Abstract
Graphene-based nanocomposites have attracted more and more attention recently in the field of biology and biomedicine. Graphene and its derivatives have been integrated with drugs, nucleic acids, antibodies, and other molecules. And these materials could be use as nanocomposite carriers or scaffold materials taking advantages of their enormous specific surface area, good elasticity and ductility, excellent biocompatibility, and outstanding mechanical strength. In addition, these composites have strong near-infrared absorbance and can act as photothermal agents to kill target cells through physical or chemical mechanisms. Along with significant advances in cell and organ transplantation, many of these materials have been explored in recent years for use in tissue engineering and regenerative medicine. Tissue engineering includes bone, nerve, heart, and muscle tissue engineering based on two-dimensional and three-dimensional graphene-based matrices or scaffolds possessing certain mechanical strengths and electrical conductivities, and the aim is to produce bioactive tissues to replace or repair natural tissue by promoting osteogenic, neuronal, and myogenic differentiation and myocardial cell growth. In this review, the basic properties of graphene-based complexes are systematically described and the biomedical applications of graphene-based materials in vivo and in vitro are summarized. This review first discusses the safety of graphene-based materials in terms of their biocompatibility and toxicity, and then it discusses these materials’ applications in biosensing, photothermal therapy, stem cell culture, and tissue engineering. This review therefore provides a comprehensive understanding of graphene and its derivatives and their present and future applications.
Keywords: Graphene, biocompatibility, toxicity, biomedical, tissue engineering
Introduction
Graphene is a 2D carbon nanomaterial composed of carbon atoms in an sp2 hybrid orbital hexagonal honeycomb crystal lattice [1]. The physicists A. Gem and K. Novoselov of the University of Manchester in the UK successfully separated graphene from graphite by micromechanical stripping, and this discovery led to their jointly winning the 2010 Nobel Prize in Physics. Traditional preparation methods of graphene and its derivatives can be generally classified as mechanical stripping [2], redox methods [3], orientation epiphysis [4], chemical vapor deposition [5], graphitization [6], solvothermal methods [7], organic synthesis [8], liquid exfoliation of graphite [9], thermal exfoliation and liquid intercalation [10], electrochemical exfoliation [11], chemical reduction of graphene oxide (GO) [12], thermal reduction of GO [13], and photothermal reduction of GO [14]. These graphene-based nanomaterials have been used in many applications such as surface-enhanced Raman scattering [15-22], electrochemistry [23-29], catalysis [30-34], fuel cells [35-38], and biomedicine [39-45].
Among the nanomaterials used for biomedical applications, graphene-based biomaterials have attracted much scientific and technological interest in recent years [46-49] and show great promise as antibacterial agents [50], biosensors [51,52], photothermal therapies (PTT) [53], bioimaging tools [54-56], and as stem cell and tissue engineering components [57,58]. Surface coatings with graphene and its derivatives involve in covalent bonds, Van der Waals forces, π-π reactions, or electrostatic interactions to improve the biocompatibility and to prolong the circulation time of these graphene-based biomaterials. Aptamers and specific antibodies can be ligated to the surface of graphene to achieve targeting of lesions, which can be modified with fluorescent molecules, quantum dots, and contrast agents to achieve multimodal imaging, combined with drugs and genes to achieve synergistic therapies, and also linked to enzymes, specific molecules, antibodies, etc., for applications in molecular detection, biomarker detection, disease diagnosis, PTT, tissue engineering, and cell or exosome capture and detection (Figure 1).
Figure 1.
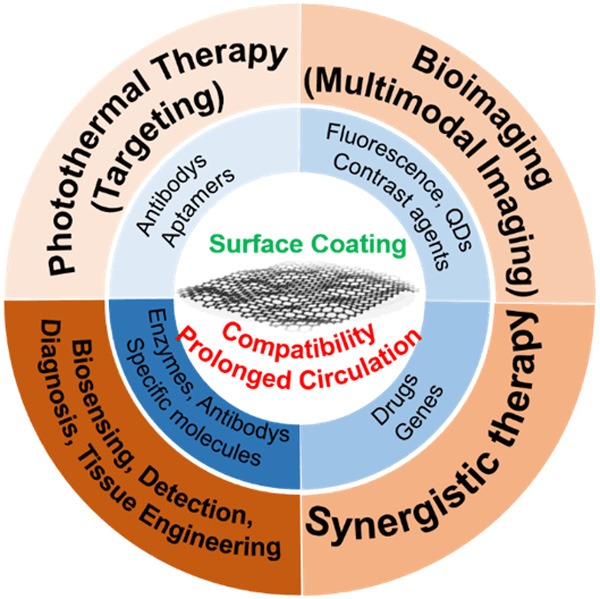
The primary applications of graphene-based nanomaterials in biomedicine.
Herein, we review the preclinical biocompatibility and cell toxicity of graphene-based nanomaterials and advanced applications in biosensing, PTT, and tissue engineering. Furthermore, we discuss the challenges and future prospects of graphene-based biomaterials.
Biocompatibility and toxicity
The biocompatibility and toxicity of a nanomaterial is the primary consideration for its biomedical applications. The properties of graphene and graphene-based nanomaterials, such as morphology, size, surface modifications, etc., can have diverse implications for cells, tissues, and even the body as a whole, so the potential toxicity must be well explored. Many recent studies have been conducted to investigate the potential toxicity of graphene-based materials in their interaction with cells and tissues in vitro and in vivo, and this is critical for identifying suitable candidates for clinical applications.
Zhou et al. [59] used large-scale all-atom molecular dynamics simulations to investigate the potential toxicity of graphene on cells at the molecular level and found that hydrophobic protein-protein interactions can be interrupted by graphene. Disruption of the cell’s metabolism or even the cell’s survival was proposed to be due to the separation of 2-deoxyribose-5-phosphate aldolase and phosphoglucose isomerase. Wang et al. [60] used A549 cells as the model to investigate the influence of GO on cell morphology, viability, mortality, and membrane integrity, and they found that the GO caused dose-dependent oxidative stress in A549 cells, leading to slightly decreased cell viability. Recently, Chu et al. [61] performed a systematic evaluation of graphene quantum dot toxicity in male mice and showed the low toxicity of graphene quantum dots in terms of sexual behaviors, reproduction, and offspring viability. Furthermore, graphene materials have been tested for their antibacterial activities, and this activity has been shown to be related to the material’s lattice size [62], number of layers [63], shape [64], surface modifications [65-67], agglomeration, and dispersion [68,69]. In addition, the interactions of the graphene materials with lipids, proteins, and DNA/RNA have been shown to lead to lipid extraction [70], protein disruption [59], and reactive oxygen species production [71], resulting in the death or inactivation of microorganisms [72-74]. These results above suggest that graphene could efficiently bring up the toxicity to bacteria and the in vitro toxicity to cells.
Although the toxicity of graphene can be applied in the field of antibacterial, this defect just hinders the development of graphene in the field of biomedicine. Appropriate biocompatibility modifications are necessary for graphene materials to be used in a wider range of applications.
The application of graphene-based materials
As emerging nanomaterials with simple, inexpensive, and good physical and chemical properties, graphene-based nanomaterials are widely used in many biomedical fields. The following mainly introduces the application of graphene-based nanomaterials in biosensing, PTT, and tissue engineering.
Biosensors
The high surface-to-volume ratio of graphene provides sufficient loading of the required ligand, and its excellent conductivity and small bandgap makes graphene useful for sensitive electrical and electrochemical sensing. In addition, its tunable optical properties make it suitable for using as a fluorescence and plasmonic sensor. In general terms, graphene-based sensors consist of a receptor, such as an antibody [75], single-stranded DNA [76], or enzyme [77] can combined with the target ions, molecules, nucleic acids, or even whole cells or microorganisms. The graphene-based nanomaterials also serve as the transducer to convert chemical information regarding the interactions between the receptor and the target molecules into a measurable signal.
Rapid and sensitive detection of glucose has always been a popular research, and many different methods for its detection have been reported over the past few decades. Graphene-based glucose biosensors generally comprise immobilized glucose oxidase and a graphene surface, such as the glucose oxidase-functionalized tilted fiber grating developed by Zhang et al. [77] to detect low concentrations of glucose. In that work, the amino groups of glucose oxidase were covalently connected to the graphene oxide via the assistance of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide and N-hydroxysulfosuccinimide, and the glucose was oxidized and the changes in the refractive index of the local microenvironment were converted into measurable information with a sensitivity of ~ 0.25 nanometers per millimolar. A non-enzymatic glucose sensor was also developed by Lai et al. [78] through the self-assembly of phenoxyl-dextran on electrochemically reduced GO (rGO) to compete with glucose for binding to the biosensor.
