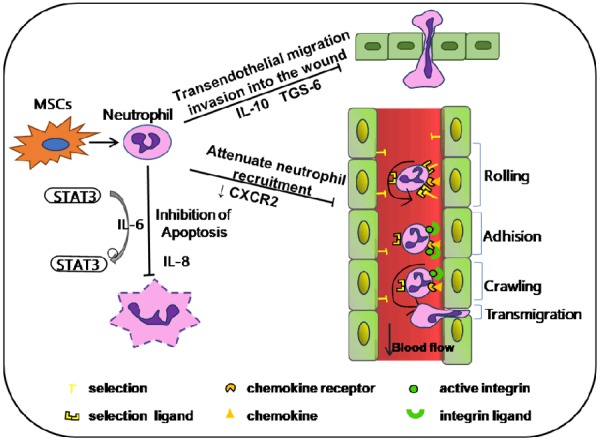
The effect of MSCs on neutrophils. Abbreviations: IL-10 (interleukin 10), IL-6 (interleukin 6), IL-8 (interleukin 8), Signal transducer and activator of transcription 3 (STAT3), TNF-stimulated gene 6 protein (TSG-6), CXC receptor 2 (CXCR2).
MSC as a new trend for cell therapy
Stem cells are unspecialized cells with the ability to renew themselves for long periods without significant changes in their general properties. They can differentiate into various specialized cell types under certain physiological or experimental conditions. Cell therapy is a sub-type of regenerative medicine. Cell therapy based on stem cells describes the process of introducing stem cells into tissue to treat a disease with or without the addition of gene therapy. MSCs exert different functions by secreting a variety of factors. They produce growth factors such as TGF-β, HGF, FGF and VEGF that induce proliferation and angiogenesis of various cell types, in particular fibroblasts, epithelial or endothelial cells [38]. Currently, there are many registered clinical trials in different clinical trial phases aimed at evaluating the potential of MSC-based cell therapy worldwide. With the advancement of preclinical studies, MSCs have been shown to be effective in the treatment of many diseases, including immune diseases and non-immune diseases.
MSC in tissue repair
The wide tissue distribution and multipotent differentiation of MSCs together with the observed reparative effects of infused MSCs in many clinical and preclinical models [98-103] strongly suggest a critical role of MSCs in injury healing. They are believed to be responsible for growth, wound healing, and replacing cells that are lost through daily wear and tear and pathological conditions. Because of these functions, they have been shown to be effective in the treatment of tissue injury and degenerative diseases. In preclinical studies, researchers have also shown that human MSCs are effective in treating myocardial infarction through the secretion of tumor necrosis factor-inducible gene 6 protein (TSG-6), which reduces inflammation and promotes tissue reconstruction. It also reduced inflammatory damage to the cornea by secreting TNF-alpha stimulated gene/protein 6 [102].
MSC in immune disorder therapy
In addition to their property of treating tissue injury, MSCs are also applied to alleviate immune disorders because of their powerful capacity of regulating immune responses. Various studies have evaluated the therapeutic effect of MSCs in preclinical animal models and demonstrated great clinical potential. For example, MSCs have been successfully applied to reverse GvHD in patients receiving bone marrow transplantation [104,105] especially in patients diagnosed with severe steroid resistance [106-108]. Similarly, in SLE and Crohn’s disease patients, both autologous and allogeneic MSCs were able to suppress inflammation and reduce damage to the kidneys and bowel through the possible induction of regulatory T cells in patients [109-111]. It has also been reported that BM-MSCs can improve multiple system atrophy (MSA) [112] multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS) [113] and stroke [114], likely through immediate immunomodulatory effects [8]. Osiris’ Prochymal, the world’s first stem cell drug approved in Canada on May 12, 2012, was successful in phase III clinical trials in treating GvHD and Crohn’s disease and has become the only stem cell-based drug approved by FDA [115,116].
The interaction between mesenchymal stem cells and neutrophils has been demonstrated in various pathologies such as GVHD and IBD. Our search on the PubMed, SCI and Google scholar database about articles that highlight the interaction between these two kinds of cells in various diseases revealed a total of 110 documents published from the discovery of MSCs till date (Figure 3).
Figure 3.
