Abstract
Purpose: Tong Sheng tablets (TSTs) have long been used for treating cerebral ischemic reperfusion injury (CIRI) in clinic, but the underlying mechanism remains unknown. Therefore, in this study, TSTs were evaluated systematically using chemical analysis, network pharmacology and classical pharmacology. Methods: The first part was TSTs quality control including TSTs fingerprint establishment and chemicals identification. In the second part, network pharmacology analysis and bioinformatics were combined to construct a compound-target-disease network, which can screen out key targets or pathways, revealing complex molecule mechanism of TSTs. The last part was experiment verification. Classical pharmacology of TSTs was investigated in vivo to verify the results of network pharmacology. Results: (1) Fingerprints of TSTs were established, and 11 characteristic peaks were identified using HPLC. (2) Network pharmacology and bioinformatics suggested that the protection of TSTs in treating CIRI might be related to regulation of oxidative stress, inflammation and apoptosis, and some key molecules such as Nrf2, IL-1β, TNF, Bcl-2 and Cyt-C involved in the pathways. (3) TSTs significantly improved neurologic behavior scores, decreased the areas of ischemic necrosis and neuronal necrosis, and increased Nissl body counts. Besides, TSTs significantly decreased pro-inflammatory cytokine (IL-1β, TNF-α) and pro-oxidative product levels (LPO, MDA) and increased anti-oxidative product levels (NO, SOD). TSTs downregulated the protein expressions of Nrf2 and HO-1. Meanwhile, TSTs reduced apoptotic cell counts, downregulated the protein expressions of Cyt-C and Bax, and upregulated the protein expression of Bcl-2. In terms of autophagy, TSTs enhanced LC-3B protein expression. Conclusion: The present results illustrated that TSTs effectively alleviated CIRI, and the underlying mechanism might be associated with multiple molecular pathways. Herein, we established a primary pattern for studying Chinese herbal compounds and provided basic guidance for future investigation.
Keywords: Chinese herbal compound, cerebral ischemia reperfusion injury, Tong Sheng tablets, molecular targets, network pharmacology
Introduction
Cerebral stroke is the second most fatal disease globally, and ischemic cerebral stroke (ICS) accounts for 85% of the cases [1]. Cerebral ischemia and reperfusion injury (CIRI) is a critical pathological process in ICS. Oxidative and inflammatory damage are important factors for neurological dysfunction. The brain is sensitive to oxidative stress. ROS overproduction induces excessive oxidative stress, thereby damaging brain tissue. Endogenous antioxidant enzymes such as NO and SOD reverse the accumulation of ROS [2]. Reducing inflammation and depleting inflammatory genes exert neuroprotective effects against brain damage following ischemic stroke [3]. Additionally, necrosis, apoptosis and autophagy are the main modes of neuronal death after CIRI. Recently, apoptosis and autophagy have become key research fields in CIRI [4].
Tissue plasminogen activator remains the only FDA-approved drug for ICS, but it only benefits 3%-8.5% of patients who experienced a stroke for its narrow time window and adverse effects [5]. Therefore, it is imperative to find novel therapeutic strategies for stroke. In fact, traditional Chinese medicine (TCM) has a long history for treating ICS with Chinese herbs. In Chinese medicine, two or more herbs, in the form of a Chinese herbal compound (CHC), are applied together to treat diseases under inherent principles. Chinese medicine or CHC possesses bioactive substances in treating diseases although there are abundant and complicated components. For example, in ancient China, Artemisia apiacea was used for treating malaria, and modern research identified artemisinin as the main bioactive substance [6-8]. Other examples include Folium Ginkgo and arsenic [9,10]. Although the specific curative mechanism has not been fully illuminated, the CHC is characterized by an overall benefit verified by clinical application. For example, Tong Sheng tablets (TSTs), a successfully transformed hospital preparation, have been commonly used to treat cerebral stroke in Dongguan Hospital of Traditional Chinese Medicine. TSTs, with some modifications of the ancient formula Angong Niuhuang Wan (ANW) and Fang Feng Tong Sheng San, have been used to treat mental dizziness, delirium, and other stroke-like symptoms [11,12]. TSTs consist of Rhei Radix et Rhizoma (Rheum officinale Baill), Saposhnikoviae Radix (Saposhnikovia divaricata [Turcz.] Schischk), Gardeniae Fructus (Gardenia jasminoides J. Ellis), Bovis Calculus Sativus (gallstones of Bos Taurus domesticus Gmelin), Bambusae Concretio Solicea (Bambusa textilis McClure), Magnoliae Officinalis Cortex (Magnolia officinalis Rehder & E.H. Wilson), Paeoniae Radix Rubra (Paeonia veitchii Lynch) and Polygoni Cuspidati Rhizoma et Radix (Polygonum cuspidatum Siebold & Zucc). In the TSTs prescription, Bovis Calculus Sativus acts as a sovereign drug that has been used to treat stroke traditionally. ANW is a representative medicine, and modern pharmacological studies revealed that it can alleviate cerebral stroke [13]. Rhei Radix et Rhizoma, Polygoni Cuspidati Rhizoma et Radix, Paeoniae Radix Rubra, Gardeniae Fructus and Magnoliae Officinalis Cortex act as ministerial drugs, and their active ingredients rheochrysin [14], chrysophanol [15], polydatin [16], paeoniflorin [17], Geniposide [18] and magnolol [19] all have therapeutic effects against CIRI. Bambusae Concretio Solicea and Saposhnikoviae Radix, as conductant drugs, can facilitate the treatment of CIRI.
Although many studies have focused on the herbs (or their monomers) involved in the effects of TSTs, the complete TSTs prescription analysis remains largely unexplored. The multiple-targets and multiple-pathways characteristics of TSTs also make research more difficult. Therefore, in this exploration, a quality control analysis of TSTs was initially performed by HPLC. Then a component-target-disease network was constructed to find the potential key targets and possible mechanism. Further, classical pharmacology of TSTs was investigated in vivo to verify the results of network pharmacology. Our research aimed to provide a scientific basis for the clinical application of TSTs.
Material and methods
Materials and reagents
Chromatographic-grade methanol was purchased from Merck & Co., Inc (Darmstadt, Germany). The other regents used in this experiment were all analytic reagents, and water was purified. Standards including geniposide, paeoniflorin, polydatin, magnolol, 4’-O-beta-glucopyranosyl-5-O-methylvisamminol, emodin, chrysophanol, aloe-emodin, physcion, rhein and prim-O-glucosylcimifugin were all obtained from Chengdu Must Bio-Technology Co., Ltd (Chengdu, Sichuan, China). ANW was bought from Tong Ren Tang Technologies Co. Ltd (Beijing, China). TSTs were provided by Dongguan Traditional Chinese Medicine Hospital (Dongguan, Guangdong, China).
