Abstract
Background: This study aimed to investigate roles of Toll-like receptor 4 (TLR4)/nuclear factor (NF)-κB signaling in triptolide (TPL)-induced sensitivity of pancreatic cancer cells to gemcitabine (GEM). Methods: In vitro, pancreatic cancer PANC-1 cells were treated with lipopolysaccharide (LPS) to activate TLR4, TLR4-siRNA, GEM alone, or GEM plus TPL. In vivo, nude mice bearing PANC-1 cell xenografts were treated with GEM, TPL, or both. Cell proliferation was detected by MTT assay and Ki-67 staining. Apoptosis was assessed by flow cytometry and TUNEL assay. A double luciferase reporter gene was used to detect NF-κB activity. Results: The sensitivity of PANC-1 cells to GEM was reduced by LPS but enhanced by TLR4-siRNA. TPL inhibited expression of TLR4/NF-κB signaling components, which was reversed by LPS. The TPL+GEM group showed more apoptosis than the LPS+TPL+GEM group. Moreover, the activity of NF-κB and the expression of TLR4, p-p65 Survivin, CyclinD1 and Bcl-2 in the TPL+GEM group were lower than in the LPS+TPL+GEM group, whereas Bax expression was higher. The volume of transplanted tumors in the TPL+GEM group was lower than that in the TPL or GEM group. Phospho-p65, Survivin, CyclinD1 and Bcl-2 expression in transplanted tumors was lower in TPL+GEM group than in either single drug group. The Ki-67 staining score of the TPL+GEM group was lower and tumor cells apoptosis rate was increased when compared with TPL or GEM alone. Conclusions: TPL enhances the sensitivity of pancreatic cancer PANC-1 cells to GEM by inhibiting TLR4/NF-κB signaling.
Keywords: Triptolide, gemcitabine, Toll-like receptor-4 protein, NF-κB, pancreatic cancer
Introduction
Pancreatic cancer is a type of malignant digestive system tumors and associated with a median survival of about 6 months in patients of all stages. Even with early resection, the five-year survival rate was less than 20% [1]. Gemcitabine (GEM) is currently used as a first-line chemotherapy for treatment of advanced pancreatic cancer; however, the overall outcome was poor. Many drugs have been used in combination with GEM for the treatment of advanced pancreatic cancer, but the overall survival rate has not been significantly improved compared with that achieved with GEM alone [2]. Therefore, it is of great interest to study the molecular mechanisms of chemotherapy resistance, to find new therapeutic targets and to develop effective intervention measures in order to improve the sensitivity of pancreatic cancer to chemotherapy.
Triptolide (TPL) is a diterpene lactone compound isolated from plants such as Tripterygium wilfordii. TPL has been shown to have a broad spectrum of anti-tumor effects with multiple targets [3]. TPL was shown to induce apoptosis and S-phase arrest in Taxol-resistant A549 cells via modulation of the MAPK and PI3K/Akt signaling pathways [4]. Moreover, it was reported that TPL inhibits the invasion and tumorigenesis of hepatocellular carcinoma MHCC-97H cells through NF-κB signaling [5]. Our previous study showed that TPL inhibits the growth of human pancreatic cancer PANC-1 cells and promotes tumor cell apoptosis, and these effects were related to the down-regulation of cyclooxygenase (COX)-2 and vascular endothelial growth factor (VEGF) expression and inhibition of tumor angiogenesis [6]. Recent studies have shown that TPL enhances the sensitivity of tumor cells to chemotherapeutic drugs, but the exact mechanism remains to be further defined [7-10].
Toll-like receptors (TLRs) are a family of transmembrane receptor proteins encoded by the dToll gene found during the development of Drosophila embryos. TLR4 was the first discovered and now is the most extensively studied TLR family member. In the TLR4 signaling pathway, the immunostimulant lipopolysaccharide (LPS) is an exogenous ligand of TLR4. LPS recognizes and binds TLR4, which in turn produces a signal that induces the immune defense response in the host. TLR4 is highly expressed in tumor cells and is closely related to the occurrence and development of a variety of cancers including colon, breast, lung, and other cancers [11-13]. In recent years, accumulating evidence suggests that TLR4 is also involved in the resistance of a variety of tumor cells to chemotherapeutic drugs [14,15]. It has been reported that TLR4 can activate NF-κB, induce a variety of cytokines, and regulate the expression of Bcl-2, Bax, VEGF, and a series of tumor-related genes, thereby promoting tumorigenesis, development, and chemotherapy resistance [16-18]. However, the relationship between TLR4/NF-κB signaling and pancreatic cancer cell resistance to GEM chemotherapy remains to be determined. In addition, whether TPL can inhibit the TLR4/NF-κB signaling pathway to increase the sensitivity of pancreatic cancer cells to GEM has not been reported.
In this study, the role of TLR4/NF-κB signaling in the sensitivity of pancreatic cancer cells to GEM was investigated; then the effect of TLR4/NF-κB signaling on the ability of TPL was further explored to increase the sensitivity of pancreatic cancer cells to GEM and examined the expression of components of the TLR4/NF-κB signaling pathway and apoptosis signaling.
Methods
Reagents and cells
The human pancreatic cancer cell line PANC-1 was purchased from ATCC (Manassas, VA, USA) and was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA), 100 U/mL penicillin, and 100 U/mL streptomycin at 37°C under 5% CO2. Human TLR4-siRNA (sense: 5’-CUUUAUCCAACCAGGUGCAUUUU-3’; antisense: 5’-AAUGCACCUGGUUGGAUAAAGUU-3’) was synthesized by Gene Pharma Company, Shanghai, China) and transected into cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction. LPS derived from O55: B5 Escherichia Coli was purchased from Sigma-Aldrich (St Louis, MO, USA). TLR4, Bcl-2, Bax, and β-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). p65, p-p65, Survivin, and CyclinD1 antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). The double luciferase reporter gene system was purchased from Promega (Madison, WI, USA). Immunohistochemical SP and DAB color kits were purchased from Fuzhou Manxing Biotechnology (Fuzhou, China). The TUNEL kit was purchased from Calbiochem (San Diego, CA, USA).
