Abstract
Zinc finger protein ZNF503 is an important regulator during developmental process and tumor initiation. ZNF503 drives tumor development and process and was cancer-specific dysregulated in cancers. However, its expression and function in hepatocellular carcinoma (HCC) still need to be studied and elucidated. In this study, we demonstrated for the first time that ZNF503 mRNA and protein was up-regulated in HCC tissues and cell lines. Clinical data showed that high ZNF503 was significantly correlated with poor prognostic features, including advanced TNM stage and venous invasion. Moreover, ZNF503 was identified as a potential 5-year prognostic marker of HCC patients. Notably, ZNF503 promoted migration, invasion and EMT progress. ZNF503 was recruited to GATA3 promoter and inhibited its expression. GATA3 inhibited HCC cells migration, invasion and EMT process. Furthermore, we demonstrated that ZNF503 expression was regulated by miR-495. In HCC tissues. MiR-495 has an inverse correlation with ZNF503 expression. Conclusively, our data revealed that ZNF503 promoted migration, invasion and EMT process through regulating GATA3 expression, which was regulated by miR-495, suggesting the potential therapeutic value for HCC.
Keywords: ZNF503, hepatocellular carcinoma, GATA3, miR-495, EMT
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancer and represents the third leading cause of cancer-related mortality worldwide [1,2]. The patients in China accounts for about half of the total number of cases and deaths due to the prevalence of hepatitis B virus [3]. In spite of advancement in diagnostic and treatment strategies, the prognosis of patients with HCC remains poor because of distant metastasis and recurrence [4-6]. Therefore, the identification of novel biomarkers involved in HCC progression is urgent and important for improving the clinical outcome.
ZNF503, also called Zeppo2 (zinc finger elbow-related proline domain protein 2, Zpo2), was evolutionary conserved zinc finger protein. It belongs NET subfamily and related to the Sp family of transcription factors, which was important mediator during developmental processes and more recently to cancer [7-10]. ZNF503 drives aggressive breast cancer progression by down-regulation of GATA3 expression [11]. LncRNA ZNF503-AS1 promotes retinal pigment epithelium differentiation by down-regulating ZNF503 expression [12]. The transcriptional repressor ZNF503 promotes mammary epithelial cell proliferation and enhances cell invasion [13]. ZNF503 also is a cell type-selective expression and performs critical role in the developing mouse striatum [14]. Moreover, expression of the NET family member ZNF503 is regulated by hedgehog and BMP signaling in the limb [15]. ZNF503 also involved in regulating spatiotemporal expression of Nolz-1 during mouse embryogenesis [16]. However, the functional effects of ZNF503 and its mechanisms are still largely unknown in HCC progression.
In this study, we demonstrated for the first time that ZNF503 expression was significantly up-regulated in human HCC tissues and cells. Its overexpression was associated with adverse clinical features and poor survival. We also confirmed that ZNF503 promoted HCC cell migration, invasion and EMT phenotype by gain- and loss-of-function in vitro and in vivo. Moreover, we identified that ZNF503 is recruited to GATA3 promoter and regulated GATA3 expression in downstream and the upstream of ZNF503 expression was regulated by miR-495 in HCC cells. Taken together, we confirmed that ZNF503 plays an oncogene role in HCC progression and identify ZNF503 as a candidate gene for future diagnostic and therapeutic strategies.
Materials and methods
Clinical specimens
95 pairs of hepatocellular carcinomas (HCC) tissues and adjacent non-tumor tissues were obtained from patients who received surgical resection at the First Affiliated Hospital of Xi’an Jiaotong University. All the specimens were snap-frozen in liquid nitrogen and kept at -80°C after surgical removal. This study was approved by the Ethics Committee of this hospital. The written informed consents had been obtained from all patients before study.
Cell culture
All cell lines used in this study, including HCC cell lines (Hep3B, Huh7, SMMC-7721 and MHCC-97H) and normal hepatic cell line (LO2) were commercially obtained from the Institute of Biochemistry and Cell Biology (Chinese Academy of Sciences, Shanghai, China). Cell culture was performed in DMEM medium (Thermo Fisher, Grand Island, NY, USA) which containing 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA) and 100 U/mL penicillin-streptomycin mixture (Beyotime Institute of Biotechnology, Haimen, China). The conditions for cell culture are as follows: a humidified atmosphere with 5% CO2 at 37°C.