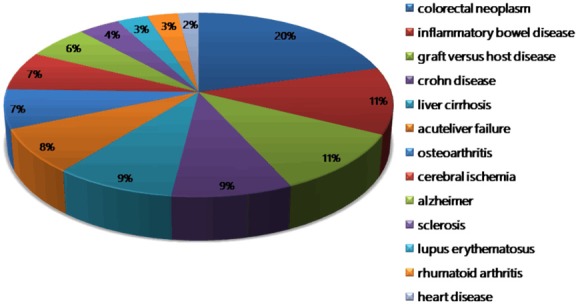
Distribution of MSC regulation on neutrophils in different diseases.
Conclusion
Mesenchymal stem cells are advantageous over other stem cell for a variety of reasons. Firstly, they avoid the ethical issues that surround the embryonic stem cells research. Secondly, repeated studies have found MSCs to be immuno-privileged, which make them an advantageous cell type for allogeneic transplantation. MSCs reduce both the risks of rejection and complications of transplantation. Thirdly, there have been advances in the use of autologous mesenchymal stem cells to regenerate human tissues, including cartilage, meniscus, tendons, and bone fractures, because MSCs can exert regenerative effects through homing to sites of damage, paracrine signaling, regulating the immune response, and affecting the microenvironment. In summary, these traits give MSCs intense therapeutic interest, because they represent a population of cells with the potential to treat a wide range of acute and degenerative diseases.
MSCs have been extensively tested for their immunomodulatory and trophic properties in the field of medical research. A growing number of journals report the role of MSCs in modulating the cells of the immune system. Although the specific mechanisms of action by which MSCs exert their immunomodulatory effects in vivo remain largely unknown, these cells also considered having therapeutic peculiarities are highly regulated by their microenvironment and paracrine signals. In this review, we discussed the effects of MSCs on some immune cells and in particular on neutrophils, thus demonstrating the importance of cellular interactions and the secretome of MSCs. A better understanding of these interactions will be crucial for the improvement and development of new clinical protocols for MSC-based cell therapy.
Existing research have identified that, cell confluence and the number of population doublings can impact a prominent therapeutic mechanism: the immunomodulatory potential of MSCs. The degree to which this effect is exerted is still largely unknown and needs to be further explored. As discussed in this review, there is also the need to futher explore the mechanisms involved in MSC and immune cells interactions in terms of their efficacy and predictability to improve their clinical potential. Furthermore, knowing the number of MSCs needed to give expected outcome for any therapy will break through a major hurdle in their application in any treatments.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Grant no. 81670502, 81672416, 81272481 and 81602883), Jiangsu Key Research and Development Project (Grant no. BE2016717), the Nature Science Foundation of Jiangsu Province (Grant no. BK20161365 and BK20161365), the Scientific Research Foundation of Jiangsu University (Grant no. FCJJ2015023), the opening project of the Key Laboratory of Embryo Molecular Biology, Ministry of Health of China, and Shanghai Key Laboratory of Embryo and Reproduction Engineering (Grant no. KF201601), and Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Disclosure of conflict of interest
None.
References
- 1.Tavassoli M, Friedenstein A. Hemopoietic stromal microenvironment. Am J Hematol. 1983;15:195–203. doi: 10.1002/ajh.2830150211. [DOI] [PubMed] [Google Scholar]
- 2.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 3.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 5.Keating A. Mesenchymal stromal cells. Curr Opin Hematol. 2006;13:419–425. doi: 10.1097/01.moh.0000245697.54887.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ti D, Hao H, Fu X, Han W. Mesenchymal stem cells-derived exosomal microRNAs contribute to wound inflammation. Sci China Life Sci. 2016;59:1305–1312. doi: 10.1007/s11427-016-0240-4. [DOI] [PubMed] [Google Scholar]
- 7.Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28:875–884. doi: 10.1016/s0301-472x(00)00482-3. [DOI] [PubMed] [Google Scholar]
