Abstract
Background/objective: Pancreatic ductal adenocarcinoma (PDA) remains the most lethal malignancy due to lack of an effective treatment. P21-activated kinases (PAKs) play a key role not only in cell proliferation and migration, but also in mediating chemo-resistance in PDA. The aim of this study was to investigate the combined effect of a PAK inhibitor PF-3758309 with multiple chemotherapeutic reagents on a panel of patient-derived PDA cell lines, and potential mechanisms involved. Methods: Cells were treated with PF-3758309 plus or minus gemcitabine, 5-fluorouracil (5-FU) or abraxane, and cell growth was determined using a cell proliferation assay kit. Protein expression profiles were measured by Western blot. PDA cells were subcutaneously injected into the flanks of SCID mice which were then treated with saline, gemcitabine, PF-3758309, gemcitabine plus PF-3758309 or abraxane. Tumour growth was measured by volume and weight. Results: PAK1 was correlated with CK19 expression, and PAK4 with α-SMA and palladin expression. Combination of PF-3758309 with 5-FU, gemcitabine or abraxane further suppressed cell growth of patient-derived PDA cell lines in vitro. The combination of PF-3758309 with gemcitabine maximally inhibited tumour growth in vivo by suppressing cell proliferation. PF-3758309 inhibited the expression of HIF-1α, palladin and α-SMA both in vitro and in vivo. Conclusions: PAK inhibitor PF-3758309 can enhance anti-tumour effects of multiple chemotherapeutic reagents on a panel of patient-derived PDA cell lines. Combination of PF-3758309 with gemcitabine achieves comparable efficacy to combination of gemcitabine with abraxane, and thus provides a potential targeted therapy in the management of PDA.
Keywords: p21-activated kinase, PF-3758309, chemotherapy, gemcitabine, pancreatic cancer
Introduction
The low survival rate of pancreatic ductal adenocarcinoma (PDA) is largely caused by resistance to chemotherapy. Due to lack of effective early diagnosis of PDA, only 20% of patients are suitable for surgical resection, which makes chemotherapy a critical component in the clinical management of PDA. Gemcitabine has been the mainstay of systemic treatment for most stages of PDA. With the discovery of active multi-agent chemotherapeutic regimens, such as FOLFIRINOX (a combination of leucovorin (folinic acid), fluorouracil (5-FU), irinotecan and oxaliplatin) and gemcitabine plus nab-paclitaxel (abraxane), the treatment landscape of PDA is slowly evolving. FOLFIRINOX and gemcitabine plus abraxane are now utilized as standard first line treatment options in local advanced and metastatic PDA [1]. FOLFIRINOX treatment offers a median survival of 11.1 months in patients with advanced disease [2], while gemcitabine plus abraxane treatment provides a median survival of 8.5 months [3]. Compared to gemcitabine monotherapy, the survival rate of PDA patients has been marginally improved with the combination treatment.
The unique desmoplastic stroma in PDA forms a barrier of fibrotic tissue which prevents the penetration of chemotherapeutic reagents and thus compromises the efficacy [4]. The stroma contains a large number of pancreatic stellate cells (PSCs), which are characterized by the expression of desmin, glial fibrillary acidic protein (GFAP), vimentin and nestin [5]. PSCs are activated by PDA cells, which is characterized by absence of vitamin A droplets in the cytoplasm and presence of myofibroblast phenotype with increased expression of alpha-smooth muscle actin (α-SMA). The interaction between PDA cells and PSCs plays an important role in tumour growth, metastasis and chemo-resistance. Palladin, an actin binding protein, has been reported to be expressed in both PDA and stroma cells. The stromal expression of palladin is an independent prognostic factor for predicting PDA patient’s survival and a surrogate indicator for the effectiveness of chemoradiation therapy [6].
P21-activated kinases (PAKs) are critical down-stream effectors of mutated Kras signalling, and contribute to initiation and progression of PDA. The six known members of the PAK family can be categorized by similarities in their sequence and structure into two groups: group I (PAK1-3) and group II (PAK4-6). Over-expression of PAK1 [7] and PAK4 [8] has been reported in PDA patients, and both PAK1 and PAK4 are involved in the proliferation and migration/invasion of PDA cells [9]. Inhibition of PAK1 synergistically enhanced the inhibitory effects of gemcitabine on the growth of PDA cells both in vitro and in vivo [10], and activation of PAK1 contributed to gemcitabine resistance of PDA cells [11]. Furthermore, inhibition of PAK1 also suppressed the activation and proliferation of PSCs while promoting the apoptosis, and deletion of stromal PAK1 increased the survival of mice with orthotopic PDA [12]. PAK4 promoted the proliferation and survival of PDA cells by activation of NF-κB pathway [13], and inhibition of PAK4 increased the sensitivity of PDA cells to gemcitabine [14]. In addition, inhibition of PAK4 also decreased PDA cell proliferation via down-regulation of Bad phosphorylation and up-regulation of tumour suppressive miRNA [15]. A novel PAK4 allosteric inhibitor suppressed PDA cell proliferation on its own or synergistically with gemcitabine and oxaliplatin while promoting the apoptosis of PDA cells [16]. These important roles of PAKs in PDA and stromal cells make PAKs emerge as promising targets in PDA treatment.
The affinity of PF-3758309, a pan-PAK inhibitor, is highest for PAK4 (IC50 2.7-4.5 nM), and second highest for PAK1 (IC50 14 nM) [17]. PF-3758309 suppressed the proliferation and migration/invasion of melanoma and lung cancer by targeting both PAK1 [18] and PAK4 [19]. PF-3758309 inhibited the growth of colorectal cancer cells both in vitro and in vivo by decreasing the activity of PAK1, and enhanced the inhibitory effect of 5-FU on the proliferation and tumourigenesis of colorectal cancer cells [20]. The aims of this study were to determine the effects of PF-3758309, in combination with multiple chemotherapeutic reagents, on a panel of patient-derived PDA cell lines, and to investigate the possible mechanisms involved.
Methods
Cells and reagents
The patient-derived cell lines (TKCC 2.1, TKCC15, TKCC18, TKCC22, TKCC23, TKCC26) used in this study were isolated at The Kinghorn Cancer Centre (TKCC) from primary patient-derived pancreatic ductal adenocarcinoma xenografts [21] and cultured under hypoxia (5% O2) as described previously [22]. Cells were tested regularly for mycoplasma contamination and were not passaged more than 30 times after resuscitation.
