Abstract
The study aims to explore the genetic predispositions and molecular pathways of low cardiorespiratory fitness (VO2max) in young Saudi females (n = 70). Young females were grouped based on the level of VO2max according to the specification of the physical fitness specialist certification as low VO2max (< 28.9; n = 19) and high VO2max (> 33; n = 14) and genotyped for 243345 putative functional exonic variants. The CYFIP2&FNDC9-rs10037485T, C1R-rs75380747G and TOP2A-rs13695C SNPs on chromosome 5, 12 and 17, respectively were found to be the most significant among young Saudi females with low VO2max (P < 8.01 × 10-05). Linkage disequilibrium (LD) analysis among the significant SNPs (P < 0.001) have revealed risk and protective haplotypes with high degree (p-value < 1.0 × 10-4) of LD. The most significant risk haplotypes with the low VO2max in young Saudi females are: Chromosome 1: LOC112268276-rs10800201G; LOC112268276-rs4657537A; rs4657583T (p-value = 2.00 × 10-04); Chromosome 3: rs978979G; CCDC66-rs7637449A; CCDC66-rs111934125T; FAM208A-rs9835332G (p-value = 5.00 × 10-04) and Chromosome 17: STX2-rs13696C; TNS4-rs1901187C (p-value = 1.00 × 10-04). Functional Enrichment Analysis revealed that the genes with SNPs P < 0.001 have significantly involved in the heart rate (P = 0.00442), body weight (P = 0.00629), breath tests (P = 0.0147), proteolysis (P = 0.00623) and cardiac muscle fiber development (P = 0.0263). In conclusion we could say that the identified genetic predispositions and gene-annotation enrichment in low VO2max in young Saudi females revealed that they are at high risk for developing cardiovascular complications.
Keywords: Cardiorespiratory fitness, genetic predispositions, VO2max, haplotyping, SNP genotyping, risk alleles, cardiovascular disease, predictor genes
Introduction
Cardiorespiratory fitness (VO2max) is a predictor of the symptoms of chronic diseases and risk of premature mortality [1]. The low VO2max is reported to be associated with development of various chronic diseases: higher risk of developing defined obesity, abdominal obesity, cardiovascular disease, hyperinsulinemia and premature mortality [1-8]. Regular exercise is always recommended to reduce the worldwide prevalence of low cardiorespiratory fitness associated diseases such as diabetes, cardiovascular disease, and stroke through various mechanism [https://www.who.int/nmh/about/chp/en/]. Studies have reported the impact of the genetic and environmental factors on the cardiorespiratory fitness [7,9,10]. Studies have identified ~100 possible genes associated with the VO2max trainability in various populations [1]. However, there were no studies on the impact of the cardiorespiratory fitness in Arab population. The current study aims to explore the possible genetic predispositions and functional molecular mechanism of low VO2max among young females from Eastern Province of Saudi Arabia.
Materials and methods
Subjects and methods
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) at Imam Abdulrahman Bin Faisal University (IRB approval number: IRB-PGS-2017-01-219). This is a cross-sectional study conducted during the period from March 2017 to March 2018. Saudi female college students (n = 70) within the age range 19-25 years were randomly selected from different health colleges (College of Medicine, Dentistry, Nursing, Applied Medical Science and Clinical Pharmacy) of Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia. Simple random selection was performed using a Microsoft Excel sheet. Each student in the campus was given a random value then values were later sorted in an ascending order. Thereafter, the required sample was chosen from the top of the list. The sample size was calculated using G*power 3.1 statistical power analysis software [11] based on the calculation of the effect size to give the study a power of 95%. The effect size: (0.39) was calculated using the mean VO2max value of the null hypothesis H0: (33.7), the standard deviation SD: (10.97) (mean VO2max and SD values were taken from earlier study) [12]. Whereas the mean VO2max value of the alternative hypothesis was H1: (40). Criteria for inclusion in the study were apparently healthy females, with normal body mass index (BMI) of 18.50-24.99 (Kg.m-2) [https://www.who.int/nutrition/databases/bmi/en/], who were sedentary according to the definition of the International Physical Activity Questionnaire (IPAQ) [https://sites.google.com/site/theipaq/home]. Participants with any contraindication of exercise stress testing [13], who has known chronic illness, who underwent major surgery or are taking any medication apart from nonsteroidal anti-inflammatory drugs were excluded from the study. Additionally, pregnancy, breastfeeding, smoking and using of ergogenic aids were also considered as exclusion criteria. All participants signed an informed written consent in English and a translated Arabic form and a form according to Standing Committee for Research Ethics on Living Creatures. All performed procedures were in agreement with the university ethical committee guidelines.
