Abstract
Background: The aim of this study was to investigate the efficacy and safety of 125I particle implantation for treating advanced non-small cell lung cancer (NSCLC). Methods: Data from 56 patients with advanced NSCLC between January 2013 and May 2016 were retrospectively analyzed. The changes of tumor size, objective response rate (ORR), disease control rate (DCR), survival rate of patients and occurrence rate of complications were calculated, and the levels of carcinoembryonic antigen (CEA) and cytokerantin-19-fragment (CYFRA21-1) before and after the treatment were evaluated. Results: The 125I particles implantation therapy significantly inhibited the tumor local growth of NSCLC (from 7.75±6.69 to 3.39±2.12 cm) (P<0.001), suggesting a better effectiveness with an RR of 55.4% and DCR of 98.2%. In addition, the 125I particle implantation down-regulated the CEA expression level of lung adenocarcinoma (LAC) patients (P<0.05). The one-year, two-year, three-year survival rate were 41.1%, 39.3% and 19.6% respectively after the implantation therapy. However, patients implanted 125I particles had no serious complications except for slight fever. Conclusions: NSCLC patients at different clinical features all can benefit from the 125I particle implantation therapy. Moreover, the level of CEA can be used as an efficacy predictor for the 125I particle implantation therapy for LAC.
Keywords: Non-small cell lung cancer, iodine-125, 125I particles implantation, efficacy, carcinoembryonic antigen
Introduction
At present, the incidence and mortality of lung cancer are among the highest in all kinds of tumors, and the incidence of lung cancer is increasing year by year [1]. One study suggests that there are about 733,000 newly diagnosed lung cancer cases in 2015 in China, and in the same year about 610,000 Chinese die from lung cancer [2]. As we all know, 5-year survival rate of lung cancer patients is relatively low. How to choose the best individual treatment for patients is an important issue facing the treatment of lung cancer. Non-small cell lung cancer (NSCLC) has become the most common disease which threats human health [3]. Due to the considerable toxicity of chemotherapy regimens and the decline of efficacy caused by tumor cell resistance, patients with advanced NSCLC have no further indications for these medications [4]. And advanced NSCLC patients generally show poor health status, can no longer tolerate further chemotherapy and radiotherapy. Therefore, some less side effects or more tolerable treatment methods continue to be developed [5]. In recent years, with 125I particles in clinical applications, interstitial permanent implantation of 125I particles for the treatment of malignant tumors has attracted widespread attention [6,7].
The 125I particle is a kind of miniature radiation source, which is made of the 0.5 × 3.0 mm silver bar infiltrated by iodine-125 is sealed in a titanium tube with 0.8 mm diameter, 4.5 mm long and 0.05 mm wall thickness. It emits C and G rays, while also emitting fluorescent C rays from the silver bar. Its half-life is 59.43 days. The effective radiation radius is 1.0 cm. The effective time in vivo is 120 days. The 125I particles emit continuous low energy radiation, which act directly on the DNA molecules of tumor cells to break up single strand of DNA molecules, break the double bond, and lead a decline in the proliferation ability of tumor cells. But at the same time, the normal tissues are not damaged or only are slight damaged [8-10]. Computed tomography (CT) guidance, the implantation of radioactive 125I particles is highly accurate and highly suitable for the treatment of malignant tumors, and has been applied clinically. Conventional treatment of lung cancer has only limited effect on long-term survival, so the treatment of 125I particles has been used for lung cancer treatment [11,12]. This study retrospectively reported the data on radioactive 125I particles implantation therapy for the patients with advanced NSCLC, evaluated its curative effect, and analyzed the relationship between clinical efficacy and tumor markers.
Methods
Ethics statement
All patients were approached based on approved ethical guidelines, and agreed to participate in this study and signed the informed consent before including the study. The study was approved by Research Ethics Committee of Gansu Provincial Hospital (Lanzhou, Gansu, China). We stated that all methods were performed in accordance with the relevant guidelines and regulations.
Enrollment criteria
The inclusion criteria: (1) patients with exact pathology and radiographic evidence of advanced NSCLC (III and IV); (2) the tumor lesions of patients could not be surgically removed or patients were not willing to receive the treatment of surgery; (3) none of the patients received radiotherapy, chemotherapy and targeted therapy before 125I particles implantation therapy or after; (4) expected survival >2 months; (5) physique status score (karnofsky score) ≥50 points; (6) without serious heart, lungs, liver, kidney, blood system and nervous system diseases; (7) no acute infection and (8) leukocytes, platelets, hemoglobin levels were not less than 3 × 109/L, 100 × 109/L, 90 g/L respectively.
Exclusion criteria
Exclusion criteria: (1) primary tumor lesions had a wide range of distant metastases; (2) accompanied by a serious mental disorder or mental illness; (3) solid tumors in the lung could not be measured; (4) could not cooperate with puncture treatment and (5) refused to accept follow-up survey.
Patients
A total of 56 patients with primary bronchial lung cancer who received the radioactive 125I particles implantation therapy at the Gansu Provincial Hospital, Lanzhou, Gansu, China from January 2013 to May 2016 were included in this retrospective study. All patients were categorized according to the tumor-node-metastasis (TNM) classification of the International Association for The Study of Lung Cancer (IASLC, Eighth edition, 2015) [13,14]. The tumors were histologically subtyped and graded according to the third edition of the World Health Organization guidelines. Clinical characteristics were retrieved from the clinical records available. Follow-up was performed after the first intervention, and the follow-up time ranged from 1 to 37 months. All patients were followed up and the patient’s death time was recorded as the last follow-up date. The detailed clinical characteristics of patients included in this study were shown in Table 1.
Table 1.