Protein biomarkers are the basis for the prediction, detection, diagnosis, etc., of many diseases and cancers. Although the detection of some biomarkers has been used clinically, there are still likely to be many unknown biomarkers for certain diseases that are only present at very low concentrations, and this poses a challenge for current biomarker detection. In addition, the need for non-invasive, fast, real-time, and accurate detection of biomarkers has also limited the development of the field. Owing to the good biocompatibility, low-cost, and high sensitivity, graphene-based nanomaterials have shown excellent performance in the selectivity of biomarker detection. A representative study was that of Nezhad et al. [79] developed a method to detect glial fibrillary acidic protein (GFAP), which is an intermediate filament protein expressed by certain cells in the central nervous system. In their study, a polyethylenimine-coated graphene electrode, covalently bound with GFAP antibody, was used for electrochemical biosensing of GFAP with a dynamic linear response in the range of 1 pg/ml to 100 ng/ml. Detection of living cells is another effective method for disease detection, and graphene-based nanomaterials are becoming more and more widely used in this field. Xing et al. [80] reported an ultra-sensitive and ultra-fast label-free method for detection of whole living cancer cells using a graphene-based optical biosensor. The corresponding sensitivity was increased, and the detection time was as short as 260 ns, and their device showed great promise for use in cell-based research. In addition, Sun et al. [81] fabricated a “turn-on” magnetic fluorescent biosensor based on graphene quantum dots, Fe3O4, and molybdenum disulfide nanosheets for rapid, efficient, and sensitive separation and detection of circulating tumor cells with a linear range of 2 to 64 nM and a limit of detection of 1.19 nM for Epithelial cell adhesion molecule detection.
Photothermal therapy
In recent years, graphene-based nanomaterials have been used in PTT due to their special physical and chemical properties. Graphene, carbon nanotubes, and their derivatives have strong near-infrared absorbance for acting as photothermal agents and have large surface areas that can be functionalized with anti-cancer drugs, biocompatibility-enhancing molecules, and specific cell-targeting biomolecules for use as anti-cancer nanodrugs. Liu et al. first reported the use of GO combined with PTT to suppress tumors [82]. When PEGylated GO absorbs near-infrared light, it releases energy, thus causing excessive heat in the cells and inducing apoptosis. Meanwhile, covalent binding of Cy7 can trace the metabolism of GO.
To further improve the efficacy of cancer treatment, PTT is being combined with other strategies. For example, a chemo-photothermal method has been reported based on PEGylated GO loaded with doxycycline (DOX) [83-85]. Wang et al. combined Fe2O3@Au with reduced-GO (rGO) to increase PTT, and the material was loaded with DOX as chemotherapy [84]. In that work, DOX was transported under the guide of a magnetic field, and synergistic results were seen with the combination of chemotherapy and PTT. The mixing of anticancer drugs loaded onto GO has also been reported [85], wherein sulfonic acid groups were loaded to enhance the stability, folic acid molecules were bound to increase the specificity, and camptothecin and DOX were used as anticancer drugs. Their work was the first to identify the ability of GO to act as a carrier for controlled loading and release of multiple drugs.
Photosensitizers have also been loaded onto GO to combine photodynamic therapy and PTT methods [86-89]. Folic acid-conjugated GO loaded with Ce6 was reported by Cui and colleagues [86]. In their study, the Ce6 was specifically delivered to targeted cells, and the controlled photohyperthermia and singlet oxygen generation worked synergistically. In addition, methylene blue [88], hypocrellin B [89], and other photosensitizers have also been used to kill cancer cells.
Due to limited laser irradiation depth, it is difficult to perform PTT and photodynamic therapy (PDT) in solid tumors in deep tissues. To overcome this obstacle, ultrasound-activated PDT was developed. By using ultrasound instead of a laser, the depth of the treatment has been greatly increased and the invasiveness of the method has been reduced [90]. Chen et al. [91] proposed a mesoporous silica (MSN) grown on reduced graphene oxide nanosheet (nrGO)-PEG-conjugated iron-oxide nanocarrier (ION) for sonodynamic therapy and ultrasound hyperthermia. This nanocarrier is an rGO, mesoporous silicon, and superparamagnetic composite structure with high photosensitivity, high biocompatibility, and magnetic targeting. PEGylated nrGO/MSN-ION loaded with Rose Bengal (sonosensitizer, maximum concentration of 0.5 µM) can generate singlet oxygen under burst-mode ultrasound irradiation. In addition to the uses mentioned above, graphene-based nanomaterials have also been combined with gene therapy [92,93], specific target molecules [94], and modifications intended to prolong circulation in the blood [95,96].
Tissue engineering
Graphene-based materials have also led to new approaches to regenerative medicine and tissue engineering [97,98]. Loss of tissue or organs caused by injury or disease is a serious health problem that negatively affects the patient’s life. Traditional surgical treatments use the patient’s own tissues to repair or replace damaged tissues or organs or use allografts of certain organs. However, these traditional methods face limitations, in particular the lack of suitable organs and tissue donors, and thus tissue engineering has been seen as a promising way forward [99]. Tissue engineering utilizes different scaffold materials to control cell proliferation, differentiation, etc., and to stimulate the extracellular matrix of the target tissue to provide a suitable microenvironment for cell adhesion, migration, proliferation, and differentiation so as to produce biologically functional tissues [100]. Tissue engineering technology has great prospects for clinical applications because it avoids immune rejection and reduces the need for suitable autologous and allogeneic materials. Tissue engineering can be applied to the skin, bone, muscle, cardiovascular system, digestive tract, liver, kidney, reproductive system, nervous system, etc., but here we mainly discuss the development of tissue engineering techniques for bone, nervous, muscle, and cardiac tissues.
Bone tissue engineering
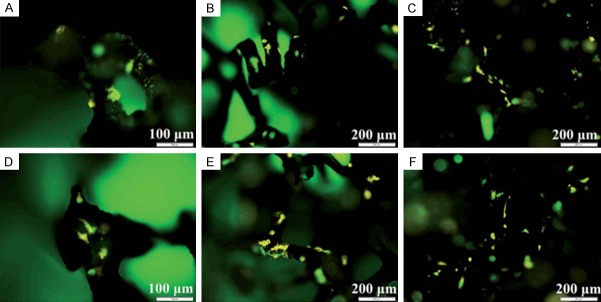
Bone tissue engineering, which aims at regenerating bone, is a promising solution for faster healing and for reconstruction of large bone defects [101], and graphene and its derivatives have a wide range of applications in bone tissue engineering. Madhu Dhar et al. [102]. evaluated the effect of graphene on in vitro growth and differentiation of goat adult mesenchymal stem cells compared with growth on polystyrene-coated tissue culture plates. In that study, they found that the materials were cytocompatible and facilitated cell adhesion and proliferation and enabled osteogenic differentiation in fetal bovine serum-containing medium without the addition of any glucocorticoids or specific growth factors. In the work of Zhai et al., capsaicin was modified on rGO with the purpose of increasing its stability and avoiding aggregation effects [103]. This graphene-based material promoted the differentiation and proliferation of osteoblasts, indicating that it has important applications in bone tissue engineering and for treating diseases such as osteoporosis. Fu et al. prepared a nanofiber matrix of poly (L-lactic-co-glycolic acid)/hydroxyapatite/graphene oxide by electrospinning and used this to promote MC3T3-E1 cell adhesion and proliferation. The role of the poly (L-lactic-co-glycolic acid) component was to provide a uniform and smooth nanofibrous substrate with a three-dimensional porous structure possessing excellent biocompatibility and biodegradation properties. GO was also used to enhance the tensile strength of the constructed poly (L-lactic-co-glycolic acid)/hydroxyapatite/graphene oxide nanofibrous matrices, and hydroxyapatite was employed to improve the biocompatibility and conductivity of the substrate. It is worth noting that the synergistic effects of the two components were demonstrated by the material’s ability to promote protein adsorption and to induce osteogenic function [104]. The material was also used to accelerate and induce proliferation and differentiation of human mesenchymal stem cells [105]. Jie et al. reported that the three-dimensional biomaterial of rGO decorated with casein phosphopeptide/polypyrrole matrix synthesized by electrospinning had remarkable hydrophilicity and water absorption capacity [106]. The biofunctionalized casein phosphopeptide that self-assembled on the surface of the scaffold could promote the formation of hydroxyapatite in a cost-effective manner, and the performance of the composite scaffold was superior to that of the conventional rGO/polypyrrole matrix (Figure 2).
Figure 2.
Fluorescence images of MC3T3-E1 cells on 3D rGO/PPY (A, D), 3D rGO/PPY/CPP10 (B, E), and 3D rGO/PPY/CPP20 (C, F) at Day 2 (A-C), and Day 4 (D-F) of culture, respectively.