Exercise testing and direct measurement of VO2max
The exercise was carried out in the Physiotherapy Research Center of the College of Applied Medical Science at the Exercise Physiology Laboratory. All tests were performed during daytime from 10:00 am to 1:00 pm using COSMED system for cardiopulmonary exercise testing (Quark CPET, COSMED, Rome, Italy). COSMED system is composed of: Gas analyzer (breath by breath analyzer) for continuous gas exchange measurements made by an open-circuit spirometry; Electronically braked cycle ergometry (Ergoline, COSMED, Bitz, Germany); Electrocardiogram (ECG) monitor (Wireless 12-lead stress PC ECG, COSMED, Rome, Italy); Arterial blood pressure cuff; Pulse oximeter (Pulse Oximetry, COSMED, Rome, Italy); and computer, online analysis software for final reports.
System calibration
The system was calibrated in accordance with the manufacturer’s instructions. Prior to calibration, system was warmed-up for 20 minutes. Analyzers calibration (fast response paramagnetic O2 analyzer and nondispersive infrared CO2 analyzer) were done every day and before each test. The appropriate analyzer response was verified by passing known gas mixture concentrations over the analyzers. Inspired gas values were verified using ambient air assumed to contain 20.93% O2 and 0.03% CO2. Appropriate expired gas values were verified using certified gas cylinders from the British Oxygen Corporation containing 16% O2, 5% CO2 and N2 for balance. Bidirectional digital turbine was calibrated every week using a 3 liter syringe.
Cardiopulmonary exercise testing
All subjects performed a maximal exercise test on a stationary bicycle ergometer using a one-minute incremental test. The selection of the work rate increase needed for the students was calculated by the formula previously published for cycle ergometry and sedentary females [14].
Work rate increase formula
It is calculated by the following formula:
Work rate increment per minute in watts = (peak VO2 in milliliters per minute - VO2 unloaded in milliliters per minute)/100.
Where VO2 unloaded in milliliters per minute is calculated as: VO2 unloaded (ml/min) = 150 + (6 × weight in Kilograms) and peak VO2 in milliliters per minute is calculated by: Peak VO2max (ml/min) = (height in centimeters - age in years) × 20 for sedentary men and × 14 for sedentary women.
Age predicted maximal heart rate was obtained from the formula (220-age) [14]. Subjects had measurement of resting arterial blood pressure (BP) taken in sitting position from the right arm manually and digitally by a mercury sphygmomanometer (diplomat-presameter®Sphygmomanometer, Riester, Jungingen, Germany) and by a vital signs monitoring device (Spot Vital Signs® Device, Welch Allyn, Skaneateles Falls, New York, USA). The height of the seat was adjusted to keep the subject’s legs at near full extension in each pedal revolution [13]. The appropriate size of an ergonomic multi-use silicone face masks (available in 3 sizes) was chosen for each subject to have a good seal about the face and to ensure an accurate test. The duration of the exercise was 6 to 12 minutes as suggested previously. This duration is considered the optimum time to give the highest maximal VO2 consumption in healthy subjects because tests that are too long will be too boring and the subjects may terminate the test prematurely while too short tests would not give sufficient informative data [14].
