Abstract
Acute graft-versus-host disease (aGVHD) is one of the major complications after liver transplantation (LTx), which is induced by over-activation of T helper lymphocytes. Cenicriviroc (CVC) exerts its anti-inflammatory effect through inhibition of C-C chemokine receptor 5 (CCR5). However, whether CVC ameliorates aGVHD after liver transplantation remains unknown. In the present study, a rat aGVHD liver transplantation model (LTx-aGVHD) was constructed. CVC was intravenously injected from day 7 to day 14 after LTx. Liver and intestine samples were harvested to evaluate GVHD severity. Peripheral blood mononuclear cells (PBMCs) were collected and CCR5 antibodies were prepared to further explore the molecular mechanism in vitro. CVC significantly decreased the severity of GVHD associated skin and intestine injury. Quality of life of the LTx-GVHD rats was improved after CVC treatment. Flow cytometry further confirmed diminished peripheral donor-derived Th cells after CVC treatment. Molecularly, CVC treatment showed similar anti-inflammatory effects to CCR5 antibody injection. The level of CCR5, C-C motif chemokine ligand 5 (CCL5), and pro-inflammatory cytokines in the liver and intestines were inhibited after CVC treatment. Thus, CVC deactivated Th lymphocytes and decreased the severity of LTx-aGVHD through inhibition of CCR5.
Keywords: Acute graft-versus-host disease, aGVHD, cenicriviroc, CCR5, liver transplantation
Introduction
Acute graft-versus-host disease (aGVHD) is one of the most dangerous complications after liver transplantation (LTx) [1]. Although the overall incidence of aGVHD is low (1-2%), the mortality of this complication is extremely high (85-90%) [2]. Patients who developed aGVHD after LTx suffer from sepsis, bone marrow suppression, and multi-organ dysfunction [3]. Although aGVHD was first proposed in 1988, its mechanism and treatment remain controversial [4]. Modification of the immunosuppressant plan shows little effect in managing aGVHD [5,6]; therefore, it is important to develop a new strategy to prevent and treat aGVHD in clinical practice.
It is widely accepted that donor T cell homing and migration is responsible for aGVHD development [7]. This process is modulated by chemokine receptors such as C-C chemokine receptor 5 (CCR5) [8]. Evidence indicated that CCR5 blockade decreased inflammation in a bone marrow transplantation GVHD model [9] and in clinical trials [10]. By contrast, inflammation caused by GVHD was inhibited after a combination of a CCR5 inhibitor and cyclosporin A treatment in a hematopoietic stem cell transplantation (HSCT) model [11]. Cenicriviroc (CVC), a CCR2/CCR5 co-inhibitor, is widely used in AIDS prevention and treatment [12]. Recently, CVC was proved to be effective in reversing liver fibrosis and steatosis through inhibition of inflammation [13]. However, whether CVC ameliorates aGVHD after LTx remains unknown.
The present study aimed to use a previously developed aGVHD model to better understand the mechanism of aGVHD. Our previous work showed that activation of T helper (Th) lymphocytes was responsible for the development of aGVHD. Th cell physiology was proven to be regulated by regulatory T cells (Tregs); the decrease in Tregs correlated with the severity of aGVHD. Therefore, the present study also aimed to explore whether CVC protects liver grafts from aGVHD and the underlying molecular mechanism.
Materials and methods
Ethical approval of the study protocol
The study was performed in accordance with the guidelines of the Ethics Committee of the Nanjing Drum Tower Hospital of Nanjing University Medical School. All in vivo experiments strictly followed the guidelines for animal care set by the Nanjing Drum Tower Hospital of Nanjing University Medical School.
GVHD liver transplantation model and groupings
The liver grafts of green fluorescent protein (GFP) transgenic Lewis male rats were harvested as liver donors. F1 generation Lewis × Brown Norway (BN) rats were chosen as LTx recipients. Rats were fasted for 1 day before LTx. Phenobarbital sodium (50 mg/kg) was injected intraperitoneally to achieve an adequate depth of anesthesia. Kamada’s two-cuff methods were used for transplantation. Briefly, the suprahepatic vena cava and infrahepatic vena cava were sutured with 8-0 microscopic vascular sutures. The two-cuff method was utilized to reconstruct the common bile duct and portal vain, without reconstruction of the hepatic artery.
To construct a GVHD model, during surgery, a donor’s spleen homogenate was prepared. The spleen homogenate was washed with phosphate-buffered saline (PBS) three times and further diluted in erythrolysis solution for 10 minutes. The homogenate was centrifuged at 2000 rpm for 5 minutes. Peripheral blood mononuclear cells (PBMCs) were counted and diluted at 8 × 108/ml. Then, 0.5 ml of PBMC solution was injected through the tail vein 30 min after LTx to construct an aGVHD-LTx model.
Rats that survived after LTx were carefully observed and randomly divided into two groups and injected with saline or CVC once a day from the 7th to the 14th day after LTx: Group 1, control saline injection; group 2, 20 mg/kg CVC injection. Body weight, activity, appetite, and symptoms of diarrhea and jaundice, were documented. Serum samples of each rat were collected at day 4 and day 12 after LTx. Skin, small intestines, and liver samples were harvested after the death of the rats.