MTT assay
PANC-1 cells were seeded in 96-well plates at a density of 5×103/well. After adherence, the cells were transfected with 100 nM TLR4-siRNA and cultured for 24 h. Then the cells were treated with LPS, GEM, and/or TPL. After incubation for 48 h, 20 μl MTT (Sigma-Aldrich) solution (5 mg/ml) was added to each well. After incubation for 4 h, the culture medium was aspirated and 150 μl DMSO was added to each well to dissolve the crystals for 10 min. The absorbance (A value) was measured at 570 nm with a microplate reader. Cell proliferation rate = (experimental A value/blank control A value) ×100%. The formula for the co-ordination of the interaction between TPL and GEM was used as reported [19]: CI = D1/Dx1 + D2/Dx2 + α (D1D2/Dx1DX2), where D1 and D2 are the required concentrations of the two drugs in combination to generate the x effect; Dx1, DX2 are the required concentrations of the two drugs alone to generate the x effect; when CI < 1, the role of the two drugs is synergistic; when CI = 1, the role of the two drugs is additive; and when CI > 1, the role of the two drugs is antagonistic.
Flow cytometry
Cell apoptosis among in vitro cultured cells was assayed by flow cytometry (Beckman Coulter, Brea, CA, USA). Cells were harvested and resuspended in phosphate-buffered saline containing 2% bovine serum albumin. After centrifugation at 1000 rpm for 5 min, the cells were resuspended in 100 μl binding buffer and mixed with 5 μl Annexin V-FITC. After incubation at room temperature for 15 min in the dark, the cells were subjected to flow cytometry.
Double luciferase reporter assay
PANC-1 cells were seeded in 24-well plates at 1×105/well and transfected with 0.5 μg pNF-κB-luc plasmid or 0.1 μg pRL-TK luciferase expression plasmid as a control using Lipofectamine 2000. After 24 h, the relative activity of NF-κB was detected using a double luciferase reporter gene system according to the kit instructions.
Western blot analysis
Total proteins from cultured PANC-1 cells or transplanted tumors were extracted with radioimmunoprecipitation assay buffer, and protein concentration was measured by BCA protein analysis (Bio-Rad Laboratories, Hercules, CA, USA). Equal total protein was separated using 10-20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. After blocking with 5% skim milk in 1× Tris-buffered saline for 2 h, the membranes were incubated with TLR4, P65, p-P65, Survivin, CyclinD1, Bcl-2, or Bax antibody overnight at 4°C. After the membranes were washed with Tris-buffered saline, the corresponding horseradish peroxidase (HRP)-labeled secondary antibody was added and incubated at room temperature for 2 h. Following enhanced chemiluminescence development, the films were scanned with a gel image analysis system. The relative level of target protein was compared with the reference β-actin.
Pancreatic cancer nude mouse xenografts
Twenty-four four- to six-week-old Balb/c nude mice (male [n = 12] and female [n = 12], average weight of 18-22 g) were purchased from the Chinese Academy of Medical Sciences Shanghai Slake Animal Center. The mice were housed in a temperature-controlled room (24°C) on a 12-h/12-h light and dark cycle at the animal experimental center. All the experimental procedures were approved by the Experimental Animal Welfare and Ethics Committee of Fudan University. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
PANC-1 cells in the logarithmic growth phase were collected, and 1×107 cells were inoculated subcutaneously in the lateral abdomen of Balb/c nude mice. After formation of transplanted tumors of about 0.5 cm in diameter, the mice were randomly divided into control, GEM, TPL, and GEM+TPL groups with six animals in each group. The control group was given 100 μl DMSO; the GEM group was given 25 mg/kg GEM; the TPL group received 0.4 mg/kg TPL; and the GEM+TPL group was given 25 mg/kg GEM plus 0.4 mg/kg TPL. The mice were treated via intraperitoneal injection every other day for 15 times. The growth of subcutaneous tumor in nude mice was observed every other day. The tumor volume was calculated as V = a×b2/2 (a is the long diameter and b is the minor axis). Animals were sacrificed on the day after the final administration, and subcutaneous transplanted tumors were removed, fixed with 10% formaldehyde and embedded in paraffin.
Immunohistochemistry
Formalin-fixed tissues were embedded in paraffin blocks, and a series of sections (4 μm) was made. The expression of Ki-67 was detected by immunohistochemical SP staining using an SP immunohistochemistry kit following the manufacturer’s instructions. Five random high power fields (×200) were observed. Semi-quantitative integration of Ki-67 expression was performed according to the staining intensity of positive cells and the positive cell rate. A positive cell rate ≤5% was given 0 points, 6%-29% was given 1 point, 30%-59% was given 2 points, and ≥60% was given 3 points. No color was graded as 0 points, light yellow as 1 point, brown as 2 points, and brown as 3 points.
TUNEL assay
The terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay was used to detect apoptosis of transplanted cells according to the manufacturer’s instructions (Roche, Indianapolis, IN, USA). Five high power fields (×400) were randomly selected in each slide. The apoptotic cells were stained brown. The apoptotic rate was expressed by calculating the apoptotic index (AI): AI (%) = (number of apoptotic cells/total number of tumor cells) ×100%.