After a period of rest, the incremental protocol allowed the subject initially to cycle for 3 minutes of unloaded pedaling as a warm-up period. Then, the power output started at 20 Watts and was increasingly incremented by 15 Watts every minute by computer control until the subject was limited by debilitating symptoms despite verbal encouragement. The cycling frequency was maintained at 50 revolutions per minute (rpm) throughout the exercise with the assistance of the digital display on the bike to maintain the required cycling pace. Finally, the participants were asked to continue cycling for 3 minutes without resistance in the recovery period. Time till exhaustion was recorded and participants were asked about the reason for stopping the exercise test. Heart rate (HR), oxygen saturation (SpO2%), arterial blood pressure and ECG were monitored at the resting baseline and during the exercise test. At the end of each stage, HR and ratings of perceived exertion (RPE) score of each participant were recorded. Specifically, The Borg Rating of Perceived Exertion scale was used. It rates the intensity of the exercise from 6 to 20 [14]. HR was monitored with the three bipolar leads of the ECG. Oxygen saturation was obtained from the pulse oximeter. The arterial blood pressure was recorded automatically by a pressure cuff attached to the bike every 2 minutes. Maximum HR, blood pressure and RPE of the last achieved stage were determined. To monitor the safety, the bipolar leads were recorded using a wireless ECG belt with a special ECG electrode (Ambu® Blue Sensor T, Ambu, Penang, Malaysia).
Based on breath by breath gas analysis system, Oxygen consumption (VO2max) in (ml.min-1) and (ml.kg-1.min-1); Carbon dioxide production (VCO2) in (ml.min-1); Minute ventilation (VE); (l.min-1); Respiratory rate (RR); (breaths.min-1); Tidal volume (TV); (l); Respiratory exchange ratio (RER) and Ventilatory anaerobic threshold (AT) in ml.min-1 and ml.kg-1.min-1 were measured. In the post-exercise time at the 3-minute recovery period, HR, manual and digital blood pressure were recorded by the same devices mentioned previously.
Maximum VO2 assessment (VO2max) and statistical analysis
VO2 values were considered maximal when two out of the four criteria (Plateau of VO2 despite an increase in workload; Respiratory exchange ratio 1:1 or higher; Heart rate within 15 beats of the age predicted maximum heart rate; An RPE ≥ 17) were achieved [14]. ACSM’s Guidelines of indications for terminating exercise testing were followed [14].
Statistical Package for the Social Sciences software version 22 (IBM SPSS Statistics) was used to perform the statistical analyses. All data were expressed as mean ± SD. Pearson correlation was applied for all the ventilator and hematological parameters. All the tested subjects were divided according to the level of VO2max into two groups, and then independent t-test was used to compare the hematological parameters. A p-value of < 0.05 was considered as significant.
DNA extraction and genotyping
QIAamp DNA Blood Mini Kit (Qiagen, Germany) was used to perform DNA extraction on blood samples collected from the study subjects with low VO2max (< 28.9 n = 19) and high VO2max (> 33; n = 14). For microarray, human Exome bead chip kit v1.0 and v1.1 Illumina (San Diego, USA), which consists of 243345 putative functional exonic markers, was utilized using Illumina iScan. All DNA samples were processed according the manufacturer’s protocol and iScan control software (Illumina, San Diego, USA) was used to acquire data. The DNA extraction, microarray genotyping, and analysis were performed at the Genetic research laboratory of the Institute for Research and Medical Consultation (IRMC), Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia. Genotyping was carried in this lab from 2016 to 2018, using the similar chip and procedures.
Statistical and functional analysis
GenomeStudio 2.0 Data Analysis Software - Illumina was used for initial quality check for the call rate, total of 0 subject were excluded from the analysis due to call rate < 0.98% and re-clustered. Hardy-Weinberg equilibrium (HWE) was tested separately among the case and control groups with 1 degree of freedom genotypic chi-squared test. Differences in clinical characteristics between cases and controls were calculated by the two-sample t-test or the χ2 test if appropriate using IBM SPSS Statistics version 23 (IBM Co., Armonk, NY, USA). Kaviar [15] and SNP-Nexus [16] were used to confirm variants reported at a base pair position at the respective chromosome as per Genome Reference Consortium Human Build 37 (GRCh37.p13). The case-control association analyses, odds ratios and 95% confidence intervals were calculated to evaluate the effects of different alleles and haplotypes using Haploview version 4.2 [17] and gPLINK version 2.050 [18] Bonferroni corrections or false discovery rate corrections were applied to correct the p values of 243345 SNPs (corrected α = 0.05/243345 = 2.05 × 10-07), to control inflation of the Type I error rate. The p-value less than 0.05 were considered as significant. The highly significant (P < 1 × 10-05) genes were annotated for functional implications through DAVID 6.7 [19], STRING 10.5 [20], SNPnexus [16], Expression Atlas [21] and Reactome [22].