Flow cytometry analysis
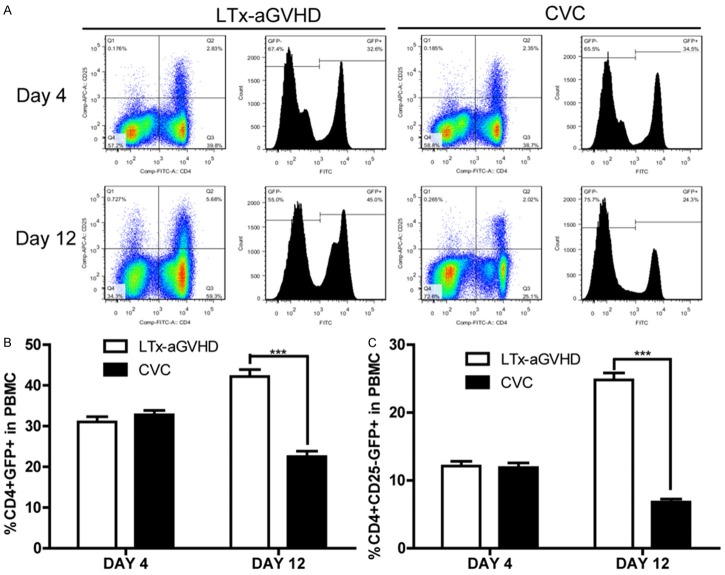
Blood samples collected on day 4 and 12 after LTx from the vena caudalis were mixed with red blood cell (RBC) lysis buffer. PMBCs were collected for flow cytometry analysis. The cells were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-CD4 antibodies (BioLegend, San Diego, CA) and allophycocyanin (APC)-conjugated anti-CD25 antibodies (BioLegend, San Diego, CA) for 30 minutes on ice. Then, the cells were fixed with fixation and permeabilization buffer for 45 minutes at room temperature. The proportions of CD4+GFP+ and CD4+CD25-GFP+ Th cells were examined using flow cytometry.
Chemicals and reagents
All primary and secondary antibodies used in western blotting, including those recognizing CCR2, CCR5, C-C motif chemokine ligand 5 (CCL5), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were obtained from Cell Signaling Technology (Danvers, MA, USA).
Chemotaxis analysis
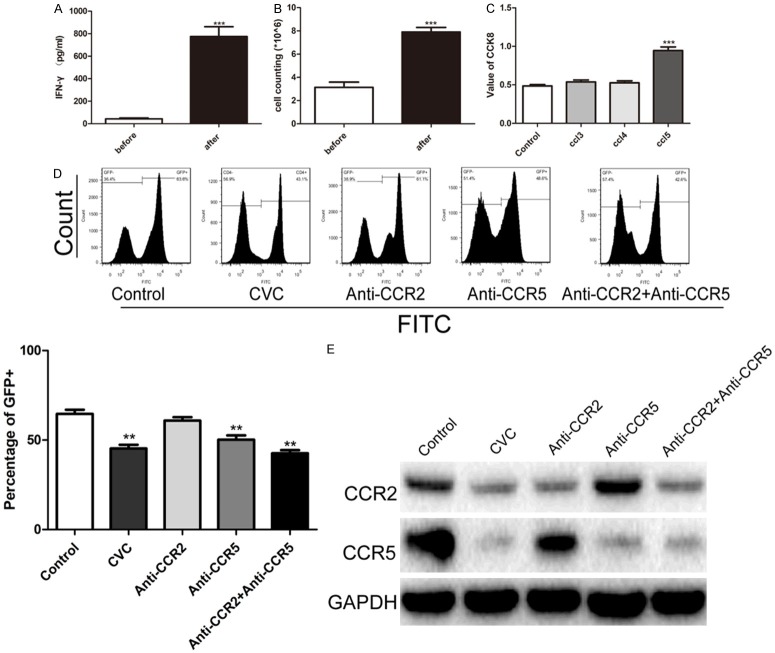
PBMCs were collected as previously described. Cells were plated into a 24-well plate at a concentration of 1.5 × 106 and treated with anti-CD3 (BioLegend, San Diego, CA) and anti-CD28 antibodies (BioLegend, San Diego, CA) for activation of Th cell. At 48 hours after incubation, the medium was changed with Roswell Park Memorial Institute (RPMI)-1640 medium with 1 ng/ml interleukin-12 (IL-12) for another 24 hours. The cells were then treated with 1) CVC (1 nM); 2) anti-CCR5 antibodies (1 ng/ml); 3) anti-CCR2 antibodies (1 ng/ml); and 4) anti-CCR5 antibody (1 ng/ml) + anti-CCR2 antibody (1 ng/ml) for 24 hours. Cells (2 × 105) were further plated in Chemo-TX plates containing CCL3, CCL4, and CCL5 and incubated at 37°C for 30 minutes. Cell counting kit 8 (CCK-8) (Dojindo, Japan) analysis was used to quantify cells that penetrated the membrane.