Clinico-pathological features of lung cancer cases (N=56)
| Group | Characteristics | Number (%) |
|---|---|---|
| Sex | Male | 37 (66.1%) |
| Female | 19 (33.9%) | |
| Age | <60 | 18 (32.1%) |
| ≥60 | 38 (67.9%) | |
| Smoking | Yes | 20 (35.7%) |
| No | 36 (64.3%) | |
| Histology | LAC | 34 (60.7%) |
| LSCC | 22 (39.3%) | |
| Pathologic grade | Poorly differentiated | 20 (35.7%) |
| Moderately differentiated | 26 (46.4%) | |
| Well-differentiated | 10 (17.9%) | |
| Lymphatic invasion | Positive | 40 (71.4%) |
| Negative | 16 (28.6%) | |
| Clinical staging | III | 33 (58.9%) |
| IV | 23 (41.1%) |
LAC, lung adenocarcinoma; LSCC, lung squamous cell carcinoma.
Collection and treatment of blood samples
Each patient received this therapy is given a serial number, and the specimen collection container is numbered according to the number. At the beginning of the treatment and 6 months after the treatment, the patient’s venous blood was taken in a fasting early morning from the patient’s elbow vein. The blood samples were immediately shaken and mixed to prevent coagulation and the serum was separated as soon as possible at 3000 rpm for 10 minutes. The collected serum was stored at -20°C under freezing conditions. The frozen serum was thawed at room temperature when testing.
Test of carcinoembryonic antigen (CEA) and cytokeratin 19 fragments (CYFRA 21-1)
The expressions of CEA (Roche Diagnostics, Cat No, 11731629) and CYFRA 21-1 (Roche Diagnostics, Cat No, 11820966) in serum of patients were tested using a Roche Elecsys E601 immunoassay analyzer. Briefly, 20 µL of sample, a biotinylated monoclonal CEA or CYFRA 21-1-specific antibody, and a monoclonal CEA or CYFRA 21-1 antibody labeled with a ruthenium complex were mixed to form a sandwich complex. Streptavidin-coated microparticles were added to the complex and allowed them bind to the microparticles through the reaction between biotin and streptavidin. The reaction mixture is sucked into the measuring pool and the particles are adsorbed onto the electrode by the magnet (unconsolidated substances were washed away by washing liquid). The electrode took place a chemiluminescence after adding voltage and was measured by photomultiplier tube. The test results were automatically detected according to a standard curve. The normal range was defined as CEA 0-5 ng/ml and CYFRA 21-1 0-3.3 ng/ml in this study.
Equipments and materials for 125I particles implantation therapy
The equipments and instruments included: 16-slice Spiral CT and Pinpoint Piercing Guidance System from Phillips, Netherlands. Radioactive particle computer treatment planning system (TPS 2.0) was from Shanghai Fudan University. The 125I radioactive particles were purchased from Shanghai Xinke Pharmaceutical Co., Ltd. The properties of 125I radioactive particles are as follows: (1) 4.5 mm in length and 0.8 mm in diameter; (2) the half-life of the particles is 59.6 days; (3) the average photon energy is 27-35 KeV; (4) the initial dose rate was 7 cGy/h; (5) the tissue penetration distance is 1.7 cm; (6) particle activity is 0.50~0.80 mCi.
Process of particle implantation
All patients enrolled were required to be hospitalized before treatment, and to be completed the basic preoperative examination, including blood tests, coagulation tests, blood chemistry and electrocardiography. Before implantation, the patient underwent a CT scan of the thorax with a layer thickness of 5 mm and a layer distance of 5 mm (when the boundaries between tumor and the adjacent blood vessels were not clear, a further enhanced scanning was needed). The CT images were subsequently transmitted to the radioactive particle computer treatment planning system for three-dimensional digital image reconstruction, and the researchers delineated the target. Based on the three mutually perpendicular diameters of the tumor target volume, the spatial distribution and number of particles that would place in patient’s tumor were determined. Finally, isodose curves and dose-volume histograms were determined based on matched peripheral dose of planning target volume.
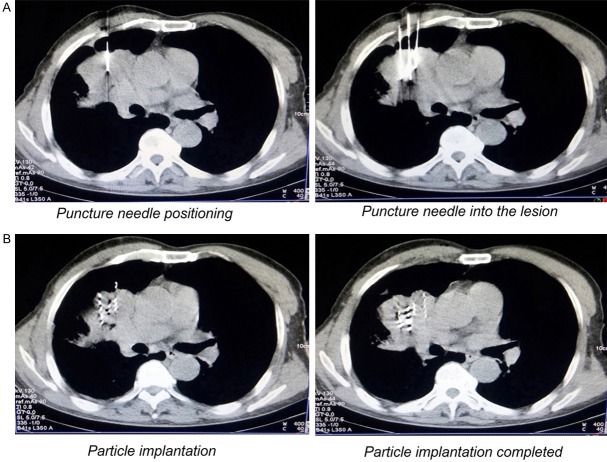
Based on the CT scan of the tumor area to measure the depth of the body surface to the tumor, the appropriate length of the puncture needle, the puncture point and needle orientation were determined. Through local anesthesia with lidocaine, the needle was punctured into the tumor and the expected location of the needle tip within the lesion was confirmed by CT scan. According to the TPS treatment plan combined with CT real-time scan images, the direction and depth of the needle was adjusted and was kept in parallel. Through pumping back, when there was no blood reflux, the particles are implanted, and the implantation interval of the particles was about 0.8 to 1.2 cm (Figure 1A and 1B).
Figure 1.
Implantation process of CT-guided 125I particles. (A) Based on the CT scan of the tumor area to measure the depth of the body surface to the tumor, the puncture point, needle orientation and depth of needle were determined; (B) According to the TPS treatment plan combined with CT real-time scan images, the particles are implanted in lesion, and the implantation interval of the particles was about 0.8 to 1.2 cm; TPS, treatment planning system.