Results
Saudi female subjects were grouped on the level of VO2max according to the specification of the physical fitness specialist certification as low VO2max (n = 19) and high VO2max (n = 14) (Table 1) [23]. Ventilation (L/min), tidal volume (L), VO2max (ml/min), VO2max (ml/min.Kg), CO2 production (VCO2) (ml/min), Respiratory exchange ratio VCO2/VO2, anaerobic threshold (ml/min), and anaerobic threshold (ml/min.Kg) were significantly different among the selected study group (Table 1). The distinct two groups of the Saudi young females were subjected for the genotyping analysis of 243345 putative functional exonic markers.
Table 1.
Ventilatory parameters obtained from the maximum cardiopulmonary exercise testing and hematological indices
| Parameter | Low VO2max (n = 19) | High VO2max (n = 14) | p value |
|---|---|---|---|
| RBC count × 106 | 4.28 ± 0.57 | 4.17 ± 0.87 | 0.649 |
| Hb g/dL | 10.59 ± 1.71 | 11.05 ± 0.91 | 0.354 |
| Hct% | 34.65 ± 5.40 | 34.57 ± 4.10 | 0.962 |
| MCH pg | 24.69 ± 2.29 | 26.37 ± 4.4 | 0.187 |
| MCHC g/dl | 30.57 ± 1.63 | 33.35 ± 6.03 | 0.085 |
| RDW% | 14.99 ± 3.18 | 15.28 | 0.865 |
| MCV fl | 80.99 ± 8.10 | 86.05 ± 18.18 | 0.327 |
| HbF% | 0.46 ± 0.50 | 0.46 ± 0.55 | 1.000 |
| HbA2% | 2.68 ± 1.04 | 2.74 ± 0.28 | 0.801 |
| Serum ferritin µg/L | 21.56 ± 23.17 | 21.44 ± 22.17 | 0.986 |
| Serum iron µg/L | 65.57 ± 31.18 | 55.47 ± 30.75 | 0.303 |
| TIBC µmol/L | 304.36 ± 90.03 | 307.09 ± 60.79 | 0.912 |
| Ventilation L/min | 54.68 ± 9.88 | 63.93 ± 10.79 | 0.002** |
| Tidal volume L | 1.20 ± 0.17 | 1.40 ± 0.19 | < 0.001** |
| Respiratory frequency | 45.72 ± 7.63 | 45.96 ± 7.06 | 0.911 |
| VO2max ml/min | 1290.00 ± 154.66 | 1740.35 ± 200.80 | < 0.001** |
| VO2max ml/min.Kg) | 23.81 ± 1.90 | 33.87 ± 2.65 | < 0.001** |
| CO2 production (VCO2) ml/min | 1422.88 ± 224.76 | 1799.45 ± 331.26 | < 0.001** |
| Respiratory exchange ratio VCO2/VO2 | 1.35 ± 0.10 | 1.26 ± 0.08 | 0.001** |
| Anaerobic threshold ml/min | 711.85 ± 298.55 | 931.40 ± 407.91 | 0.028* |
| Anaerobic threshold ml/min.Kg | 13.16 ± 5.61 | 18.20 ± 7.90 | < 0.009** |