- 8.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 9.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 10.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 11.Chen CP, Chen YY, Huang JP, Wu YH. The effect of conditioned medium derived from human placental multipotent mesenchymal stromal cells on neutrophils: possible implications for placental infection. Mol Hum Reprod. 2014;20:1117–1125. doi: 10.1093/molehr/gau062. [DOI] [PubMed] [Google Scholar]
- 12.Chen PM, Liu KJ, Hsu PJ, Wei CF, Bai CH, Ho LJ, Sytwu HK, Yen BL. Induction of immunomodulatory monocytes by human mesenchymal stem cell-derived hepatocyte growth factor through ERK1/2. J Leukoc Biol. 2014;96:295–303. doi: 10.1189/jlb.3A0513-242R. [DOI] [PubMed] [Google Scholar]
- 13.Chiesa S, Morbelli S, Morando S, Massollo M, Marini C, Bertoni A, Frassoni F, Bartolome ST, Sambuceti G, Traggiai E, Uccelli A. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc Natl Acad Sci U S A. 2011;108:17384–17389. doi: 10.1073/pnas.1103650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh JY, Ko JH, Lee HJ, Yu JM, Choi H, Kim MK, Wee WR, Prockop DJ. Mesenchymal stem/stromal cells inhibit the NLRP3 inflammasome by decreasing mitochondrial reactive oxygen species. Stem Cells. 2014;32:1553–1563. doi: 10.1002/stem.1608. [DOI] [PubMed] [Google Scholar]
- 15.Yen BL, Yen ML, Hsu PJ, Liu KJ, Wang CJ, Bai CH, Sytwu HK. Multipotent human mesenchymal stromal cells mediate expansion of myeloid-derived suppressor cells via hepatocyte growth factor/c-met and STAT3. Stem Cell Reports. 2013;1:139–151. doi: 10.1016/j.stemcr.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teng TS, Ji AL, Ji XY, Li YZ. Neutrophils and immunity: from bactericidal action to Being conquered. J Immunol Res. 2017;2017:9671604. doi: 10.1155/2017/9671604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sionov RV, Fridlender ZG, Granot Z. The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron. 2015;8:125–158. doi: 10.1007/s12307-014-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 20.Mao F, Xu WR, Qian H, Zhu W, Yan YM, Shao QX, Xu HX. Immunosuppressive effects of mesenchymal stem cells in collagen-induced mouse arthritis. Inflamm Res. 2010;59:219–225. doi: 10.1007/s00011-009-0090-y. [DOI] [PubMed] [Google Scholar]
- 21.Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, Genrich K, Mehrotra S, Setty S, Smith B, Bartholomew A. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38:1745–1755. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdel Salam AG, Ata HM, Salman TM, Rashed LA, Sabry D, Schaalan MF. Potential therapeutic utility of mesenchymal stem cells in inflammatory bowel disease in mice. Int Immunopharmacol. 2014;22:515–521. doi: 10.1016/j.intimp.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 23.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Brighton CT, Hunt RM. Early histological and ultrastructural changes in medullary fracture callus. J Bone Joint Surg Am. 1991;73:832–847. [PubMed] [Google Scholar]
- 25.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45:115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cananzi M, Atala A, De Coppi P. Stem cells derived from amniotic fluid: new potentials in regenerative medicine. Reprod Biomed Online. 2009;18(Suppl 1):17–27. doi: 10.1016/s1472-6483(10)60111-3. [DOI] [PubMed] [Google Scholar]
- 27.Gregory CA, Prockop DJ, Spees JL. Non-hematopoietic bone marrow stem cells: molecular control of expansion and differentiation. Exp Cell Res. 2005;306:330–335. doi: 10.1016/j.yexcr.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 28.Krampera M, Sartoris S, Liotta F, Pasini A, Angeli R, Cosmi L, Andreini A, Mosna F, Bonetti B, Rebellato E, Testi MG, Frosali F, Pizzolo G, Tridente G, Maggi E, Romagnani S, Annunziato F. Immune regulation by mesenchymal stem cells derived from adult spleen and thymus. Stem Cells Dev. 2007;16:797–810. doi: 10.1089/scd.2007.0024. [DOI] [PubMed] [Google Scholar]
- 29.Reger RL, Tucker AH, Wolfe MR. Differentiation and characterization of human MSCs. Methods Mol Biol. 2008;449:93–107. doi: 10.1007/978-1-60327-169-1_7. [DOI] [PubMed] [Google Scholar]
- 30.Branch MJ, Hashmani K, Dhillon P, Jones DR, Dua HS, Hopkinson A. Mesenchymal stem cells in the human corneal limbal stroma. Invest Ophthalmol Vis Sci. 2012;53:5109–5116. doi: 10.1167/iovs.11-8673. [DOI] [PubMed] [Google Scholar]