Tumor efficacy evaluation
CT scan was employed to measure the size of NSCLC lesions before the 125I particles implantation therapy and after six months of implantation. All patients adopted the same criteria recommended by Response Evaluation Criteria in Solid Tumors (RECIST) for evaluating the treatment efficacy [15,16]. CR: all target lesions disappear, and at least 4 weeks or more; PR: the longest diameter sum of baseline lesions is reduced by more than 30% at least 4 weeks; SD: the baseline lesions have a decrease in the sum of the longest diameter but did not reach PR or increase but did not reach PD; PD: baseline lesions increase the total length of 20% or new lesions appear. Objective response rate (ORR)=CR + PR/overall cases; disease control rate (DCR)=CR + PR + SD/overall cases.
Statistical analysis
The patient’s clinical characteristics were analysed by descriptive statistics. The efficacy of 125I particles implantation therapy was analyzed using the Student’s t test. The associations between the expressions of CEA and CYFRA 21-1 and the efficacy of 125I particles implantation were determined by Student’s t test and nonparametric rank sum test. The survival time was measured from the date of 125I particles implantation to the date of finally follow-up patients and the occurrence rate of complications of patients were displayed through descriptive statistics. All tests were two-sided, and p-values <0.05 were considered to be statistically significant. The SPSS 20.0 software package (Chicago, USA) was used to perform the statistical analysis.
Results
The 125I particles implantation therapy showed a better tumor local control rate
All patients with advanced NSCLC were followed up to 6 months and underwent a chest CT scan. By comparing with pre-treatment CT, we performed a short-term efficacy evaluation. Although none of the cases were shown as complete response (CR), a total of 31 cases showed as partial response (PR), and 24 cases showed as stable disease (SD). In particular, only one patient appeared disease progression (Figures 2A-C, 3A). The 125I particles implantation therapy suggested a better tumor local control rate, which showed an objective response rate (ORR) of 55.4% and a disease control rate (DCR) of 98.2%.
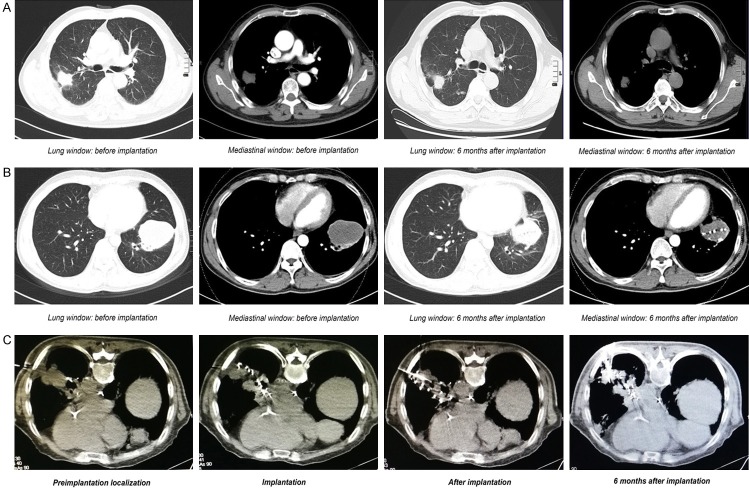
Figure 2.
Images before and after CT-guided 125I particles implantation for treating NSCLC. (A) Compared with before implantation, the lesions of LAC of the right lung were significantly reduced after implantation therapy for 6 months; (B) Compared with before implantation, the lesions of LSCC of the left lung were significantly reduced after implantation therapy for 6 months; (C) The 125I particles implantation therapy significantly inhibited the growth of local tumor; NSCLC, non-small cell lung cancer; LAC, lung adenocarcinoma; LSCC, lung squamous cell carcinoma.
Figure 3.
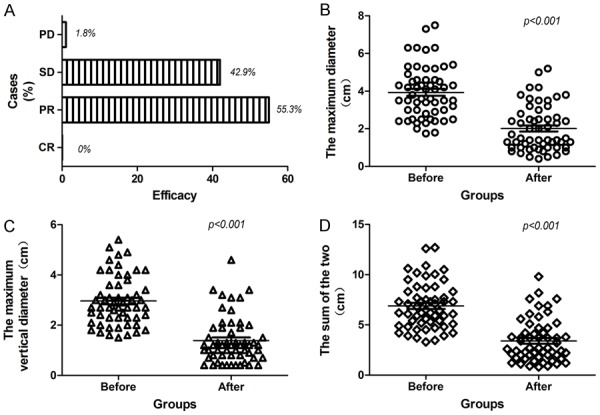
Efficacy of 125I particles implantation therapy for treating advanced NSCLC. (A) The 125I particles implantation therapy showed a better tumor local control rate, which suggested an ORR of 55.4% and DCR of 98.2%; (B) Compared before implantation with after six months of implantation, the maximum diameter of the tumor displayed a obvious decrease (3.93±1.37 vs. 2.01±1.21 cm) (P<0.001); (C) Compared before implantation with after six months of implantation, the maximum vertical diameter displayed a obvious decrease (2.96±0.98 vs. 1.38±0.93 cm) (P<0.001); (D) Compared before implantation with after six months of implantation, the sum of the maximum diameter and the maximum vertical diameter displayed a obvious decrease (7.75±6.69 vs. 3.39±2.12 cm) (P<0.001); NSCLC, non-small cell lung cancer; ORR, objective response rate; DCR, disease control rate.
The 125I particles implantation therapy significantly inhibited the local growth of advanced NSCLC
As shown in Table 2, compared before implantation with after six months of implantation, no matter the maximum diameter of the tumor (3.93±1.37 vs. 2.01±1.21 cm) (Figure 3B) or the maximum vertical diameter (2.96±0.98 vs. 1.38±0.93 cm) (Figure 3C), they all displayed a obvious decrease (P<0.001), showing that the 125I particles implantation therapy significantly inhibited the local growth o NSCLC (the sum of the two: from 7.75±6.69 to 3.39±2.12 cm) (Figure 3D).
Table 2.