| Weight Kg | 54.27 ± 5.60 | 51.41 ± 4.95 | 0.067 |
| Body Mass Index BMI | 21.53 ± 1.70 | 21.10 ± 1.82 | 0.393 |
| Resting heart rate (beats/min) | 99.12 ± 13.57 | 91.80 ± 13.10 | 0.059 |
| Resting systolic blood pressure (mmHg) | 100.45 ± 11.00 | 98.25 ± 10.63 | 0.477 |
| Resting diastolic blood pressure (mmHg) | 60.03 ± 11.25 | 57.10 ± 8.40 | 0.319 |
| Maximum heart rate (beats/min) | 183.61 ± 6.10 | 178.96 ± 7.08 | 0.023* |
| Systolic blood pressure (mmHg) at VO2Max | 146.33 ± 11.79 | 140.65 ± 18.26 | 0.174 |
| Diastolic blood pressure (mmHg) at VO2Max | 82.06 ± 17.75 | 70.80 ± 16.00 | 0.024* |
| Post exercise heart rate at 3 minutes recovery (beats/min) | 142.36 ± 11.78 | 138.74 ± 12.24 | 0.297 |
| Post exercise diastolic blood pressure (mmHg) | 110.94 ± 13.06 | 111.85 ± 13.69 | 0.811 |
| Post exercise systolic blood pressure (mmHg) | 58.97 ± 10.12 | 53.70 ± 8.44 | 0.058 |
Significant;
Highly significant.
A total of 106 SNPs was found to be significant with the low VO2max at the p-value below 0.001 among the 24,3345 variants (Supplementary Table 1). Three CYFIP2 and FNDC9-rs10037485, and C1R-rs75380747 and TOP2A-rs13695 SNPs on chromosome 5, 12 and 17, respectively were found to be associated significantly in young Saudi females with low VO2max (P < 8.01 × 10-05) among the 24,3345 variants (Figure 1). The most significant (P < 0.000979) SNPs that are associated with low VO2max in young Saudi females from the Arab-ancestry are summarized (Table 2). All these SNPs obeyed the hardy-Weinberg equilibrium.
Figure 1.
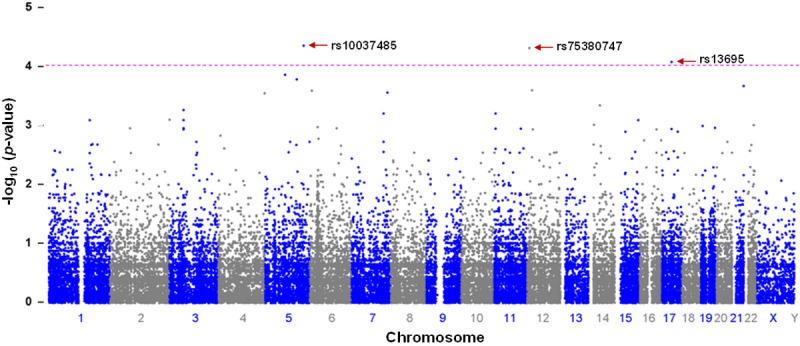
Manhattan plot of putative functional exonic (n = 243,345) variants in the molecular genetics of low VO2max from association study. The-log10 (p-values) from the association are plotted according to variant position on each chromosome. Positions of candidate SNP biomarkers of functional exonic variants at CYFIP2 and FNDC9 (rs10037485), and C1R (rs75380747) and TOP2A (rs13695) on chromosome 5, 12 and 17, respectively for low VO2max are indicated by arrows. The horizontal dotted line indicates the suggestive threshold of p 1.00 × 10-4.
Table 2.