Gemcitabine and 5-fluorouracil were purchased from Sigma-Aldrich (Melbourne, Australia), PF-3758309 from Active Biochemical Co. (Maplewood, NJ), and Nab-paclitaxel (Abraxane) from Abraxis Bioscience Australia Pty Ltd (East Kew, Victoria, Australia).
Cell proliferation
Cells (5 × 103 cells/well) were seeded in the 96-well plate, and were incubated with different concentrations of PF-3758309 for 48 h or pre-treated with PF-3758309 at the IC50 calculated for proliferation (Table 1) for 48 h, followed by treatment with gemcitabine, 5-FU or Abraxane for another 48 h in the absence of PF-3758309. Cell proliferation was measured using a CyQUANT Direct Cell Proliferation Assay Kit (Thermo Fisher, Melbourne, Australia) according to the manufacturer’s instruction. Fluorescence intensity was determined using a plate reader (FLUOstar OPTIMA, BMG Labtech, Melbourne, Australia).
Table 1.
IC50 values for a panel of patient-derived PDA cell lines
| PF-3758309 (nM) | 5-FU (μM) | Gemcitabine (nM) | Abraxane (nM) | |
|---|---|---|---|---|
| TKCC 15 | 5 | 10 | 10 | 10 |
| TKCC 18 | 5 | 5 | 20 | 25 |
| TKCC 2.1 | 400 | 5 | 5 | 15 |
| TKCC 22 | 400 | 100 | 60 | 40 (μM) |
| TKCC 23 | 200 | 150 | 1300 (μM) | 70 |
| TKCC 26 | 5 | 150 | 45 | 10 (μM) |
Western blot
Cells were lysed in SDS sample buffer. Proteins extracts were obtained from tumour tissues by homogenising in 1% Triton X100 lysis buffer (50 mM HEPES, 150 mM NaCl, 10 mM EDTA, 10 mM Na4P2O7, 100 mM NaF, 5 μg/ml aprotinin, 5 μg/ml leupeptin and 1 mM PMSF). All the samples were subjected to SDS-PAGE for immunoblotting and were detected with antibodies against GAPDH, total and phospho-PAK1 and PAK4 (Cell Signaling Technology, Arundel, Australia), cytokeratin 19 (CK19), alpha-smooth muscle actin (α-SMA), palladin (Abcam, Cambridge, MA) and hypoxia-inducible factor 1-alpha (HIF-1α, BD Biosciences, North Ryde, Australia. Bound antibodies were visualized using ECL reagents (GE Healthcare, Amersham, UK), and the density of each band was analysed using Multigauge computer software (Berthold, Bundoora, Australia). The relative amount of each protein was calculated as the ratio of the density of its band to the density of the GAPDH band.
Mouse study
All mouse experiments were approved by the Austin Health Animal Ethics Committee (A2015/05269). SCID mice were purchased from the Animal Resource Centre (Perth, Australia). TKCC 15 cells (3 × 106 cells/100 μl/site) were injected subcutaneously into the opposite flanks of 8-week old SCID mice. On day 25 after tumour induction, the mice were randomly divided into 5 groups (3 mice/group): control (saline), gemcitabine alone (40 mg/kg), PF-3758309 alone (25 mg/kg), gemcitabine plus abraxane (10 mg/kg), and gemcitabine plus PF-3758309. The mice were treated by intra-peritoneal injection (i.p.) as shown in Figure 5A for four weeks. Tumour dimensions were measured twice/week using a caliper, and volumes were calculated using the formula w2*L/2, where w and L are the shortest and longest tumour diameters, respectively. The tumour volumes on day 25 in each group were taken as 1. Mice were euthanized on day 53, and tumour tissues were weighed and collected for paraffin-imbedding for the following immuno-staining.
Figure 5.
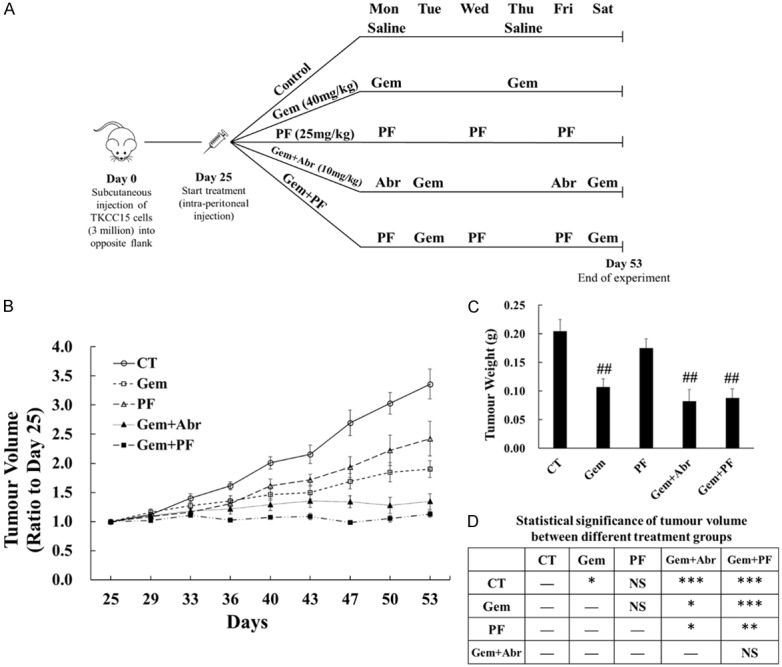
Combination of gemcitabine with PF-3758309 maximally inhibited tumour growth in vivo. TKCC 15 cells (3 × 106/100 µl/site) were injected subcutaneously into the opposite flanks of 8-week old male SCID mice. On day 25 after tumour induction, when the xenografted tumours had grown to approximate 80 mm3, the mice were randomly divided into the following 5 treatment groups: control (CT), PF-3758309 (PF), gemcitabine (Gem), gemcitabine plus abraxane (Gem+Abr) and gemcitabine plus PF-3758309 (Gem+PF). Each group had 3 mice bearing 2 tumours each mouse, making 6 tumours in total. The treatments were given by intra-peritoneal (i.p.) injection for four weeks, and the mice were euthanized on day 53. The schematic diagram of tumour induction and treatment was summarized and presented (A). The tumour volume (B) and weight (C) were measured and calculated as described in Material and Methods. The average tumour volume in each group on day 25 were taken as 1 (B). The statistical significance of tumour volumes between different groups were summarized in the table (D). NS, not significant; CT, control treatment with saline; Gem, gemcitabine; PF, PF-3758309; Abr, abraxane. ##P < 0.01 compared to CT. *P < 0.05, **P < 0.01, ***P < 0.001 as indicated in the table.