The effect of 125I particles implantation therapy to local growth of advanced NSCLC by CT measurement
| Groups | Measurement of tumors by CT scan (mean ± SD, cm) | ||
|---|---|---|---|
|
| |||
| The maximum diameter | The maximum vertical diameter | The sum of the two | |
| Before therapy | 3.93±1.37 | 2.96±0.98 | 7.75±6.69 |
| After therapy | 2.01±1.21 | 1.38±0.93 | 3.39±2.12 |
| Degree of free | 55 | 55 | 55 |
| T value | 15.529 | 18.01 | 4.83 |
| P value | <0.001 | <0.001 | <0.001 |
NSCLC, non-small cell lung cancer; CT, computed tomography; SD, standard deviation; cm, centimeter; The sum of the two, the maximum diameter + the maximum vertical diameter.
Advanced NSCLC patients with different clinico-pathological features all could benefit from the 125I particle implantation therapy
As shown in Table 3, compared before implantation with after six months of implantation, whether in male patients (P<0.001) or in women patients (P<0.001), in patients with smoking (P<0.001) or non-smokers (P<0.001), in lung adenocarcinoma (LAC) patients (P<0.001) or lung squamous cell carcinoma (LSCC) patients (P<0.001), in patients with poor differentiation cancer (P<0.001) or moderate and well differentiation patients (P<0.001), in patients with lymphatic metastasis (P<0.001) or those without lymphatic metastasis (P<0.001) and in patients at III stage (P<0.001) or IV stage (P<0.001), the 125I particles implantation therapy all obviously decreased the size of local tumor of advanced NSCLC (Figure 4A-F).
Table 3.
Relationship between efficacy of 125I particles implantation therapy and clinical-pathological features of NSCLC
| Items | Groups | Measurement of tumors by CT scan (cm) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | The maximum diameter + the maximum vertical diameter (mean ± SD) | DF | T value | P value | |||
|
| |||||||
| Before implantation | After implantation | ||||||
| Gender | Male | 37 | 7.07±2.11 | 3.53±2.05 | 36 | 15.618 | <0.001 |
| Female | 19 | 6.51±2.60 | 3.13±2.27 | 18 | 18.715 | <0.001 | |
| Smoking | Yes | 20 | 7.09±2.56 | 3.54±2.62 | 19 | 15.556 | <0.001 |
| No | 36 | 6.76±2.14 | 3.32±1.81 | 35 | 15.222 | <0.001 | |
| Histology | LAC | 34 | 6.80±2.20 | 3.15±1.91 | 33 | 16.080 | <0.001 |
| LSCC | 22 | 7.01±2.35 | 3.78±2.39 | 21 | 14.065 | <0.001 | |
| Pathologic grade | Poor | 20 | 7.01±2.57 | 3.53±2.53 | 19 | 15.014 | <0.001 |
| Moderate and Well | 36 | 6.81±2.14 | 3.32±1.88 | 35 | 15.154 | <0.001 | |
| Lymphatic metastasis | Yes | 40 | 6.86±2.08 | 3.39±1.98 | 39 | 16.995 | <0.001 |
| No | 16 | 6.91±2.81 | 3.41±2.48 | 15 | 12.368 | <0.001 | |
| TNM stage | III | 33 | 6.67±2.11 | 3.38±1.94 | 32 | 18.376 | <0.001 |
| IV | 23 | 7.18±2.53 | 3.43±2.38 | 22 | 12.216 | <0.001 | |
NSCLC, non-small cell lung cancer; SD, standard deviation; DF, degree of free; LAC, adenocarcinoma of the lung; LSCC, squamous cell carcinoma of the lung; TNM, Tumor-Node-Metastasis Staging system.
Figure 4.
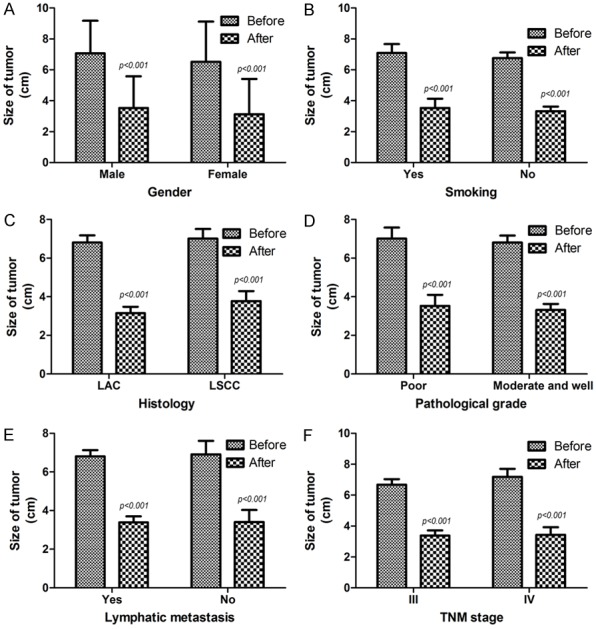
Correlation between different clinico-pathological features and the efficacy of 125I particles implantation therapy. (A) Whether in male patients (P<0.001) or in women patients (P<0.001); (B) whether in patients with smoking (P<0.001) or non-smokers (P<0.001); (C) whether in LAC patients (P<0.001) or LSCC patients (P<0.001); (D) whether in patients with poor differentiation cancer (P<0.001) or moderate and well differentiation patients (P<0.001); (E) whether in patients with lymphatic metastasis (P<0.001) or those without lymphatic metastasis (P<0.001) and (F) whether in patients at III stage (P<0.001) or IV stage (P<0.001), the 125I particles implantation therapy all showed an obvious inhibition efficacy on the local growth of NSCLC; Before, before the 125I particles implantation therapy; After, after the 125I particles implantation therapy; LAC, lung adenocarcinoma; LSCC, lung squamous cell carcinoma.