Statistical analysis
Measurement data are presented as mean ± standard deviation (x ± SD). Statistical significance was determined by unpaired two-tailed t tests or two-way analysis of variance (ANOVA) with post-hoc test carried out via lsd-t test. All data were analyzed using the SPSS 11.0 statistical software package. P < 0.05 showed the difference statistically significant.
Results
Activation of TLR4/NF-κB signaling reduces the sensitivity of PANC-1 cells to GEM
Our previous studies demonstrated that LPS activates the TLR4 pathway in PANC-1 cells at 1 μg/ml LPS for PANC-1 cells. To assess the role of TLR4 signaling in the GEM sensitivity of PANC-1 cells, we first pretreated PANC-1 cells with 1 μg/ml LPS or TLR4-siRNA and then added different concentrations of GEM. Compared with that in the GEM group, the inhibition rate of PANC-1 cells in the LPS+GEM group was significantly decreased as the GEM concentration increased, and the inhibition rate of PANC-1 cells was significantly increased in the TLR4-siRNA+GEM group (Figure 1A). The IC50 of the LPS+GEM group was significantly higher than that of the GEM group (P < 0.05), while the IC50 of the TLR4-siRNA+GEM group was significantly lower than that of the GEM group (P < 0.05; Figure 1B). In addition, compared with that in the GEM group, the apoptotic rate in the LPS+GEM group was significantly decreased (P < 0.05), while that of the TLR4-siRNA+GEM group was significantly increased (P < 0.01; Figure 1C and 1D). These results indicate that abnormal activation of the TLR4 pathway may inhibit PANC-1 cell sensitivity to GEM.
Figure 1.
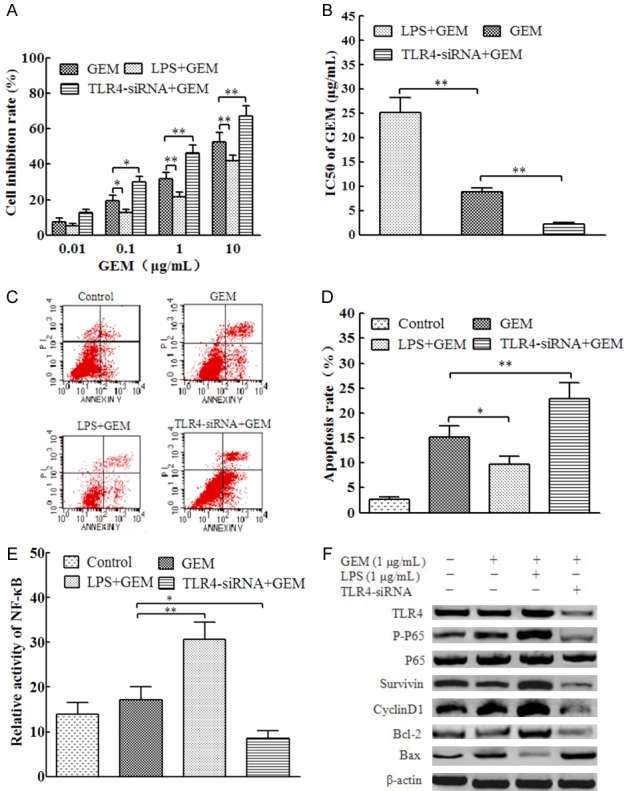
Activation of TLR4/NF-κB signaling suppressed the sensitivity of PANC-1 cells to GEM. (A) PANC-1 cells were transfected with 100 nM TLR4-siRNA or pretreated with 1 μg/mL LPS for 4 h, followed by incubation with 0.01, 0.1, 1, or 10 μg/mL GEM for 48 h. Cell proliferation was assessed by MTT assay. (B) The IC50 calculated from (A). (C) PANC-1 cells were transfected with 100 nM TLR4-siRNA or pretreated with 1 μg/mL LPS for 4 h, followed by incubation with 1 μg/mL GEM for 24 h. Cell apoptosis was determined by Annexin V staining followed by flow cytometry. (D) Quantitation of apoptotic cells in each group of (C). (E) PANC-1 cells were transfected with 100 nM TLR4-siRNA or pretreated with 1 μg/mL LPS for 4 h, followed by incubation with 1 μg/mL GEM for 24 h. The relative NF-κB activity was detected using a double luciferase reporter gene. (F) Cells were treated as in (E), and total proteins were extracted for immnoblotting of the indicated proteins with β-actin as the loading control. *P < 0.05; **P < 0.01.
Further experiments showed that the activity of NF-κB (P < 0.05) and the expression of p-p65, Survivin, CyclinD1, and Bcl-2 in the LPS+GEM group were higher and the expression of Bax was lower than those in the GEM group. In contrast, the activity of NF-κB and the expression of TLR4, p-p65, Survivin, CyclinD1, and Bcl-2 in the TLR4-siRNA+GEM group were lower than those in the GEM group, while the expression of Bax was higher (Figure 1E and 1F). These results suggest that TLR4 activates NF-κB signaling to promote the expression of Survivin, CyclinD1, and Bcl-2 and inhibits the expression of Bax, thereby reducing the sensitivity of PANC-1 cells to GEM.
TPL inhibits TLR4/NF-κB signaling in PANC-1 cells
It has been reported that TPL suppresses the TLR4/NF-κB signaling pathway in the process of inflammation, but whether TPL affects TLR4/NF-κB signaling in tumor cells is unknown. In this study, we treated PANC-1 cells with different concentrations of TPL and found that TPL significantly inhibited NF-κB activity (Figure 2A). Moreover, TPL decreased the expression of TLR4, p-p65, Survivin, CyclinD1, and Bcl-2 and increased the expression of Bax in a concentration-dependent manner (Figure 2B). In addition, in comparison to those after LPS treatment alone, NF-κB activity (P < 0.01, Figure 2C) and TLR4, p-p65, Survivin, CyclinD1, and Bcl-2 expression were lower, whereas Bax expression was higher in the TPL+LPS group (Figure 2D). These results indicate that TPL inhibits TLR4/NF-κB signaling in PANC-1 cells.