HPLC analysis
The HPLC analysis of TSTs was performed using a SHIMADZU LC-20A, which was equipped with a photodiode array ultraviolet-visible detector. An Agilent ZORBAX SB-C18 column was used in the analysis. Mobile phase A was methanol, and mobile phase B was 0.1% phosphate-water solution. The mobile phase gradient was set at a flow rate of 1 mL/min, and the column temperature was 30°C. The injection volume was 20 μL. The detection wavelength was 254 nm. The gradient elution was as follows: 0-5 min, 5% A → 30% A; 5-40 min, 30% A → 60% A; 50-55 min, 60% A → 100% A; and 55-60 min, 100% A.
Mixed standards were prepared as follows: all standards were weighed accurately, dissolved in 10 mL of methanol, and filtered through a 0.45-μm membrane. Samples were extracted as follows: TSTs were crushed into powder, and then 1.0 g of powder was accurately weighed and ultrasonically extracted (250 W, 40 kHz, 25°C) with 50 mL of methanol for 30 min. If necessary, methanol was added to replenish the weight lost during extraction. Samples were finally obtained after filtering the extracts through a 0.45-μm membrane.
Experimental animals
Ninety male Sprague-Dawley rats (250-270 g, approval No. SYXK 2018-0001) were provided by the Experimental Animal Center of Guangzhou University of Chinese Medicine. All rats had free access to a standard diet and drinking water, and they were housed in a room at 24.0 ± 0.5°C with a 12 h/12 h cyclic lighting schedule. The experiment was performed in compliance with the Animal Ethics Committee of Guangzhou University of Chinese Medicine.
Cerebral artery occlusion (MCAO)-induced focal CIRI model
MCAO-induced CIRI was established as follows [20]. Rats were anesthetized via an intraperitoneal injection of pentobarbital sodium. Then, the left common carotid artery (CCA), internal carotid artery (ICA), and external carotid artery (ECA) were carefully exposed. The ECA needed to be ligated at a proximal location. The CCA and ICA were temporarily nipped. A suture was inserted from an incision on the CCA into the ICA until the head of the suture arrived at the bifurcation of the ICA and middle cerebral artery (MCA). The length of the nylon suture was approximately 18 mm from the bifurcation of the CCA/ECA. After 1.5 h, the suture was removed to restore blood supply to the MCA area via reperfusion. After the surgery, neurological deficits were evaluated and scored. The rats in the sham group underwent the same surgical procedure without a suture occlusion.
Study design
All animals were randomly and averagely divided into six groups (n = 15) as follows: sham (distilled water, p.o.), model (distilled water, p.o.), ANW (ANW at a dosage of 0.3 g/kg, p.o.), TST-L (TSTs at a dosage of 0.15 g/kg, p.o.), TST-M (TSTs at a dosage of, 0.3 g/kg, p.o.), and TST-H groups (TSTs at the dosage of 0.6 g/kg, p.o.). Distilled water, ANW or TSTs were administered once daily for a week after surgery.
Application of laser speckle imaging
After successful surgery, rats (one from each group) were randomly chosen from the sham and model groups for laser speckle imaging. Rats were anesthetized with air-mixed isoflurane (RWD, Shenzhen, Guangdong, China) throughout surgery. The cranial region between the coronal and lambdoid sutures was thinned using a 1.6-mm-diameter drill until the cortex vessel was clearly visible. The imager was installed on the thinned region polished for data collection.
Neurological behavior assessment
Neurologic deficit scoring was performed on days 1, 3 and 7 after surgery. The criteria were as follows: (1) lifting rats by the tail and observing the flexion-extension of their front legs (0-4 points) [21]; (2) placing rats on the ground and examining the resistance force of their shoulders (0-3 points); (3) placing rats on a metal net and examining the muscular tension of their front legs (0-3 points); and (4) circling to the left or right only (1 point). Higher scores indicated more severe neurological deficits.
Cerebral infarction size
Rats were sacrificed using anesthesia, and then whole heads were removed via decapitation for sample collection. Cerebrums were completely peeled off, and other tissues were carefully removed, including cerebella, olfactory bulbs, and low brain stems. The sample-collecting process was performed on ice. Samples were quickly frozen at -20°C for 30 min and later removed to be made into coronal sections. These sections were further preprocessed as follows: stained in 2% TTC (2,3,5-triphenyl-2H-tetrazolium chloride, Amresco, center valley, PA, USA), incubated in the dark for 20 min, and then fixed in 4% paraformaldehyde (Servicebio, Wuhan, Hubei, China) for 1 h. The TTC staining results were digitally scanned and analyzed using Image-Pro Plus 6.0.
Pathological staining
Six rats from each group were used for pathological analysis. The cerebral tissue around the optic chiasma were sliced into coronal sections, fixed in 4% paraformaldehyde, dehydrated through a graded alcohol series, mounted in paraffin, cut into cross-sections at 4 μm, and stained with hematoxylin & eosin (HE staining) or 0.1% cresyl violet-luxol (Nissl staining). HE staining was used to evaluate the pathological changes of cerebral tissue, and Nissl staining was used to evaluate the morphology, amount, and distribution changes of Nissl bodies.
Biochemical indices
The impact of TSTs on oxidative stress in acute ischemic stroke models was assessed by lipid peroxidation (LPO), superoxide dismutase (SOD), malondialdehyde (MDA) and nitric oxide (NO). The measurements and calculations were strictly detected with commercial kits (Jiancheng, Nanjing, Jiangsu, China). Meanwhile, the serum levels of inflammatory factors (IL-1β and TNF-α) were measured with ELISA kits (Abbkine, Wuhan, Hubei, China).
Western blot
Western blot was applied to detect protein expressions of Bax, Bcl-2, Cyt-C, LC-3, Nrf2 and HO-1 (Abcam, MA, USA). Briefly, tissues were lysed in RIPA buffer (Cwbio, Beijing, China) and centrifuged at 12,000×g, 4°C for 10 min. After dilution, degeneration and centrifugation, the supernatant was added to a prepared sodium dodecyl sulfate-polyacrylamide gel to separate proteins via electrophoresis. Proteins were then transferred to a polyvinylidene difluoride membrane. After blocking with 5% bovine serum albumin for 1 h, the membrane was probed overnight at 4°C with anti-Bcl-2, anti-LC3B, anti-Bax, anti-active caspase-3, anti-Cyt-C, anti-Nrf2, anti-heme oxygenase 1 (Abcam, MA, USA), anti-GAPDH or anti-β-actin antibodies (Cwbio, Beijing, China). The membrane was incubated with secondary antibody (IRDye 800CW Goat anti-Rabbit or IRDye 800CW Goat anti-Mouse were purchased from LI-COR, Inc (Lindon, Nebraska, USA)). Bands were quantified using Image-Pro Plus 6.0.