The degree of tumor reduction by 125I particle implantation therapy did not correlated with different clinico-pathological parameters of advanced NSCLC patients
We further calculated the degree of tumor reduction [(before treatment: the maximum diameter + the maximum vertical diameter) - (after treatment: the maximum diameter + the maximum vertical diameter)] in each patient after the 125I particle implantation therapy. The following clinico-pathological factors were assessed retrospectively: gender (male vs. female), smoking (yes vs. no), histological classification (LAC vs. LSCC), grade of differentiation (poor vs. moderate and well), lymph node metastasis (yes vs. no) and clinical staging (III vs. IV). As shown in Table 4, in terms of different clinico-pathological factors of NSCLC patients, the anti-tumor effect of 125I particle implantation therapy did not show a statistic difference (P>0.05), indicating that advanced NSCLC patients of different clinical features all could choose the 125I particle implantation as an treatment as long as the physical condition of patients permitted.
Table 4.
Relationship between efficacy of 125I particles implantation therapy and clinical-pathological features of NSCLC
| Items | Groups | Measurement of tumors by CT scan (cm) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | The degree of tumor reduction (mean ± SD) | DF | T value | P value | ||
| Gender | Male | 37 | 3.53±1.37 | 54 | 0.466 | 0.643 |
| Female | 19 | 3.37±0.93 | ||||
| Smoking | Yes | 20 | 3.55±1.02 | 54 | -0.303 | 0.763 |
| No | 36 | 3.44±1.35 | ||||
| Histology | LAC | 34 | 3.64±1.32 | 54 | 1.256 | 0.215 |
| LSCC | 22 | 3.22±1.07 | ||||
| Pathologic grade | Poor | 20 | 3.47±1.03 | 54 | -0.032 | 0.975 |
| Moderate and Well | 36 | 3.48±1.35 | ||||
| Lymphatic metastasis | Yes | 40 | 3.47±1.29 | 54 | 0.068 | 0.946 |
| No | 16 | 3.50±1.13 | ||||
| TNM stage | III | 33 | 3.29±1.03 | 54 | 1.361 | 0.179 |
| IV | 23 | 3.75±1.47 | ||||
NSCLC, non-small cell lung cancer; SD, standard deviation; DF, degree of free; LAC, adenocarcinoma of the lung; LSCC, squamous cell carcinoma of the lung; TNM, Tumor-Node-Metastasis Staging system.
The efficacy of 125I particle implantation therapy was negatively correlated with the serum level of carcinoembryonic antigen (CEA) in treating advanced LAC patients
As shown in Table 5, as to LAC patients, whether in patients with smoking (P=0.046) or non-smokers (P=0.001), in patients with poor differentiation cancer (P=0.020) or moderate and well differentiation patients (P=0.007), in patients with lymphatic metastasis (P=0.005) or those without lymphatic metastasis (P=0.041) and in patients at III stage (P=0.010) or IV stage (P=0.020), the 125I particles implantation therapy all down-regulated the expression level of CEA (Figure 5A-D). The results indicated that the efficacy of 125I particle implantation therapy was negatively correlated with the level of CEA in treating LAC patients.
Table 5.
Impact of 125I particles implantation therapy on serum levels of CEA and CYFRA 21-1 in treating NSCLC
| Items | Groups | Level of CEA for LAC | Level of CYFRA 21-1 for LSCC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| N | M ± SD (ng/ml) | T | P value | N | M ± SD (ng/ml) | T | P value | ||||
|
|
|
||||||||||
| Before of therapy | After of therapy | Before of therapy | After of therapy | ||||||||
| Smoking | Yes | 10 | 35.87±32.44 | 18.61±15.44 | 1.588 | 0.046 | 10 | 34.85±26.02 | 17.46±14.13 | 1.858 | 0.080 |
| No | 24 | 56.03±40.94 | 24.83±17.68 | 3.427 | 0.001 | 12 | 30.17±30.10 | 18.13±19.80 | 1.058 | 0.302 | |
| T | 1.383 | -0.967 | 0.484 | -0.090 | |||||||
| P value | 0.176 | 0.341 | 0.632 | 0.929 | |||||||
| Pathologic grade | Poor | 15 | 59.44±44.83 | 28.67±17.45 | 2.477 | 0.020 | 5 | 19.16±10.81 | 9.02±5.26 | 1.886 | 0.096 |
| Moderate and well | 19 | 42.73±33.68 | 18.54±15.79 | 2.835 | 0.007 | 17 | 37.08±32.19 | 20.42±18.54 | 1.849 | 0.074 | |
| T | 1.242 | 1.773 | -1.206 | -1.337 | |||||||
| P value | 0.223 | 0.086 | 0.242 | 0.196 | |||||||
| Lymphatic metastasis | Yes | 24 | 46.65±38.59 | 21.71±16.02 | 2.920 | 0.005 | 16 | 33.26±27.92 | 17.63±17.22 | 1.905 | 0.066 |
| No | 10 | 58.39±41.72 | 26.12±19.93 | 2.207 | 0.041 | 6 | 32.34±36.29 | 18.34±18.23 | 0.844 | 0.418 | |
| T | -0.790 | -0.681 | 0.064 | -0.085 | |||||||
| P value | 0.435 | 0.501 | 0.950 | 0.933 | |||||||
| TNM stage | III | 17 | 47.51±36.42 | 20.66±17.85 | 2.729 | 0.010 | 16 | 36.49±33.15 | 20.15±19.11 | 1.708 | 0.098 |
| IV | 17 | 52.69±42.89 | 25.35±16.46 | 2.453 | 0.020 | 6 | 23.71±14.74 | 11.63±7.93 | 1.767 | 0.108 | |
| T | -0.380 | -0.797 | 0.901 | 1.046 | |||||||
| P value | 0.707 | 0.431 | 0.378 | 0.308 | |||||||
CEA, carcinoembryonic antigen; CYFRA 21-1, cytokeratin 19 fragments; LAC, lung adenocarcinoma; LSCC, lung squamous cell carcinoma; TNM, Tumor-Node-Metastasis Staging system.
Figure 5.