Figure 2.
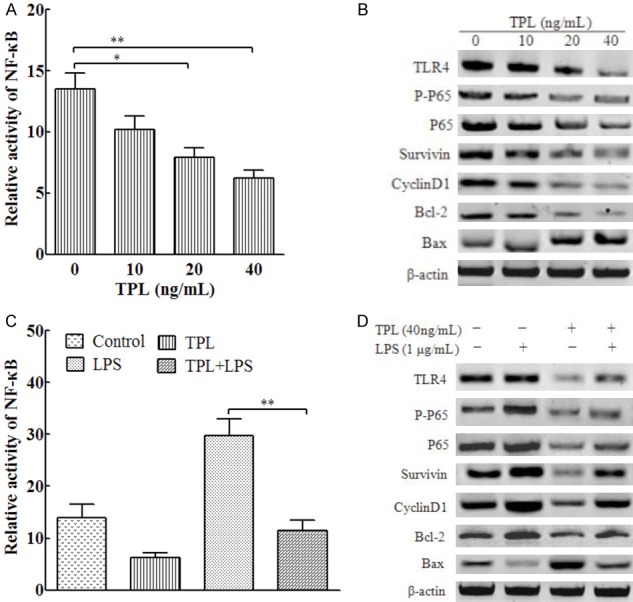
TPL inhibited TLR4/NF-κB signaling in PANC-1 cells. (A) PANC-1 cells were incubated with 0, 10, 20, or 40 ng/mL TPL for 24 h. The relative activity of NF-κB was detected using a double luciferase reporter gene. (B) Cells were treated as in (A), and total proteins were extracted for immunoblotting of the indicated proteins with β-actin as the loading control. (C) PANC-1 cells were pretreated with 1 μg/mL LPS for 4 h, followed by incubation with 40 ng/mL TPL for 24 h. The relative activity of NF-κB was detected using a double luciferase reporter gene. (D) Cells were treated as in (C), and total proteins were extracted for western blotting of the indicated proteins with β-actin as the loading control. *P < 0.05; **P < 0.01.
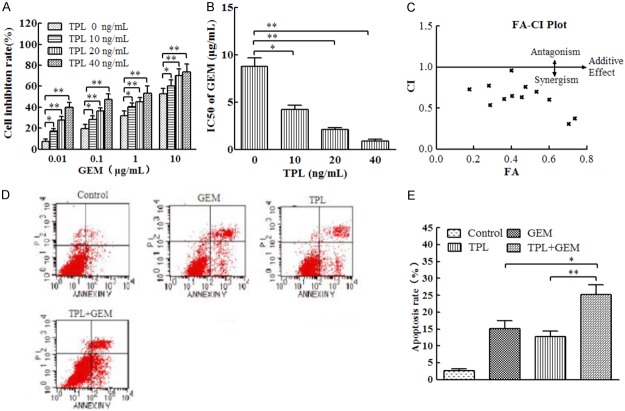
TPL enhances the sensitivity of PANC-1 cells to GEM
Our previous study showed that TPL inhibited proliferation and promoted apoptosis of PANC-1 cell, but whether TPL can enhance the sensitivity of PANC-1 cells to GEM is unknown. Therefore, we treated PANC-1 cells with different concentrations of GEM in the presence of 0, 10, 20, or 40 ng/mL TPL and analyzed cell proliferation and apoptosis. We found that the inhibition of PANC-1 cell proliferation by GEM was enhanced by TPL in a concentration-dependent manner (Figure 3A). Accordingly, the IC50 of GEM was significantly reduced by TPL in a concentration-dependent manner (Figure 3B). The combined index of TPL and GEM was less than 1.0, indicating that the two drugs had a synergistic effect (Figure 3C). Then, the cells were treated with 100 μg/mL GEM combined with 40 ng/mL TPL and measured cell apoptosis by flow cytometry. The results showed that the apoptotic rates were significantly higher than that in the single drug group (Figure 3D and 3E).
Figure 3.
TPL enhanced the sensitivity of PANC-1 cells to GEM by inhibiting TLR4/NF-κB signaling. (A) PANC-1 cells were incubated with 0.01, 0.1, 1, or 10 μg/mL GEM and 0, 10, 20, or 40 ng/mL TPL for 48 h. Cell proliferation was assessed by MTT assay. (B) The IC50 values of GEM were calculated from the data in (A). (C) The combination of GEM and TPL was used to calculate the combined drug index (CI). (D) PANC-1 cells were treated with 1 μg/mL GEM or 40 ng/mL TPL alone or in combination for 24 h. Cell apoptosis was determined by Annexin V staining followed by flow cytometry. (E) Quantitation of apoptotic cells in each group in (D). *P < 0.05; **P < 0.01.
TPL enhances the sensitivity of PANC-1 cells to GEM by inhibiting TLR4/NF-κB signaling
To explore whether TPL enhances the sensitivity of PANC-1 cells to GEM by inhibiting TLR4/NF-κB signaling, PANC-1 cells were pretreated with LPS followed by treatment with GEM in the presence or absence of TPL. The results revealed that both the cell inhibition rate (Figure 4A) and the apoptotic rate (Figure 4B and 4C) of the TPL+GEM group were significantly higher than those of the group pretreated with LPS. Further analysis showed that NF-κB activity in the LPS+GEM+TRP group was significantly lower than that in TPL+GEM group (P < 0.05, Figure 4D). The protein levels of TLR4, p-p65, Survivin, CyclinD1, and Bcl-2 were lower, whereas that of Bax was higher in the LPS+GEM+TRP group relative to those in the TPL+GEM group (Figure 4E). These results suggest that inhibition of TLR4/NF-κB signaling plays an important role in enhancing the sensitivity of PANC-1 cells to TPL.