TUNEL assay
An apoptotic cell detection kit (Roche, Mannheim, Germany) was used to stain the dewaxed sections according to the manufacturer’s recommendations. The results were scanned using a microscope, and the results were calculated using Image-Pro Plus 6.0.
Immunofluorescence assay
Bax, Cyt-C and LC-3B, which are mainly expressed in the cytoplasm and mitochondria, were tagged with fluorescent labels and directly visualized using a laser-scanning confocal microscope. 0.5% Triton-X-100 was used to increase the permeability of fixed cerebral tissue in the dewaxed and repaired sections. Later, they were blocked with goat serum and then sequentially incubated with primary and secondary antibodies. The images were scanned using a laser-scanning confocal microscope using excitation and emission wavelengths of 488 and 518 nm, respectively.
Disease-drug-target network construction
ChemDIS is a tool for retrieving the chemical-protein interactions for referencing the chemical-disease relations, and PubChem’s BioAssay database is a repository of small-molecule screening data that provides the biological effects of chemical compounds [22]. To identify potential targets of TSTs, we retrieved interacting proteins and biological protein targets of 10 major compounds from the STITCH database v5.0 using ChemDIS V2.4 and from PubChem’s BioAssay database, respectively. The score cutoff for ChemDIS results was set as 0.4 to increase the confidence. Both chemical-protein interactions and small-molecule targets were integrated into a drug-target network using Cytoscape 2.8.3 and visualized using Cytoscape 3.6.1.
The Open Targets Validation Platform is a platform providing evidence about the association of known and potential drug targets with diseases [23]. To associate a therapeutic target with disease, we retrieved ischemia reperfusion injury (IRI)-associated therapeutic targets. The IRI targets were further integrated with the drug-target network and used to form a comprehensive disease-drug-target network.
Network analysis
To understand the associations of drug targets with IRI, we extracted the common targets of 10 compounds and IRI and constructed an IRI-drug-common target subnetwork. We next identified functional clusters within this network using the graph-clustering algorithm TFit (for iterated Transfer-Fusion) in Cluster & See and enriched the pathways implicated in this network using the Reactome FI Cytoscape plug-in [24].
Statistical analysis
Data were analyzed using SPSS 22.0 statistical software and presented as the mean ± SD. A t-test was used to compare two independent samples, whereas one-way analysis of variance was applied to analyze between-group differences. In addition, a least significant difference test was used for two-sample comparisons between groups. P < 0.05 was considered statistically significant. Graphs were created using GraphPad Prism 6.0.
Results
Chemical profile analysis of TSTs by HPLC
The chemical fingerprint of TSTs was shown in Figure 1. The HPLC analysis revealed 11 compounds of TSTs including geniposide, paeoniflorin, prim-O-glucosylcimifugin, 5-O-methylvisammioside, aloe-emodin, physcion, rhein, magnolol, emodin, chrysophanol and polydatin, and their retention times were 17.109, 18.293, 19.691, 21.184, 28.447, 43.579, 49.171, 50.259, 50.760, 51.518, and 52.254 min, respectively. To investigate the stability and ensure the quality of the formula, we analyze fingerprints of TSTs from six batches. Results as Figure 1B showed, the similarity among six batches were 0.983-0.996.
Figure 1.
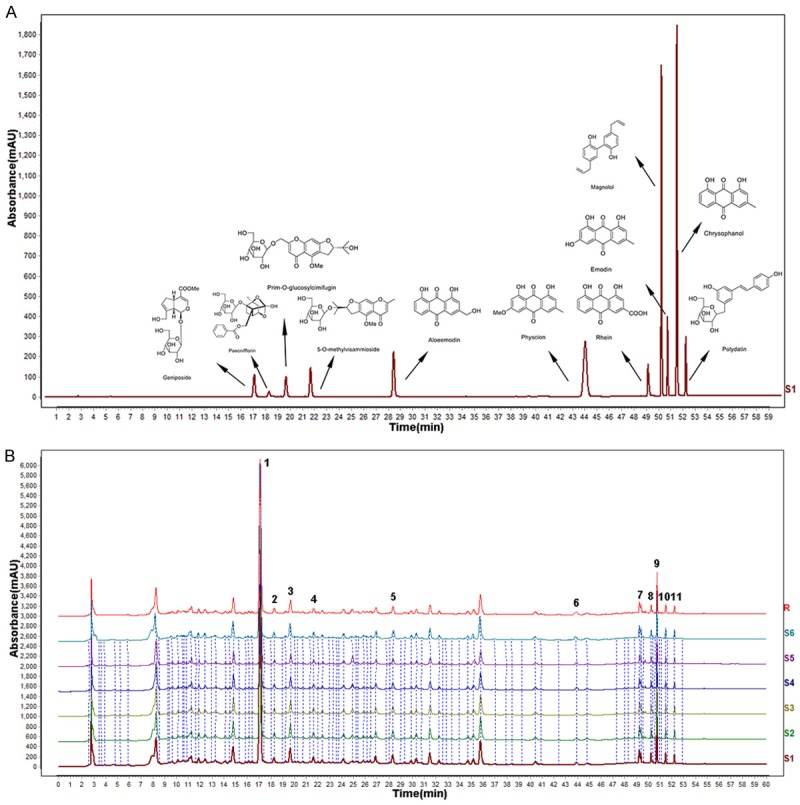
The chemical profile of TSTs determined at 254 nm via HPLC-UV analysis. Notes: A. The standard curves for each compound. B. The chemical contents of TSTs.
Network analysis
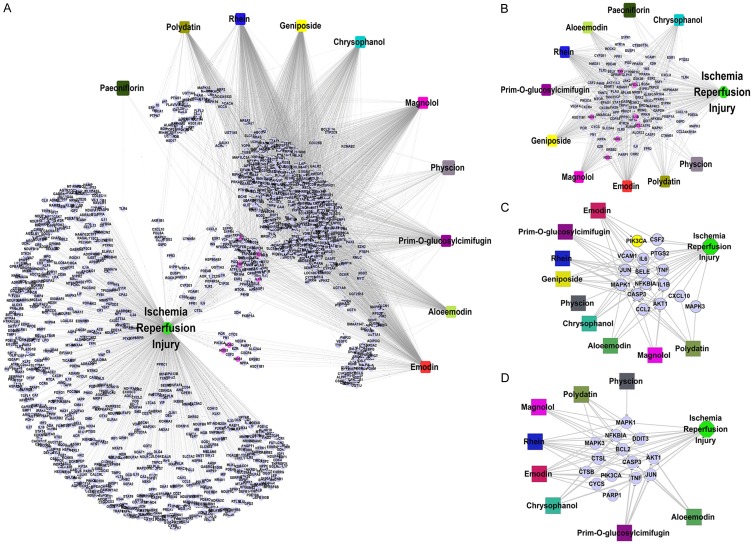
We retrieved 621 potential targets of 10 components of TSTs and 890 ISI-associated proteins. The drug-target and IRI-associated protein pairs formed a comprehensive disease-drug-target network (Figure 2A). All nodes and edges of this network were listed in Table S1.