Immunohistochemistry
Tumour tissues, collected from the animal model as described above, were cut into 4-μm thick sections. Antigens were retrieved by treating sections in boiling citrate buffer (10 mM, citric acid, pH6, Sigma-Aldrich) for 30 min. Sample sections were incubated with hydrogen peroxidase for 15 min and 5% normal goat serum for 30 min at room temperature for endogenous peroxidase quenching and protein blocking, respectively. After incubation with Ki67 antibody (ThermoFisher Scientific, Australia) for proliferation, cleaved caspase 3 antibody (Cell Signaling Technology, Arundel, Australia) for apoptosis, sections were visualized by using an EnVision System kit (Dako, Botany, Australia) and were counterstained with hematoxylin. Samples were imaged using a Leica microscope for at least 10 fields at × 20 magnification. For quantification, the percentage of positive stained cells in each field was calculated.
Immunofluorescent staining
TKCC cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and blocked with 5% normal goat serum in 1% bovine serum albumin (BSA) TBST. For mouse tumour sections, samples were quenched, blocked and antigen retrieved as described above. Then samples were incubated with primary antibodies against CK19 (1:300), α-SMA (1:300) (Abcam, Cambridge, MA) overnight at 4°C, followed by incubation with secondary AlexaFlour 488 anti-rabbit or AlexaFlour 546 anti-mouse antibodies (1:1000) in 1% BSA TBST in the dark. After counterstained with DAPI (Life Technologies, Scoresby, Australia) (1:5000), samples were mounted using fluorescence mounting medium. All images were captured using a Nikon fluorescent microscope (Eclipse Ti-E Model) at × 40 magnification.
Statistical analysis
All values were expressed as mean ± standard error. The results were summarized from three independent experiments. Results were analysed by one-way ANOVA or student’s t-test. The correlation of PAK1 or PAK4 expression with CK19, α-SMA and palladin expression was determined by regression analysis. Differences between two means with P < 0.05 were considered significant. All the statistical analyses were performed using SPSS software (v16.0, SPSS Inc., Chicago, IL).
Results
PAK1 expression is correlated with CK19, and PAK4 expression is correlated with α-SMA and palladin
The protein expression profile of six TKCC cell lines were determined by Western blots. The expression of total and phospho-PAK1 and PAK4 varied in the panel of six TKCC cell lines. As an epithelial marker, CK19 was highly expressed, meanwhile the stromal markers including α-SMA, palladin were also observed in all these cell lines. The whole protein profile is shown in Figure 1A, 1B. Additionally, immunofluorescent staining was performed to further confirm the expression and localization of CK19, α-SMA in two selected cell lines (TKCC 15, 18) (Figure 1C). Regression analysis showed that the expression of PAK1 was positively correlated with the expression of CK19, whereas the protein level of PAK4 was positively correlated with α-SMA, palladin (Figure 1D). These results indicate that PAK1 and PAK4 mediate the regulation of proteins differently in these TKCC cell lines. The interaction between PAKs proteins and the tumoural and stromal marker proteins requires further investigation.
Figure 1.
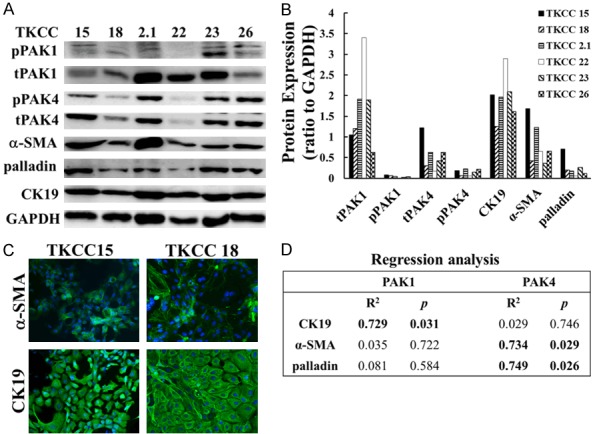
PAK1 expression was correlated with CK19, while PAK4 expression was correlated with α-SMA and palladin. The expression of multiple proteins in six TKCC cell lines was determined by Western blot (A), and results were analysed and quantified (B) as described in Materials and Methods. The intracellular localization of CK19, α-SMA were determined by immunofluorescent staining in two selected TKCC cell lines (TKCC 15, 18) (C). The fluorescent images were taken using a Nikon fluorescent microscope at × 40 magnification. The correlations between PAK1 or PAK4 expression with the expression of CK19, α-SMA and palladin were assessed by regression analysis, and R2 and p values were presented (D). pPAK1, phospho-PAK1; tPAK1, total PAK1; pPAK4, phospho-PAK4; tPAK4, total PAK4; CK19, cytokeratin 19; α-SMA, alpha-smooth muscle actin.
PF-3758309 enhanced the inhibitory effects of 5-FU, gemcitabine and abraxane on the proliferation of TKCC cells in vitro
The TKCC cells were incubated with increasing concentrations of PF-3758309 for 48 h under hypoxia (5% O2). PF-3758309 inhibited the proliferation of all TKCC cell lines in a dose-dependent manner (data not shown). The IC50 values of PF-3758309 for each cell line were calculated and listed in Table 1. Similarly, the IC50 values for 5-FU, gemcitabine and abraxane were also determined and were listed in Table 1. Then all six TKCC cell lines were pre-treated with PF-3758309 at concentrations around the IC50 values for 48 h, and followed by another 48 h treatment with 5-FU, gemcitabine or abraxane at concentrations around the IC50 values in the absence of PF-3758309. PF-3758309 pre-treatment enhanced the inhibitory effects of 5-FU on TKCC 15, 18, 22, 23 and 26 cells, of gemcitabine on TKCC 18, 2.1, 22, 23 and 26 cells, and of abraxane on TKCC 15, 2.1, 22, 23 and 26 cells (Figure 2) compared to each corresponding single-agent treatment. These results indicate that PF-3758309 inhibited TKCC cell growth synergistically with multiple chemotherapeutic reagents.
Figure 2.