- 31.Wang S, Qu X, Zhao RC. Clinical applications of mesenchymal stem cells. J Hematol Oncol. 2012;5:19. doi: 10.1186/1756-8722-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caplan AI. The mesengenic process. Clin Plast Surg. 1994;21:429–435. [PubMed] [Google Scholar]
- 33.Dennis JE, Merriam A, Awadallah A, Yoo JU, Johnstone B, Caplan AI. A quadripotential mesenchymal progenitor cell isolated from the marrow of an adult mouse. J Bone Miner Res. 1999;14:700–709. doi: 10.1359/jbmr.1999.14.5.700. [DOI] [PubMed] [Google Scholar]
- 34.Lue J, Lin G, Ning H, Xiong A, Lin CS, Glenn JS. Transdifferentiation of adipose-derived stem cells into hepatocytes: a new approach. Liver Int. 2010;30:913–922. doi: 10.1111/j.1478-3231.2010.02231.x. [DOI] [PubMed] [Google Scholar]
- 35.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 36.Portmann-Lanz CB, Schoeberlein A, Portmann R, Mohr S, Rollini P, Sager R, Surbek DV. Turning placenta into brain: placental mesenchymal stem cells differentiate into neurons and oligodendrocytes. Am J Obstet Gynecol. 2010;202:294.e291–294.e211. doi: 10.1016/j.ajog.2009.10.893. [DOI] [PubMed] [Google Scholar]
- 37.Screven R, Kenyon E, Myers MJ, Yancy HF, Skasko M, Boxer L, Bigley EC 3rd, Borjesson DL, Zhu M. Immunophenotype and gene expression profile of mesenchymal stem cells derived from canine adipose tissue and bone marrow. Vet Immunol Immunopathol. 2014;161:21–31. doi: 10.1016/j.vetimm.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 39.Kim DH, Yoo KH, Choi KS, Choi J, Choi SY, Yang SE, Yang YS, Im HJ, Kim KH, Jung HL, Sung KW, Koo HH. Gene expression profile of cytokine and growth factor during differentiation of bone marrow-derived mesenchymal stem cell. Cytokine. 2005;31:119–126. doi: 10.1016/j.cyto.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, Noel D, Jorgensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 41.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 42.Kim JH, Lee YT, Hong JM, Hwang YI. Suppression of in vitro murine T cell proliferation by human adipose tissue-derived mesenchymal stem cells is dependent mainly on cyclooxygenase-2 expression. Anat Cell Biol. 2013;46:262–271. doi: 10.5115/acb.2013.46.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 44.Chen K, Wang D, Du WT, Han ZB, Ren H, Chi Y, Yang SG, Zhu D, Bayard F, Han ZC. Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanism. Clin Immunol. 2010;135:448–458. doi: 10.1016/j.clim.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 45.Ling W, Zhang J, Yuan Z, Ren G, Zhang L, Chen X, Rabson AB, Roberts AI, Wang Y, Shi Y. Mesenchymal stem cells use IDO to regulate immunity in tumor microenvironment. Cancer Res. 2014;74:1576–1587. doi: 10.1158/0008-5472.CAN-13-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 47.Xu C, Yu P, Han X, Du L, Gan J, Wang Y, Shi Y. TGF-beta promotes immune responses in the presence of mesenchymal stem cells. J Immunol. 2014;192:103–109. doi: 10.4049/jimmunol.1302164. [DOI] [PubMed] [Google Scholar]
- 48.English K, Barry FP, Mahon BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett. 2008;115:50–58. doi: 10.1016/j.imlet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Spaggiari GM, Moretta L. Interactions between mesenchymal stem cells and dendritic cells. Adv Biochem Eng Biotechnol. 2013;130:199–208. doi: 10.1007/10_2012_154. [DOI] [PubMed] [Google Scholar]
- 50.Schurch CM, Riether C, Ochsenbein AF. Cytotoxic CD8+ T cells stimulate hematopoietic progenitors by promoting cytokine release from bone marrow mesenchymal stromal cells. Cell Stem Cell. 2014;14:460–472. doi: 10.1016/j.stem.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 52.Benvenuto F, Ferrari S, Gerdoni E, Gualandi F, Frassoni F, Pistoia V, Mancardi G, Uccelli A. Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem Cells. 2007;25:1753–1760. doi: 10.1634/stemcells.2007-0068. [DOI] [PubMed] [Google Scholar]
- 53.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 54.Xue Q, Luan XY, Gu YZ, Wu HY, Zhang GB, Yu GH, Zhu HT, Wang M, Dong W, Geng YJ, Zhang XG. The negative co-signaling molecule b7-h4 is expressed by human bone marrow-derived mesenchymal stem cells and mediates its T-cell modulatory activity. Stem Cells Dev. 2010;19:27–38. doi: 10.1089/scd.2009.0076. [DOI] [PubMed] [Google Scholar]