Figure 4.
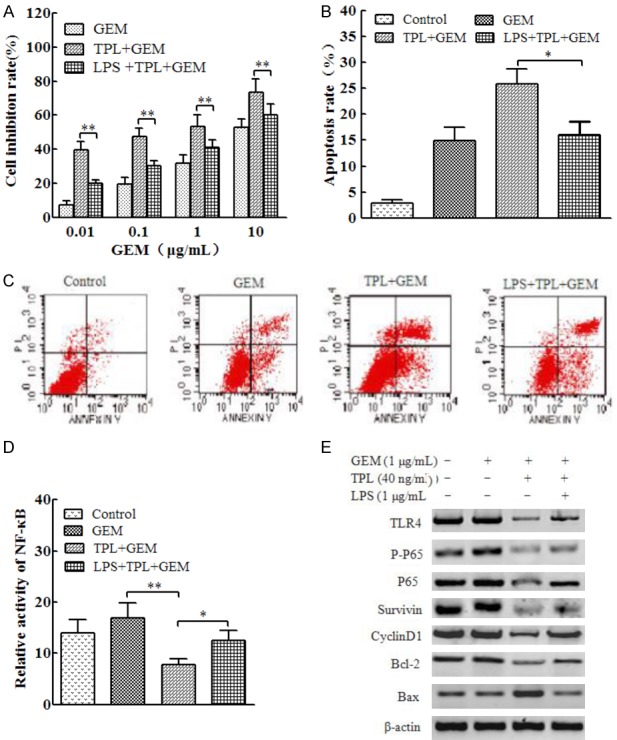
TPL enhanced GEM-mediated apoptosis of PANC-1 cells by inhibiting TLR4/NF-κB signaling. (A) PANC-1 cells were incubated with 0.01, 0.1, 1, or 10 μg/mL GEM and 40 ng/mL TPL for 48 h or pretreated with 1 μg/mL of LPS for 4 h, followed by incubation with TPL or GEM for 48 h. Cell proliferation was assessed by MTT assay. (B) PANC-1 cells were incubated with 1 μg/mL GEM and 40 ng/mL TPL for 24 h or pretreated with 1 μg/mL of LPS for 4 h, followed by incubation with TPL or GEM for 24 h. Cell apoptosis was determined by Annexin V staining followed by flow cytometry. (C) Representative histograms for the flow cyctometric data in (B). (D) Cells were treated as in (B), and the relative activity of NF-κB was detected using a double luciferase reporter gene. (E) Cells were treated as (B), and total proteins were extracted for western blotting of the indicated proteins with β-actin as the loading control. *P < 0.05; **P < 0.01.
TPL enhances the growth-inhibitory effects of GEM in PANC-1 cell mouse xenografts
To further verify that TPL promotes the sensitivity of pancreatic cancer PANC-1 cells to GEM by inhibiting TLR4/NF-κB signaling, we administered TPL, GEM, or both in nude mouse models of pancreatic cancer xenografts and monitored tumor growth with time (Figure 5A). The tumor volume of the TPL+GEM group was significantly lower than that of the TPL or GEM single drug group (Figure 5B) at the end of the treatment. Compared with levels in the GEM monotherapy group, the expression levels of p-p65, Survivin, CyclinD1, and Bcl-2 were lower in the TPL+GEM group, and the expression level of Bax was higher (Figure 5C). In addition, Ki-67 expression and tumor cell apoptosis were detected by immunohistochemistry and TUNEL, respectively (Figure 5D). The results showed that Ki-67 staining in the TPL+GEM group was significantly lower than that in the TPL or GEM single drug group (Figure 5E), whereas the apoptosis rate of tumor cells was significantly greater in the TPL+GEM group compared with that in the TPL or GEM single drug group (Figure 5F).
Figure 5.