Figure 2.
Network Analysis of TSTs. Notes: A. IRI-drug-common target network. B. IRI-drug-common target network. C. IRI-drug-TNF pathway network. D. IRI-drug-apoptosis pathway network. Abbreviations: TSTs, Tong Sheng tablets; IRI, ischemia reperfusion injury.
A total of 111 common targets of 10 TST components overlapped with the IRI-associated proteins (Figure 2B).
We enriched 372 pathways from the IRI-drug-common target network and predicted that signaling pathways could be potential targets of TSTs in the treatment of IRI (the full pathway list can be found in Table S1). Figure 2C, 2D showed two representative TNF and apoptosis signaling pathways. Major identified components of TSTs were involved in these two pathways.
Pharmacodynamic evaluation
Laser speckle imaging in model assessment
The images of cerebral blood flow were presented in Figure 3. Compared with the sham group findings, the model group exhibited narrower or absent dextral blood flow, which indicated that the MCAO model was established successfully.
Figure 3.
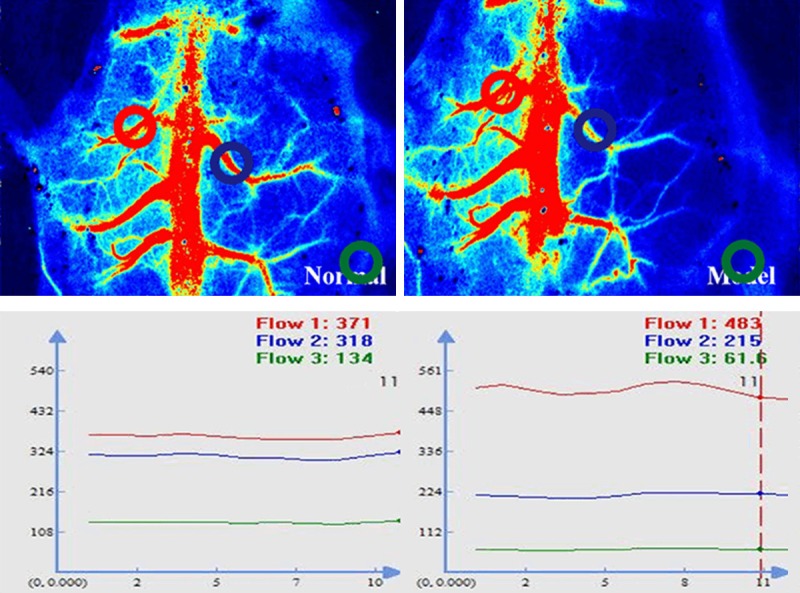
Cerebral blood flow assessed via laser speckle imaging.
Neurological behavior assessment and cerebral infarction size
As shown in Figure 4A, on day 1, the scores were significantly lower in the sham group than in the other five groups. However, on day 7, the scores for all TST groups were lower than that of the model group.
Figure 4.
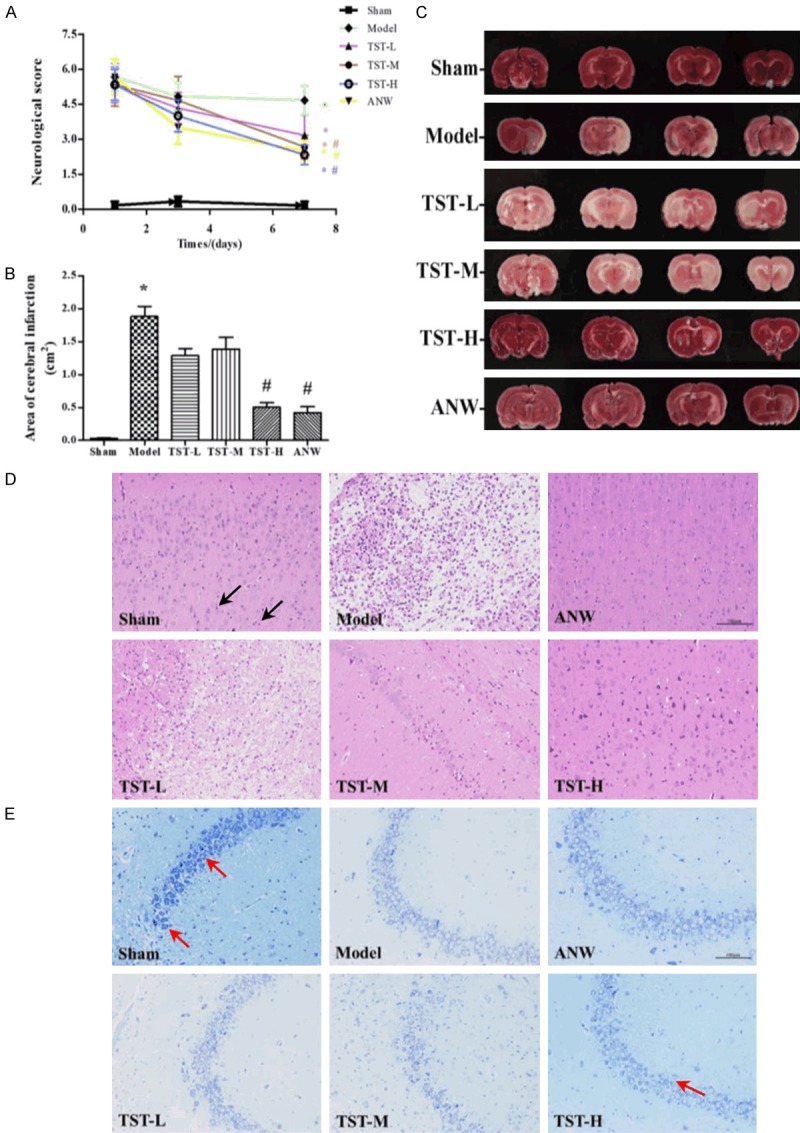
Neurological behavior assessment and cerebral infarction size. Notes: A. Effect of TSTs on the neurological score of MCAO change in the groups. B and C. Effect of TSTs on the area of cerebral infarction of MCAO rats. D. Effect of TSTs on the brain tissue of MCAO rats, Black arrows point to pyramidal cells, HE staining, ×200. E. Effect of TSTs on the brain tissue of MCAO rats, red arrows point to Nissl bodies, Nissl staining, ×200. Data are expressed as the mean ± SD (n = 6). *P < 0.05 vs. sham group; #P < 0.05 vs. model group. Abbreviations: TSTs, Tong Sheng tablets; ANW, Angong Niuhuang Wan; MCAO, middle cerebral artery occlusion; HE, hematoxylin-eosin.
In Figure 4B, 4C, a large area of ischemic infarction was appeared in the model group. Compared with these findings, the ischemic areas of the TST-H and ANW groups were significantly smaller.