RNA extraction and quantitative real-time PCR (qRT-PCR)
The total RNA from GC cells and tissues was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. cDNA was synthesized by TaqMan miRNA reverse transcription (Applied Biosystems, Foster City, CA, USA) and a PrimeScript Reverse Transcriptase kit (Takara, Dalian, China). The relative expression of miR-495 and ZNF503 mRNA were quantified using miRNA-specific TaqMan miRNA Assay Kit (Applied Biosystems) and the SYBR Premix Ex TaqTM Kit (Takara, Shiga, Japan) in the Applied Biosystems 7500 Sequence Detection system. Primers for miR-495 and ZNF503 were obtained from Genecopoeia (Guangzhou, China).
Western blot
Immunohistochemical analysis status was performed as our previously publication [17-19]. Celll lysates were prepared using RIPA lysis Buffer (RIPA; Pierce, Rockford, IL). Then protease inhibitors were added into lysates. A bicinchoninic acid (BCA) kit (Beyotime Institute of Biotechnology, Shanghai, China) was used to determine protein concentration. Subsequently, protein was separated in 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) membrane and then transferred onto a polyvinylidene difluoride (PVDF) membrane (Mippore, Merck KGaA, Germany). Tris-buffered saline (TBS) containing 5% nonfat milk was used to block the membranes for an hour at room temperature. Thereafter, the membranes were incubated with specific primary antibodies at 4°C overnight. Subsequently, HRP-conjugated secondary antibody goat anti-rabbit IgG (1:2000, Abcam) was added and incubated for 2 hours at room temperate. GAPDH was considered as the internal control. Bands signal were detected using the enhanced chemiluminescence (ECL; Millipore, Merck KGaA, Germany).
Transwell assay
The capacity of cells migration and invasion was evaluated via transwell assay. The upper chamber was pre-coated with BioCoat Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) for invasion assay, whereas migration assay was not pre-coated with BioCoat Matrigel. Subsequently, 200 μl cells were seeded into upper chamber which mixed with serum-free medium, 500 μl DMEM medium containing 10% FBS was added into the lower chamber. After 24 hours incubation, cells on the upper chamber surface were removed using cotton swabs. Cells on the lower surface were fixed in 4% paraformaldehyde and stained with 0.5% crystal violet. Finally, NIS Elements image software (Nikon, Tokyo, Japan) was used to detect the number of migrating and invading cells.
Immunohistochemistry (IHC) staining and scoring
Immunohistochemical analysis status was performed as our previously publication [20,21].
Luciferase reporter assay
The sequence of ZNF503 3’-UTR containing the putative miR-495 binding region was amplified from human genomic DNA. Then the sequence was cloned into pGL3 luciferase reporter vector (Promega, Madison, WI, USA). The potential miR-495 binding sites were mutated by the Quick-change site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA, USA). The wild type (wt) ZNF503 3’-UTR vector or mutant (mt) ZNF503 3’-UTR vector and miR-495 mimics or miR-495 inhibitors were co-transfected into MHCC-97H cells by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). The luciferase activity was measured using Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) under luminometer (Berthold Detection System, Pforzheim, Germany), and luciferase activity was normalized to Renilla activity.
Statistical analysis
In this study, statistical analyses were performed with SPSS 17.0 software (Abbott Laboratories, Chicago, IL). All experiments were repeated for at least 3 times. Data represent mean ± standard deviation (SD) of more than two independent experiments. A two-sided Student’s t-test and one-way ANOVA were separately used to compare the statistical differences. The Kaplan-Meier method was used to estimate the overall survival (OS) of HCC patients with high or low level of ZNF503. Expression association of genes in HCC tissues was analyzed with Pearson’s correlation analysis. Difference was considered statistically significant when P<0.05.
Results
ZNF503 was up-regulated in human HCC tissues and cell lines
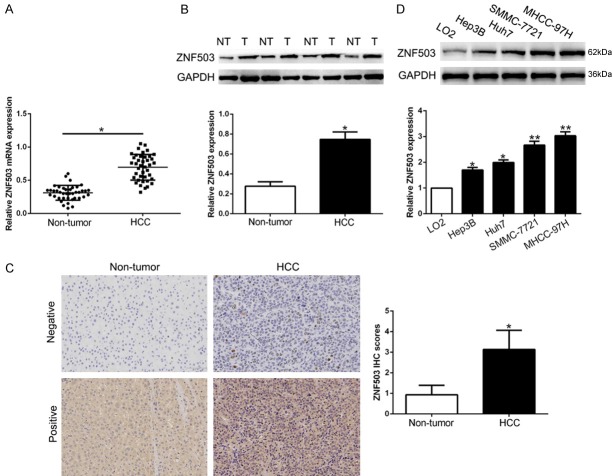
To confirm the expression of ZNF503 in HCC, we performed qRT-PCR and Western blot in randomly selected 40 HCC tissues and corresponding adjacent non-tumor tissues. The data showed that ZNF503 mRNA and protein expression was significantly higher in HCC tissues than that in non-tumor tissues (P<0.05, Figures 1A, 1B, S1). Furthermore, IHC staining showed that ZNF503 in HCC tissues was remarkedly increased compared to normal tissues (P<0.05, Figure 1C). In addition, the WB data revealed that ZNF503 was obviously up-regulated in a panel of HCC cells compared with normal immortalized hepatic cell LO2 (P<0.05, Figures 1D, S1). These data indicated that ZNF503 may be an oncogene in HCC progression.