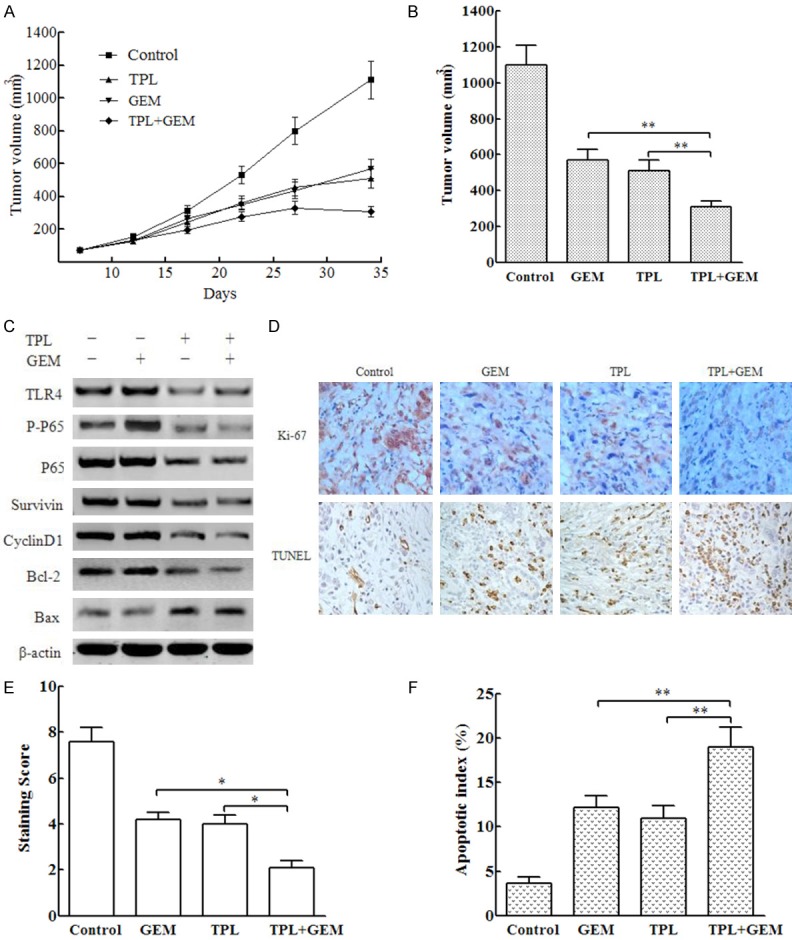
TPL enhanced the growth-inhibitory effects of GEM in PANC-1 cell mouse xenografts. PANC-1 cell xenograft-bearing mice were randomly divided into control (100 μl DMSO), GEM, TPL, and GEM+TPL groups with 6 animals in each group. The control group was given phosphate-buffered saline; the GEM group was given 25 mg/kg GEM; the TPL group received 0.4 mg/kg TPL; and the GEM+TPL group was given 25 mg/kg GEM plus 0.4 mg/kg TPL. The mice were treated via intraperitoneal injection every other day for 15 times. The growth of subcutaneous tumors in nude mice was observed every other day. A. Comparison of tumor volume during the course of treatments. B. Comparison of tumor volume at the end of treatments. C. Western blot detection of the indicated proteins in transplanted tumors. D. Detection of Ki-67 by immunohistochemistry and apoptosis by TUNEL assay in tumor cells. E. Comparison of Ki-67 staining points in each group. F. Comparison of the apoptotic index in each group. *P < 0.05; **P < 0.01.
Discussion
TLR4 is mainly expressed on the surface of the host defense-related immune and endothelial cells. The cell surface distribution of TLR4 can make it easier to identify extracellular bacteria and their ligands and to induce a strong inflammation response [20]. Emerging data have shown that TLR4 is expressed in immune cells as well as in a variety of tumor cells, and its expression is closely related not only to the tumor occurrence and development, but also to resistance to chemotherapy drugs. Zhang et al. [21] found that TLR4 activation by LPS significantly decreases the sensitivity of prostate cancer PC-3 cells to docetaxel, whereas down-regulation of TLR4 expression via siRNA significantly increases the sensitivity of PC-3 cells to docetaxel. Sun et al. [22] showed that TLR4 is highly expressed in human oral squamous cell carcinoma and that high expression of TLR4 is associated with oral squamous cell carcinoma resistance to cisplatin. In addition, studies have shown that paclitaxel is also an important ligand for TLR4 and can induce the expression of various cytokines and anti-apoptotic molecules by activating TLR4, thereby reducing the chemosensitivity of tumor cells to paclitaxel [23,24]. In the present study, it was found that activation of the TLR4 pathway by LPS reduced the sensitivity of PANC-1 cells to GEM, while knockdown of TLR4-1 by siRNA significantly enhanced the sensitivity of PANC-1 cells to GEM. Our findings suggest that TLR4 signaling plays an important role in the resistance of pancreatic cancer cells to GEM.
NF-κB is widely expressed and is an important nuclear transcription factor involved in a variety of cellular biological processes including immune, inflammation, apoptosis, and proliferation. In recent years, increasing numbers of studies suggest that NF-κB is closely linked to tumor chemotherapy resistance, making NF-κB a new target for the treatment of cancers [25,26]. It has been reported that TLR4 activates NF-κB under the stimulation of the corresponding ligands, which plays a role in promoting chemotherapeutic drug resistance in tumor cells [16-18]. Zhou et al. [26] found that inhibition of the TLR4/NF-κB signaling pathway leads to reduced expression of COX-2, CyclinD1, Survivin, cIAP-1, XIAP, Bcl-2, and other genes that is accompanied by increased GEM sensitivity. Our study also found that TLR4 activated NF-κB; promoted the expression of Survivin, CyclinD1, and Bcl-2; down-regulated Bax expression; and played an important role in reducing PANC-1 cell sensitivity to GEM.
TPL is the main active ingredient of various Tripterygium wilfordii preparations widely used in China for the prevention and treatment of a variety of diseases. TPL has been recently identified as a multi-target anti-tumor natural agent in addition to being known for its anti-inflammatory and immunosuppressive effects. Moreover, TPL can also improve the effect of chemotherapy drugs on tumor cells and reduce tumor cell drug resistance [6-10]. It was reported that TPL could enhance the growth inhibition of doxorubicin in breast cancer cells [27]. It has also been shown that the combination of TPL and heat shock protein 90 inhibitor BIIB021 has a strong synergy, significantly enhancing the effectiveness of BIIB021 for thyroid cancer cell proliferation inhibition and apoptosis induction [28]. Recent studies have shown that TPL can enhance the sensitivity of pancreatic cancer cells to GEM [29]. In agreement with these previous studies, it was found that TPL combined with GEM can produce a synergistic effect and enhance the effect of GEM on pancreatic cancer cell proliferation inhibition and apoptosis induction, indicating that TPL can enhance the sensitivity of pancreatic cancer cells to GEM chemotherapy. Further animal experiments confirmed that TPL could enhance the inhibitory effect of GEM on the growth of pancreatic cancer xenografts in nude mice and promote the apoptosis of tumor cells in collaboration with GEM.