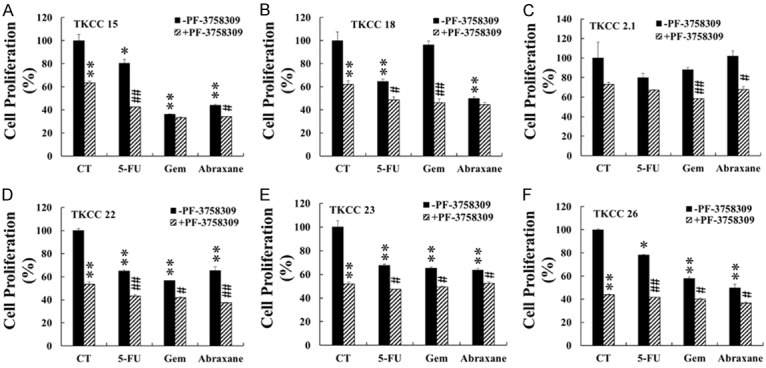
PF-3758309 enhanced the inhibitory effects of 5-FU, gemcitabine and abraxane on TKCC cell lines. Six TKCC cell lines (TKCC 15 (A); TKCC 18 (B); TKCC 2.1 (C); TKCC 22 (D); TKCC 23 (E); TKCC 26 (F)) were pre-incubated with PF-3758309 at the IC50 concentrations listed in Table 1 for 48 h, followed by another 48 h incubation with 5-FU, gemcitabine (Gem) or abraxane at the IC50 concentrations listed in Table 1. Cell proliferation was determined as described in Materials and Methods. The values obtained from controls (CT) without any treatment were taken as 100%. The data were summarized from at least three independent experiments. *P < 0.05, **P < 0.01, compared to the values obtained for the controls of non-PF-3758309 pre-treated cells. #P < 0.05, ##P < 0.01, compared to the values obtained from individual chemo-reagent (non-PF-3758309)-treated cells. 5-FU, 5-fluorouracil; Gem, gemcitabine.
PAKs inhibition by PF-3758309 caused reduced expression of HIF-1α, palladin and α-SMA in TKCC cell lines in vitro
After 48 h treatment with PF-3758309 at the IC50 concentrations determined in a proliferation assay (Table 1), the expression of pPAK1 (TKCC 15, 18, 2.1, 23) and pPAK4 (TKCC 18, 2.1, 22, 23, 26) were significantly reduced in the majority of these cell lines (Figure 3A, 3B, 3D), whereas no significant reduction in tPAK1 and tPAK4 were observed except for tPAK4 in TKCC 22, 23 and 26 cell lines (Figure 3A, 3C, 3E). All six TKCC cell lines were cultured under hypoxia (5% O2), the inhibitory effect of PF-3758309 on the expression of HIF-1α which is critical for cell survival under hypoxia, was determined. PF-3758309 significantly inhibited the expression of HIF-1α in all six TKCC lines (Figure 4A, 4B). Additionally, decreased expression of palladin (TKCC 15, 18, 2.1) and α-SMA (TKCC 15, 26) were also observed in the selected TKCC cell lines (Figure 4A, 4C, 4D). These data suggest that PAK1 and/or PAK4 play an important role in regulating the expression of HIF-1α, palladin and α-SMA. Inhibition of PAKs by PF-3758309 leads to down-regulation of HIF-1α, palladin and α-SMA expression.
Figure 3.
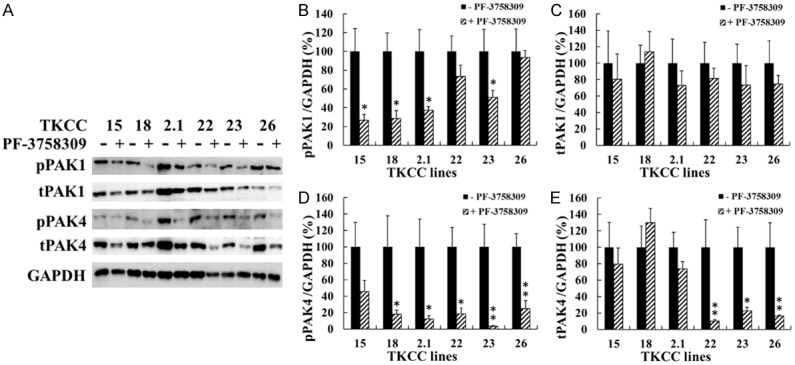
PF-3758309 suppressed the activities of PAK1 and PAK4. The six TKCC cell lines were incubated with PF-3758309 at the concentrations listed in Table 1 under hypoxia (5% O2) for 48 h. The protein levels of total and phospho-PAK1 and PAK4 were determined by Western blot, and representative images were presented (A). Relative protein amount was calculated as described in Materials and Methods, and the values for untreated cells were taken as 100% (B-E). The results were summarized from three independent experiments. *P < 0.05, **P < 0.01, compared to the values for non-PF-3758309-treated cells. pPAK1, phospho-PAK1; tPAK1, total PAK1; pPAK4, phospho-PAK4; tPAK4, total PAK4.
Figure 4.
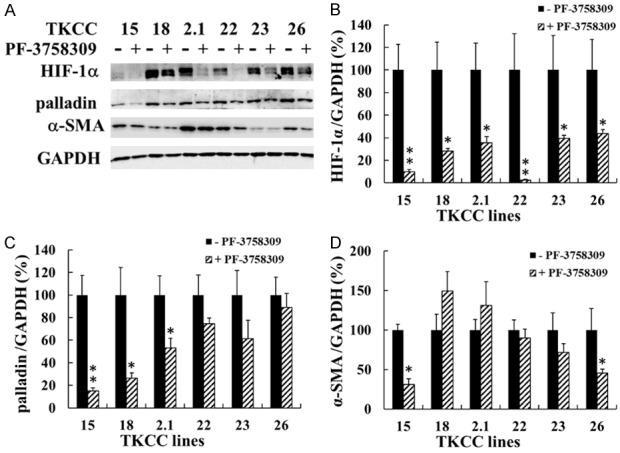
PF-3758309 inhibited the expression of HIF-1α, palladin and α-SMA in vitro. The six TKCC cell lines were incubated with PF-3758309 at the concentrations listed in Table 1 under hypoxia (5% O2) for 48 h. The protein levels of HIF-1α, palladin and α-SMA were determined by Western blot, and representative images were presented (A). Relative protein amount was calculated as described in Materials and Methods, and the values for untreated cells were taken as 100% (B-D). The results were summarized from three independent experiments. *P < 0.05, **P < 0.01, compared to the values for non-PF-3758309-treated cells. HIF-1α, hypoxia-inducible factor 1-alpha; α-SMA, alpha-smooth muscle actin.