Western blot analysis
Samples of cell lysate pro-teins (40 mg) were separated by 10% sodium dodecylsulfate-polyacrylamide gel electrophoresis and transferred topolyvinylidene difluoride membranes. The membranes were blocked with Tris-buffered saline/0.1% Tween 20 containing 5% bovine serum albumin and incubated with specific primary antibodies (all 1:1000 dilution) for 6 hours. The membranes were then washed (3 times; 30 minutes per wash) with Tris-buffered saline/0.1% Tween 20 and incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (all 1:2000 dilution). All antibodies were bought from Cell Signaling Technology except the HRP-conjugated secondary antibodies (Beyo-time Institute of Biotechnology, Shanghai, China).
ELISA
The supernatants were collected and stored at 4°C. Concentrations of IL-2, IL-6, IFN-ã, IL-10, TGF-â were detected using commercial ELISA kits (R&D Systems, Inc.).
Hematoxylin and eosin staining
The tissue samples of skin, intestine, and liver harvested from the rat were fixed with 4% paraformaldehyde for 24 h. After conventional dehydration, wax infiltration, and paraffin embedding, the tissues were sectioned at intervals of 4 µm and stained with hematoxylin and eosin.
Statistical analysis
The results were analyzed using SPSS v11.0 (IBM Corp., Armonk, NY, USA) and data are presented as the mean ± standard deviation (SD). All in vitro experiments were repeated at least three times. One-way analysis of variance was used to compare group variables, followed by least-significant difference post hoc testing if required. P < 0.05 was considered significant.
Results
CVC inhibited inflammation in the LTx-aGVHD model
The LTx-aGVHD model was constructed as previously described [14]. Donor livers were harvested from GFP transgenic Lewis rats, and transplanted to Lewis × BN F1 rats. Rats that survived the operation were randomly divided into two groups (n = 11), one receiving 20 mg/kg CVC and one receiving saline injected through tail vein once a day from day 7 to 14 after LTx. The survival time of each LTx rat was documented. As shown in Table 1, the mean survival time of the CVC group was longer than that of the control group (50 days vs. 27 days, P < 0.05). Kaplan-Meier survival analysis was performed and displayed in Figure 1A. The rats in the control group suffered from severe diarrhea and body weight loss after LTx; however, the rats that received CVC had normal body shapes and no diarrhea. Representative photographs of the two groups were taken and are shown in Figure 1B. Skin, small intestine, and liver samples of each rat were collected after death and mounted on slides. H&E staining was performed for each slide and the results are displayed in Figure 1C. Diffusive infiltration of neutrophils was observed in the skin and intestine samples of the GVHD rats. The number of infiltrated neutrophils decreased after CVC treatment. However, no obvious differences in the hepatic microstructure were observed between the groups. Additional, histology assay showed that the expression of CCR2 decreased after CVC treatment (Figure 6).
Table 1.
Mortality of acute graft-versus-host disease and survival time in different groups
| Groups | n | Mortality | Survival Time [days (numbers of animals)] | Mean Survival Time (days) |
|---|---|---|---|---|
| Control | 11 | 100% | 22 (1), 25 (2), 27 (3), 30 (1), 31 (1), 33 (1), 38 (1), 42 (1) | 27 |
| CVC | 11 | 100% | 25 (2), 29 (1), 37 (1), 40 (1), 50 (2), 57 (1), 60 (2), 63 (1) | 50* |
P < 0.05 Versus Control Group; CVC, Cenicriviroc.
Rats were randomly divided into two groups: 1) Control and 2) CVC. Each group contained 11 pairs of experimental rats. All liver transplant recipients died within 100 days after surgery. The survival time of each rat was documented. Mean survival times were calculated and displayed in Table 1.
Figure 1.
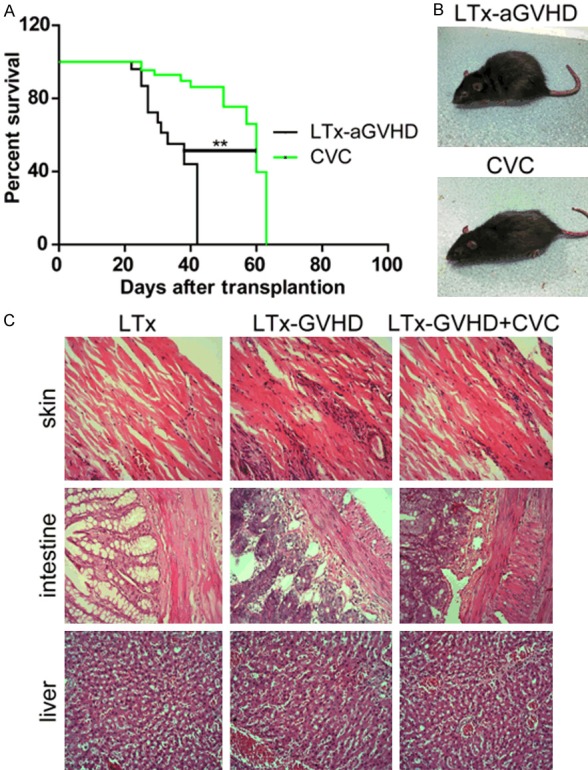
CVC treatment ameliorated LTx-aGVHD in a rat model of liver transplantation. A. Kaplan-Meier survival analysis of LTx rats. CVC treatment induced a significant elongation of survival time after LTx (**P < 0.01). B. Representative images of each group. Rats were carefully observed after LTx. Representative images of each rat taken 21 days after liver transplantation. C. Hematoxyin and eosin (H&E) staining of skin, intestine, and liver samples. Rats that received LTx, aGVHD LTx, and CVC + LTx-aGVHD were observed after LTx. Skin, intestine, and liver samples were collected after death. H&E staining was conducted as previously described.