Although it has been shown that TPL enhanced the sensitivity of pancreatic cancer cells to GEM and other chemotherapeutic drugs, the underlying mechanism remained to be determined. TPL has been shown to inhibit the TLR4/NF-κB signaling pathway during immune response and inflammation, suggesting that inhibition of TLR4/NF-κB signaling pathway may play a role in the TPL-based enhancement of PANC-1 cell sensitivity to GEM [30]. In this study, it was showed that TPL dose-dependently inhibited the expression of TL4 and the downstream targets of the TLR4/NF-κB signaling pathway in PANC-1 cells, which was however abolished by LPS. Besides, LPS pretreatment significantly reduced the cell inhibition rate and apoptosis rate that was induced by TPL combined with GEM, and these effects were accompanied by attenuation of NF-κB activity and TLR4, p-p65, Survivin, CyclinD1, Bcl-2, and Bax expression. The results of animal experiments further confirmed that TPL could inhibit the expression of TLR4, p-p65, Survivin, CyclinD1, and Bcl-2 in nude mice and promote the expression of Bax. Our findings suggest that inhibition of TLR4/NF-κB signaling is an important mechanism by which TPL enhances PANC-1 cell sensitivity to GEM. Nevertheless, though TPL has been shown to have a broad spectrum of anti-tumor effects with multiple targets [3], including our present finding of TPL inhibition of TLR4/NF-κB signaling pathway, the molecular mechanisms of the regulation the specific targets by TPL is unknown, which needs further exploration. In addition, whether TPL reduces TLR4 expression at transcriptional, post transcriptional, or post translational levels warrants future investigation.
Conclusions
Taken together, our results obtained using both in vitro and in vivo models showed that TPL, an active ingredient of Tripterygium wilfordii, enhanced the sensitivity of human pancreatic cancer PANC-1 cells to GEM by inhibiting TLR4/NF-κB signaling. Nevertheless, the abnormal activation of TLR4/NF-κB signaling may be only one mechanism of the resistance of pancreatic cancer cells to chemotherapy such as GEM. Other possible mechanisms by which TPL enhances the sensitivity of PANC-1 cells to GEM warrant further investigation.
Disclosure of conflict of interest
None.
References
- 1.Tempero MA, Berlin J, Ducreux M, Haller D, Harper P, Khayat D, Schmoll HJ, Sobrero A, Van Cutsem E. Pancreatic cancer treatment and research: an international expert panel discussion. Ann Oncol. 2011;22:1500–1506. doi: 10.1093/annonc/mdq545. [DOI] [PubMed] [Google Scholar]
- 2.Thota R, Pauff JM, Berlin JD. Treatment of metastatic pancreatic adenocarcinoma: a review. Oncology (Williston Park) 2014;28:70–74. [PubMed] [Google Scholar]
- 3.Tan W, Lu J, Huang M, Li Y, Chen M, Wu G, Gong J, Zhong Z, Xu Z, Dang Y, Guo J, Chen X, Wang Y. Anti-cancer natural products isolated from chinese medicinal herbs. Chin Med. 2011;6:27. doi: 10.1186/1749-8546-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie CQ, Zhou P, Zuo J, Li X, Chen Y, Chen JW. Triptolide exerts pro-apoptotic and cell cycle arrest activity on drug-resistant human lung cancer A549/Taxol cells via modulation of MAPK and PI3K/Akt signaling pathways. Oncol Lett. 2016;12:3586–3590. doi: 10.3892/ol.2016.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Ma D, Wang C, Zhao S, Liu C. Triptolide inhibits invasion and tumorigenesis of hepatocellular carcinoma MHCC-97H cells through NF-kappaB signaling. Med Sci Monit. 2016;22:1827–1836. doi: 10.12659/MSM.898801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma JX, Sun YL, Wang YQ, Wu HY, Jin J, Yu XF. Triptolide induces apoptosis and inhibits the growth and angiogenesis of human pancreatic cancer cells by downregulating COX-2 and VEGF. Oncol Res. 2013;20:359–368. doi: 10.3727/096504013X13657689382932. [DOI] [PubMed] [Google Scholar]
- 7.Ho JN, Byun SS, Lee S, Oh JJ, Hong SK, Lee SE, Yeon JS. Synergistic antitumor effect of triptolide and cisplatin in cisplatin resistant human bladder cancer cells. J Urol. 2015;193:1016–1022. doi: 10.1016/j.juro.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Jiang N, Dong XP, Zhang SL, You QY, Jiang XT, Zhao XG. Triptolide reverses the Taxol resistance of lung adenocarcinoma by inhibiting the NF-kappaB signaling pathway and the expression of NF-kappaB-regulated drug-resistant genes. Mol Med Rep. 2016;13:153–159. doi: 10.3892/mmr.2015.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li CJ, Chu CY, Huang LH, Wang MH, Sheu LF, Yeh JI, Hsu HY. Synergistic anticancer activity of triptolide combined with cisplatin enhances apoptosis in gastric cancer in vitro and in vivo. Cancer Lett. 2012;319:203–213. doi: 10.1016/j.canlet.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Xiao E, Yuan L, Li G. Triptolide synergistically enhances antitumor activity of oxaliplatin in colon carcinoma in vitro and in vivo. DNA Cell Biol. 2014;33:418–425. doi: 10.1089/dna.2014.2356. [DOI] [PubMed] [Google Scholar]