A recently developed method of preparing a free-standing rGO matrix takes advantage of chemical reduction, and the GO solution is filtered through nanofiber membranes under vacuum. The proteins decorating the synthesized rGO film produce a rough surface, and this roughness can be controlled by changing the parameters of the electrospinning. In addition, the synergistic effects of surface-functionalized protein and rGO have been shown to improve the biocompatibility, roughness, and specific surface area of the basement membrane with the aim of promoting cell adsorption and the proliferation of human bone marrow mesenchymal stem cells [107].
Nervous tissue engineering
Central nervous system damage, including traumatic brain injury and spinal cord injury and neurological diseases such as Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease, is difficult to repair due to the limited regenerative capacity of nerve tissue. Neural stem cells (NSCs) have the ability to differentiate into neurons, and they have high neuronal protein expression, extended axon projection and arrangement after differentiation, and secretion of various neurotrophic factors, indicating that NSCs therapy is a promising approach to repairing nervous system damage. Graphene has also been widely used in nervous tissue engineering due to its superior performance (Table 1).
Table 1.
Summary of the main results reported based on graphene and its derivatives for nervous tissue engineering
| Material | Coating | Cells | Results | Ref. |
|---|---|---|---|---|
| Graphene film | Poly-L-lysine | Mouse hippocampal neurons | Promoted neurite sprouting and outgrowth and the expression of growth-associated protein-43 | [111] |
| Graphene rod | Gelatin | MSCs | Profound effect on the differentiation of MSCs to Schwann cell-like phenotypes and on their paracrine activity | [112] |
| 3D hybrid graphene | None | NSCs | Facilitated the differentiated NSCs to bridge the spacing between skeletons and promoted the formation of neural networks | [113] |
| Graphene oxide | Polyvinylidene fluoride | PC12 cells | Promoted cell proliferation and could easily be converted into a nerve guidance conduit | [114] |
| Graphene oxide | None | NSCs | Selective differentiation of NSCs into mature oligodendrocytes | [117] |
| Reduced graphene oxide | None | Schwann cells, PC12 cells | Enhanced Schwann cell migration, proliferation, and myelination and induced the differentiation of PC12 cells | [115] |
| Reduced graphene oxide | None | SH-SY5Y cells | Oriented the growth of SH-SY5Y cells and enhanced SH-SY5Y cell neuronal differentiation and the formation of neural networks | [116] |
| Reduced graphene oxide | Collagen | MSCs | Supported MSC attachment, maintained cells in a more active proliferation and neural differentiation state, and promoted neurite sprouting and outgrowth | [118] |
| Reduced graphene oxide | Polycaprolac-tone | PC12 cells | Supported neural differentiation of PC12 cells | [119] |
| Graphene foam | None | NSCs | Supported NSC growth, maintained cells in an active proliferation state, and enhanced the NSC differentiation towards astrocytes and especially neurons | [120] |
MSC = mesenchymal stem cell, NSC = neural stem cell.
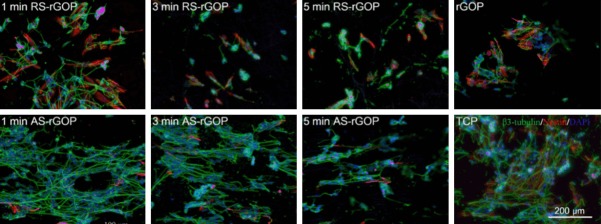
At present, the most important difficulty in the regeneration of nervous system damage is how to effectively differentiate NSCs into uniaxially arranged neurons. Recently, a novel composite scaffold structure including aligned electrospun silk nanofibers and conductive reduced graphene paper was used to enhance directional SH-SY5Y cell growth and neuron differentiation [116]. This neatly arranged directional matrix of nanofibers supported the cultivation of cells and stimulated the growth of neurites. The rGO paper contributed of good biocompatibility and excellent conductivity in the complex, and it increased neurological activity under electrical stimulation. The formation of extended axons and the expression of β3-tubulin and Nsetin as the neuronal markers were observed by immunolabeling and confocal imaging, indicating that the conductivity and aligned silk nanofiber-reduced graphene oxide paper (ASrGOP) could effectively induce SH-SY5Y cells to differentiate into neurons and then guide the arrangement of the resulting neurons to form a functional neural network (Figure 3). Another graphene-based hybrid nanofibrous scaffold has been proposed to guide NSCs differentiation into oligodendrocytes [117]. In that work, GO was coated on nanofibers, and by varying the amount of GO coating changes in the expression and concentration of key neuronal markers were observed, where a high concentration of GO coating promoted the differentiation of NSCs into mature oligodendrocytes. Furthermore, Gao et al. investigated composites of aligned and aminated poly-L-lactide coated with GO without destroying the alignment [121]. Scanning electronic microscopy was used to characterize the topological structure, and X-ray photoelectron spectroscopy and water contact angle showed the modification and GO coating. The material promoted the differentiation and proliferation of rat pheochromocytoma 12 (PC12) cells.
Figure 3.
Immunostaining images of SH-SY5Y cells growing on the arranged directional matrix of nanofibers with staining for DAPI (blue), β3-tubulin (green), and Nestin (red). The upper row of images shows the differentiated cells that were supported on randomly oriented silk nanofiber-reduced graphene oxide paper (RS-rGOP) after electrospinning for 1, 3, and 5 min, with rGOP as the control. The lower row of images shows the differentiated cells growing on AS-rGOP after different electrospinning for 1, 3, and 5 min, with tissue culture plate as the control. The neuron-specific marker β3-tubulin was expressed to the greatest extent on AS-rGOP with an electrospinning time of 1 min.
Muscle tissue engineering
Muscles tissue engineering has great clinical value for the regeneration and reconstruction of muscle tissue in the treatment of muscle damage and muscle atrophy and for the repair of smooth muscles in the heart, intestines, and urinary system, and graphene-based nanomaterials have been widely used in this field. For example, Park et al. investigated myotube formation on graphene-based nanomaterials [122]. In their work, GO was covalently linked to an aminated glass surface via a carboxyl group to act as the substrate for the culture of mouse C2C12 myoblasts. They studied the adhesion, proliferation, and differentiation on bare and laminin-coated graphene foam (GF) by measuring the expression of myogenic proteins and differentiation-specific genes and the formation of multinucleated myotubes and demonstrated GO’s ability to stimulate myogenic differentiation. Estrada et al. used laminin-coated GF as a biocompatible platform to study the cellular activity of C2C12 myoblasts [123]. The expression of myosin heavy chain protein was used as a marker for demonstrating that the laminin-coated GF could induce C2C12 myoblasts cells to differentiate into myotubes (Figure 4).
Figure 4.
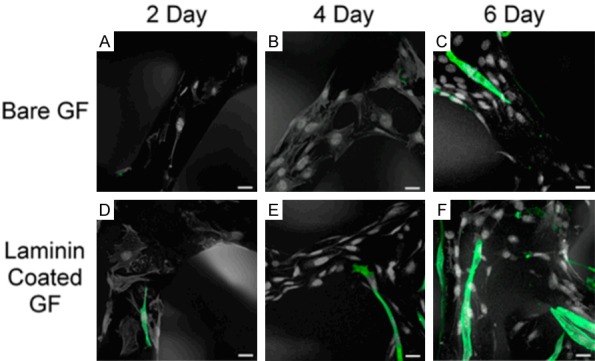
Immunofluorescence of MHCs indicative of C2C12 differentiation. Differentiation of C2C12 grown on bare GF (A-C) for 2, 4, and 6 days and on laminin-coated GF (D-F) for 2, 4, and 6 days. The scale bars are all 20 μm.
Cardiac tissue engineering
Cardiac tissue damage is associated with high mortality because damage to heart tissue is irreversible due to the inability of the organ to carry out tissue regeneration and to repair itself [124]. Clinical treatment is limited to heart transplants, but this has the drawbacks of a lack of donors, immune rejection, etc. The development of cardiac tissue engineering technology brings the promise of expanded therapeutic options.
The development of traditional biomaterials is limited by low conductivity, but new graphene-based nanomaterials meet the need for both biocompatibility and conductivity. Sonkusale et al. proposed a three-dimensional GF deposited with a 10 nm layer of titanium and a 50 nm layer of gold to create a scaffold for recording the electrical activity of cardiac cells [125]. The results demonstrated that the scaffold had good biocompatibility and excellent conductivity. Another electronically conductive hybrid hydrogel was engineered based on gelatin methacryloyl (GelMA) hybrid hydrogels composed of homogeneously dispersed rGO nanoparticles [126]. The efficiency of UV-induced crosslinking of GelMA was decreased with the GO incorporation, thus the GO was reduced with ascorbic acid to improve the electrical conductivity and biocompatibility, and the GelMA acted as the biocompatible substrate to provide active chemical groups. This composition provided a natural cell microenvironment for cardiomyocyte culture and maintained proper cell attachment. Overall, these results suggest that GO is a promising material in cardiac tissue engineering applications (Figure 5).