Figure 6.
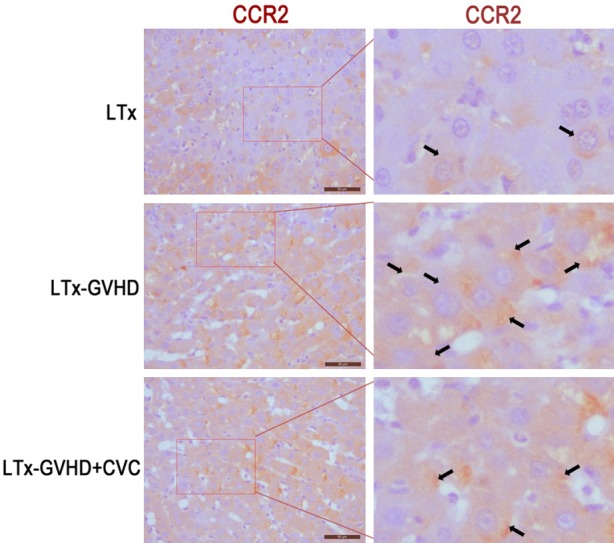
Histology assay detected the expression of CCR2 after CVC treatment.
CVC treatment decreased the percentage of donor CD4+/CD4+CD25- Th cells
Serum samples of LTx rats were collected before (4 days after LTx) and after (12 days after LTx) CVC treatment. Flow cytometry was performed to quantify CD4, CD25, and GFP positive Th lymphocytes (Figure 2A). The percentages of donor T lymphocytes and Th cells among PBMCs was calculated and are displayed in Figure 2B and 2C. CD4+GFP+ and CD+CD25-GFP+ cell numbers decreased after CVC treatment. Taken together, CVC reduced the numbers of donor T lymphocytes and Th cells.
Figure 2.
The proportion of CD4+GFP+/CD4+CD25-GFP+ T effector lymphocytes among PBMCs. CVC treatment was given for 7 days after LTx. PBMC samples were collected at day 4 and 12 after LTx and prepared for flow cytometry. A. Flow cytometry analysis of CD4+CD25-GFP+ lymphocytes in the control group and CVC treatment group. B. The proportion of CD4+GFP+ donor-derived Th lymphocytes in PBMCs at day 4 and 12. C. The proportion of CD4+CD25-GFP+ donor-derived T effector cells in PBMCs 4 days and 12 days after LTx.
CVC decreased donor T lymphocytes in vitro via blockage of CCR5
Th lymphocytes were activated in vitro. As shown in Figure 3A, isolated PBMCs were incubated with CD3 (5 µg/ml) and CD28 (2 µg/ml) antibodies, and increased levels of interferon-gamma (IFN-ã) were observed. Then, isolated PBMCs were treated with Interleukin-12 (IL-12) and the cell number was counted. Th lymphocytes proliferated markedly after IL-12 treatment (Figure 3B). The chemotaxis of activated Th cells was evaluated using Chemo-TX plates incubated with CCL3, CCL4, and CCL5. The CCK-8 method was used to quantify amount of cells penetrating Chemo-TX plate. As displayed in Figure 3C, the CCL5 Chemo-TX plate showed the most Th cells among all the groups. Furthermore, we mixed donor and recipient Th cells in a ratio of 2:1. Mixed cells were then treated with CVC (1 nM), anti-CCR5 antibodies (1 ng/ml), anti-CCR2 antibodies (1 ng/ml), and anti-CCR5 antibodies (1 ng/ml) + anti-CCR2 antibodies (1 ng/ml). GFP+ cells were quantified using flow cytometry (Figure 3D). CVC treatment showed a similar decrease in GFP+ cells to those treated with anti-CCR5 antibodies. The anti-CCR2 antibodies had no obvious effect on proportion of GFP+ cells. Protein samples were collected from each group and western blotting was conducted to evaluate the levels of CCR2 and CCR5. The levels of CCR2 and CCR5 decreased significantly after CVC treatment.
Figure 3.