- 11.Ehsan N, Murad S, Ashiq T, Mansoor MU, Gul S, Khalid S, Younas M. Significant correlation of TLR4 expression with the clinicopathological features of invasive ductal carcinoma of the breast. Tumour Biol. 2013;34:1053–1059. doi: 10.1007/s13277-013-0645-y. [DOI] [PubMed] [Google Scholar]
- 12.Fu HY, Li C, Yang W, Gai XD, Jia T, Lei YM, Li Y. FOXP3 and TLR4 protein expression are correlated in non-small cell lung cancer: implications for tumor progression and escape. Acta Histochem. 2013;115:151–157. doi: 10.1016/j.acthis.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Hsu RY, Chan CH, Spicer JD, Rousseau MC, Giannias B, Rousseau S, Ferri LE. LPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasis. Cancer Res. 2011;71:1989–1998. doi: 10.1158/0008-5472.CAN-10-2833. [DOI] [PubMed] [Google Scholar]
- 14.Cai G, Ma X, Chen B, Huang Y, Liu S, Yang H, Zou W. Galectin-3 induces ovarian cancer cell survival and chemoresistance via TLR4 signaling activation. Tumour Biol. 2016;37:11883–11891. doi: 10.1007/s13277-016-5038-6. [DOI] [PubMed] [Google Scholar]
- 15.Hsiao CC, Chen PH, Cheng CI, Tsai MS, Chang CY, Lu SC, Hsieh MC, Lin YC, Lee PH, Kao YH. Toll-like receptor-4 is a target for suppression of proliferation and chemoresistance in HepG2 hepatoblastoma cells. Cancer Lett. 2015;368:144–152. doi: 10.1016/j.canlet.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Chung YH, Kim D. Enhanced TLR4 expression on colon cancer cells after chemotherapy promotes cell survival and epithelial-mesenchymal transition through phosphorylation of GSK3beta. Anticancer Res. 2016;36:3383–3394. [PubMed] [Google Scholar]
- 17.Huang JM, Zhang GN, Shi Y, Zha X, Zhu Y, Wang MM, Lin Q, Wang W, Lu HY, Ma SQ, Cheng J, Deng BF. Atractylenolide-I sensitizes human ovarian cancer cells to paclitaxel by blocking activation of TLR4/MyD88-dependent pathway. Sci Rep. 2014;4:3840. doi: 10.1038/srep03840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J, Chen X, Gilvary DL, Tejera MM, Eksioglu EA, Wei S, Djeu JY. HMGB1 induction of clusterin creates a chemoresistant niche in human prostate tumor cells. Sci Rep. 2015;5:15085. doi: 10.1038/srep15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 20.Nijland R, Hofland T, van Strijp JA. Recognition of LPS by TLR4: potential for anti-inflammatory therapies. Mar Drugs. 2014;12:4260–4273. doi: 10.3390/md12074260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Wang Y, Yuan J, Qin W, Liu F, Wang F, Zhang G, Yang X. Toll-like receptor 4 ligation confers chemoresistance to docetaxel on PC-3 human prostate cancer cells. Cell Biol Toxicol. 2012;28:269–277. doi: 10.1007/s10565-012-9221-2. [DOI] [PubMed] [Google Scholar]
- 22.Sun Z, Luo Q, Ye D, Chen W, Chen F. Role of Toll-like receptor 4 on the immune escape of human oral squamous cell carcinoma and resistance of cisplatin-induced apoptosis. Mol Cancer. 2012;11:33. doi: 10.1186/1476-4598-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajput S, Volk-Draper LD, Ran S. TLR4 is a novel determinant of the response to paclitaxel in breast cancer. Mol Cancer Ther. 2013;12:1676–1687. doi: 10.1158/1535-7163.MCT-12-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szajnik M, Szczepanski MJ, Czystowska M, Elishaev E, Mandapathil M, Nowak-Markwitz E, Spaczynski M, Whiteside TL. TLR4 signaling induced by lipopolysaccharide or paclitaxel regulates tumor survival and chemoresistance in ovarian cancer. Oncogene. 2009;28:4353–4363. doi: 10.1038/onc.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monisha J, Padmavathi G, Roy NK, Deka A, Bordoloi D, Anip A, Kunnumakkara AB. NF-kappaB blockers gifted by mother nature: prospectives in cancer cell chemosensitization. Curr Pharm Des. 2016;22:4173–4200. doi: 10.2174/1381612822666160609110231. [DOI] [PubMed] [Google Scholar]
- 26.Zhou L, Qi L, Jiang L, Zhou P, Ma J, Xu X, Li P. Antitumor activity of gemcitabine can be potentiated in pancreatic cancer through modulation of TLR4/NF-kappaB signaling by 6-shogaol. AAPS J. 2014;16:246–257. doi: 10.1208/s12248-013-9558-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SH, Kang JG, Kim CS, Ihm SH, Choi MG, Yoo HJ, Lee SJ. Synergistic cytotoxicity of BIIB021 with triptolide through suppression of PI3K/Akt/mTOR and NF-kappaB signal pathways in thyroid carcinoma cells. Biomed Pharmacother. 2016;83:22–32. doi: 10.1016/j.biopha.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Xiong J, Su T, Qu Z, Yang Q, Wang Y, Li J, Zhou S. Triptolide has anticancer and chemosensitization effects by down-regulating Akt activation through the MDM2/REST pathway in human breast cancer. Oncotarget. 2016;7:23933–23946. doi: 10.18632/oncotarget.8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiao Z, He M, He MU, Li W, Wang X, Wang Y, Kuai Q, Li C, Ren S, Yu Q. Synergistic antitumor activity of gemcitabine combined with triptolide in pancreatic cancer cells. Oncol Lett. 2016;11:3527–3533. doi: 10.3892/ol.2016.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang Q, Zhang J, Tang W, Geng Q, Xu X, Jiang W. Triptolide attenuates acute small-for-size liver graft injury in rats by inhibition of Toll-like receptor 4. Transplant Proc. 2014;46:3303–3308. doi: 10.1016/j.transproceed.2014.07.077. [DOI] [PubMed] [Google Scholar]