Morphological changes of the cerebrum
H&E staining revealed normomorphic neurons in the cerebral cortex, hippocampus, and ischemic penumbra in the sham group. However, in the model group, ischemic necrosis occurred in the left area of the cerebral cortex, along with large malacia, liquefactive necrosis of nerve tissues, the disappearance of neuronal nuclei, and significant infiltration of microglial cells. In the hippocampus, some pyramidal cells were found to have hyperchromatic nuclei or necrosis in the CA1 region, and they were lower in number of absent in the CA3 region, surrounded by glial cell proliferation and infiltration. Neurons or pyramidal cells in the DG region, with pyknosis and hyperchromatism, exhibited triangular or irregular shapes with invisible nucleoli. Compared with the model group findings, the abnormal cell morphology was alleviated, neural necrosis was improved, and the number of living cells was increased in the TST-H and ANW groups (Figure 4D).
As shown in the Nissl staining results, in the sham group, numerous blue-dyed neurons were densely arranged, accompanied by abundant Nissl bodies. However, in the model group, the layer consisting of neurons became thinner. Most of the soma were swelled, deformed, necrosed, and even disintegrated. Meanwhile, Nissl body counts decreased sharply. However, more cells were alive and arranged neatly and normally in the TST-H and ANW groups (Figure 4E).
Oxidative stress
LPO, MDA, NO, and SOD levels in serum were measured as indicators of oxidative stress. As shown in Figure 5A-D, the serum levels of LPO and MDA were significantly higher in the model group than in the sham group. Compared with the model group findings, the serum level of LPO was decreased in the TST-H group, and that of MDA was lower in the TST-L and ANW groups. Meanwhile, the serum level of NO was significantly increased in the TST-L, TST-H, and ANW groups, and the serum level of SOD displayed a similar trend.
Figure 5.
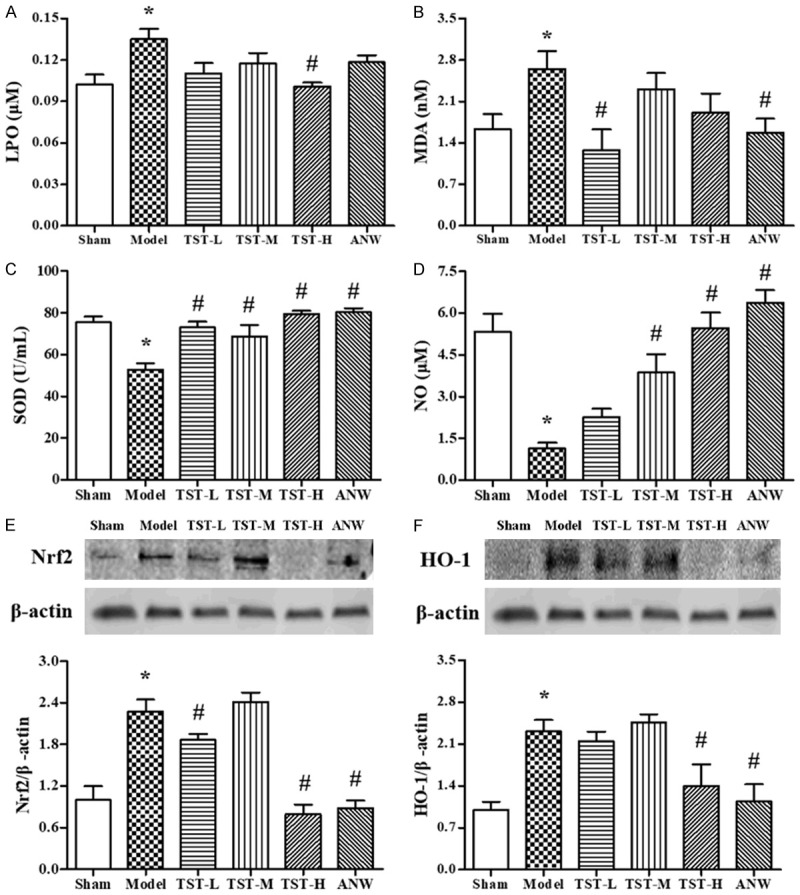
The impact of TSTs on oxidative stress. Notes: (A) LPO serum level, (B) MDA serum level, (C) the activity of SOD serum level and (D) NO serum level in MCAO rats after TST treatment. Data are expressed as the mean ± SD (n = 6). (E) Nrf2 and (F) HO-1 protein expressions in MCAO rats after TST treatment. Data are expressed as the mean ± SD (n = 3). *P < 0.05 vs. sham group; #P < 0.05 vs. model group. Abbreviations: TSTs, Tong Sheng tablets; ANW, Angong Niuhuang Wan; MCAO, middle cerebral artery occlusion; LPO, lipid peroxidation; MDA, malondialdehyde; SOD, superoxide dismutase; NO, nitric oxide; Nrf2, nuclear factor-erythroid 2 related factor 2; HO-1, heme oxygenase-1.
Meanwhile, Nrf2 and HO-1, two proteins related to oxidative stress in the cerebrum, were detected via western blot (Figure 5E, 5F). The results revealed that the protein expressions of Nrf2 and HO-1 in the model group were increased by more 100% compared with the sham group findings. At the same time, Nrf2 and HO-1 expressions were lower in the TST-L, TST-H, and ANW groups than in the model group.
Inflammatory cytokines
The serum levels of IL-1β and TNF-α were significantly higher in the model group than in the sham group. However, compared with the model group findings, serum IL-1β and TNF-α levels were remarkable decreased in the TST-L, TST-H, and ANW groups (Figure 6).
Figure 6.
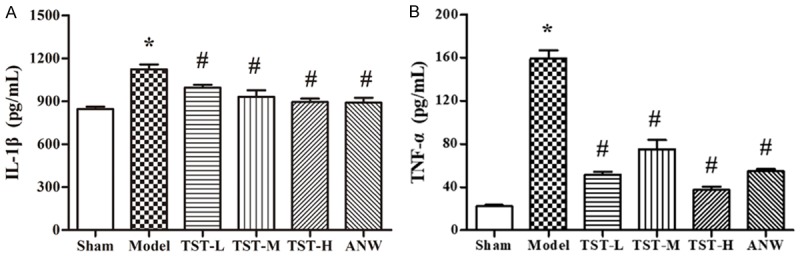
The impact of TSTs on inflammatory cytokines. Notes: (A) IL-1β serum level and (B) TNF-α serum level in MCAO rats after TSTs treatment. Data are expressed as the mean ± SD (n = 6). *P < 0.05 vs. sham group; #P < 0.05 vs. model group. Abbreviations: TSTs, Tong Sheng tablets; ANW, Angong Niuhuang Wan; MCAO, middle cerebral artery occlusion; IL-1β, interleukin1β; TNF-α, tumor necrosis factor-α.