Figure 1.
ZNF503 is up-regulated in HCC tissues and cell lines. A. Relative ZNF503 mRNA expression levels in HCC tissues and matched adjacent non-tumor tissues were measured by qRT-PCR. B. Representative Western blot analysis of ZNF503 expression in the HCC (T) and non-tumor tissues (NT). C. Representative ZNF503 IHC staining in differentiated HCC and adjacent non-tumor tissues. D. Comparing differences in the expression level of ZNF503 protein between HCC cell lines and the normal immortalized hepatic cell line LO2. n = three repeats with similar results; *P<0.05 by ANOVA. *P<0.05.
Clinical importance of ZNF503 in pathological features and prognosis of HCC
To confirm the clinical importance of ZNF503 in HCC, we determine the median value as the cut-off and the high-expression ZNF503 was significantly associated with tumor-node-metastasis (TNM) stage (III+IV, P=0.008) and venous invasion (P=0.023) (Table 1). Kaplan-Meier survival cure showed that patients with high ZNF503 had worse disease-free survival (DFS, P=0.0005, Figure 2A) and overall survival rates (OS, P=0.0002, Figure 2B). These findings suggest that ZNF503 had significant pathological roles in HCC progression.
Table 1.
Clinical correlation of ZNF503 expression in HCC (n=95)
| Clinical parameters | Cases (n) | Expression level | P value (* P<0.05) | |
|---|---|---|---|---|
|
| ||||
| ZNF503high (n=48) | ZNF503low (n=47) | |||
| Age (years) | ||||
| <50 years | 41 | 21 | 20 | 0.906 |
| ≥50 years | 54 | 27 | 27 | |
| Gender | ||||
| Male | 78 | 40 | 38 | 0.752 |
| Female | 17 | 8 | 9 | |
| Tumor size (cm) | 0.218 | |||
| <5 cm | 76 | 36 | 40 | |
| ≥5 cm | 19 | 12 | 7 | |
| Tumor number | 0.526 | |||
| Solitary | 85 | 42 | 43 | |
| Multiple | 10 | 6 | 4 | |
| Edmondson | ||||
| I+II | 67 | 33 | 34 | 0.701 |
| III+IV | 28 | 15 | 13 | |
| TNM stage | 0.008* | |||
| I+II | 74 | 32 | 42 | |
| III+IV | 21 | 16 | 5 | |
| Capsular infiltration | 0.509 | |||
| Present | 72 | 35 | 37 | |
| Absent | 23 | 13 | 10 | |
| Venous infiltration | 0.023* | |||
| Present | 14 | 11 | 3 | |
| Absent | 81 | 37 | 44 | |
| AFP | 0.963 | |||
| <400 ng/ml | 16 | 8 | 8 | |
| ≥400 ng/ml | 79 | 40 | 39 | |
| HBsAg | 0.975 | |||
| Positive | 87 | 44 | 43 | |
| Negative | 8 | 4 | 4 | |
P<0.05.
Figure 2.
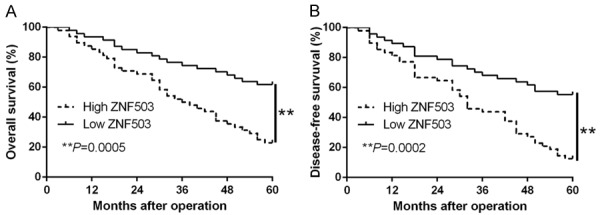
The prognostic value of ZNF503 for HCC patients. HCC patients with higher expression of ZNF503 had worse (A) overall survival and (B) disease-free survival. **P<0.01.