- 55.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 56.Kong QF, Sun B, Bai SS, Zhai DX, Wang GY, Liu YM, Zhang SJ, Li R, Zhao W, Sun YY, Li N, Wang Q, Peng HS, Jin LH, Li HL. Administration of bone marrow stromal cells ameliorates experimental autoimmune myasthenia gravis by altering the balance of Th1/Th2/Th17/Treg cell subsets through the secretion of TGF-beta. J Neuroimmunol. 2009;207:83–91. doi: 10.1016/j.jneuroim.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–1213. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 58.Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–1490. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 59.Morandi F, Raffaghello L, Bianchi G, Meloni F, Salis A, Millo E, Ferrone S, Barnaba V, Pistoia V. Immunogenicity of human mesenchymal stem cells in HLA-class I-restricted T-cell responses against viral or tumor-associated antigens. Stem Cells. 2008;26:1275–1287. doi: 10.1634/stemcells.2007-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 61.Rafei M, Hsieh J, Fortier S, Li M, Yuan S, Birman E, Forner K, Boivin MN, Doody K, Tremblay M, Annabi B, Galipeau J. Mesenchymal stromal cell-derived CCL2 suppresses plasma cell immunoglobulin production via STAT3 inactivation and PAX5 induction. Blood. 2008;112:4991–4998. doi: 10.1182/blood-2008-07-166892. [DOI] [PubMed] [Google Scholar]
- 62.Li L, Yang M, Wang C, Zhao Q, Liu J, Zhan C, Liu Z, Li X, Wang W, Yang X. Effects of cytokines and chemokines on migration of mesenchymal stem cells following spinal cord injury. Neural Regen Res. 2012;7:1106–1112. doi: 10.3969/j.issn.1673-5374.2012.14.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee DS, Yi TG, Lee HJ, Kim SN, Park S, Jeon MS, Song SU. Mesenchymal stem cells infected with Mycoplasma arginini secrete complement C3 to regulate immunoglobulin production in B lymphocytes. Cell Death Dis. 2014;5:e1192. doi: 10.1038/cddis.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan H, Wu M, Yuan Y, Wang ZZ, Jiang H, Chen T. Priming of toll-like receptor 4 pathway in mesenchymal stem cells increases expression of B cell activating factor. Biochem Biophys Res Commun. 2014;448:212–217. doi: 10.1016/j.bbrc.2014.04.097. [DOI] [PubMed] [Google Scholar]
- 65.Ungerer C, Quade-Lyssy P, Radeke HH, Henschler R, Konigs C, Kohl U, Seifried E, Schuttrumpf J. Galectin-9 is a suppressor of T and B cells and predicts the immune modulatory potential of mesenchymal stromal cell preparations. Stem Cells Dev. 2014;23:755–766. doi: 10.1089/scd.2013.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosado MM, Bernardo ME, Scarsella M, Conforti A, Giorda E, Biagini S, Cascioli S, Rossi F, Guzzo I, Vivarelli M, Dello Strologo L, Emma F, Locatelli F, Carsetti R. Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev. 2015;24:93–103. doi: 10.1089/scd.2014.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fan L, Hu C, Chen J, Cen P, Wang J, Li L. Interaction between mesenchymal stem cells and B-cells. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17050650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 69.Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 70.Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 72.Craddock CG Jr, Perry S, Ventzke LE, Lawrence JS. Evaluation of marrow granulocytic reserves in normal and disease states. Blood. 1960;15:840–855. [PubMed] [Google Scholar]
- 73.Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, Le AD. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 75.Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 76.Berkow RL, Dodson RW. Purification and functional evaluation of mature neutrophils from human bone marrow. Blood. 1986;68:853–860. [PubMed] [Google Scholar]
- 77.Paust S, Senman B, von Andrian UH. Adaptive immune responses mediated by natural killer cells. Immunol Rev. 2010;235:286–296. doi: 10.1111/j.0105-2896.2010.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hayashi Y, Tsuji S, Tsujii M, Nishida T, Ishii S, Iijima H, Nakamura T, Eguchi H, Miyoshi E, Hayashi N, Kawano S. Topical implantation of mesenchymal stem cells has beneficial effects on healing of experimental colitis in rats. J Pharmacol Exp Ther. 2008;326:523–531. doi: 10.1124/jpet.108.137083. [DOI] [PubMed] [Google Scholar]