Cell apoptosis
As shown in the TUNEL result (Figure 7A), the number of positive neurons was much higher in the model group than in the sham group. Compared with the model group findings, the number of positive neurons was significantly lower in all treatment groups, especially in TST-H and ANW groups. These results indicated that TSTs reduced apoptotic neuron counts in the cerebrum in MCAO rats.
Figure 7.
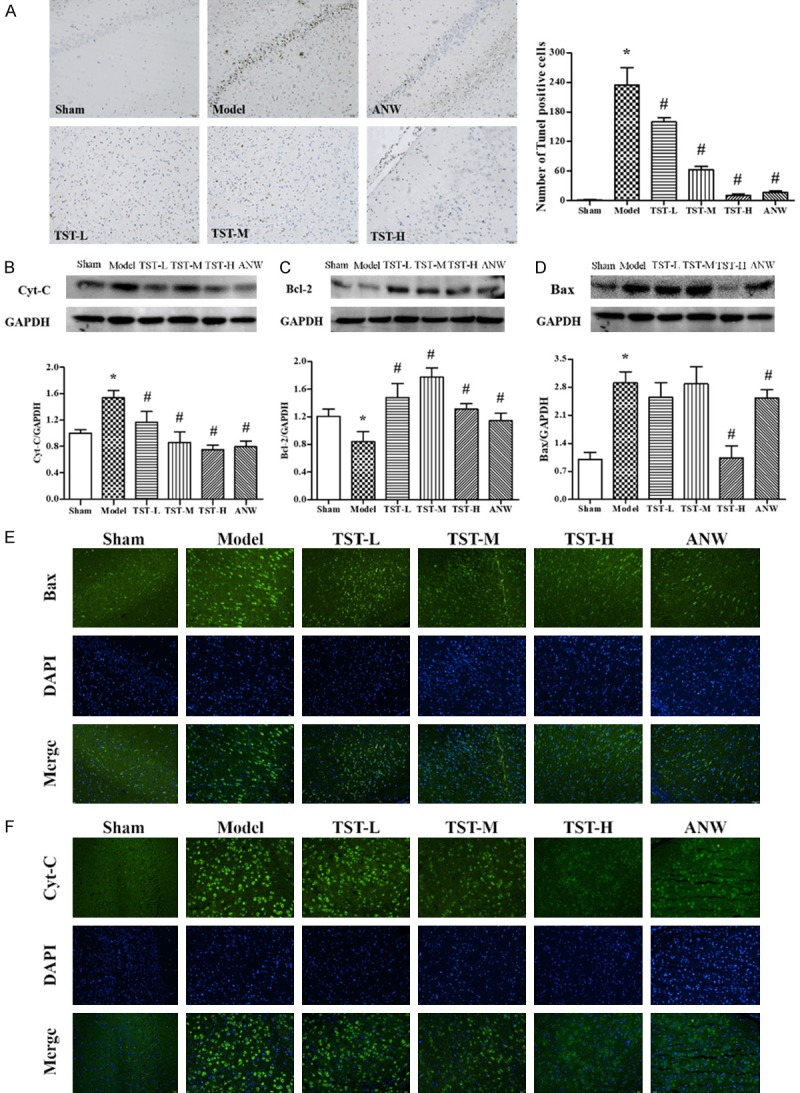
The impact of TSTs on cell apoptosis. Notes: (A) Cell apoptosis in MCAO rat brain tissue after TST treatment as displayed using TUNEL staining, ×200. Data are expressed as the mean ± SD (n = 6). (B) Cyt-C, (C) Bcl-2 and (D) Bax protein expressions in MCAO rat brain tissue after TSTs treatment. Data are expressed as the mean ± SD (n = 3). (E) Bax and (F) Cyt-C protein expressions in MCAO rat brain tissue after TSTs treatment as displayed via immunofluorescence, ×200. Scale bar: 50 μm. *P < 0.05 vs. sham group; #P < 0.05 vs. model group. Abbreviations: TSTs, Tong Sheng tablets; ANW, Angong Niuhuang Wan; MCAO, middle cerebral artery occlusion; Cyt-C, cytochrome C; Bcl-2, B-cell lymphoma-2; Bax, BCL2-associated X.
Bax, Bcl-2, and Cyt-C have close relationships with cell apoptosis. Compared with the sham group findings, the protein expressions of Cyt-C and Bax were increased in the model group, whereas Bcl-2 expression was decreased. The changes of these proteins were reversed in the TST-H and ANW groups (Figure 7B-D). Immunofluorescence assays confirmed the results of western blot for protein expressions of Bax and Cyt-C.
Autophagy
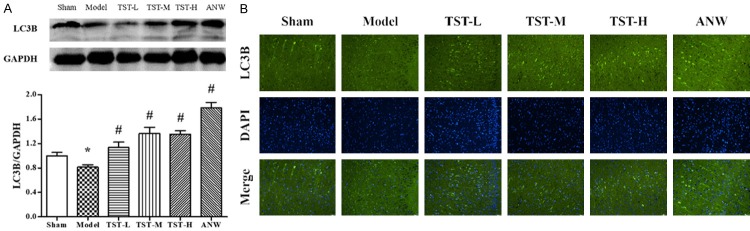
The expression of LC-3B, a protein related to autophagy, was decreased significantly in the model group compared with the sham group level. Its expression was remarkably increased in the ANW and TST-H groups (Figure 8A).
Figure 8.
The impact of TSTs on Autophagy. Notes: A and B. LC3B protein expression in MCAO rat brain tissue after TST treatment. Data are expressed as the mean ± SD (n = 3). *P < 0.05 vs. sham group; #P < 0.05 vs. model group. Scale bar: 50 μm. Abbreviations: TSTs, Tong Sheng tablets; ANW, Angong Niuhuang Wan; MCAO, middle cerebral artery occlusion; LC-3B, microtubule-associated protein 1 light chain 3B.
Fewer fluorescent and positive cells were found in the model group. However, in the treatment groups, both the fluorescent intensity and number of positive cells were obviously increased, especially in the TST-H and ANW groups (Figure 8B).
Subnetwork of validated signaling pathways
Among the predicted signaling pathways, experimental pharmacological studies have validated the involvement of oxidative stress response, inflammatory regulation, and apoptotic pathways in IRI treatment with TSTs. Figure 9 showed the potential relationship between drugs and IRS with testable targets in these pathways.
Figure 9.
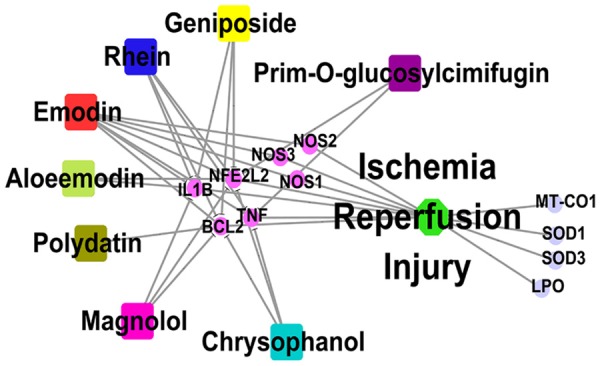
TST drug-target subnetwork associated with IRI.