The combination of gemcitabine with PF-3758309 maximally inhibited tumour growth by suppressing cell proliferation in vivo
SCID mice bearing TKCC 15 xenografted tumours were divided into the following five groups: control, gemcitabine alone, PF-3758309 alone, gemcitabine plus abraxane and gemcitabine plus PF-3758309. The schematic diagram of tumour induction and treatment was shown in Figure 5A. At the beginning of the treatments (day 25), the tumour volumes (measured in mm3) were 72 ± 12, 82 ± 11, 80 ± 13, 81 ± 11, and 83 ± 13 for control, gemcitabine alone, PF-3758309 alone, gemcitabine plus abraxane and gemcitabine plus PF-3758309, respectively. Tumour volume on day 25 was taken as 1 in each group. The following tumour volume was calculated as the ratio to that on day 25 (Figure 5B). After 4-week treatment, tumour volumes in all groups (except for PF-3758309 alone) were significantly reduced compared to control. No significant difference was observed between gemcitabine alone and PF-3758309 alone treatment group. Both combination treatment groups (gemcitabine plus abraxane or gemcitabine plus PF-3758309) showed further inhibition on tumour growth compared to both single-agent treatment groups (gemcitabine or PF-3758309) (Figure 5B, 5D). Among all four drug treatment groups, gemcitabine plus PF-3758309 inhibited the tumour growth maximally, but no significant difference was observed when compared to gemcitabine plus abraxane. All treatment groups except for PF-3758309 alone showed significantly decreased tumour weights compared to control. However, neither of combination treatment showed further significant decrement in tumour weight compared to gemcitabine alone treatment (Figure 5C). Presumably, the difference between the tumour volume and tumour weight was caused by necrosis where liquification was presented leading to weight loss in some tumours.
Proliferation and apoptosis within xenograft tumour were determined by immunohistochemical staining of Ki67 and cleaved caspase-3, respectively. Gemcitabine alone, but not PF-3758309 alone, significantly inhibited cell proliferation to 83% of control. Combination treatment further reduced proliferation to 74% and 67% of control for gemcitabine plus abraxane and gemcitabine plus PF-3758309, respectively, and the reductions were significantly greater than that of gemcitabine alone (Figure 6A, 6B). There was no significant difference in apoptosis in all four treatment groups compared to control (Figure 6C, 6D). These results indicated that combination of gemcitabine with PF-3758309 synergistically reduced tumour growth via suppressing cell proliferation. This combination could achieve a comparable or even greater therapeutic efficacy compared to gemcitabine plus abraxane.
Figure 6.
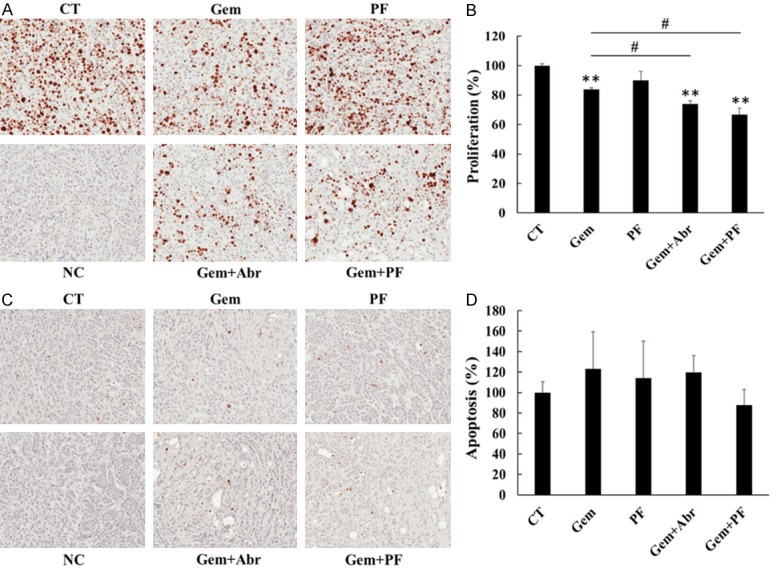
PF-3758309 promoted anti-tumour effect of gemcitabine in vivo by decreasing cell proliferation without affecting apoptosis. Tumour sections originated from TKCC 15 xenograft were immunohistochemically stained with antibody against Ki67 (A) and cleaved caspase-3 (C) to determine cell proliferation and apoptosis, respectively. The images were taken using a Leica microscope at × 20 magnification. The data were analysed as described in the Materials and Methods. The values for CT in both Ki67 (B) and cleaved caspase-3 (D) were taken as 100%. NC, negative control stained without the primary antibody; CT, control treatment with saline; Gem, gemcitabine; PF, PF-3758309; Abr, abraxane. **P < 0.01 compared to CT. #P < 0.05 compared to gemcitabine alone.
PF-3758309 reduced the expression HIF-1α, palladin and α-SMA in vivo
Co-expression of CK19 and α-SMA was observed by dual immunofluorescent staining in tumour sections of TKCC15 xenograft (Figure 7A). Consistent to the in vitro results where PF-3758309 treatment significantly reduced the expression of HIF-1α, palladin and α-SMA in TKCC 15 cells (Figure 4), PF-3758309 (PF-3758309 alone and gemcitabine plus PF-3758309 treatment group) also greatly decreased the expression of HIF-1α, palladin and α-SMA in protein extracts of tumour tissues originated from TKCC 15 xenograft. Gemcitabine alone treatment slightly decreased α-SMA, but had no effects on HIF-1α and palladin expression. Gemcitabine plus abraxane treatment suppressed the level of palladin and α-SMA, but not HIF-1α. These results further confirmed the expression of α-SMA in PDA cells (TKCC 15), and PAK inhibition by PF-3758309 treatment also down-regulated HIF-1α, palladin and α-SMA expression in vivo.
Figure 7.
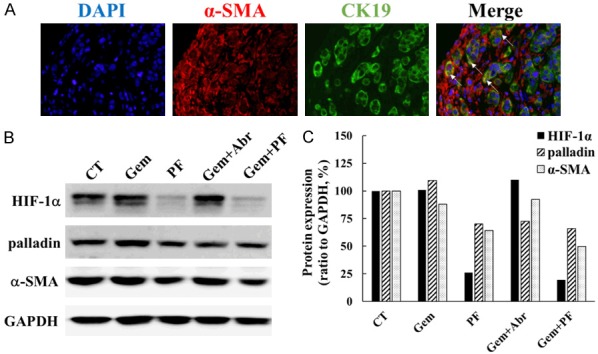
PF-3758309 reduced the expression of HIF-1α, palladin and α-SMA in vivo. A dual immunofluorescent staining of CK19 (green) and α-SMA (red) was conducted on tumour tissue sections generated from TKCC 15 xenografts (A). Co-localization of CK19 and α-SMA (yellow, arrows) was observed in several tumour cells. The images were taken using a Nikon fluorescent microscope at × 40 magnification. The amounts of HIF-1α, palladin and α-SMA were determined by Western blotting in protein extracts obtained from TKCC 15 xenograft tumour tissue, and representative images were presented (B). Relative protein amount was calculated as described in Materials and Methods, and the values for CT were taken as 100% (C). CK19, cytokeratin 19; α-SMA, alpha-smooth muscle actin; CT, control treatment with saline; Gem, gemcitabine; PF, PF-3758309; Abr, abraxane; HIF-1α, hypoxia-inducible factor 1-alpha; α-SMA, alpha-smooth muscle actin.