CVC inhibited the proliferative ability of T effector cells in vitro. A. PBMCs from the spleen were isolated and cultured in vitro. Anti-CD3 antibodies (5 µg/ml) and anti-CD28 antibody (2 µg/ml) treatment was to activate T effector lymphocytes. A significant increase in the IFN-ã level in the supernatants was detected using ELISA (***P < 0.001). B. 1 ng/ml IL-12 was added to isolated PBMCs to stimulate proliferation of T effector cell. C. Chemotaxis analysis of isolated T effector lymphocytes. The cells were cultured in a Chemo-TX plate embedded with CCL3, CCL4, and CCL5. The amounts of cells penetrating the membrane were measured using the CCK-8 method. An elevated amount of penetrating cells was observed in the CCL5 group (***P < 0.001). Activated T effector lymphocytes were mixed with F1 T effector cells in a ratio of 2:1 and treated with CVC (1 nM), anti-CCR5 antibodies (1 ng/ml), anti-CCR2 antibodies (1 ng/ml), and anti-CCR5 antibodies (1 ng/ml) + anti-CCR2 antibodies (1 ng/ml). D. Flow cytometry was performed to examine the proportion of GFP+ cells. E. Western blotting was used to evaluate levels of CCR2 and CCR5 in each group.
CVC inhibited the inflammatory response through blockage of CCR5
We mixed donor and recipient Th lymphocytes and treated them with CVC, anti-CCR5 antibodies, anti-CCR2 antibodies, anti-CCR5 + anti-CCR2 antibodies as previously described. The supernatant of each group was collected and prepared for ELISA detection. As shown in Figure 4, CVC significantly decreased the levels of pro-inflammatory cytokines, including IL-2 (Figure 4A), IL-6 (Figure 4B), and IFN-ã (Figure 4C). By contrast, the levels of anti-inflammatory factors IL-10 and TGF-â were increased after CVC treatment. The anti-CCR2 antibodies showed no significant difference compared with the control group. The anti-CCR5 antibodies had a similar effect to that of CVC treatment.
Figure 4.

Secretion of pro-inflammatory and anti-inflammatory cytokines after CVC treatment. Activated T effector lymphocytes were mixed with F1 T effector cells in a ratio of 2:1 and treated with CVC (1 nM), anti-CCR5 antibodies (1 ng/ml), anti-CCR2 antibodies (1 ng/ml), and anti-CCR5 antibodies (1 ng/ml) + anti-CCR2 antibodies (1 ng/ml). Cytokine secretion levels were measured using ELISA. A. IL-2, B. IL-6. C. IFN-ã. D. IL-10. E. TGF-â. CVC significantly decreased the expression of pro-inflammatory cytokines and increased the expression of anti-inflammatory cytokines through inhibition of CCR5 (**P < 0.01, ***P < 0.001).
CVC inhibited the aGVHD-induced inflammatory reaction in vivo
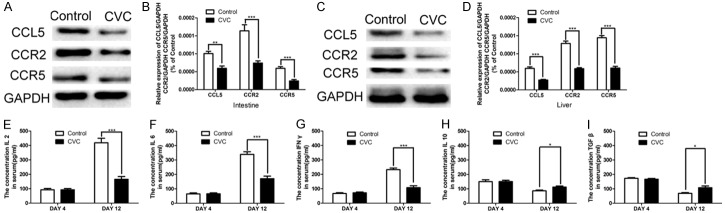
We collected small intestine and liver samples for western blotting analysis. CVC treatment downregulated the levels of CCL5, CCR2, and CCR5 in the intestine (Figure 5A) and liver (Figure 5C). The relative levels of CCL5, CCR2, and CCR5 in the intestine (Figure 5B) and liver (Figure 5D) were calculated and displayed. CVC treatment significantly decreased the levels of CCL5, CCR2, and CCR5 (P < 0.001). Moreover, we collected serum samples of each LTx rat before (4 days after LTx) and after (12 days after LTx) CVC treatment. The supernatant of each sample was prepared for enzyme linked immunosorbent assay (ELISA) detection. The secretion of pro-inflammatory cytokines IL-2, IL-6, and IFN-ã was abrogated by CVC treatment. However, the levels of anti-inflammatory chemokines IL-10 and TGF-â chemokines increased after CVC treatment (P < 0.05).
Figure 5.
CVC inhibited the expression of CCL5, CCR2, CCR5, and pro-inflammatory cytokines, and increased the expression of anti-inflammatory cytokines in vivo. Twenty-one days after LTx, intestine (A and B) and liver (C and D) samples were collected, and western blotting was conducted to evaluate the expression of CCL5, CCR2, and CCR5. CVC treatment decreased the levels of pro-inflammatory cytokines IL-2 (E), IL-6 (F), and IFN-ã (G); and induced the expression of anti-inflammatory cytokines IL-10 (H) and TGF-â (I).
Discussion
Although it has been 30 years since aGVHD after LTx was firstly proposed [4], the mechanism and treatment of aGVHD after LTx remains undetermined. The most widely accepted theory of LTx-aGVHD is disordered immune regulation, similar to the development of GVHD after bone marrow transplantation [15]. Recently, the relationship between T lymphocytes and GVHD was studied. FOXP3+ T regulatory (Tregs) cells derived from the donor were proven to be protective by decreasing the probability of developing GVHD after allogeneic stem cell transplantation [16]; and CD4+CD25+CD62L+ T regulators protected recipients from lethal aGVHD after allogeneic bone marrow transplantation [17]. By contrast, Th lymphocytes showed an adverse effect by exacerbating the severity of GVHD. CXCR3-induced Th lymphocyte migration was responsible for the induction of aGVHD after bone marrow transplantation [7]; and GVHD was prevented by blockage of CD28-induced Th lymphocyte deactivation [18]. Thus, the balance of Tregs and Th cells play a pivotal role in development of GVHD [19].