Discussion
ICS is one of the most dangerous diseases threatening the health and lives of humans [25]. Cerebral ischemia blocks the delivery of oxygen and nutrients, so restoration of blood supply (reperfusion) remains the standard therapy for ischemia; however, reperfusion contributes to delayed secondary brain injury. CIRI often causes irreversible brain damage that leads to functional impairment and/or neuronal death. Hence, inhibition of reperfusion might be an effective approach for treating cerebral IRI.
Inflammation and oxidative stress, as the key factors involved in IRI, can interact with each other and further create a damaging cascade effect. On the one hand, numerous radicals will break through the endogenous anti-oxidative system under an oxidative stress condition. Some endogenous products such as LPO or MDA can further damage surrounding tissue, resulting in an inflammatory reaction and even influencing cell apoptosis and autophagy [26]. On the other hand, when brain tissue damaged, the levels of some pro-inflammatory cytokines such as TNF-α and IL-1β would increase, enhancing other inflammatory cytokines production such as ICAM-1 and ILs. At the same time, abnormally high levels of inflammatory cytokines might trigger the excessive production of radicals and LPO [27]. In addition, IRI was reported to result in neuronal damage or death in ischemic regions. Neuron autophagy and apoptosis are activated in stroke [28]. Therefore, enhancing neuron autophagy and reducing apoptosis might be the potential treatment strategies to rescue damaged neurons.
An MCAO-induced model, which was regarded as a classic animal model of CIRI, was adapted in this study because of its reliable reproducibility. In this model, the reperfusion time can be controlled artificially and precisely to minimize adverse effects on the whole body. Therefore, the model induced via MCAO is suitable for investigating the cerebral functional changes of CIRI and neuroprotective effects of potential agents [29]. In this study, we discovered that the serum levels of IL-1β, TNF-α, LPO and MDA were increased in model rats, whereas NO and SOD were decreased (Figures 5A-D and 6). The expression of oxidative stress-associated proteins (Nrf2 and HO-1, Figure 5E, 5F) and pro-apoptosis proteins (Cyt-C, Bax) were all upregulated, whereas anti-apoptosis (Bcl-2) and autophagy-associated proteins (LC-3B) were downregulated (Figures 7 and 8). These results indicate that oxidative stress, inflammatory response and apoptosis were enhanced whereas autophagic activity was inhibited in the MCAO-induced model.
TSTs, as a multicomponent formula containing eight Chinese herbs, the quality control analysis was conducted firstly using HPLC in the study. Along with referencing the Similarity Evaluation System for Chromatographic Fingerprint of TCM published by the Chinese Pharmacopoeia Commission (Version 2004A), we successfully identified 11 compounds in TSTs. Then, a network pharmacological method and integrated bioinformatics analysis were applied. Network pharmacological theory, which is based on systematic biology and poly-pharmacology, was first proposed by Hopkins in 2008 [30]. It tries to investigate a mechanism from an established network of drugs and genes as well as targets and diseases, which have complex interactions with each other. In fact, the integrality and complexity of this giant bioinformatics network are also similar to the characteristics of TCM [31]. At present, network pharmacology is widely used in the exploration of pathogenesis, drug development, identification of drug targets and many other areas [32]. In this study, we screened 10 possible components from the 11 identified chemicals by HPLC to examine their working targets and construct a compound-target-disease network. Then, we verified the possible key signaling pathways and mechanism using pharmacological experiments.
Herein, the protective effect of TSTs on CIRI was evaluated in a MCAO rat. TSTs alleviated dysneuria caused by IRI and decreased the cerebral infarct volume, as displayed in pathological sections in which ischemic damage of neurons was obviously improved (Figure 4). Further, TSTs alleviated inflammation by reducing the serum levels of IL-1β and TNF-α (Figure 6). TSTs ameliorated oxidization by decreasing the serum levels of LPO and MDA, increasing the serum levels of NO and SOD (Figure 5A-D), and upregulating the protein expressions of Nrf2 and HO-1 (Figure 5E, 5F). In addition, TSTs regulated apoptosis by reducig apoptotic neuron counts, inhibiting the protein expression of Bax, promoting the protein expression of Bcl-2, and decreasing the protein expression of Cyt-C (Figure 9). TSTs promoted autophagy through upregulating LC-3B (Figure 8). Therefore, TSTs reduced oxidative stress, inhibited inflammation, and regulated cell apoptosis and autophagic activity.
Collectively, the most effective components mainly functioned through comprehensive pathways and targets, including NO and Nrf2 pathways associated with oxidative stress, IL-1β and TNF pathways related to inflammation, and Bcl-2 and Cyt-C pathways associated with apoptosis.
Conclusion
Taken together, our study found that TSTs have notable effect on alleviating MCAO-induced CIRI in rats. Multiple factors may be partly responsible for the therapeutic effect of TSTs, including reducing oxidative stress, inhibiting inflammation, and regulating cell apoptosis and autophagic activity. This study suggests TSTs could be a potential agent for treating cerebral IRI.