Figure 5.
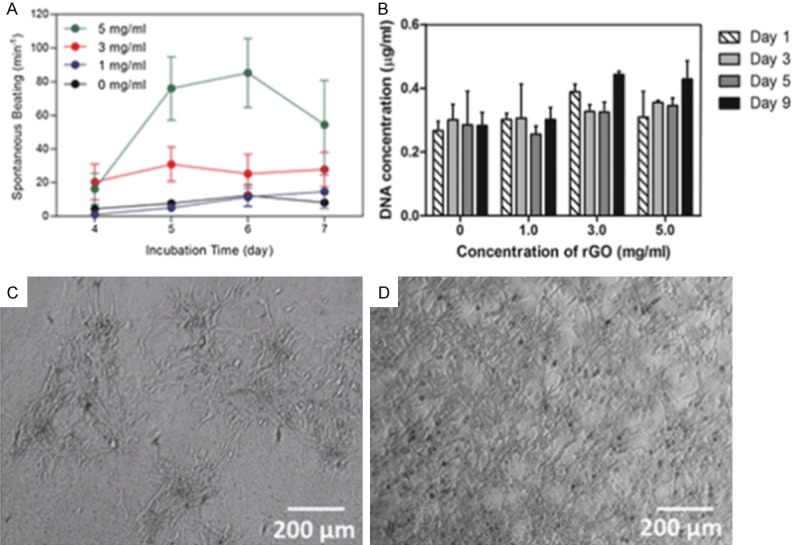
Characterization of the electrophysiological properties and compatibility of the graphene-based material. (A) Spontaneous beating rates of cardiomyocytes seeded on GelMA incorporated with different amounts of rGO (0, 1, 3, 5 mg/ml) plotted against incubation time. (B) Stability of cells (in terms of DNA content) cultured on GelMA alone and on rGO-GelMA hydrogels. Phase contrast images of cells cultured on (C) GelMA and (D) rGO-GelMA hydrogels at 6 days after seeding.
Conclusion and future perspectives
Graphene-based nanomaterials have been widely used in the biomedical field because of their various unique properties. The large specific surface area of graphene and its derivatives supports the absorption and binding of antibodies, adapters, drugs, genes, enzymes, and other molecules. Among these processes, aptamers and specific antibodies have been employed to target binding to the lesion site, and modifications involving fluorescent molecules, quantum dots, and contrast agents have been used for achieving multimodal imaging. In addition, the combinations of drugs and genes that are possible with graphene nanomaterials have been shown to have synergistic therapeutic effects. High priority should be given to linkages of enzymes, specific molecules, and antibodies for applications involving biomarker detection, disease diagnosis, PTT, tissue engineering, and cell or exosome capture and detection. At the same time, a large number of active groups on the surface of graphene-based materials can be functionalized by physical or chemical reactions, and this can improve the specific properties of the material to promote the material’s biocompatibility, thus reducing the biological toxicity and achieving targeted functions. The graphene substrates can be modified with functional groups or molecules through covalent bonds, Van der Waals forces, π-π reactions, or electrostatic interactions with the purpose of improving the material’s biocompatibility and prolonging its time in circulation. In addition, graphene has unique optical properties and can be used in PTT. Given this premise, these composites with strong near-infrared absorbance capabilities can act as photothermal agents, and these materials can rapidly release their cargo at specific targets when exposed to near-infrared irradiation, and this enables them to kill target cells by increasing cell permeability.
What needs to be noted in particular is that the excellent mechanical strength, biocompatibility, and electrical conductivity of graphene and its derivatives need to be taken into consideration when constructing matrices or scaffold materials with different morphologies and surface roughness. The interaction between cells and two-dimensional and three-dimensional graphene-based matrixes or scaffolds can stimulate stem cells to proliferate and differentiate into specific lineages, indicating their applications in biosensing, PTT, and tissue engineering. With regard to these applications, developments in tissue engineering have attracted more and more attention and have benefited from recent advances in materials science and bioengineering. In this review we have systematically elucidated the progress in bone, nerve, muscle, and cardiac tissue engineering with the goal of producing bioactive tissues to replace or repair damaged or degraded tissue function by promoting osteogenic differentiation, neuronal differentiation, myogenic differentiation, and myocardial cell growth.
Although most of the reported studies are still in the primary stage, great progress has been made. Many efforts have been made to develop a better understanding of the basic mechanisms and signaling pathways involved in the interaction between graphene-based materials and cells, and the material’s biotoxicity needs to be further studied in pre-clinical applications. These efforts to overcome problems and the advances that have been made have pushed the development of these graphene-based materials for clinical application.
With an in-depth understanding of graphene and its derivatives, more and more studies have been devoted to reducing the biological toxicity of these materials. Unfortunately, the findings of material toxicology research have not yet provided an authoritative conclusion because the different production processes that have been used have led to significant differences in the size, active groups, thickness, stability, and other parameters of the prepared graphene-based materials, and this has affected the interaction between the materials and the cultured cells. Moreover, different experimental methods and performance evaluations of substrates and scaffolds have aroused heated discussions in academic circles. It is exciting to note that current research in the field is focused on improving the properties of graphene-based materials through the formation of composite materials or functional modifications in order to overcome current limitations of the materials and to open the way for further developments in clinical research. All in all, continued efforts in these areas are in keeping with the goal of solving all of the current problems with graphene-based materials, and thus it is not difficult for us to predict that graphene and its derivatives will pave the way to significant breakthroughs in future clinical and biomedical research.
Acknowledgements
This work was supported by grants from the Strategic Priority Research Program of the Chinese Academy of Science (XDA16010303), the National Key R&D Program of China (No. 2017YFA0103903), the National Natural Science Foundation of China (No. 81622013), Boehringer Ingelheim Pharma GmbH, the Yingdong Huo Education Foundation, the Fundamental Research Funds for the Central Universities, and Open Research Fund of State Key Laboratory of Genetic Engineering, Fudan University (No. SKLGE1809), Shuangba He was supported by Jiangsu Provincial Natural Science Foundation (No. BK20161116) and Nanjing Medical Science and Technique Development Foundation (No. QRX17033).
Disclosure of conflict of interest
None.