Discussion
The data presented here indicates that the pan-PAK inhibitor PF-3758309 [23] is an attractive therapeutic reagent for targeting pancreatic cancer. In this study, PF-3758309 not only inhibited cell proliferation on its own, but also enhanced the inhibitory effects of multiple chemotherapeutic reagents including 5-FU, gemcitabine and abraxane in a panel of established cell lines derived from patients with pancreatic cancer [22]. In xenograft animal model, combination of gemcitabine with PF-3758309 achieved a comparable or even greater therapeutic efficacy compared to gemcitabine plus abraxane via suppression of cell proliferation rather than apoptosis. In agreement, our previous studies also demonstrated that genetically or pharmaceutically inhibition of PAK1 and/or PAK4 synergistically enhanced gemcitabine sensitivity both in vitro and in vivo [10,24]. Additionally, it has been reported that both PAK1 and PAK4 play pivotal roles in mediating gemcitabine resistance [11,14]. Here, our data showing that PF-3758309 enhanced the inhibitory effects of multiple chemotherapeutic reagents on a panel of patient’s derived PDA cell lines in vitro and in vivo, make PF-3758309 a potential reagent in adjunctive or combination therapy in the management of pancreatic cancer.
Protein profiling by Western blot showed that TKCC cell lines not only expressed the cancer cell marker CK19, but also stromal cell markers including α-SMA and palladin. Total and phospho-PAK1 and PAK4 were also expressed in all six cell lines. Interestingly, the expression of PAK1 was significantly correlated with CK19, while the expression of PAK4 was significantly correlated with palladin and α-SMA (Figure 1D). Presumably, this result suggests that PAK1 and PAK4 contribute to regulation or maintenance of an “epithelial” or “mesenchymal”-like state differently in tumour development. Furthermore, a dual immunofluorescent staining also confirmed the co-expression of CK19 and α-SMA within tumour tissues. To our knowledge, this is the first study showing the expression of α-SMA in PDA cells in vitro and in vivo. α-SMA is an established marker for PSC activation [25], and is reported to be a negative prognostic factor for patients with resectable PDA [26,27]. In PSCs, all-trans retinoic acid induced down-regulation of α-SMA attenuated its pro-tumour interaction with PDA cells and suppressed the tumour progression [28]. Our previous study also demonstrated that inhibition of PAK1 suppressed α-SMA expression in PSCs, leading to decreased cell proliferation and increased apoptosis [12]. In this study, PF-3758309 significantly reduced the expression of α-SMA both in vitro and in vivo. The different inhibitory effect of PF-3758309 on α-SMA expression in selected TKCC cell lines may be due to the heterogeneous nature of pancreatic cancer. Colocalization of CK19 and α-SMA in some tumour cells in vivo, particularly in the interphase where tumour cells meet stromal cells (Figure 7A), suggested that these dual stained cells might play an important role in metastasis. Similarly, α-SMA has also been reported to be involved in tumour cell metastasis in lung cancer [29]. Targeting α-SMA in both PSC and PDA cells by PAK inhibition might be a potential approach in PDA treatment. The role of α-SMA in PDA tumour biology is still not fully understood, further investigation is required to unravel the underlying mechanisms linking PAKs to α-SMA regulation.
The expression of HIF-1α and palladin, which was involved in cell survival and cytoskeletal re-organization, was also significantly reduced by PF-3758309 treatment in vitro and in vivo. HIF-1α was reported to mediate gemcitabine resistance in pancreatic cancer [30]. Inhibition of HIF-1α could sensitize cancer cells to gemcitabine treatment [31], whereas high expression and activity of HIF-1α inhibited the transcription of hENT1 and hENT2, leading to decreased gemcitabine uptake [32]. The critical role of PAK1 in regulating HIF-1α has been demonstrated in our previous studies, showing that PAK1 enhanced cancer cell survival by up-regulation of HIF-1α [33] and PAK1 knockdown by shRNA decreased expression of HIF-1α and tumour growth [10]. Another recent study also reported that PAK4 inhibition reduced expression of HIF-1α via the AKT-mTOR-4E-BP1 axis [34]. Therefore, PAKs could mediate cell survival and gemcitabine resistance by up-regulation of HIF-1α signalling. It was reported that palladin stimulated cell invasion of PDA by promoting invadopodia formation in cancer-associated fibroblasts [35]. One recent clinical study analysed tissue microarrays of resected PDA samples from 167 patients, and found that palladin expression was correlated with a significantly lower survival rate and was an independent poor prognostic factor [6]. Although the mechanisms involved in the relationship between PAKs and palladin have not been well elucidated, the inhibitory effect of PAK inhibitor on palladin expression was observed in this study. Treatment with PAK inhibition could suppress tumour metastasis and improve patient’s survival via down-regulation of palladin.
In conclusion, we have demonstrated here that the combination of the PAK inhibitor PF-3758309 with multiple chemotherapeutic reagents further suppressed the growth of a panel of patient-derived pancreatic cancer cell lines compared to single-agent chemotherapies. The combination of PF-3758309 with gemcitabine showed a comparable or even greater inhibition of the tumour growth in vivo when compared to gemcitabine plus abraxane, and thus provided a potential treatment regime in the management of PDA. Furthermore, we firstly reported that PDA tumour cells not only expressed epithelial marker, but also expressed stromal marker both in vitro and in vivo. PAK inhibition by PF-3758309 reduced the expression of HIF-1α, palladin and α-SMA, which were critical effector proteins in cell growth, survival and chemo-resistance in PDA.
Acknowledgements
This work was supported by the Austin Medical Research Foundation (HH, No. He007), the National Health and Medical Research Council of Australia (GSB, No. 1041831) and the Pancare Foundation (https://www.pancare.org.au). The authors would like to thank Dr. Dannel Yeo for his help and suggestion in TKCC cell culture. Dr. Kai Wang is supported by scholarships from the University of Melbourne and Pancare foundation (Moshe Sambor Scholarship).