ZNF503 promotes HCC cell migration and invasion
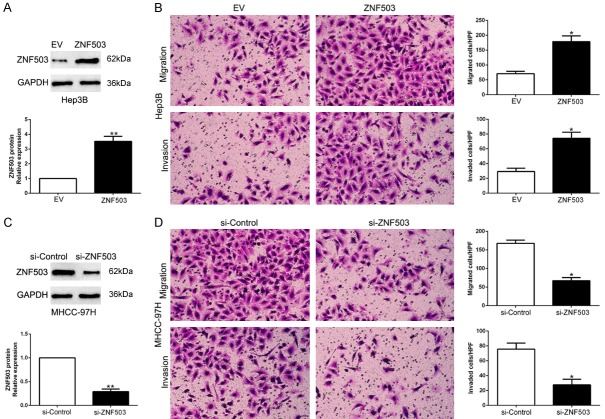
To determine the biological effects of ZNF503 in HCC, we transfected Hep3B or MHCC-97H cells whose endogenous ZNF503 level was lowest and highest with corresponding vectors (P<0.05, Figures 3A, 3C, S2). We confirmed that ZNF503 overexpression significantly promoted HCC cell migration and invasion in Hep3B (P<0.05, Figure 3B). On the contrary, ZNF503 knockdown led to a prominent reduction of cell migration and invasion (P<0.05, Figure 3D). These results suggest that ZNF503 regulates the migration and invasion of HCC cells.
Figure 3.
ZNF503 promotes HCC cell migration and invasion. A. Hep3B cells that were transfected with empty vector (EV) or ZNF503 retroviruses were subjected to western blot for ZNF503. *P<0.05 by t test. B. Cell migration and invasion as measured by Transwell assays were increased by ZNF503 overexpression in Hep3B cells as compared with control cells. *P<0.05 by t test. HPF: high power field. C. MHCC-97H cells that were transfected with scrambled siRNA or ZNF503 siRNA were subjected to western blot for ZNF503. *P<0.05 by t test. D. ZNF503 knockdown MHCC-97H cells conferred a smaller number of migrated and invaded cells as compared with control cells. *P<0.05 by t test. HPF: high power field.
ZNF503 promotes epithelial-to-mesenchymal transition in HCC cells
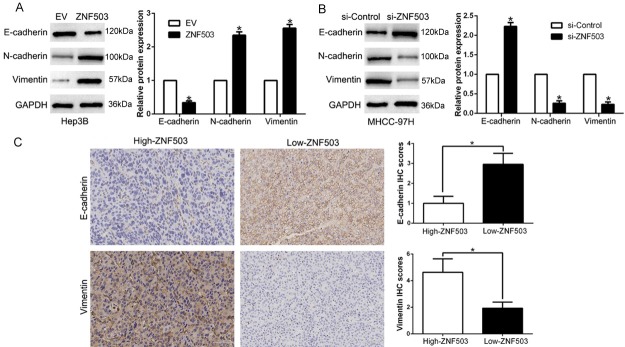
EMT has been identified as a critical phenotype in the initiation of metastasis progression of HCC [22,23]. We confirmed that ZNF503 overexpression decreased the epithelial marker E-cadherin and increased the mesenchymal marker N-cadherin and Vimentin (P<0.05, Figures 4A, S3). ZNF503 knockdown showed opposite effects in MHCC-97H cells (P<0.05, Figures 4B, S3). Moreover, in HCC tissues, we found that the E-cadherin expression in high-ZNF503 group was lower than that in low-ZNF503 group. Conversely, the expression level of Vimentin in the high-ZNF503 was obviously higher than that in low-ZNF503 group (P<0.05, Figure 4C). In conclusion, these data indicated that ZNF503 function as a promoter of EMT in HCC cells.
Figure 4.
ZNF503 promotes epithelial-to-mesenchymal transition in HCC cell. The overexpression of ZNF503 in Hep3B cells reduced the expression of the epithelial cell marker E-cadherin and increased the expression of the mesenchymal cell marker N-cadherin and Vimentin determined by western blot (A). In contrast, ZNF503 knockdown increased E-cadherin expression and reduced N-cadherin and Vimentin expression (B). (C) Immunohistochemical analysis of E-cadherin and Vimentin in HCC samples. In cases of high ZNF503 expression; there was no detectable E-cadherin (left, up) and strong Vimentin protein (down, right) expression in tissue section. In contrast, in the case of low ZNF503 expression, there was strong E-cadherin (right, up) and no detectable Vimentin protein (right, down) expression. Values are depicted as Mean ± SD; *P<0.05 by t test.