List of the most significant SNPs associated with low VO2max
| S. No | RS id | CHR | BP | MA | MAF | CHISQ | P | OR (L95-U95) | Gene | AA | CCF | HWpval |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | rs10037485 | 5 | 156770133 | A | 0.6053 | 16.75 | 4.26E-05 | 12.78 (3.27-49.92) | CYFIP2 and FNDC9 | T | 0.605, 0.107 | 1 |
| 2 | rs75380747 | 12 | 7188562 | A | 0.1053 | 16.59 | 4.65E-05 | 0.09 (0.02-0.32) | C1R | G | 0.895, 0.429 | 0.365 |
| 3 | rs13695 | 17 | 38545193 | A | 0.05263 | 15.56 | 8.01E-05 | 0.06 (0.01-0.32) | TOP2A | C | 0.947, 0.536 | 1 |
| 4 | rs7726099 | 5 | 79521704 | G | 0.2105 | 14.61 | 0.0001323 | 0.13 (0.04-0.38) | SERINC5 | A | 0.789, 0.321 | 1 |
| 5 | rs45074 | 5 | 126993249 | C | 0.6842 | 14.25 | 0.0001598 | 7.94 (2.56-24.66) | CTXN3 and CTC-548H10.2 | C | 0.684, 0.214 | 0.002 |
| 6 | rs743346 | 21 | 47851636 | A | 0.02778 | 13.78 | 0.0002056 | 0.04 (0.01-0.37) | PCNT | G | 0.972, 0.607 | 0.003 |
| 7 | rs4959505 | 6 | 8869571 | A | 0.6316 | 13.43 | 0.0002475 | 7.89 (2.45-25.42) | A | 0.632, 0.179 | 1 | |
| 8 | rs2462603 | 7 | 145813854 | A | 0.1944 | 13.3 | 0.0002648 | 0.13 (0.04-0.42) | CNTNAP2 | G | 0.806, 0.357 | 0.859 |
| 9 | rs11726022 | 4 | 188170467 | A | 0.2632 | 13.23 | 0.0002751 | 0.14 (0.05-0.43) | G | 0.737, 0.286 | 1 | |
| 10 | rs9835332 | 3 | 56667682 | C | 0.6053 | 12.02 | 0.0005274 | 7.05 (2.2-22.62) | FAM208A | G | 0.605, 0.179 | 1 |
| 11 | rs11033800 | 11 | 4790948 | A | 0.1111 | 11.78 | 0.0005977 | 0.13 (0.03-0.45) | CPA5 | C | 0.889, 0.500 | 0.345 |
| 12 | rs17854248 | 7 | 130007388 | A | 0.1111 | 11.78 | 0.0005977 | 0.13 (0.03-0.45) | MMP26 and OR51F1 | G | 0.889, 0.500 | 0.345 |
| 13 | rs4675801 | 2 | 242493511 | G | 0.6316 | 11.32 | 0.0007657 | 6.29 (2.06-19.22) | BOK-AS1 | C | 0.632, 0.214 | 0.98 |
| 14 | rs7178698 | 15 | 94945719 | C | 0.02941 | 11.3 | 0.0007748 | 0.05 (0.01-0.46) | MCTP2 | T | 0.971, 0.643 | 0.321 |
| 15 | rs978979 | 3 | 56533016 | A | 0.2632 | 11.29 | 0.0007776 | 0.17 (0.06-0.49) | G | 0.737, 0.321 | 0.297 | |
| 16 | rs4657537 | 1 | 166268048 | G | 0.2632 | 11.29 | 0.0007776 | 0.17 (0.06-0.49) | A | 0.737, 0.321 | 1 | |
| 17 | rs1263811 | 14 | 21993498 | G | 0.4118 | 10.95 | 0.0009359 | 17.5 (2.12-144.7) | SALL2 | G | 0.412, 0.038 | 1 |
| 18 | rs55989856 | 22 | 39493294 | G | 0.02632 | 10.92 | 0.0009507 | 0.06 (0.01-0.48) | APOBEC3H | T | 0.974, 0.679 | 1 |
| 19 | rs7258236 | 19 | 6760974 | G | 0.1053 | 10.87 | 0.0009793 | 0.14 (0.04-0.49) | SH2D3A | T | 0.895, 0.536 | 0.126 |
CHR: Chromosome; SNP ID: Single nucleotide polymorphism ID; BP: Base pair position at the respective chromosome as per GRCh37.p13; MA: Minor allele name; MAF: Frequency of minor allele in controls; ChisQ: Basic allelic test chi-square; P: p-value; OR: Odd ratio; SE: Standard error; L95: Lower bound of 95% confidence interval for odds ratio; U95: Upper bound of 95% confidence interval for odds ratio. AA: Associated Allele; CCF: Case, Control Frequencies; HWpval: p-value of Hardy-Weinberg equilibrium.