Disclosure of conflict of interest
None.
References
- 1.Lau SC, Cheung WY. Evolving treatment landscape for early and advanced pancreatic cancer. World J Gastrointest Oncol. 2017;9:281–292. doi: 10.4251/wjgo.v9.i7.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, Lolkema MP, Buchholz M, Olive KP, Gress TM, Tuveson DA. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–868. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 5.Apte MV, Pirola RC, Wilson JS. Pancreatic stellate cells: a starring role in normal and diseased pancreas. Front Physiol. 2012;3:344. doi: 10.3389/fphys.2012.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato D, Tsuchikawa T, Mitsuhashi T, Hatanaka Y, Marukawa K, Morooka A, Nakamura T, Shichinohe T, Matsuno Y, Hirano S. Stromal palladin expression is an independent prognostic factor in pancreatic ductal adenocarcinoma. PLoS One. 2016;11:e0152523. doi: 10.1371/journal.pone.0152523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou W, Jubb AM, Lyle K, Xiao Q, Ong CC, Desai R, Fu L, Gnad F, Song Q, Haverty PM, Aust D, Grutzmann R, Romero M, Totpal K, Neve RM, Yan Y, Forrest WF, Wang Y, Raja R, Pilarsky C, de Jesus-Acosta A, Belvin M, Friedman LS, Merchant M, Jaffee EM, Zheng L, Koeppen H, Hoeflich KP. PAK1 mediates pancreatic cancer cell migration and resistance to MET inhibition. J Pathol. 2014;234:502–513. doi: 10.1002/path.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahlamaki EH, Kauraniemi P, Monni O, Wolf M, Hautaniemi S, Kallioniemi A. High-resolution genomic and expression profiling reveals 105 putative amplification target genes in pancreatic cancer. Neoplasia. 2004;6:432–439. doi: 10.1593/neo.04130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeo D, He H, Baldwin GS, Nikfarjam M. The role of p21-activated kinases in pancreatic cancer. Pancreas. 2015;44:363–369. doi: 10.1097/MPA.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 10.Yeo D, He H, Patel O, Lowy AM, Baldwin GS, Nikfarjam M. FRAX597, a PAK1 inhibitor, synergistically reduces pancreatic cancer growth when combined with gemcitabine. BMC Cancer. 2016;16:24. doi: 10.1186/s12885-016-2057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jagadeeshan S, Subramanian A, Tentu S, Beesetti S, Singhal M, Raghavan S, Surabhi RP, Mavuluri J, Bhoopalan H, Biswal J, Pitani RS, Chidambaram S, Sundaram S, Malathi R, Jeyaraman J, Nair AS, Venkatraman G, Rayala SK. P21-activated kinase 1 (Pak1) signaling influences therapeutic outcome in pancreatic cancer. Ann Oncol. 2016;27:1546–1556. doi: 10.1093/annonc/mdw184. [DOI] [PubMed] [Google Scholar]
- 12.Yeo D, Phillips P, Baldwin GS, He H, Nikfarjam M. Inhibition of group 1 p21-activated kinases suppresses pancreatic stellate cell activation and increases survival of mice with pancreatic cancer. Int J Cancer. 2017;140:2101–2111. doi: 10.1002/ijc.30615. [DOI] [PubMed] [Google Scholar]
- 13.Tyagi N, Bhardwaj A, Singh AP, McClellan S, Carter JE, Singh S. p-21 activated kinase 4 promotes proliferation and survival of pancreatic cancer cells through AKT- and ERK-dependent activation of NF-kappaB pathway. Oncotarget. 2014;5:8778–8789. doi: 10.18632/oncotarget.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon SU, Kim JW, Sung JH, Kang MH, Kim SH, Chang H, Lee JO, Kim YJ, Lee KW, Kim JH, Bang SM, Lee JS. p21-activated kinase 4 (PAK4) as a predictive marker of gemcitabine sensitivity in pancreatic cancer cell lines. Cancer Res Treat. 2015;47:501–508. doi: 10.4143/crt.2014.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohammad RM, Li Y, Muqbil I, Aboukameel A, Senapedis W, Baloglu E, Landesman Y, Philip PA, Azmi AS. Targeting Rho GTPase effector p21 activated kinase 4 (PAK4) suppresses p-Bad-microRNA drug resistance axis leading to inhibition of pancreatic ductal adenocarcinoma proliferation. Small GTPases. 2017 doi: 10.1080/21541248.2017.1329694. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aboukameel A, Muqbil I, Senapedis W, Baloglu E, Landesman Y, Shacham S, Kauffman M, Philip PA, Mohammad RM, Azmi AS. Novel p21-activated kinase 4 (PAK4) allosteric modulators overcome drug resistance and stemness in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2017;16:76–87. doi: 10.1158/1535-7163.MCT-16-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray BW, Guo C, Piraino J, Westwick JK, Zhang C, Lamerdin J, Dagostino E, Knighton D, Loi CM, Zager M, Kraynov E, Popoff I, Christensen JG, Martinez R, Kephart SE, Marakovits J, Karlicek S, Bergqvist S, Smeal T. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc Natl Acad Sci U S A. 2010;107:9446–9451. doi: 10.1073/pnas.0911863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong CC, Jubb AM, Jakubiak D, Zhou W, Rudolph J, Haverty PM, Kowanetz M, Yan Y, Tremayne J, Lisle R, Harris AL, Friedman LS, Belvin M, Middleton MR, Blackwood EM, Koeppen H, Hoeflich KP. P21-activated kinase 1 (PAK1) as a therapeutic target in BRAF wild-type melanoma. J Natl Cancer Inst. 2013;105:606–607. doi: 10.1093/jnci/djt054. [DOI] [PubMed] [Google Scholar]
- 19.Ryu BJ, Lee H, Kim SH, Heo JN, Choi SW, Yeon JT, Lee J, Lee J, Cho JY, Kim SH, Lee SY. PF-3758309, p21-activated kinase 4 inhibitor, suppresses migration and invasion of A549 human lung cancer cells via regulation of CREB, NF-kappaB, and beta-catenin signalings. Mol Cell Biochem. 2014;389:69–77. doi: 10.1007/s11010-013-1928-8. [DOI] [PubMed] [Google Scholar]