ZNF503 down-regulates GATA3 expression through directly binding to its promoter
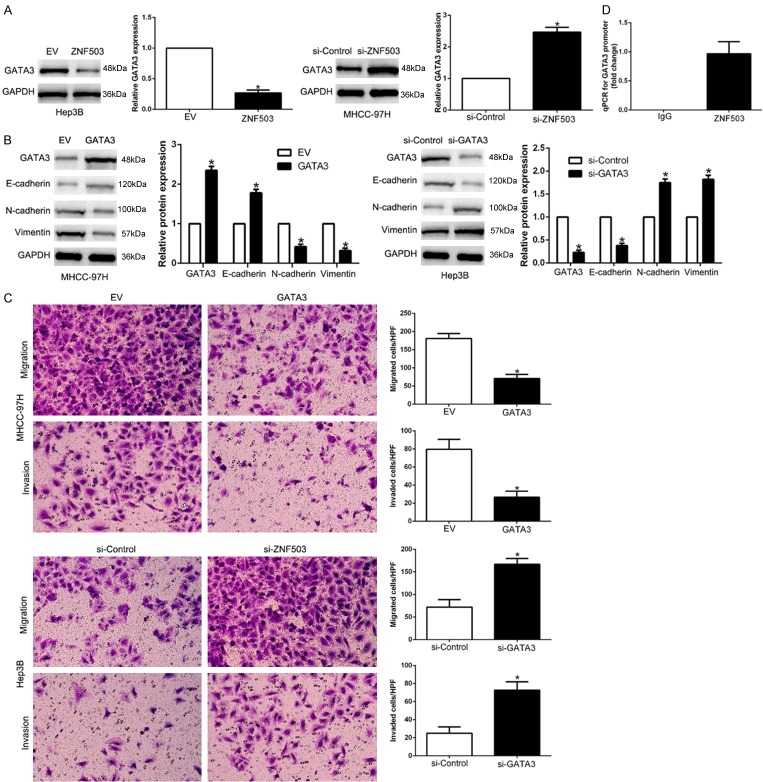
To unveil the downstream signaling pathway of ZNF503, we found ZNF503 could regulate GATA3 expression to participate EMT phenotype. We found that ZNF503 down-regulated GATA3 levels and ZNF503 knockdown enhanced GATA3 expression (P<0.05, Figures 5A, S4). Previous studies confirmed that GATA3 functions as a tumor suppressor partly blocks EMT-associated gene and interfere with cellular invasiveness [24]. Here, our data showed that GATA3 regulated EMT-associated gene expression (P<0.05, Figures 5B, S4) and cellular migration, invasion (P<0.05, Figure 5C). To confirm that ZNF503 inhibits GATA3 transcriptional activity, we performed ChIP analysis to validate that ZNF503 occupy the GATA3 promoter region (P<0.05, Figure 5D). Taken together, our data indicated that ZNF503 down-regulated GATA3 through binding to its promoter.
Figure 5.
ZNF503 down-regulates GATA3 expression through directly binding to its promoter. A. ZNF503 overexpression inhibited GATA3 expression while ZNF503 knockdown increased GATA3 expression. B. GATA3 overexpression promoted EMT process. C. GATA3 overexpression inhibited HCC migration and invasion. D. ChIP analysis indicating the presence of ZNF503 on the GATA3 promoter. qRT-PCR analysis was performed using primers specific to the GATA3 promoter. *P<0.05.
ZNF503 expression was regulated by miR-495
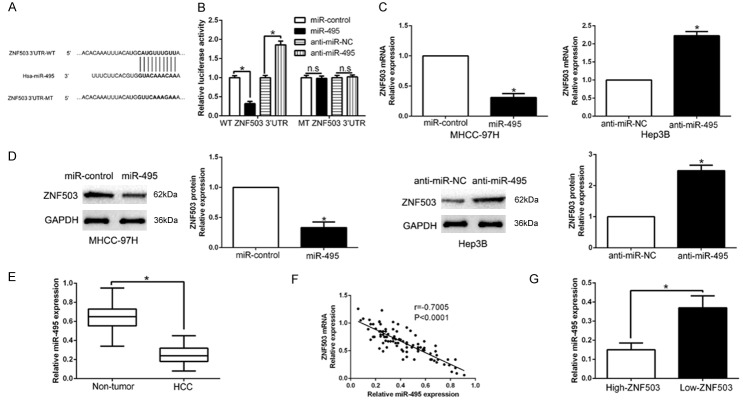
Previous studies reported that dysregulated miRNAs were involved in HCC progression [25]. To elucidate the upstream that ZNF503 was up-regulated, we searched the database Targetscan to predict that miR-495 could bind to the 3’UTR of ZNF503 (Figure 6A). We performed luciferase reporter assays to confirm that miR-495 overexpression significantly inhibited whereas miR-495 knockdown promoted the luciferase activity of HCC cells with wild-type ZNF503 3’UTR (P<0.05, Figure 6B). However, the activity in mild-type ZNF503 3’UTR had no change (Figure 6B). Moreover, miR-495 overexpression significantly suppressed while miR-495 knockdown promoted ZNF503 mRNA and protein in HCC cells (P<0.05, Figures 6C, 6D, S5). In HCC tissues, our results showed that miR-495 was down-regulated in HCC tissues compared to adjacent non-tumor tissues (P<0.05, Figure 6E), which was consistent with previous studies [26]. We also found an inverse correlation between ZNF503 mRNA and miR-495 in HCC tissues (r=-0.7005, P<0.05, Figure 6F). In addition, miR-495 in high ZNF503 group was lower than that in low-ZNF503 group (P<0.05, Figure 6G). Collectively, we disclosed that miR-495 regulated ZNF503 expression in HCC tissues.