Previously, Tregs, which are regarded as negative modulators of T lymphocyte activity in LTx-aGVHD, were studied and the severity of aGVHD was associated with a decrease in CD4+CD25+FOXP3+ Tregs [20]; rapamycin, tacrolimus [21] and OSI-027 [22] rescued LTx-aGVHD through the induction of Tregs. However, less attention has been paid to the physiology of Th cells in LTx-aGVHD. In the present study, we constructed a rat model of LTx-aGVHD and found that over-activated Th cells were responsible for severity of LTx-aGVHD.
CCR are widely recognized as key mediators in T-cell-associated liver diseases including Hepatitis B [23], Hepatitis C [24], and non-alcoholic fatty liver disease (NAFLD) [25]. Recently, attention has been paid to connect CCRs with liver fibrosis. Seki et al. discovered that CCR1 and CCR5 in Kupffer and stellate cells are directly involved in the development of hepatic fibrosis [26]. Lefebvre et al. found the CCR2 and CCR5 antagonist CVC reversed fibrosis in the liver and kidney in a rat model [27]. Ambade et al. proved that CVC was effective in ameliorating alcohol-induced liver steatosis and fibrosis [13]. In addition, CVC is now under observation in a Phase 2b clinical trial to treat adults with non-alcoholic steatohepatitis (NASH) and liver fibrosis [28]. Among the CCRs, CCR2 exerts its effect by regulating monocyte chemotaxis [29]. CCR5, by contrast, is believed to be closely related to Th lymphocyte activation [30]. Donor-derived Th lymphocytes play a very important role in the development of GVHD [9,31]. Therefore, in the present study, we focused on modulation of Th lymphocyte activation through CVC-mediated CCR5 inhibition. Several studies have already connected CCR5 with GVHD physiology. Deletion of CCR5 may induce or accelerate the development of GVHD in bone marrow transplantation [32,33]; however, CCR5 blockade showed promising results in the prophylaxis and treatment of GVHD [11,34,35]. Our research favors the hypothesis that CCR5 blockade results in attenuated GVHD-induced organ injury after liver transplantation.
CVC was first discovered as an anti-HIV drug [12]. It has also been shown as effective to attenuate NASH and reversing liver fibrosis. However, little attention has been paid to CVC’s function in an LTx-GVHD model. We demonstrated that CVC ameliorated the severity of GVHD through inhibition of CCR5, which further decreased inflammatory cytokine secretion. CVC inhibited Th lymphocyte activation, which was independent of CCR2 inhibition. The expression of CCR2 was significantly inhibited in the liver and intestines, which are the target organs of GVHD-associated injury, after CVC treatment. It is undeniable that CVC participates in the inhibition of LTx-GVHD; however, the exact role of CVC in CCR2 inhibition and the underlying molecular mechanism remained unknown. Further study focusing on CVC blockade of CCR2-associated macrophage chemotaxis and activation is required.
In the present study, we found that CVC injection rescued LTx-GVHD in a rat model of liver transplantation. A decrease in CD4+CD25+ Th lymphocytes was observed after treatment with CVC. In terms of the mechanism, CVC deactivated Th lymphocytes through CCR5 inhibition but not CCR2 inhibition. Taken together, the results showed that the clinical application of CVC is promising to prevent and treat LTx-GVHD.
Acknowledgements
This work was sponsored by the National Science Foundation for Young Scholars of China [Grant number 81501380] and the Natural Science Foundation for Young Scholars of Jiangsu Province, China [Grant number BK20150110].
Disclosure of conflict of interest
None.