- 20.Huynh N, Shulkes A, Baldwin G, He H. Up-regulation of stem cell markers by P21-activated kinase 1 contributes to 5-fluorouracil resistance of colorectal cancer. Cancer Biol Ther. 2016;17:813–823. doi: 10.1080/15384047.2016.1195045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, Quinn MC, Robertson AJ, Fadlullah MZ, Bruxner TJ, Christ AN, Harliwong I, Idrisoglu S, Manning S, Nourse C, Nourbakhsh E, Wani S, Wilson PJ, Markham E, Cloonan N, Anderson MJ, Fink JL, Holmes O, Kazakoff SH, Leonard C, Newell F, Poudel B, Song S, Taylor D, Waddell N, Wood S, Xu Q, Wu J, Pinese M, Cowley MJ, Lee HC, Jones MD, Nagrial AM, Humphris J, Chantrill LA, Chin V, Steinmann AM, Mawson A, Humphrey ES, Colvin EK, Chou A, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Pettitt JA, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N, Jamieson NB, Graham JS, Niclou SP, Bjerkvig R, Grutzmann R, Aust D, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Falconi M, Zamboni G, Tortora G, Tempero MA Australian Pancreatic Cancer Genome Initiative. Gill AJ, Eshleman JR, Pilarsky C, Scarpa A, Musgrove EA, Pearson JV, Biankin AV, Grimmond SM. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chou A, Froio D, Nagrial AM, Parkin A, Murphy KJ, Chin VT, Wohl D, Steinmann A, Stark R, Drury A, Walters SN, Vennin C, Burgess A, Pinese M, Chantrill LA, Cowley MJ, Molloy TJ Australian Pancreatic Cancer Genome Initiative (APGI) Waddell N, Johns A, Grimmond SM, Chang DK, Biankin AV, Sansom OJ, Morton JP, Grey ST, Cox TR, Turchini J, Samra J, Clarke SJ, Timpson P, Gill AJ, Pajic M. Tailored first-line and second-line CDK4-targeting treatment combinations in mouse models of pancreatic cancer. Gut. 2018;67:2142–2155. doi: 10.1136/gutjnl-2017-315144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo C, McAlpine I, Zhang J, Knighton DD, Kephart S, Johnson MC, Li H, Bouzida D, Yang A, Dong L, Marakovits J, Tikhe J, Richardson P, Guo LC, Kania R, Edwards MP, Kraynov E, Christensen J, Piraino J, Lee J, Dagostino E, Del-Carmen C, Deng YL, Smeal T, Murray BW. Discovery of pyrroloaminopyrazoles as novel PAK inhibitors. J Med Chem. 2012;55:4728–4739. doi: 10.1021/jm300204j. [DOI] [PubMed] [Google Scholar]
- 24.Wang K, Huynh N, Wang X, Baldwin G, Nikfarjam M, He H. Inhibition of p21 activated kinase enhances tumour immune response and sensitizes pancreatic cancer to gemcitabine. Int J Oncol. 2018;52:261–269. doi: 10.3892/ijo.2017.4193. [DOI] [PubMed] [Google Scholar]
- 25.Han S, Delitto D, Zhang D, Sorenson HL, Sarosi GA, Thomas RM, Behrns KE, Wallet SM, Trevino JG, Hughes SJ. Primary outgrowth cultures are a reliable source of human pancreatic stellate cells. Lab Invest. 2015;95:1331–1340. doi: 10.1038/labinvest.2015.117. [DOI] [PubMed] [Google Scholar]
- 26.Erkan M, Michalski CW, Rieder S, Reiser-Erkan C, Abiatari I, Kolb A, Giese NA, Esposito I, Friess H, Kleeff J. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2008;6:1155–1161. doi: 10.1016/j.cgh.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Sinn M, Denkert C, Striefler JK, Pelzer U, Stieler JM, Bahra M, Lohneis P, Dorken B, Oettle H, Riess H, Sinn BV. alpha-Smooth muscle actin expression and desmoplastic stromal reaction in pancreatic cancer: results from the CONKO-001 study. Br J Cancer. 2014;111:1917–1923. doi: 10.1038/bjc.2014.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Froeling FE, Feig C, Chelala C, Dobson R, Mein CE, Tuveson DA, Clevers H, Hart IR, Kocher HM. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-beta-catenin signaling to slow tumor progression. Gastroenterology. 2011;141:1486–1497. 1497.e1481–1414. doi: 10.1053/j.gastro.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 29.Lee HW, Park YM, Lee SJ, Cho HJ, Kim DH, Lee JI, Kang MS, Seol HJ, Shim YM, Nam DH, Kim HH, Joo KM. Alpha-smooth muscle actin (ACTA2) is required for metastatic potential of human lung adenocarcinoma. Clin Cancer Res. 2013;19:5879–5889. doi: 10.1158/1078-0432.CCR-13-1181. [DOI] [PubMed] [Google Scholar]
- 30.Wang K, Baldwin GS, Nikfarjam M, He H. p21-activated kinase signalling in pancreatic cancer: new insights into tumour biology and immune modulation. World J Gastroenterol. 2018;24:3709–3723. doi: 10.3748/wjg.v24.i33.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao T, Ren H, Jia L, Chen J, Xin W, Yan F, Li J, Wang X, Gao S, Qian D, Huang C, Hao J. Inhibition of HIF-1alpha by PX-478 enhances the anti-tumor effect of gemcitabine by inducing immunogenic cell death in pancreatic ductal adenocarcinoma. Oncotarget. 2015;6:2250–2262. doi: 10.18632/oncotarget.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schonfeld C, Loffler M, Reyes G, Duszenko M, Karhausen J, Robinson A, Westerman KA, Coe IR, Colgan SP. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202:1493–1505. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu KH, Huynh N, Patel O, Shulkes A, Baldwin G, He H. P21-activated kinase 1 promotes colorectal cancer survival by up-regulation of hypoxia-inducible factor-1alpha. Cancer Lett. 2013;340:22–29. doi: 10.1016/j.canlet.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 34.Kim H, Woo DJ, Kim SY, Yang EG. p21-activated kinase 4 regulates HIF-1alpha translation in cancer cells. Biochem Biophys Res Commun. 2017;486:270–276. doi: 10.1016/j.bbrc.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 35.Goicoechea SM, Garcia-Mata R, Staub J, Valdivia A, Sharek L, McCulloch CG, Hwang RF, Urrutia R, Yeh JJ, Kim HJ, Otey CA. Palladin promotes invasion of pancreatic cancer cells by enhancing invadopodia formation in cancer-associated fibroblasts. Oncogene. 2014;33:1265–1273. doi: 10.1038/onc.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]