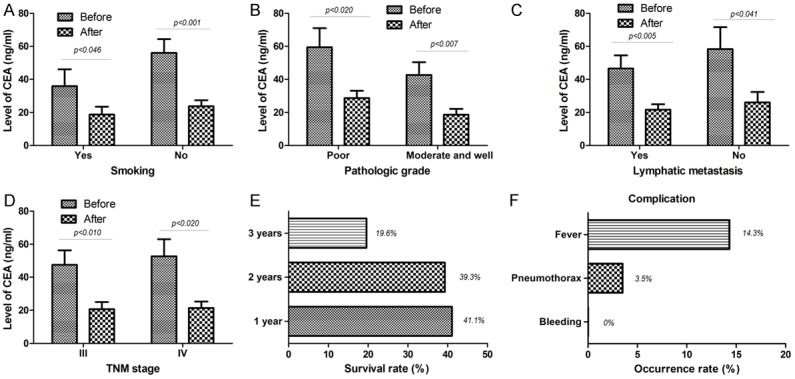
Impact of 125I particle implantation therapy on the expression of CEA in LAC, on the survival rate, complications and side effects of patients with advanced NSCLC. (A) As to LAC patients, whether in patients with smoking (P=0.046) or non-smokers (P=0.001); (B) whether in patients with poor differentiation cancer (P=0.020) or moderate and well differentiation patients (P=0.007); (C) whether in patients with poor differentiation cancer (P=0.020) or moderate and well differentiation patients (P=0.007); and (D) whether in patients at III stage (P=0.010) or IV stage (P-0.020), the 125I particles implantation therapy all down-regulated the expression level of CEA; (E) Of the 56 patients, One year survival rate was 41.1% the 2-year survival rate was 39.3%, the 3-year survival rate even reached 19.6%; (F) No significant hemorrhage occurred at the puncture site of the patient during operation; occurence rate of the pneumothorax and the fever was 3.5% and 14.3%; NSCLC, non-small cell lung cancer; LAC, lung adenocarcinoma; CEA, carcinoembryonic antigen; Before, before the 125I particles implantation therapy; After, after the 125I particles implantation therapy.
The level of cytokeratin 19 fragments (CYFRA 21-1) expression in advanced LSCC patients was not related to the efficacy of 125I particle implantation therapy
As shown in Table 5, when compared the level of CYFRA 21-1 expression on various clinical parameters between before implantation therapy and after, there was no a statistical significance (P>0.05). In addition, when we only compared the level of CYFRA 21-1 between various clinical parameters before the 125I particle implantation therapy or after, it still did not show the statistical difference (P>0.05). These results suggested that the efficacy of 125I particle implantation therapy for treating LSCC patients was not related to the level of CYFRA 21-1 expression.
Effect of 125I particle implantation therapy on advanced NSCLC patient survival
We further analyzed the impact of 125I particle implantation therapy on the survival of patients with advanced NSCLC. Valid survival data was obtained from all 56 patients. Of the 56 patients, 23 patients had a survival time of more than one year (one-year survival rate was 41.1%). Twenty-two of 56 patients survived for more than 2 years, and the 2-year survival rate was 39.3%. Finally, among the 56 patients, the 3-year survival rate even also reached 19.6% (11/56) (Figure 5E).
Side effects and complications of 125I particle implantation therapy
As shown in Figure 5F, no significant hemorrhage occurred at the puncture site of the patient during operation. However, pneumothorax was found in 2 cases (3.5%) and compression of lung tissue was 5%~20%, but there was no obvious symptom of shortness of breath in patients and the pneumothorax was obviously absorbed after conservative treatment (high concentrations of inhaled oxygen) in two weeks. In addition, eight patients (14.3%) had s symptom of fever, the degree of fever was mild to moderate. After symptomatic treatment, the fever disappeared in a few days. Especially, postoperative imaging follow-up did not find that particles migrated from implantation site.
Discussion
NSCLC is one of the most common malignancies and is the leading cause of cancer-related deaths in China [17]. Unfortunately, in China, the majority of NSCLC patients are diagnosed at an advanced stage of disease. At this point, most of the treatments can not be given due to the poor physique of the patient, such as surgical removal and chemotherapy. Stereotactic body radiotherapy (SBRT), which is characterized by high dose and low frequency of treatment, has become one of the treatments for NSCLC patients who can not be surgically treated in recent years [18]. In particular, in recent years, the 125I particle implantation therapy has gradually been applied clinically and has achieved certain therapeutic effects. We conducted a retrospective study in order to disclose the short-term efficacy, feasibility and safety of 125I particle implantation in the treatment of NSCLC. Our research data showed that the 125I particles implantation therapy had a better tumor local control rate, which responded an ORR of 55.4% and DCR of 98.2%. Because the patients included in our study all belonged to advanced stage of disease (III and IV). We believe CT-guided 125I particle implantation therapy appears to be ideally suited for patients who can not afford surgery and chemotherapy, suggesting that this method especially is suited to the patients with advanced disease. A new study showed that the intraoperative implantation of 125I particles during R2 resection of NSCLC may be a safer and more reliable method to reduce the local recurrence rate compared with conventional radiotherapy and the overall survival rate of patients in the 125I seed implantation group was higher compared with the postoperative radiotherapy group [19]. Patients with advanced NSCLC have limited options for treatment because the progress of the disease and physical deterioration, the emergence of 125I particle implantation therapy undoubtedly bring a new help to these patients.
In order to accurately reflect the effect of treatment, we measured the size of NSCLC lesions before the 125I particles implantation therapy and after six months of implantation. We found that, no matter the maximum diameter of the tumor or the maximum vertical diameter, they all displayed an obvious reduction. Especially, we analysed the correlation between the different clinico-pathological features and the efficacy of 125I particles implantation therapy and found that the 125I particles implantation therapy could inhibit the local growth of advanced NSCLC patients in any clinical state. We also further determined the correlation between the different clinicopathological features and the degree of tumor lesion reduction treated through treating by 125I particles implantation. The results suggested that NSCLC patients of different clinical features all benefited from 125I particles implantation therapy, indicating that any advanced NSCLC patient, as long as the physical condition is acceptable, all can choose it as an alternative treatment. Recent basic research points out CT-guided 125I particle implantation therapy markedly suppressed rabbit VX2 transplanted tumor cell proliferation, promoted tumor regression, induced tumor cell apoptosis, reduced Bcl-2 expression, and up-regulated Bax expression level, suggesting that CT-guided 125I particle implantation therapy can inhibit tumor proliferation and growth by regulating the expression of apoptosis-related genes and proteins [7]. Of the patients we treated, the largest lung lesion was 7.5 × 5.1 cm and the smallest is 1.8 × 1.5 cm. We did not find the size of the lesion has an effect on the efficacy of 125I particle implantation therapy. Due to the limited number of patients we included in this study, we can not give a positive answer to this phenomenon and further research is still needed in the future.