- 79.Tzaribachev N, Vaegler M, Schaefer J, Reize P, Rudert M, Handgretinger R, Muller I. Mesenchymal stromal cells: a novel treatment option for steroid-induced avascular osteonecrosis. Isr Med Assoc J. 2008;10:232–234. [PubMed] [Google Scholar]
- 80.Malygin AM, Meri S, Timonen T. Regulation of natural killer cell activity by transforming growth factor-beta and prostaglandin E2. Scand J Immunol. 1993;37:71–76. doi: 10.1111/j.1365-3083.1993.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 81.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 82.Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deng P, Zhou C, Alvarez R, Hong C, Wang CY. Inhibition of IKK/NF-kappaB signaling enhances differentiation of mesenchymal stromal cells from human embryonic stem cells. Stem Cell Reports. 2016;6:456–465. doi: 10.1016/j.stemcr.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46:e70. doi: 10.1038/emm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Steele RW, Steele CR, Pilkington NS Jr, Charlton RK. Functional capacity of marginated and bone marrow reserve granulocytes. Infect Immun. 1987;55:2359–2363. doi: 10.1128/iai.55.10.2359-2363.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Inoue Y, Iriyama A, Ueno S, Takahashi H, Kondo M, Tamaki Y, Araie M, Yanagi Y. Subretinal transplantation of bone marrow mesenchymal stem cells delays retinal degeneration in the RCS rat model of retinal degeneration. Exp Eye Res. 2007;85:234–241. doi: 10.1016/j.exer.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 87.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 88.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 89.Hall SR, Tsoyi K, Ith B, Padera RF Jr, Lederer JA, Wang Z, Liu X, Perrella MA. Mesenchymal stromal cells improve survival during sepsis in the absence of heme oxygenase-1: the importance of neutrophils. Stem Cells. 2013;31:397–407. doi: 10.1002/stem.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luo HR, Loison F. Constitutive neutrophil apoptosis: mechanisms and regulation. Am J Hematol. 2008;83:288–295. doi: 10.1002/ajh.21078. [DOI] [PubMed] [Google Scholar]
- 91.Dallegri F, Ottonello L. Tissue injury in neutrophilic inflammation. Inflamm Res. 1997;46:382–391. doi: 10.1007/s000110050208. [DOI] [PubMed] [Google Scholar]
- 92.Fadeel B, Ahlin A, Henter JI, Orrenius S, Hampton MB. Involvement of caspases in neutrophil apoptosis: regulation by reactive oxygen species. Blood. 1998;92:4808–4818. [PubMed] [Google Scholar]
- 93.Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, Ottonello L, Pistoia V. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. 2008;26:151–162. doi: 10.1634/stemcells.2007-0416. [DOI] [PubMed] [Google Scholar]
- 94.Cassatella MA, Mosna F, Micheletti A, Lisi V, Tamassia N, Cont C, Calzetti F, Pelletier M, Pizzolo G, Krampera M. Toll-like receptor-3-activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils. Stem Cells. 2011;29:1001–1011. doi: 10.1002/stem.651. [DOI] [PubMed] [Google Scholar]
- 95.Maqbool M, Vidyadaran S, George E, Ramasamy R. Human mesenchymal stem cells protect neutrophils from serum-deprived cell death. Cell Biol Int. 2011;35:1247–1251. doi: 10.1042/CBI20110070. [DOI] [PubMed] [Google Scholar]
- 96.El Kebir D, Filep JG. Modulation of neutrophil apoptosis and the resolution of inflammation through beta2 integrins. Front Immunol. 2013;4:60. doi: 10.3389/fimmu.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Murphy MP, Caraher E. Mcl-1 is vital for neutrophil survival. Immunol Res. 2015;62:225–233. doi: 10.1007/s12026-015-8655-z. [DOI] [PubMed] [Google Scholar]