References
- 1.Perri R, Assi M, Talwalkar J, Heimbach J, Hogan W, Moore SB, Rosen CB. Graft vs. host disease after liver transplantation: a new approach is needed. Liver Transpl. 2007;13:1092–1099. doi: 10.1002/lt.21203. [DOI] [PubMed] [Google Scholar]
- 2.Taylor AL, Gibbs P, Bradley JA. Acute graft versus host disease following liver transplantation: the enemy within. Am J Transplant. 2004;4:466–474. doi: 10.1111/j.1600-6143.2004.00406.x. [DOI] [PubMed] [Google Scholar]
- 3.Smith DM, Agura E, Netto G, Collins R, Levy M, Goldstein R, Christensen L, Baker J, Altrabulsi B, Osowski L, McCormack J, Fichtel L, Dawson DB, Domiati-Saad R, Stone M, Klintmalm G. Liver transplant-associated graft-versus-host disease. Transplantation. 2003;75:118–126. doi: 10.1097/00007890-200301150-00022. [DOI] [PubMed] [Google Scholar]
- 4.Burdick JF, Vogelsang GB, Smith WJ, Farmer ER, Bias WB, Kaufmann SH, Horn J, Colombani PM, Pitt HA, Perler BA, et al. Severe graft-versus-host disease in a liver-transplant recipient. N Engl J Med. 1988;318:689–691. doi: 10.1056/NEJM198803173181107. [DOI] [PubMed] [Google Scholar]
- 5.Kuball J, Theobald M, Ferreira EA, Hess G, Burg J, Maccagno G, Barreiros AP, Luth S, Schimanski CC, Schuchmann M, Schwarting A, Neurath M, Otto G, Galle PR, Lohse AW. Control of organ transplant-associated graft-versus-host disease by activated host lymphocyte infusions. Transplantation. 2004;78:1774–1779. doi: 10.1097/01.tp.0000144183.77279.ec. [DOI] [PubMed] [Google Scholar]
- 6.Lehner F, Becker T, Sybrecht L, Luck R, Schwinzer R, Slateva K, Blasczyk R, Hertenstein B, Klempnauer J, Nashan B. Successful outcome of acute graft-versus-host disease in a liver allograft recipient by withdrawal of immunosuppression. Transplantation. 2002;73:307–310. doi: 10.1097/00007890-200201270-00030. [DOI] [PubMed] [Google Scholar]
- 7.Duffner U, Lu B, Hildebrandt GC, Teshima T, Williams DL, Reddy P, Ordemann R, Clouthier SG, Lowler K, Liu C, Gerard C, Cooke KR, Ferrara JL. Role of CXCR3-induced donor T-cell migration in acute GVHD. Exp Hematol. 2003;31:897–902. doi: 10.1016/s0301-472x(03)00198-x. [DOI] [PubMed] [Google Scholar]
- 8.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murai M, Yoneyama H, Harada A, Yi Z, Vestergaard C, Guo B, Suzuki K, Asakura H, Matsushima K. Active participation of CCR5(+)CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J Clin Invest. 1999;104:49–57. doi: 10.1172/JCI6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reshef R, Luger SM, Hexner EO, Loren AW, Frey NV, Nasta SD, Goldstein SC, Stadtmauer EA, Smith J, Bailey S, Mick R, Heitjan DF, Emerson SG, Hoxie JA, Vonderheide RH, Porter DL. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med. 2012;367:135–145. doi: 10.1056/NEJMoa1201248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan J, Ren HY, Shi YJ, Liu W. Prophylaxis of acute graft-versus-host disease by CCR5 blockade combined with cyclosporine A in a murine model. Inflamm Res. 2015;64:137–144. doi: 10.1007/s00011-014-0793-6. [DOI] [PubMed] [Google Scholar]
- 12.Klibanov OM, Williams SH, Iler CA. Cenicriviroc, an orally active CCR5 antagonist for the potential treatment of HIV infection. Curr Opin Investig Drugs. 2010;11:940–950. [PubMed] [Google Scholar]
- 13.Ambade A, Lowe P, Kodys K, Catalano D, Gyongyosi B, Cho Y, Iracheta Vellve A, Adejumo A, Saha B, Calenda C, Mehta J, Lefebvre E, Vig P, Szabo G. Pharmacological inhibition of CCR2/5 signaling prevents and reverses alcohol-induced liver damage, steatosis and inflammation in mice. Hepatology. 2019;69:1105–1121. doi: 10.1002/hep.30249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue F, Chen W, Wang XG, Liang L, Bai XL, Wang LY, Wang HP, Liang TB. Establishment of an acute graft-versus-host disease model following liver transplantation in donor-dominant one-way major histocompatibility complex matching rats. Transplant Proc. 2009;41:1914–1920. doi: 10.1016/j.transproceed.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Domiati-Saad R, Klintmalm GB, Netto G, Agura ED, Chinnakotla S, Smith DM. Acute graft versus host disease after liver transplantation: patterns of lymphocyte chimerism. Am J Transplant. 2005;5:2968–2973. doi: 10.1111/j.1600-6143.2005.01110.x. [DOI] [PubMed] [Google Scholar]
- 16.Rezvani K, Mielke S, Ahmadzadeh M, Kilical Y, Savani BN, Zeilah J, Keyvanfar K, Montero A, Hensel N, Kurlander R, Barrett AJ. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108:1291–1297. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ermann J, Hoffmann P, Edinger M, Dutt S, Blankenberg FG, Higgins JP, Negrin RS, Fathman CG, Strober S. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105:2220–2226. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 18.Watkins BK, Tkachev V, Furlan SN, Hunt DJ, Betz K, Yu A, Brown M, Poirier N, Zheng HB, Taraseviciute A, Colonna L, Mary C, Blancho G, Soulillou JP, Panoskaltsis-Mortari A, Sharma P, Garcia A, Strobert E, Hamby K, Garrett A, Deane T, Blazar BR, Vanhove B, Kean LS. CD28 blockade controls T cell activation to prevent graft-versus-host disease in primates. J Clin Invest. 