References
- 1.Geim AK. Graphene: status and prospects. Science. 2009;324:1530–1534. doi: 10.1126/science.1158877. [DOI] [PubMed] [Google Scholar]
- 2.Nair RR, Blake P, Grigorenko AN, Novoselov KS, Booth TJ, Stauber T, Peres NM, Geim AK. Fine structure constant defines visual transparency of graphene. Science. 2008;320:104–106. doi: 10.1126/science.1156965. [DOI] [PubMed] [Google Scholar]
- 3.Tromp RM, Hannon JB. Thermodynamics and kinetics of graphene growth on SiC. PRL. 2009;102:106104. doi: 10.1103/PhysRevLett.102.106104. [DOI] [PubMed] [Google Scholar]
- 4.Sutter P. Epitaxial graphene: how silicon leaves the scene. Nat Mater. 2009;8:171. doi: 10.1038/nmat2392. [DOI] [PubMed] [Google Scholar]
- 5.Kim KS, Zhao Y, Jang H, Lee SY, Kim JM, Kim KS, Ahn JH, Kim P, Choi JY, Hong BH. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature. 2009;457:706–710. doi: 10.1038/nature07719. [DOI] [PubMed] [Google Scholar]
- 6.Emtsev KV, Bostwick A, Horn K, Jobst J, Kellogg GL, Ley L, McChesney JL, Ohta T, Reshanov SA, Rohrl J, Rotenberg E, Schmid AK, Waldmann D, Weber HB, Seyller T. Towards wafer-size graphene layers by atmospheric pressure graphitization of silicon carbide. Nat Mater. 2009;8:203–207. doi: 10.1038/nmat2382. [DOI] [PubMed] [Google Scholar]
- 7.Choucair M, Thordarson P, Stride JA. Gram-scale production of graphene based on solvothermal synthesis and sonication. Nat Nanotechnol. 2009;4:30–33. doi: 10.1038/nnano.2008.365. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Zhi L, Tsao N, Tomovic Z, Li J, Mullen K. Transparent carbon films as electrodes in organic solar cells. Angew Chem Int Ed. 2008;47:2990–2992. doi: 10.1002/anie.200704909. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez Y, Nicolosi V, Lotya M, Blighe FM, Sun ZY, De S, McGovern IT, Holland B, Byrne M, Gun’ko YK, Boland JJ, Niraj P, Duesberg G, Krishnamurthy S, Goodhue R, Hutchison J, Scardaci V, Ferrari AC, Coleman JN. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat Nanotechnol. 2008;3:563–568. doi: 10.1038/nnano.2008.215. [DOI] [PubMed] [Google Scholar]
- 10.Li XL, Zhang GY, Bai XD, Sun XM, Wang XR, Wang E, Dai HJ. Highly conducting graphene sheets and Langmuir-Blodgett films. Nat Nanotechnol. 2008;3:538–542. doi: 10.1038/nnano.2008.210. [DOI] [PubMed] [Google Scholar]
- 11.Liu N, Luo F, Wu H, Liu Y, Zhang C, Chen J. One-step ionic-liquid-assisted electrochemical synthesis of ionic-liquid-functionalized graphene sheets directly from graphite. Adv Funct Mater. 2008;18:1518–1525. [Google Scholar]
- 12.Fan X, Peng W, Li Y, Li X, Wang S, Zhang G, Zhang F. Deoxygenation of exfoliated graphite oxide under alkaline conditions: a green route to graphene preparation. Adv Mater. 2008;20:4490–4493. [Google Scholar]
- 13.Zangmeister CD. Preparation and evaluation of graphite oxide reduced at 220 degrees C. Chem Mater. 2010;22:5625–5629. [Google Scholar]
- 14.Matsumoto Y, Koinuma M, Kim SY, Watanabe Y, Taniguchi T, Hatakeyama K, Tateishi H, Ida S. Simple photoreduction of graphene oxide nanosheet under mild conditions. ACS Appl Mater Interfaces. 2010;2:3461–3466. doi: 10.1021/am100900q. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Zhang L, Chen B, Ji N, Chen F, Zhang Y, Zhang Z. Nanocomposites of size-controlled gold nanoparticles and graphene oxide: Formation and applications in SERS and catalysis. Nanoscale. 2010;2:2733–2738. doi: 10.1039/c0nr00473a. [DOI] [PubMed] [Google Scholar]
- 16.Yu X, Cai H, Zhang W, Li X, Pan N, Luo Y, Wang X, Hou JG. Tuning chemical enhancement of SERS by controlling the chemical reduction of graphene oxide nanosheets. ACS Nano. 2011;5:952–958. doi: 10.1021/nn102291j. [DOI] [PubMed] [Google Scholar]
- 17.Ren W, Fang Y, Wang E. A binary functional substrate for enrichment and ultrasensitive SERS spectroscopic detection of folic acid using graphene oxide/ag nanoparticle hybrids. ACS Nano. 2011;5:6425–33. doi: 10.1021/nn201606r. [DOI] [PubMed] [Google Scholar]
- 18.Xu W, Ling X, Xiao J, Dresselhaus MS, Kong J, Xu H, Liu Z, Zhang J. Surface enhanced Raman spectroscopy on a flat graphene surface. PNAS. 2012;109:9281–9286. doi: 10.1073/pnas.1205478109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Liu S, Wang L, Qin X, Tian J, Lu W, Chang G, Sun X. One-pot green synthesis of Ag nanoparticles-graphene nanocomposites and their applications in SERS, H2O2, and glucose sensing. RSC Advances. 2012;2:538–545. [Google Scholar]
- 20.Xu W, Mao N, Zhang J. Graphene: a platform for surface-enhanced raman spectroscopy. Small. 2013;9:1206–1224. doi: 10.1002/smll.201203097. [DOI] [PubMed] [Google Scholar]
- 21.Lee J, Novoselov KS, Shin HS. Interaction between metal and graphene: dependence on the layer number of graphene. ACS Nano. 2011;5:608–612. doi: 10.1021/nn103004c. [DOI] [PubMed] [Google Scholar]
- 22.Dutta S, Ray C, Sarkar S, Pradhan M, Negishi Y, Pal T. Silver nanoparticle decorated reduced graphene oxide (rGO) nanosheet: a platform for SERS based low-level detection of uranyl ion. ACS Appl Mater Interfaces. 2013;5:8724–8732. doi: 10.1021/am4025017. [DOI] [PubMed] [Google Scholar]
- 23.Shan C, Yang H, Song J, Han D, Ivaska A, Niu L. Direct electrochemistry of glucose oxidase and biosensing for glucose based on graphene. Anal Chem. 2009;81:2378–2382. doi: 10.1021/ac802193c. [DOI] [PubMed] [Google Scholar]
- 24.Kang X, Wang J, Wu H, Aksay IA, Liu J, Lin Y. Glucose Oxidase-graphene-chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens Bioelectron. 2009;25:901–905. doi: 10.1016/j.bios.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Pumera M. Graphene-based nanomaterials and their electrochemistry. Chem Soc Rev. 2010;39:4146–4157. doi: 10.1039/c002690p. [DOI] [PubMed] [Google Scholar]
- 26.Chen D, Tang L, Li J. Graphene-based materials in electrochemistry. Chem Soc Rev. 2010;39:3157–3180. doi: 10.1039/b923596e. [DOI] [PubMed] [Google Scholar]
- 27.Pumera M. Electrochemistry of graphene, graphene oxide and other graphenoids: review. Electrochem Commun. 2013;36:14–18. [Google Scholar]
- 28.Brownson DA, Kampouris DK, Banks CE. Graphene electrochemistry: fundamental concepts through to prominent applications. Chem Soc Rev. 2012;41:6944–6976. doi: 10.1039/c2cs35105f. [DOI] [PubMed] [Google Scholar]
- 29.Ambrosi A, Chua CK, Bonanni A, Pumera M. Electrochemistry of graphene and related materials. Chem Rev. 2014;114:7150–7188. doi: 10.1021/cr500023c. [DOI] [PubMed] [Google Scholar]
- 30.Zheng Y, Jiao Y, Ge L, Jaroniec M, Qiao SZ. Two-step boron and nitrogen doping in graphene for enhanced synergistic catalysis. Angew Chem Int Ed. 2013;125:3192–3198. doi: 10.1002/anie.201209548. [DOI] [PubMed] [Google Scholar]
- 31.Deng J, Ren P, Deng D, Bao X. Enhanced electron penetration through an ultrathin graphene layer for highly efficient catalysis of the hydrogen evolution reaction. Angew Chem Int Ed. 2015;54:2100–2104. doi: 10.1002/anie.201409524. [DOI] [PubMed] [Google Scholar]
- 32.Deng ZH, Li L, Ding W, Xiong K, Wei ZD. Synthesized ultrathin MoS2 nanosheets perpendicular to graphene for catalysis of hydrogen evolution reaction. Chem Commun. 2015;51:1893–1896. doi: 10.1039/c4cc08491h. [DOI] [PubMed] [Google Scholar]
- 33.Machado BF, Serp P. Graphene-based materials for catalysis. Catal Sci Technol. 2012;2:54–75. [Google Scholar]
- 34.Kong X, Chen C, Chen Q. Doped graphene for metal-free catalysis. Chem Soc Rev. 2014;43:2841–2857. doi: 10.1039/c3cs60401b. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Xia Z. Mechanisms of oxygen reduction reaction on nitrogen-doped graphene for fuel cells. J Phys Chem C. 2011;115:11170–11176. [Google Scholar]
- 36.Qu L, Liu Y, Baek JB, Dai L. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano. 2010;4:1321–1326. doi: 10.1021/nn901850u. [DOI] [PubMed] [Google Scholar]