- 98.Kim JM, Lee ST, Chu K, Jung KH, Song EC, Kim SJ, Sinn DI, Kim JH, Park DK, Kang KM, Hyung Hong N, Park HK, Won CH, Kim KH, Kim M, Kun Lee S, Roh JK. Systemic transplantation of human adipose stem cells attenuated cerebral inflammation and degeneration in a hemorrhagic stroke model. Brain Res. 2007;1183:43–50. doi: 10.1016/j.brainres.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 99.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muller I, Kordowich S, Holzwarth C, Isensee G, Lang P, Neunhoeffer F, Dominici M, Greil J, Handgretinger R. Application of multipotent mesenchymal stromal cells in pediatric patients following allogeneic stem cell transplantation. Blood Cells Mol Dis. 2008;40:25–32. doi: 10.1016/j.bcmd.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 101.Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roddy GW, Oh JY, Lee RH, Bartosh TJ, Ylostalo J, Coble K, Rosa RH Jr, Prockop DJ. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-alpha stimulated gene/protein 6. Stem Cells. 2011;29:1572–1579. doi: 10.1002/stem.708. [DOI] [PubMed] [Google Scholar]
- 103.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 104.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 105.Wu KH, Chan CK, Tsai C, Chang YH, Sieber M, Chiu TH, Ho M, Peng CT, Wu HP, Huang JL. Effective treatment of severe steroid-resistant acute graft-versus-host disease with umbilical cord-derived mesenchymal stem cells. Transplantation. 2011;91:1412–1416. doi: 10.1097/TP.0b013e31821aba18. [DOI] [PubMed] [Google Scholar]
- 106.Carrion F, Nova E, Ruiz C, Diaz F, Inostroza C, Rojo D, Monckeberg G, Figueroa FE. Autologous mesenchymal stem cell treatment increased T regulatory cells with no effect on disease activity in two systemic lupus erythematosus patients. Lupus. 2010;19:317–322. doi: 10.1177/0961203309348983. [DOI] [PubMed] [Google Scholar]
- 107.Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, Minelli A, Alvisi C, Vanoli A, Calliada F, Dionigi P, Perotti C, Locatelli F, Corazza GR. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut. 2011;60:788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 108.Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H, Hua B, Liu B, Ye S, Hu X, Xu W, Zeng X, Hou Y, Gilkeson GS, Silver RM, Lu L, Shi S. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467–2475. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]
- 109.Connick P, Kolappan M, Patani R, Scott MA, Crawley C, He XL, Richardson K, Barber K, Webber DJ, Wheeler-Kingshott CA, Tozer DJ, Samson RS, Thomas DL, Du MQ, Luan SL, Michell AW, Altmann DR, Thompson AJ, Miller DH, Compston A, Chandran S. The mesenchymal stem cells in multiple sclerosis (MSCIMS) trial protocol and baseline cohort characteristics: an open-label pre-test: post-test study with blinded outcome assessments. Trials. 2011;12:62. doi: 10.1186/1745-6215-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, Kooy-Winkelaar EM, Koning F, Zwaginga JJ, Fidder HH, Verhaar AP, Fibbe WE, van den Brink GR, Hommes DW. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut. 2010;59:1662–1669. doi: 10.1136/gut.2010.215152. [DOI] [PubMed] [Google Scholar]
- 111.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JW, Petrou P, Ben-Hur T, Abramsky O, Slavin S. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee PH, Kim JW, Bang OY, Ahn YH, Joo IS, Huh K. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther. 2008;83:723–730. doi: 10.1038/sj.clpt.6100386. [DOI] [PubMed] [Google Scholar]
- 113.Choi MR, Kim HY, Park JY, Lee TY, Baik CS, Chai YG, Jung KH, Park KS, Roh W, Kim KS, Kim SH. Selection of optimal passage of bone marrow-derived mesenchymal stem cells for stem cell therapy in patients with amyotrophic lateral sclerosis. Neurosci Lett. 2010;472:94–98. doi: 10.1016/j.neulet.2010.01.054. [DOI] [PubMed] [Google Scholar]
- 114.Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, Waxman SG, Kocsis JD. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134:1790–1807. doi: 10.1093/brain/awr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kebriaei P, Isola L, Bahceci E, Holland K, Rowley S, McGuirk J, Devetten M, Jansen J, Herzig R, Schuster M, Monroy R, Uberti J. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:804–811. doi: 10.1016/j.bbmt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 116.Mannon PJ. Remestemcel-L: human mesenchymal stem cells as an emerging therapy for Crohn’s disease. Expert Opin Biol Ther. 2011;11:1249–1256. doi: 10.1517/14712598.2011.602967. [DOI] [PubMed] [Google Scholar]