2018;128:3991–4007. doi: 10.1172/JCI98793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alho AC, Kim HT, Chammas MJ, Reynolds CG, Matos TR, Forcade E, Whangbo J, Nikiforow S, Cutler CS, Koreth J, Ho VT, Armand P, Antin JH, Alyea EP, Lacerda JF, Soiffer RJ, Ritz J. Unbalanced recovery of regulatory and effector T cells after allogeneic stem cell transplantation contributes to chronic GVHD. Blood. 2016;127:646–657. doi: 10.1182/blood-2015-10-672345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia X, Liang C, Liu H, Xue F, Hu Q, Chen W, Ma T, Zhang Y, Bai X, Liang T. Effects of trichostatin A in a rat model of acute graft-versus-host disease after liver transplantation. Transplantation. 2013;96:25–33. doi: 10.1097/TP.0b013e318295c04d. [DOI] [PubMed] [Google Scholar]
- 21.Xu G, Wang L, Chen W, Xue F, Bai X, Liang L, Shen X, Zhang M, Xia D, Liang T. Rapamycin and tacrolimus differentially modulate acute graft-versus-host disease in rats after liver transplantation. Liver Transpl. 2010;16:357–363. doi: 10.1002/lt.22003. [DOI] [PubMed] [Google Scholar]
- 22.Zhi X, Xue F, Chen W, Liang C, Liu H, Ma T, Xia X, Hu L, Bai X, Liang T. OSI-027 modulates acute graft-versus-host disease after liver transplantation in a rat model. Liver Transpl. 2017;23:1186–1198. doi: 10.1002/lt.24797. [DOI] [PubMed] [Google Scholar]
- 23.Thio CL, Astemborski J, Bashirova A, Mosbruger T, Greer S, Witt MD, Goedert JJ, Hilgartner M, Majeske A, O’Brien SJ, Thomas DL, Carrington M. Genetic protection against hepatitis B virus conferred by CCR5Delta32: evidence that CCR5 contributes to viral persistence. J Virol. 2007;81:441–445. doi: 10.1128/JVI.01897-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hellier S, Frodsham AJ, Hennig BJ, Klenerman P, Knapp S, Ramaley P, Satsangi J, Wright M, Zhang L, Thomas HC, Thursz M, Hill AV. Association of genetic variants of the chemokine receptor CCR5 and its ligands, RANTES and MCP-2, with outcome of HCV infection. Hepatology. 2003;38:1468–1476. doi: 10.1016/j.hep.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Martinez L, Perez-Matute P, Aguilera-Lizarraga J, Rubio-Mediavilla S, Narro J, Recio E, Ochoa-Callejero L, Oteo JA, Blanco JR. Maraviroc, a CCR5 antagonist, ameliorates the development of hepatic steatosis in a mouse model of non-alcoholic fatty liver disease (NAFLD) J Antimicrob Chemother. 2014;69:1903–1910. doi: 10.1093/jac/dku071. [DOI] [PubMed] [Google Scholar]
- 26.Seki E, De Minicis S, Gwak GY, Kluwe J, Inokuchi S, Bursill CA, Llovet JM, Brenner DA, Schwabe RF. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119:1858–1870. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lefebvre E, Moyle G, Reshef R, Richman LP, Thompson M, Hong F, Chou HL, Hashiguchi T, Plato C, Poulin D, Richards T, Yoneyama H, Jenkins H, Wolfgang G, Friedman SL. Antifibrotic effects of the dual CCR2/CCR5 antagonist cenicriviroc in animal models of liver and kidney fibrosis. PLoS One. 2016;11:e0158156. doi: 10.1371/journal.pone.0158156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman S, Sanyal A, Goodman Z, Lefebvre E, Gottwald M, Fischer L, Ratziu V. Efficacy and safety study of cenicriviroc for the treatment of non-alcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR Phase 2b study design. Contemp Clin Trials. 2016;47:356–365. doi: 10.1016/j.cct.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1310–1321. doi: 10.1152/ajpgi.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanford MM, Issekutz TB. The relative activity of CXCR3 and CCR5 ligands in T lymphocyte migration: concordant and disparate activities in vitro and in vivo. J Leukoc Biol. 2003;74:791–799. doi: 10.1189/jlb.1102547. [DOI] [PubMed] [Google Scholar]
- 31.Wysocki CA, Jiang Q, Panoskaltsis-Mortari A, Taylor PA, McKinnon KP, Su L, Blazar BR, Serody JS. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood. 2005;106:3300–3307. doi: 10.1182/blood-2005-04-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welniak LA, Wang Z, Sun K, Kuziel W, Anver MR, Blazar BR, Murphy WJ. An absence of CCR5 on donor cells results in acceleration of acute graft-vs-host disease. Exp Hematol. 2004;32:318–324. doi: 10.1016/j.exphem.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Bogunia-Kubik K, Duda D, Suchnicki K, Lange A. CCR5 deletion mutation and its association with the risk of developing acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Haematologica. 2006;91:1628–1634. [PubMed] [Google Scholar]
- 34.Palmer LA, Sale GE, Balogun JI, Li D, Jones D, Molldrem JJ, Storb RF, Ma Q. Chemokine receptor CCR5 mediates alloimmune responses in graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16:311–319. doi: 10.1016/j.bbmt.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang B, Ren H, Liu H, Shi Y, Liu W, Dong Y, Yin Y, Miao S. CCR5 blockade combined with cyclosporine A attenuates liver GVHD by impairing T cells function. Inflamm Res. 2016;65:917–924. doi: 10.1007/s00011-016-0974-6. [DOI] [PubMed] [Google Scholar]