- 37.Yong Y, Dong X, Chan-Park MB, Song H, Chen P. Macroporous and monolithic anode based on polyaniline hybridized three-dimensional graphene for high-performance microbial fuel cells. ACS Nano. 2012;6:2394–2400. doi: 10.1021/nn204656d. [DOI] [PubMed] [Google Scholar]
- 38.Liu M, Zhang R, Chen W. Graphene-supported nanoelectrocatalysts for fuel cells: synthesis, properties, and applications. Chem Rev. 2014;114:5117–5160. doi: 10.1021/cr400523y. [DOI] [PubMed] [Google Scholar]
- 39.Bitounis D, Ali-Boucetta H, Hong BH, Min D, Kostarelos K. Prospects and challenges of graphene in biomedical applications. Adv Mater. 2013;25:2258–2268. doi: 10.1002/adma.201203700. [DOI] [PubMed] [Google Scholar]
- 40.Shen H, Zhang L, Liu M, Zhang Z. Biomedical applications of graphene. Theranostics. 2012;2:283–294. doi: 10.7150/thno.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Nayak TR, Hong H, Cai W. Graphene: a versatile nanoplatform for biomedical applications. Nanoscale. 2012;4:3833–3842. doi: 10.1039/c2nr31040f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y, Asiri AM, Tang Z, Du D, Lin Y. Graphene based materials for biomedical applications. Mater Today. 2013;16:365–373. [Google Scholar]
- 43.Singh SK, Singh MK, Kulkarni PP, Sonkar VK, Gracio JJA, Dash D. Amine-modified graphene: thromboprotective safer alternative to graphene oxide for biomedical applications. ACS Nano. 2012;6:2731–2740. doi: 10.1021/nn300172t. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Tan C, Zhang H, Wang L. Two-dimensional graphene analogues for biomedical applications. Chem Soc Rev. 2015;44:2681–2701. doi: 10.1039/c4cs00300d. [DOI] [PubMed] [Google Scholar]
- 45.Dong H, Li Y, Yu J, Song Y, Cai X, Liu J, Zhang J, Ewing RC, Shi D. A Versatile Multicomponent assembly via β-cyclodextrin host-guest chemistry on graphene for biomedical applications. Small. 2013;9:446–456. doi: 10.1002/smll.201201003. [DOI] [PubMed] [Google Scholar]
- 46.Orecchioni M, Moyon CM, Delogu LG, Bianco A. Graphene and the immune system: challenges and potentiality. Adv Drug Deliver Rev. 2016;105:163–175. doi: 10.1016/j.addr.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Su Y, Hu S, Chen S. Functionalized graphene nanocomposites for enhancing photothermal therapy in tumor treatment. Adv Drug Deliver Rev. 2016;105:190–204. doi: 10.1016/j.addr.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 48.Shadjou N, Hasanzadeh M. Graphene and its nanostructure derivatives for use in bone tissue engineering: recent advances. J Biomed Mater Res A. 2016;104A:1250–1275. doi: 10.1002/jbm.a.35645. [DOI] [PubMed] [Google Scholar]
- 49.Qu Y, He F, Yu C, Liang X, Liang D, Ma L, Zhang Q, Lv J, Wu J. Advances on graphene-based nanomaterials for biomedical applications. Mat Sci Eng C. 2018;90:764–780. doi: 10.1016/j.msec.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 50.Karahan HE, Wiraj C, Xu C, Wei J, Wang Y, Wang L, Liu F, Chen Y. Graphene materials in antimicrobial nanomedicine: current status and future perspectives. Adv Healthcare Mater. 2018;7:1701406. doi: 10.1002/adhm.201701406. [DOI] [PubMed] [Google Scholar]
- 51.Bahamonde JP, Nguyen HN, Fanourakis SK, Rodrigues DF. Recent advances in graphene-based biosensor technology with applications in life sciences. J Nanobiotechnol. 2018;16:75. doi: 10.1186/s12951-018-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shao Y, Wang J, Wu H, Liu J, Aksay IA, Lin Y. Graphene based electrochemical sensors and biosensors: a review. Electroanal. 2010;22:1027–1036. [Google Scholar]
- 53.Murugana C, Sharma V, Murugan RK, Malaimegu G, Sundaramurthy A. Two-dimensional cancer theranostic nanomaterials: synthesis, surface functionalization and applications in photothermal therapy. J Control Release. 2019;299:1–20. doi: 10.1016/j.jconrel.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 54.Gu H, Tang H, Xiong P, Zhou Z. Biomarkers-based biosensing and bioimaging with graphene for cancer diagnosis. Nanomaterials. 2019;9:130. doi: 10.3390/nano9010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luo Y, Li Z, Zhu C, Cai X, Qu L, Du D, Lin Y. Graphene-like metal-free 2d nanosheets for cancer imaging and theranostics. Trends Biotechnol. 2018;36:1145–1156. doi: 10.1016/j.tibtech.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 56.Wang H, Chen Q, Zhou S. Carbon-based hybrid nanogels: a synergistic nanoplatform for combined biosensing, bioimaging, and responsive drug delivery. Chem Soc Rev. 2018;47:4198–4232. doi: 10.1039/c7cs00399d. [DOI] [PubMed] [Google Scholar]
- 57.Bei HP, Yang Y, Zhang Q, Tian Y, Luo X, Yang M, Zhao X. Graphene-based nanocomposites for neural tissue engineering. Molecules. 2019;24:658. doi: 10.3390/molecules24040658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mohammadrezaei D, Golzar H, Rad MR, Omidi M, Rashedi H, Yazdian F, Khojasteh A, Tayebi L. In vitro effect of graphene structures as an osteoinductive factor in bone tissue engineering: a systematic review. J Biomed Mater Res A. 2018;106A:2284–2243. doi: 10.1002/jbm.a.36422. [DOI] [PubMed] [Google Scholar]
- 59.Luan B, Huynh T, Zhao L, Zhou R. Potential toxicity of graphene to cell functions via disrupting protein-protein interactions. ACS Nano. 2015;9:663–669. doi: 10.1021/nn506011j. [DOI] [PubMed] [Google Scholar]
- 60.Chang Y, Yang S, Liu J, Dong E, Wang Y, Cao A, Liu Y, Wang H. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol Lett. 2011;200:201–210. doi: 10.1016/j.toxlet.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 61.Zhang D, Zhang Z, Wu Y, Fu K, Chen Y, Li W, Chua M. Systematic evaluation of graphene quantum dot toxicity to male mouse sexual behaviors, reproductive and offspring health. Biomaterials. 2019;194:215–232. doi: 10.1016/j.biomaterials.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 62.Perreault F, de Faria AF, Nejati S, Elimelech M. Antimicrobial properties of graphene oxide nanosheets: why size matters. ACS Nano. 2015;9:7226. doi: 10.1021/acsnano.5b02067. [DOI] [PubMed] [Google Scholar]
- 63.Wang J, Wei Y, Shi X, Gao H. Cellular entry of graphene nanosheets: the role of thickness, oxidation and surface adsorption. RSC Adv. 2013;3:15776. [Google Scholar]
- 64.Kang S, Herzberg M, Rodrigues DF, Elimelech M. Antibacterial effects of carbon nanotubes: size does matter! Langmuir. 2008;24:6409–6413. doi: 10.1021/la800951v. [DOI] [PubMed] [Google Scholar]
- 65.Musico YLF, Santos CM, Dalida MLP, Rodrigues DF. Surface modification of membrane filters using graphene and graphene oxide-based nanomaterials for nacterial inactivation and removal. ACS Sustainable Chem Eng. 2014;2:1559–1565. [Google Scholar]
- 66.Henriques PC, Borges I, Pinto AM, Magalhães FD, Gonçalves IC. Fabrication and antimicrobial performance of surfaces integrating graphene-based materials. Carbon. 2018;32:709–732. [Google Scholar]
- 67.Sadhukhan S, Ghosh TK, Roy I, Rana D, Bhattacharyya A, Saha R, Chattopadhyay S, Khatua S, Acharya K, Chattopadhyay D. Green synthesis of cadmium oxide decorated reduced graphene oxide nanocomposites and its electrical and antibacterial properties. Mater Sci Eng C. 2019;99:696–709. doi: 10.1016/j.msec.2019.01.128. [DOI] [PubMed] [Google Scholar]
- 68.Matharu RK, Porwal H, Ciric L, Edirisinghe M. The effect of graphene-poly (methyl methacrylate) fibres on microbial growth. Interface Focus. 2018;8:20170058. doi: 10.1098/rsfs.2017.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Akbari M, Niassar MS, Matsuura T, Ismail AF. Janus graphene oxide nanosheet: a promising additive for enhancement of polymeric membranes performance prepared via phase inversion. J Colloid Interf Sci. 2018;527:10–24. doi: 10.1016/j.jcis.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 70.Tu Y, Lv M, Xiu P, Huynh T, Zhang M, Castelli M, Liu Z, Huang Q, Fan C, Fang H, Zhou R. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat Nanotechnol. 2013;125:594–601. doi: 10.1038/nnano.2013.125. [DOI] [PubMed] [Google Scholar]