Carcinoembryonic antigen (CEA) is the earliest serum tumor marker for the diagnosis of LAC. It is a complex acid glycoprotein, which is produced by normal embryonic tissues and gradually disappears after birth. Only very low expression can be detected in the body [17]. Some studies have pointed out that CEA can be used as an indicator for treatment efficacy evaluation and prognosis in LAC patients [20,21]. In our study, we investigated whether the 125I particle implantation therapy affects the level of serum CEA expression in LAC patients and found that the efficacy of 125I particle implantation therapy was negatively correlated with the level of CEA in treating LAC patients, which directly demonstrated that the 125I particle implantation therapy down-regulated the CEA expression level of LAC patients. In other words, the better the efficacy of 125I particle implantation therapy, the lower the level of CEA, which means that CEA can be used as a predictor of treatment outcome for treating LAC patients by the 125I particle implantation therapy. However, in analyzing the relationship between the efficacy of 125I particle implantation therapy and CYFRA 21-1 in LSCC patients, we found that the efficacy of 125I particle implantation therapy was not related to the level of CYFRA 21-1 expression in LSCC patients. The CYFRA 21-1 has been reported to be expressed in histological types of NSCLC, especially in LSCC. Now the CYFRA 21-1 assay has been developed for the detection in serum and one study even shows that the CYFRA 21-1 level was higher in pleural effusion of LSCC patients than that in LAC patients [22]. Similarly, this conclusion can not be affirmed due to the limited sample size of this study and further large sample research is still needed in the future.
Analysis of survival data for patients undergoing the 125I particle implantation therapy showed that 1 year, 2 years, 3 years survival rates were 41.1%, 39.3% and 19.6% after the 125I particle implantation therapy. Survival does not seem to be as expected. We thought that the possible causes included: (1) most patients could not tolerate chemotherapy or were not suitable for targeted drug therapy due to their physical condition, so only received the 125I particle implantation therapy. Although the local lesion control was better, pulmonary metastasis or distant metastasis had taken place, thus cutting down the patient’s survival time; (2) because the patients included in our study all belonged to advanced stage of disease (III and IV), which also affected the survival of patients; (3) the number of patients in this study was less, the result would inevitably have a possibility of bias. Previous study reports that the combination of chemoradiotherapy with 125I particle implantation obtains a higher local control rate and better quality of life than when using chemoradiatherapy alone [23]. Moreover, CT-guided radioactive seed 125I implantation has been found to be effective and safety in treating metastatic lymph nodes, with minimal damage and few complications [24]. Radioactive seed implantation has its unique advantages, but also has many risks. Percutaneous implantation of radioactive particles may cause adverse reactions such as pneumothorax, hemothorax, hemoptysis and particle translocation. In our study, no significant hemorrhage occurred at the puncture site of the patient during operation. Although pneumothorax was found in 2 cases (3.5%) and eight patients (14.3%) had symptom of fever. After symptomatic treatment, the above abnormity gradually improved. In our study, the patients had no serious complications such as severe bleeding and cardiopulmonary failure, which further illustrated that the 125I particle implantation therapy is safe and effective. The limitation of this study is mainly that the number of patients included in the study was small, so the future clinical studies require a larger sample size and long-term follow-up to confirm our conclusions.
Conclusions
In summary, the 125I particle implantation therapy significantly inhibited the local growth of advanced NSCLC, suggesting a better disease control rate. Moreover, the therapy of 125I particle implantation down-regulated the CEA expression level of LAC patients, indicating that CEA can be used as an efficacy predictor of this therapy. After the 125I particle implantation therapy, the 1 year, 2 years, 3 years survival rates of patients were 41.1%, 39.3% and 19.6% respectively. Especially, this treatment did not show obvious complications and side effects.
Acknowledgements
This work was supported by Gansu provincial natural science foundation (1606RJZA154).
Disclosure of conflict of interest
None.