Figure 6.
ZNF503 is identified as a direct target of miR-495 in HCC. A. miR-495 and its putative binding sequence in the 3’-UTR of ZNF503. The mutant binding site was generated in the complementary site for the seed region of miR-495. B. miR-495 significantly suppresses the luciferase activity that carried wild-type (wt) but not mutant (mt) 3’-UTR of ZNF503. Anti-miR-495 led to a notable increase in the luciferase activity of wt 3’-UTR of ZNF503. N.S means no significance. C. qRT-PCR analysis of ZNF503 mRNA expression in MHCC-97H cells with miR-495 or miR-control vector transfection and Hep3B cells with anti-miR-495 or anti-miR-NC vector transfection. D. Overexpression of miR-495 reduced the expression of ZNF503 protein in MHCC-97H cells and knockdown of miR-495 increases the level of ZNF503 protein in Hep3B cells. E. The expression level of miR-495 in HCC tissues and adjacent non-tumor tissues. F. A significant inverse correlation between the mRNA levels of ZNF503 and miR-495 was observed in HCC tissues. G. The expression of miR-495 in ZNF503 high-expressing tumors was significantly lower than that in ZNF503 low-expressing tumors. n = six repeats with similar results, *P<0.05.
Discussion
Members of the NET subfamily of zinc-finger proteins are related to the Sp-family of transcription factors and are required during embryogenesis and more recently to cancer. Among them, ZNF703 acts as a repressor of transcription and hypothesize that other NET family members function in a similar manner [27]. In particular, ZNF703 and ZNF503 are required for formation of rhombomere 4 of the vertebrate hindbrain [28]. However, the role of ZNF503 in HCC remains largely unknown. Here, we demonstrated for the first time that ZNF503 mRNA and protein were up-regulated in HCC tissues and cells. Its overexpression was significantly correlated with TNM stage and venous invasion. Moreover, ZNF503 was a useful prognostic marker for OS and DFS survival in HCC patients. Taken together, these data suggest that ZNF503 acts as an oncogene in progression of HCC.
To confirm the biological function of ZNF503 in HCC, we performed gain- and loss-of-function experiment to show that ZNF503 promoted HCC migration and invasion. EMT has been recognized as a physiological process in the invasion and metastasis of various cancers through transformation of polarized and adherent cells into an invasive mesenchymal cell phenotype. Our data showed ZNF503 promoted EMT phenotype. Moreover, ZNF503 was positively correlated with EMT process in HCC tissues. GATA3 was an important transcription factor to regulate cell differentiation. Here, we showed that ZNF503 can regulated GATA3 expression and GATA3 inhibited HCC migration, invasion and EMT. ChIP assays revealed that ZNF503 recruited to GATA3 promoter. Our data provide insight that ZNF503 promoted HCC progression via GATA3 regulation. Previous studies reported that aberrant miRNAs were crucial for initiation and progression of human cancers. We searched database and showed that miR-495 could bind with ZNF503 3’UTR. Luciferase assays showed that miR-495 directly bind to the 3’UTR of ZNF503. In addition, miR-495 negatively regulated the expression of ZNF503 mRNA and protein in HCC cells. In HCC tissues, miR-495 showed an inverse correlation of ZNF503 expression. This data was same to previous studies. miR-495 suppresses cell proliferation and invasion of hepatocellular carcinoma by directly targeting insulin-like growth factor receptor-1 [26]. miR-495 inhibits proliferation, migration and invasion and induces apoptosis via inhibiting PBX3 in melanoma cells [29]. miR-495 inhibits proliferation, metastasis and promotes apoptosis by targeting Twist1 in gastric cancer cells [30]. These data further confirm that miR-495 exerts a tumor suppressor in different cancers. In conclusion, these data suggest that ZNF503 was a downstream target of miR-495 in HCC.
In conclusion, we reported for the first time that ZNF503 was up-regulated in HCC tissues and cells. Its overexpression was correlated with malignant clinical features and unfavorable prognosis. Gain- and loss-of-function experiment confirm that ZNF503 promotes migration, invasion and EMT progress. Moreover, miR-495 regulated ZNF503 expression in HCC cells. These data indicated that ZNF503 was an important biomarker of HCC progression and a novel and attractive therapeutic target for HCC treatment.