- 71.Ullaha S, Ahmada A, Subhanb F, Janc A, Razaa M, Khana AU, Rahmana AU, Khana UA, Tariqa M, Yuan Q. Tobramycin mediated silver nanospheres/graphene oxide composite for synergistic therapy of bacterial infection. J Photoch Photobio B. 2018;183:342–348. doi: 10.1016/j.jphotobiol.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 72.Feng Y, Chen Q, Yin Q, Pan G, Tu Z, Liu L. Reduced graphene oxide functionalized with gold nanostar nanocomposites for synergistically killing bacteria through intrinsic antimicrobial activity and photothermal ablation. ACS Appl Bio Mater. 2019;2:747–756. doi: 10.1021/acsabm.8b00608. [DOI] [PubMed] [Google Scholar]
- 73.Ko K, Kim M, Lee J, Kim W, Chung H. Effects of graphene oxides and silver-graphene oxides on aquatic microbial activity. Sci Total Environ. 2019;651:1087–1095. doi: 10.1016/j.scitotenv.2018.09.124. [DOI] [PubMed] [Google Scholar]
- 74.El-Shafai N, El-Khouly ME, El-Kemary M, Ramadan M, Eldesoukey I, Masoud M. Graphene oxide decorated with zinc oxide nanoflower, silver and titanium dioxide nanoparticles: fabrication, characterization, DNA interaction, and antibacterial activity. RSC Adv. 2019;9:3704–3714. doi: 10.1039/c8ra09788g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Afsahia S, Lerner MB, Goldstein JM, Lee J, Tang X, Bagarozzi DA Jr, Pan D, Locascio L, Walker A, Barron F, Goldsmith BR. Novel graphene-based biosensor for early detection of Zika virus infection. Biosens Bioelectron. 2018;100:85–88. doi: 10.1016/j.bios.2017.08.051. [DOI] [PubMed] [Google Scholar]
- 76.Fan X, Qi Y, Shi Z, Lv Y, Guo Y. A graphene-based biosensor for detecting microRNA with augmented sensitivity through helicase-assisted signal amplification of hybridization chain reaction. Sensor Actuat B. 2018;255:1582–1586. [Google Scholar]
- 77.Jiang B, Zhou K, Wang C, Sun Q, Yin G, Tai Z, Wilson K, Zhao J, Zhang L. Label-free glucose biosensor based on enzymatic graphene oxide-functionalized tilted fiber grating. Sensor Actuat B. 2018;254:1033–1039. [Google Scholar]
- 78.Li B, Yu A, Lai G. Self-assembly of phenoxyl-dextran on electrochemically reduced graphene oxide for nonenzymatic biosensing of glucose. Carbon. 2018;127:202–208. [Google Scholar]
- 79.Khetani S, Kollath VO, Kundra V, Nguyen MD, Debert C, Sen A, Karan K, Nezhad AS. Polyethylenimine modified graphene-oxide electrochemical immunosensor for the detection of glial fibrillary acidic protein in central nervous system injury. ACS Sens. 2018;3:844–851. doi: 10.1021/acssensors.8b00076. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, Zhang S, Xu T, Zhang T, Mo Y, Liu J, Yan L, Xing F. Ultra-sensitive and ultra-fast detection of whole unlabeled living cancer cell responses to paclitaxel with a graphene-based biosensor. Sensor Actuat B. 2018;263:417–425. [Google Scholar]
- 81.Cui F, Ji J, Sun J, Wang J, Wang H, Zhang Y, Ding H, Lu Y, Xu D, Sun X. A novel magnetic fluorescent biosensor based on graphene quantum dots for rapid, efficient, and sensitive separation and detection of circulating tumor cells. Anal Bioanal Chem. 2019;411:985–995. doi: 10.1007/s00216-018-1501-0. [DOI] [PubMed] [Google Scholar]
- 82.Yang K, Zhang S, Zhang G, Sun X, Lee ST, Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010;10:3318–3323. doi: 10.1021/nl100996u. [DOI] [PubMed] [Google Scholar]
- 83.Zhang W, Guo Z, Huang D, Liu Z, Guo X, Zhong H. Synergistic effect of chemo-photothermal therapy using PEGylated graphene oxide. Biomaterials. 2011;32:8555–8561. doi: 10.1016/j.biomaterials.2011.07.071. [DOI] [PubMed] [Google Scholar]
- 84.Chen H, Liu F, Lei Z, Ma L, Wang Z. Fe2O3@Au core@shell nanoparticle-graphene nanocomposites as theranostic agents for bioimaging and chemo-photothermal synergistic therapy. RSC Adv. 2015;5:84980–84987. [Google Scholar]
- 85.Zhang L, Xia J, Zhao Q, Liu L, Zhang Z. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small. 2010;6:537–544. doi: 10.1002/smll.200901680. [DOI] [PubMed] [Google Scholar]
- 86.Tian B, Wang C, Zhang S, Feng L, Liu Z. Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano. 2011;5:7000–7009. doi: 10.1021/nn201560b. [DOI] [PubMed] [Google Scholar]
- 87.Huang P, Xu C, Lin J, Wang C, Wang X, Zhang C, Zhou X, Guo S, Cui D. Folic acid-conjugated graphene oxide loaded with photosensitizers for targeting photodynamic therapy. Theranostics. 2011;1:240–250. doi: 10.7150/thno/v01p0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sahu A, Choi W, Lee JH, Tae G. Graphene oxide mediated delivery of methylene blue for combined photodynamic and photothermal therapy. Biomaterials. 2013;34:6239–6248. doi: 10.1016/j.biomaterials.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 89.Zhou L, Jiang H, Wei S, Ge X, Zhou J, Shen J. High-efficiency loading of hypocrellin B on graphene oxide for photodynamic therapy. Carbon. 2012;50:5594–5604. [Google Scholar]
- 90.Bai WK, Shen E, Hu B. The induction of the apoptosis of cancer cell by sonodynamic therapy: a review. Chin J Cancer Res. 2012;24:368–373. doi: 10.3978/j.issn.1000-9604.2012.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen Y, Liu T, Chang P, Hsu P, Liu H, Lina H, Chen S. A theranostic nrGO@MSN-ION nanocarrier developed to enhance the combination effect of sonodynamic therapy and ultrasound hyperthermia for treating tumor. Nanoscale. 2016;8:12648–12657. doi: 10.1039/c5nr07782f. [DOI] [PubMed] [Google Scholar]
- 92.Feng L, Yang X, Shi X, Tan X, Peng R, Wang J, Liu Z. Polyethylene glycol and polyethylenimine dualfunctionalized nano-graphene oxide for photothermally enhanced gene delivery. Small. 2013;9:1989–1997. doi: 10.1002/smll.201202538. [DOI] [PubMed] [Google Scholar]
- 93.Kim H, Kim WJ. Photothermally controlled gene delivery by reduced graphene oxide-polyethylenimine nanocomposite. Small. 2014;10:117–126. doi: 10.1002/smll.201202636. [DOI] [PubMed] [Google Scholar]
- 94.Wang Y, Li Z, Hu D, Lin CT, Li J, Lin Y. Aptamer/Graphene oxide nanocomplex for in situ molecular probing in living cells. J Am Chem Soc. 2010;132:9274–9276. doi: 10.1021/ja103169v. [DOI] [PubMed] [Google Scholar]
- 95.Sasidharan A, Panchakarla LS, Sadanandan AR, Ashokan A, Chandran P, Girish CM, Menon D, Nair SV, Rao CN, Koyakutty M. Hemocompatibility and macrophage response of pristine and functionalized graphene. Small. 2012;8:1251–1263. doi: 10.1002/smll.201102393. [DOI] [PubMed] [Google Scholar]
- 96.Singh SK, Singh MK, Kulkarni PP, Sonkar VK, Grácio JA, Dash D. Amine-modified graphene: thrombo-protective safer alternative to graphene oxide for biomedical applications. ACS Nano. 2012;6:2731–2740. doi: 10.1021/nn300172t. [DOI] [PubMed] [Google Scholar]
- 97.Amani H, Mostafavi E, Arzaghi H, Davaran S, Akbarzadeh A, Akhavan O, Toroudi HP, Webster TJ. Three-dimensional graphene foams: synthesis, properties, biocompatibility, biodegradability, and applications in tissue engineering. ACS Biomater Sci Eng. 2019;5:193–214. doi: 10.1021/acsbiomaterials.8b00658. [DOI] [PubMed] [Google Scholar]
- 98.Bai RG, Ninan N, Muthoosamy K, Manickam S. Graphene: a versatile platform for nanotheranostics and tissue engineering. Prog Mater Sci. 2018;91:24–69. [Google Scholar]