Acknowledgements
This study was supported financially by Dongguan social science and technology development project (Grants no. 2016108101006).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Gouriou Y, Demaurex N, Bijlenga P, De MU. Mitochondrial calcium handling during ischemia-induced cell death in neurons. Biochimie. 2011;93:2060–2067. doi: 10.1016/j.biochi.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC. Roles of oxidative stress, apoptosis, PGC-1α and mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci. 2011;12:7199–215. doi: 10.3390/ijms12107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.del Zoppo GJ. Acute anti-inflammatory approaches to ischemic stroke. Ann N Y Acad Sci. 2010;1207:143–8. doi: 10.1111/j.1749-6632.2010.05761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun YJ. Effects of resveratrol on regulating apoptosis and autophagy in cerebral ischemia reperfusion in rats. Journal of Hainan Medical University. 2018 [Google Scholar]
- 5.Kim EJ, Kim SY, Lee JH, Kim JM, Kim JS, Byun JI, Koo BN. Effect of isoflurane post-treatment on tPA-exaggerated brain injury in a rat ischemic stroke model. Korean J Anesthesiol. 2015;68:281–6. doi: 10.4097/kjae.2015.68.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klayman DL. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985;228:1049–1055. doi: 10.1126/science.3887571. [DOI] [PubMed] [Google Scholar]
- 7.Corson TW, Crews CM. Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell. 2007;130:769–774. doi: 10.1016/j.cell.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller L, Su X. Artemisinin: discovery from the Chinese herbal garden. Cell. 2011;146:855–858. doi: 10.1016/j.cell.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vellas B, Coley N, Ousset PJ, Berrut G, Dartigues JF, Dubois B, Grandjean H, Pasquier F, Piette F, Robert P, Touchon J, Garnier P, Mathiex-Fortunet H, Andrieu S GuidAge Study Group. Long-term use of standardised ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): a randomised placebo-controlled trial. Lancet Neurol. 2012;11:851–9. doi: 10.1016/S1474-4422(12)70206-5. [DOI] [PubMed] [Google Scholar]
- 10.Sanz MA, Lococo F. Modern approaches to treating acute promyelocytic leukemia. J. Clin. Oncol. 2011;29:495–503. doi: 10.1200/JCO.2010.32.1067. [DOI] [PubMed] [Google Scholar]
- 11.Liu XS, Song NS. Huangdi Suwen Xuanming Lunfang. 2007 [Google Scholar]
- 12.Wu T. Wen Bing Tiao Bian. Science and Technology Literature Press; 2010. [Google Scholar]
- 13.Wang GH, Lan R, Zhen XD, Zhang W, Xiang J, Cai DF. An-Gong-Niu-Huang Wan protects against cerebral ischemia induced apoptosis in rats: up-regulation of Bcl-2 and down-regulation of Bax and caspase-3. J Ethnopharmacol. 2014;154:156–62. doi: 10.1016/j.jep.2014.03.057. [DOI] [PubMed] [Google Scholar]
- 14.Guan Q, Liang S, Wang Z, Yang Y, Wang S. 1H NMR-based metabonomic analysis of the effect of optimized rhubarb aglycone on the plasma and urine metabolic fingerprints of focal cerebral ischemia-reperfusion rats. J Ethnopharmacol. 2014;154:65–75. doi: 10.1016/j.jep.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Zhang N, Zhang X, Liu X, Wang H, Xue J, Yu J, Kang N, Wang X. Chrysophanol inhibits NALP3 inflammasome activation and ameliorates cerebral ischemia/reperfusion in mice. Mediators Inflamm. 2014;2014:370530. doi: 10.1155/2014/370530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miao Q, Wang S, Miao S, Wang J, Xie Y, Yang Q. Cardioprotective effect of polydatin against ischemia/reperfusion injury: roles of protein kinase C and mito K(ATP) activation. Phytomedicine. 2011;19:8–12. doi: 10.1016/j.phymed.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Qiao L, Xu W, Wang X, Li H, Xu W, Chu K, Lin Y. Paeoniflorin attenuates cerebral ischemia-induced injury by regulating Ca2+/CaMKII/CREB signaling pathway. Molecules. 2017;22:359. doi: 10.3390/molecules22030359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang PR, Wang JS, Yang MH, Kong LY. Neuroprotective effects of Huang-Lian-Jie-Du-Decoction on ischemic stroke rats revealed by (1)H NMR metabolomics approach. J Pharm Biomed Anal. 2014;88:106–16. doi: 10.1016/j.jpba.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 19.Chen JH, Kuo HC, Lee KF. Magnolol protects neurons against ischemia injury via the downregulation of p38/MAPK, CHOP and nitro tyrosine. Toxicol Appl Pharmacol. 2014;279:294–302. doi: 10.1016/j.taap.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, Bogousslavsky J, Bonny C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med. 2003;9:1180–6. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Bryant SH, Cheng T, Wang J, Gindulyte A, Shoemaker BA, Thiessen PA, He S, Zhang J. PubChem BioAssay: 2017 update. Nucleic Acids Res. 2017;45:D955–D963. doi: 10.1093/nar/gkw1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle artery occlusion without craniectomy. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 23.Koscielny G, An P, Carvalho-Silva D, Cham JA, Fumis L, Gasparyan R, Hasan S, Karamanis N, Maguire M, Papa E, Pierleoni A, Pignatelli M, Platt T, Rowland F, Wankar P, Bento AP, Burdett T, Fabregat A, Forbes S, Gaulton A, Gonzalez CY, Hermjakob H, Hersey A, Jupe S, Kafkas Ş, Keays M, Leroy C, Lopez FJ, Magarinos MP, Malone J, McEntyre J, Munoz-Pomer Fuentes A, O’Donovan C, Papatheodorou I, Parkinson H, Palka B, Paschall J, Petryszak R, Pratanwanich N, Sarntivijal S, Saunders G, Sidiropoulos K, Smith T, Sondka Z, Stegle O, Tang YA, Turner E, Vaughan B, Vrousgou O, Watkins X, Martin MJ, Sanseau P, Vamathevan J, Birney E, Barrett J, Dunham I. Open targets: a platform for therapeutic target identification and validation. Nucleic Acids Res. 2017;45:D985–D994. doi: 10.1093/nar/gkw1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spinelli L, Gambette P, Chapple CE, Robisson B, Baudot A, Garreta H, Tichit L, Guénoche A, Brun C. Clust&See: a cytoscape plugin for the identification, visualization and manipulation of network clusters. Bio Systems. 2013;113:91–95. doi: 10.1016/j.biosystems.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Li G, Morris-Blanco KC, Lopez MS, Yang T, Zhao H, Vemuganti R, Luo Y. Impact of microRNAs on ischemic stroke: from pre- to post-disease. Prog Neurobiol. 2018;163-164:59–78. doi: 10.1016/j.pneurobio.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu J, Chen J, Yang N, Hou X, Wang J, Tan X, Feng L, Jia X. Combination of ligusticum chuanxiong and radix paeoniae ameliorate focal cerebral ischemic in MCAO rats via endoplasmic reticulum stress-dependent apoptotic signaling pathway. J Ethnopharmacol. 2016;187:313–324. doi: 10.1016/j.jep.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 27.Chamorro Á, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15:869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 28.Wang P, Shao BZ, Deng Z, Chen S, Yue Z, Miao CY. Autophagy in ischemic stroke. Prog Neurobiol. 2018;163-164:98–117. doi: 10.1016/j.pneurobio.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Shi J, Bui JD, Yang SH, He Z, Lucas TH, Buckley DL, Blackband SJ, King MA, Day AL, Simpkins JW. Estrogens decrease reperfusion-associated cortical ischemic damage an MRI analysis in a transient focal ischemia model. Stroke. 2001;32:987–992. doi: 10.1161/01.str.32.4.987. [DOI] [PubMed] [Google Scholar]
- 30.Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682–90. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 31.Shao LI, Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med. 2013;11:110–20. doi: 10.1016/S1875-5364(13)60037-0. [DOI] [PubMed] [Google Scholar]
- 32.Kibble M, Saarinen N, Tang J, Wennerberg K, Mäkelä S, Aittokallio T. Network pharmacology applications to map the unexplored target space and therapeutic potential of natural products. Natural Product Reports. 2015;32:1249–1266. doi: 10.1039/c5np00005j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.