Conclusions
We reported for the first time that ZNF503 was up-regulated in HCC tissues and cells. Its overexpression was correlated with malignant clinical features and unfavorable prognosis. Gain- and loss-of-function experiment confirm that ZNF503 promotes migration, invasion and EMT progress. Moreover, miR-495 regulated ZNF503 expression in HCC cells. These data indicated that ZNF503 was an important biomarker of HCC progression and a novel and attractive therapeutic target for HCC treatment.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81572847, 81874069), the Natural Science Basic Research Plan in Shaanxi Province of China (2017JM8002) and Key clinical research fund of The First Affliated Hospital of Xi’an Jiaotong University (XJTU1AF-CRF-2016-002).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Chen E, Xu X, Liu R, Liu T. Small but heavy role: microRNAs in hepatocellular carcinoma progression. Biomed Res Int. 2018;2018:6784607. doi: 10.1155/2018/6784607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 5.Forner A, Bruix J. Biomarkers for early diagnosis of hepatocellular carcinoma. Lancet Oncol. 2012;13:750–751. doi: 10.1016/S1470-2045(12)70271-1. [DOI] [PubMed] [Google Scholar]
- 6.Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394–399. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 7.Holland DG, Burleigh A, Git A, Goldgraben MA, Perez-Mancera PA, Chin SF, Hurtado A, Bruna A, Ali HR, Greenwood W, Dunning MJ, Samarajiwa S, Menon S, Rueda OM, Lynch AG, McKinney S, Ellis IO, Eaves CJ, Carroll JS, Curtis C, Aparicio S, Caldas C. ZNF703 is a common Luminal B breast cancer oncogene that differentially regulates luminal and basal progenitors in human mammary epithelium. EMBO Mol Med. 2011;3:167–180. doi: 10.1002/emmm.201100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sircoulomb F, Nicolas N, Ferrari A, Finetti P, Bekhouche I, Rousselet E, Lonigro A, Adelaide J, Baudelet E, Esteyries S, Wicinski J, Audebert S, Charafe-Jauffret E, Jacquemier J, Lopez M, Borg JP, Sotiriou C, Popovici C, Bertucci F, Birnbaum D, Chaffanet M, Ginestier C. ZNF703 gene amplification at 8p12 specifies luminal B breast cancer. EMBO Mol Med. 2011;3:153–166. doi: 10.1002/emmm.201100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang G, Ma F, Zhong M, Fang L, Peng Y, Xin X, Zhong J, Yuan F, Gu H, Zhu W, Zhang Y. ZNF703 acts as an oncogene that promotes progression in gastric cancer. Oncol Rep. 2014;31:1877–1882. doi: 10.3892/or.2014.2997. [DOI] [PubMed] [Google Scholar]
- 10.Ma F, Bi L, Yang G, Zhang M, Liu C, Zhao Y, Wang Y, Wang J, Bai Y, Zhang Y. ZNF703 promotes tumor cell proliferation and invasion and predicts poor prognosis in patients with colorectal cancer. Oncol Rep. 2014;32:1071–1077. doi: 10.3892/or.2014.3313. [DOI] [PubMed] [Google Scholar]
- 11.Shahi P, Wang CY, Lawson DA, Slorach EM, Lu A, Yu Y, Lai MD, Gonzalez Velozo H, Werb Z. ZNF503/Zpo2 drives aggressive breast cancer progression by down-regulation of GATA3 expression. Proc Natl Acad Sci U S A. 2017;114:3169–3174. doi: 10.1073/pnas.1701690114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Jiang C, Qin B, Liu G, Ji J, Sun X, Xu M, Ding S, Zhu M, Huang G, Yan B, Zhao C. LncRNA ZNF503-AS1 promotes RPE differentiation by downregulating ZNF503 expression. Cell Death Dis. 2017;8:e3046. doi: 10.1038/cddis.2017.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahi P, Slorach EM, Wang CY, Chou J, Lu A, Ruderisch A, Werb Z. The transcriptional repressor ZNF503/Zeppo2 promotes mammary epithelial cell proliferation and enhances cell invasion. J Biol Chem. 2015;290:3803–3813. doi: 10.1074/jbc.M114.611202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ko HA, Chen SY, Chen HY, Hao HJ, Liu FC. Cell type-selective expression of the zinc finger-containing gene Nolz-1/Zfp503 in the developing mouse striatum. Neurosci Lett. 2013;548:44–49. doi: 10.1016/j.neulet.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 15.McGlinn E, Richman JM, Metzis V, Town L, Butterfield NC, Wainwright BJ, Wicking C. Expression of the NET family member Zfp503 is regulated by hedgehog and BMP signaling in the limb. Dev Dyn. 2008;237:1172–1182. doi: 10.1002/dvdy.21508. [DOI] [PubMed] [Google Scholar]