- 99.Saburi E, Islami M, Hosseinzadeh S, Moghadam AS, Mansour RN, Azadianc E, Joneidif Z, Nikpoor AR, Ghadianih MH, Khodaii Z, Ardeshirylajimi A. In vitro osteogenic differentiation potential of the human induced pluripotent stem cells augments when grown on Graphene oxide-modified nanofibers. Gene. 2019;696:72–79. doi: 10.1016/j.gene.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 100.Shin SR, Li Y, Jang HL, Khoshakhlagh P, Akbari M, Nasajpour A, Zhang YS, Tamayol A, Khademhosseini A. Graphene-based materials for tissue engineering. Adv Drug Deliver Rev. 2016;105:255–274. doi: 10.1016/j.addr.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gu M, Liu Y, Chen T, Du F, Zhao X, Xiong C, Zhou Y. Is graphene a promising nano-material for promoting surface modification of implants or scaffold materials in bone tissue engineering. Tissue Engineering: Part B. 2014;20:447–491. doi: 10.1089/ten.teb.2013.0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Elkhenany H, Amelse L, Lafont A, Bourdo S, Caldwell M, Neilsen N, Dervishi E, Derek O, Biris AS, Anderson D, Dhar M. Graphene supports in vitro proliferation and osteogenic differentiation of goat adult mesenchymal stem cells: potential for bone tissue engineering. J Appl Toxicol. 2015;35:367–374. doi: 10.1002/jat.3024. [DOI] [PubMed] [Google Scholar]
- 103.Zhai L, Li L, Zhang Q. Fabrication of capsaicin functionalized reduced graphene oxide and its effect on proliferation and differentiation of osteoblasts. Environ Toxicol Phar. 2018;57:41–45. doi: 10.1016/j.etap.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 104.Fu C, Bai H, Zhu J, Niu Z, Wang Y, Li J, Yang X, Bai Y. Enhanced cell proliferation and osteogenic differentiation in electrospun PLGA/hydroxyapatite nanofibre scaffolds incorporated with graphene oxide. PLoS One. 2017;12:e0188352. doi: 10.1371/journal.pone.0188352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luo Y, Shen H, Fang Y, Cao Y, Huang J, Zhang M, Dai J, Shi X, Zhang Z. Enhanced proliferation and osteogenic differentiation of mesenchymal stem cells on graphene oxide-incorporated electrospun poly (lactic-co-glycolic acid) nanofibrous mats. ACS Appl Mater Interfaces. 2015;7:6331–6339. doi: 10.1021/acsami.5b00862. [DOI] [PubMed] [Google Scholar]
- 106.Jie W, Song F, Li X, Li W, Wang R, Jiang Y, Zhao L, Fan Z, Wang J, Liu B. Enhancing the proliferation of MC3T3-E1 cells on casein phosphopeptide-biofunctionalized 3D reduced-graphene oxide/polypyrrole scaffolds. RSC Adv. 2017;7:34415–34424. [Google Scholar]
- 107.Jin L, Zeng Z, Kuddannaya S, Yue D, Bao J, Wang Z, Zhang Y. Synergistic effects of a novel free-standing reduced graphene oxide film and surface coating fibronectin on morphology, adhesion and proliferation of mesenchymal stem cells. J Mater Chem B. 2015;3:4338–4344. doi: 10.1039/c5tb00295h. [DOI] [PubMed] [Google Scholar]
- 108.Lee JH, Lee Y, Shin YC, Kim MJ, Park JH, Hong SW, Kim B, Oh JW, Park KD, Han DW. In situ forming gelatin/graphene oxide hydrogels for facilitated C2C12 myoblast differentiation. Appl Spectrosc Rev. 2016;51:527–539. [Google Scholar]
- 109.Saburi E, Islami M, Hosseinzadeh S, Moghadam AS, Mansour RN, Azadian E, Joneidi Z, Nikpoor AR, Ghadiani MH, Khodaii Z, Ardeshirylajimi A. In vitro osteogenic differentiation potential of the human induced pluripotent stem cells augments when grown on Graphene oxide-modified nanofibers. Gene. 2019;696:72–79. doi: 10.1016/j.gene.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 110.Xie H, Cao T, Franco-Obregón A, Rosa V. Graphene-induced osteogenic differentiation is mediated by the integrin/FAK axis. Int J Mol Sci. 2019;20:574. doi: 10.3390/ijms20030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li N, Zhang X, Song Q, Su R, Zhang Q, Kong T, Liu L, Jin G, Tang M, Cheng G. The promotion of neurite sprouting and outgrowth of mouse hippocampal cells in culture by graphene substrates. Biomaterials. 2011;32:9374–9382. doi: 10.1016/j.biomaterials.2011.08.065. [DOI] [PubMed] [Google Scholar]
- 112.Uz M, Donta M, Mededovic M, Sakaguchi DS, Mallapragada S. Development of gelatin and graphene-based nerve regeneration conduits using three-dimensional (3D) printing strategies for electrical transdifferentiation of mesenchymal stem cells. Ind Eng Chem Res. 2019 [Google Scholar]
- 113.Ma X, Xiao M, Hao Y, Cheng G. Precisely controllable hybrid graphene scaffold reveals size effects on differentiation of neural progenitor cells in mimicking neural network. Carbon. 2019;145:90–99. [Google Scholar]
- 114.Abzan N, Kharaziha M, Labbaf S. Development of three-dimensional piezoelectric polyvinylidene fluoride-graphene oxide scaffold by non-solvent induced phase separation method for nerve tissue engineering. Mater Design. 2019;167:107636. [Google Scholar]
- 115.Wang J, Cheng Y, Chen L, Zhu T, Ye K, Jia C, Wang H, Zhu M, Fan C, Mo X. In vitro and in vivo studies of electroactive reduced graphene oxide-modified nanofiber scaffolds for peripheral nerve regeneration. Acta Biomater. 2019;84:98–113. doi: 10.1016/j.actbio.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 116.Qing H, Jin G, Zhao G, Huang G, Ma Y, Zhang X, Sha B, Luo Z, Lu T, Xu F. Heterostructured Silk-nanofiber-reduced graphene oxide composite scaffold for sh-sy5y cell alignment and differentiation. ACS Appl Mater Interfaces. 2018;10:39228–39237. doi: 10.1021/acsami.8b12562. [DOI] [PubMed] [Google Scholar]
- 117.Shah S, Yin PT, Uehara TM, Chueng SD, Yang L, Lee K. Guiding stem cell differentiation into oligodendrocytes using graphene-nanofiber hybrid scaffolds. Adv Mater. 2014;26:3673–3680. doi: 10.1002/adma.201400523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guo W, Wang S, Yu X, Qiu J, Li J, Tang W, Li Z, Mou X, Liu H, Wang Z. Construction of a 3D rGO-collagen hybrid scaffold for enhancement of the neural differentiation of mesenchymal stem cells. Nanoscale. 2016;8:1897–1904. doi: 10.1039/c5nr06602f. [DOI] [PubMed] [Google Scholar]
- 119.Vijayavenkataraman S, Thaharah S, Zhang S, Lu WF, Fuh JYH. 3D-printed PCL/rGO conductive scaffolds for peripheral nerve injury repair. Artif Organs. 2019;43:515–523. doi: 10.1111/aor.13360. [DOI] [PubMed] [Google Scholar]
- 120.Li N, Zhang Q, Gao S, Song Q, Huang R, Wang L, Liu L, Dai J, Tang M, Cheng G. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci Rep. 2013;3:1604. doi: 10.1038/srep01604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang K, Zheng H, Liang S, Gao C. Aligned PLLA nanofibrous scaffolds coated with graphene oxide for promoting neural cell growth. Acta Biomater. 2016;37:131–142. doi: 10.1016/j.actbio.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 122.Ku SH, Park CB. Myoblast differentiation on graphene oxide. Biomaterials. 2013;34:2017–2023. doi: 10.1016/j.biomaterials.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 123.Krueger E, Chang AN, Brown D, Eixenberger J, Brown R, Rastegar S, Yocham KM, Cantley KD, Estrada D. Graphene foam as a three-dimensional platform for myotube growth. ACS Biomater Sci Eng. 2016;2:1234–1241. doi: 10.1021/acsbiomaterials.6b00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Coulombe KL, Bajpai VK, Andreadis ST, Murry CE. Heart regeneration with engineered myocardial tissue. Annu Rev Biomed Eng. 2014;16:1–28. doi: 10.1146/annurev-bioeng-071812-152344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ameri SK, Singh PK, D’Angelo R, Stoppel W, Black L, Sonkusale SR. Three dimensional graphene scaffold for cardiac tissue engineering and in-situ electrical recording. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:4201–4203. doi: 10.1109/EMBC.2016.7591653. [DOI] [PubMed] [Google Scholar]
- 126.Shin SR, Zihlmann C, Akbari M, Assawes P, Cheung L, Zhang K, Manoharan V, Zhang YS, Yüksekkaya M, Wan K, Nikkhah M, Dokmeci MR, Tang X, Khademhosseini A. Reduced graphene Oxide-GelMA hybrid hydrogels as scaffolds for cardiac tissue engineering. Small. 2016;12:3677–3689. doi: 10.1002/smll.201600178. [DOI] [PMC free article] [PubMed] [Google Scholar]