Abbreviations
- CEA
carcinoembryonic antigen
- CR
complete response
- CT
computed tomography
- CYFRA21-1
cytokerantin-19-fragment
- DCR
disease control rate
- LAC
lung adenocarcinoma
- LSCC
lung squamous cell carcinoma
- NSCLC
non-small cell lung cancer
- ORR
objective response rate
- OS
overall survival
- PD
progressive disease
- PR
partial response
- RECIST
response evaluation criteria in solid tumors
- SD
stable disease
- SBRT
stereotactic body radiotherapy
- TNM
tumor-node-metastasis
- TPS
treatment planning system
References
- 1.Biaoxue R, Xiling J, Shuanying Y, Wei Z, Xiguang C, Jinsui W, Min Z. Upregulation of Hsp90-beta and annexin A1 correlates with poor survival and lymphatic metastasis in lung cancer patients. J Exp Clin Cancer Res. 2012;31:70. doi: 10.1186/1756-9966-31-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Biaoxue R, Hua L, Tian F, Wenlong G. Increased stathmin in serum as a potential tumor marker for lung adenocarcinoma. Jpn J Clin Oncol. 2017:1–8. doi: 10.1093/jjco/hyx078. [DOI] [PubMed] [Google Scholar]
- 4.Rong B, Zhao C, Gao W, Yang S. Matrine promotes the efficacy and safety of platinum-based doublet chemotherapy for advanced non-small cell lung cancer. Int J Clin Exp Med. 2015;8:14701–14717. [PMC free article] [PubMed] [Google Scholar]
- 5.Rong B, Yang S, Li W, Zhang W, Ming Z. Systematic review and meta-analysis of Endostar (rh-endostatin) combined with chemotherapy versus chemotherapy alone for treating advanced non-small cell lung cancer. World J Surg Oncol. 2012;10:170. doi: 10.1186/1477-7819-10-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiang GL, Zhu XH, Lin CZ, Wang LJ, Sun Y, Cao YW, Wang FF. 125I seed irradiation induces apoptosis and inhibits angiogenesis by decreasing HIF-1alpha and VEGF expression in lung carcinoma xenografts. Oncol Rep. 2017;37:3075–3083. doi: 10.3892/or.2017.5521. [DOI] [PubMed] [Google Scholar]
- 7.Cheng J, Ma S, Yang G, Wang L, Hou W. The mechanism of computed tomography-guided 125I particle in treating lung cancer. Med Sci Monit. 2017;23:292–299. doi: 10.12659/MSM.898526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odagaki Y, Ohori M, Yoshimura M, Nakshima J, Ohno Y, Mikami R, Nakayama H, Tokuuye K, Tachibana M. Is scintigraphy necessary to detect migration of 125I seeds after brachytherapy for early prostate cancer? Int J Clin Oncol. 2016;21:397–401. doi: 10.1007/s10147-015-0901-9. [DOI] [PubMed] [Google Scholar]
- 9.Gesztesi L, Agoston P, Major T, Godeny M, Andi J, Lengyel Z, Polgar C. Salvage 125I brachytherapy of locally recurrent prostate cancer. Magy Onkol. 2014;58:219–224. [PubMed] [Google Scholar]
- 10.Beydoun N, Bucci J, Malouf D. Iodine-125 prostate seed brachytherapy in renal transplant recipients: an analysis of oncological outcomes and toxicity profile. J Contemp Brachytherapy. 2014;6:15–20. doi: 10.5114/jcb.2014.40769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parashar B, Arora S, Wernicke AG. Radiation therapy for early stage lung cancer. Semin Intervent Radiol. 2013;30:185–190. doi: 10.1055/s-0033-1342960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colonias A, Betler J, Trombetta M, Bigdeli G, Gayou O, Keenan R, Werts ED, Parda DS. Mature follow-up for high-risk stage I non-small-cell lung carcinoma treated with sublobar resection and intraoperative iodine-125 brachytherapy. Int J Radiat Oncol Biol Phys. 2011;79:105–109. doi: 10.1016/j.ijrobp.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 14.Detterbeck FC, Chansky K, Groome P, Bolejack V, Crowley J, Shemanski L, Kennedy C, Krasnik M, Peake M, Rami-Porta R. The IASLC lung cancer staging project: methodology and validation used in the development of proposals for revision of the stage classification of NSCLC in the forthcoming (eighth) edition of the TNM classification of lung cancer. J Thorac Oncol. 2016;11:1433–1446. doi: 10.1016/j.jtho.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Mazumdar M, Smith A, Schwartz LH. A statistical simulation study finds discordance between WHO criteria and RECIST guideline. J Clin Epidemiol. 2004;57:358–365. doi: 10.1016/j.jclinepi.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Kim JH, Kim BJ, Jang HJ, Kim HS. Comparison of the RECIST and EORTC PET criteria in the tumor response assessment: a pooled analysis and review. Cancer Chemother Pharmacol. 2017;80:729–735. doi: 10.1007/s00280-017-3411-9. [DOI] [PubMed] [Google Scholar]
- 17.Biaoxue R, Hua L, Tian F, Wenlong G. Increased stathmin in serum as a potential tumor marker for lung adenocarcinoma. Jpn J Clin Oncol. 2017;47:342–349. doi: 10.1093/jjco/hyx005. [DOI] [PubMed] [Google Scholar]
- 18.Alphonse-Sullivan N, Taksler GB, Lycan T, Weaver KE, McTyre ER, Shenker RF, Page BR, Isom S, Johnson A, Munley MT, Laxton AW, Tatter SB, Watabe K, Chan MD, Ruiz J. Sociodemographic predictors of patients with brain metastases treated with stereotactic radiosurgery. Oncotarget. 2017;8:101005–101011. doi: 10.18632/oncotarget.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Zheng Y, Li Y, Guan J, Jiang J, Yu Y, Zheng X, Yang L. Effectiveness of (125)I seed implantation in the treatment of non-small cell lung cancer during R2 resection. Oncol Lett. 2017;14:6690–6700. doi: 10.3892/ol.2017.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi N, Suzuki K, Takamochi K, Oh S. Prognosis of surgically resected lung cancer with extremely high preoperative serum carcinoembryonic antigen level. Gen Thorac Cardiovasc Surg. 2011;59:699–704. doi: 10.1007/s11748-011-0797-x. [DOI] [PubMed] [Google Scholar]
- 21.Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76:138–143. doi: 10.1016/j.lungcan.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Biaoxue R, Shuanying Y, Xiguang C, Wei Z, Wei L. Differential diagnostic CYFRA 21-1 level for benign and malignant pleural effusions: a meta-analysis in the Chinese population. Arch Med Sci. 2012;8:756–766. doi: 10.5114/aoms.2012.30831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu YP, Yu Q, Guo JM, Jiang HT, Di XY, Zhu Y. (125)I particle implantation combined with chemoradiotherapy to treat advanced pancreatic cancer. Br J Radiol. 2014;87:20130641. doi: 10.1259/bjr.20130641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang Y, Huang ZL, Wu PH, Zhang FJ, Zhao M, Huang JH, Fan WJ, Li CX, Gu YK, Zhang L, Gao F, Li W. Short-term efficacy of ct-guided radioactive seed 125I implantation on residual or relapsing metastatic lymph nodes in advanced tumor patients after multi-modality treatment. Ai Zheng. 2008;27:1082–1087. [PubMed] [Google Scholar]