- 16.Chang SL, Liu YC, Chen SY, Huang TH, Liu PT, Liu FC. Identification of two evolutionarily conserved 5’ cis-elements involved in regulating spatiotemporal expression of Nolz-1 during mouse embryogenesis. PLoS One. 2013;8:e54485. doi: 10.1371/journal.pone.0054485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z, Wang Y, Dou C, Sun L, Li Q, Wang L, Xu Q, Yang W, Liu Q, Tu K. MicroRNA-1468 promotes tumor progression by activating PPAR-gamma-mediated AKT signaling in human hepatocellular carcinoma. J Exp Clin Cancer Res. 2018;37:49. doi: 10.1186/s13046-018-0717-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Liu Z, Wang Y, Dou C, Xu M, Sun L, Wang L, Yao B, Li Q, Yang W, Tu K, Liu Q. Hypoxia-induced up-regulation of VASP promotes invasiveness and metastasis of hepatocellular carcinoma. Theranostics. 2018;8:4649–4663. doi: 10.7150/thno.26789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Tu K, Wang Y, Yao B, Li Q, Wang L, Dou C, Liu Q, Zheng X. Hypoxia accelerates aggressiveness of hepatocellular carcinoma cells involving oxidative stress, epithelial-mesenchymal transition and non-canonical hedgehog signaling. Cell Physiol Biochem. 2017;44:1856–1868. doi: 10.1159/000485821. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Dou C, Yao B, Xu M, Ding L, Wang Y, Jia Y, Li Q, Zhang H, Tu K, Song T, Liu Q. Ftx non coding RNA-derived miR-545 promotes cell proliferation by targeting RIG-I in hepatocellular carcinoma. Oncotarget. 2016;7:25350–25365. doi: 10.18632/oncotarget.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z, Dou C, Yao B, Xu M, Ding L, Wang Y, Jia Y, Li Q, Zhang H, Tu K, Song T, Liu Q. Methylation-mediated repression of microRNA-129-2 suppresses cell aggressiveness by inhibiting high mobility group box 1 in human hepatocellular carcinoma. Oncotarget. 2016;7:36909–36923. doi: 10.18632/oncotarget.9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guarino M. Epithelial-mesenchymal transition and tumour invasion. Int J Biochem Cell Biol. 2007;39:2153–2160. doi: 10.1016/j.biocel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Ma L, Xue W, Ma X. GATA3 is downregulated in osteosarcoma and facilitates EMT as well as migration through regulation of slug. Onco Targets Ther. 2018;11:7579–7589. doi: 10.2147/OTT.S176534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tu K, Liu Z, Yao B, Han S, Yang W. MicroRNA-519a promotes tumor growth by targeting PTEN/PI3K/AKT signaling in hepatocellular carcinoma. Int J Oncol. 2016;48:965–974. doi: 10.3892/ijo.2015.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye Y, Zhuang J, Wang G, He S, Zhang S, Wang G, Ni J, Wang J, Xia W. MicroRNA-495 suppresses cell proliferation and invasion of hepatocellular carcinoma by directly targeting insulin-like growth factor receptor-1. Exp Ther Med. 2018;15:1150–1158. doi: 10.3892/etm.2017.5467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Nakamura M, Choe SK, Runko AP, Gardner PD, Sagerstrom CG. Nlz1/Znf703 acts as a repressor of transcription. BMC Dev Biol. 2008;8:108. doi: 10.1186/1471-213X-8-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pereira F, Duarte-Pereira S, Silva RM, da Costa LT, Pereira-Castro I. Evolution of the NET (NocA, Nlz, Elbow, TLP-1) protein family in metazoans: insights from expression data and phylogenetic analysis. Sci Rep. 2016;6:38383. doi: 10.1038/srep38383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen G, Xie Y. miR-495 inhibits proliferation, migration, and invasion and induces apoptosis via inhibiting PBX3 in melanoma cells. Onco Targets Ther. 2018;11:1909–1920. doi: 10.2147/OTT.S152362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu C, Jian M, Qi H, Mao WZ. MicroRNA-495 inhibits proliferation, metastasis and promotes apoptosis by targeting Twist1 in gastric cancer cells. Oncol Res. 2019;27:389–397. doi: 10.3727/096504018X15223159811838. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.