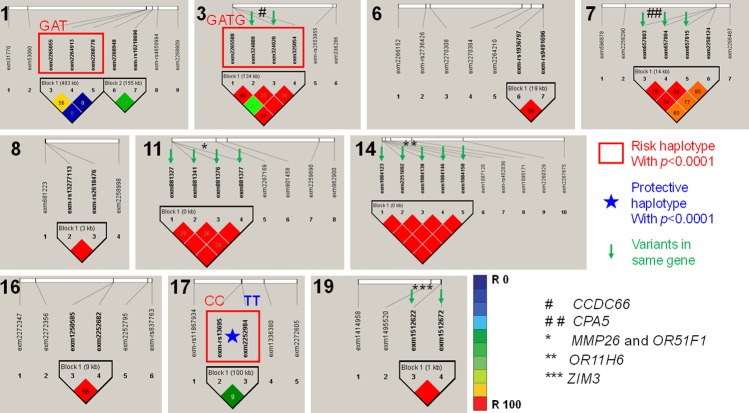
All association tests were screened using p-value of Hardy-Weinberg equilibrium, and frequency of minor allele in controls to conclude the strongest genetic predisposition (P < 0.001) and imputed for linkage disequilibrium in HapMap, separately for regions of chromosomes with more than one SNP (Figure 2). Linkage disequilibrium analysis among SNPs with significance level P < 0.001 identified risk (lowering VO2max) and protective (keeping VO2max normal) haplotypes with high degree (p-value < 1.0 × 10-4) of linkage disequilibrium (Table 3). The most significant haplotypes such as Chromosome 1: LOC112268276-rs10800201G; LOC112268276-rs4657537A; rs4657583T (p-value = 2.00 × 10-04); Chromosome 3: rs978979G; CCDC66-rs7637449A; CCDC66-rs111934125T; FAM208A-rs9835332G (p-value = 5.00 × 10-04) and Chromosome 17: STX2-rs13696C; TNS4-rs1901187C (p-value = 1.00 × 10-04) were found to be risk haplotypes associated with the low VO2max in young Saudi females (Table 2; Figure 2). The opposite alleles of the most significant haplotypes such as Chromosome 1: LOC112268276-rs10800201A; LOC112268276-rs4657537G; rs4657583C (p-value = 0.0077); Chromosome 3: rs978979A; CCDC66-rs7637449G; CCDC66-rs111934125C; FAM208A-rs9835332C (p-value = 0.0014) and Chromosome 17: STX2-rs13696T; TNS4-rs1901187T (p-value = 1.00 × 10-04) were found to be protective haplotypes associated with the low VO2max in young Saudi females (Tables 2, 3; Figure 2). Haplotypes MMP26 and OR51F1-rs1030726C; rs1030723G; rs11033800C; rs11033801A (Risk haplotype; p-value = 0.0011), MMP26 and OR51F1-rs1030726A; rs1030723A; rs11033800A; rs11033801G (Protective haplotype; p-value = 0.0024), OR11H6-rs12891553T; rs34693535C; rs17211285G; rs17277221T; rs17277228T (Risk haplotype; p-value = 0.002) and OR11H6-rs12891553C; rs34693535T; rs17211285T; rs17277221C; rs17277228C (Protective haplotype; p-value = 0.002) were identified in single gene (Table 2; Figure 2).
Figure 2.
Genetic association and linkage disequilibrium of the significant variants at selected chromosomes in Arab-ancestry females with low VO2max against high VO2max. The numbers at the left top corner of each sector indicates chromosome number. The SNPs indicated with green arrow in a block denotes variants in same gene in the respective chromosome. * and #: Gene names; Red square box: Indicates the most significant risk haplotypes of significant variants; Star indicates the most significant protective haplotype associated with the Arab-ancestry females with low VO2max, the details of the haplotypes and alleles are shown in Table 3.
Table 3.
Haplotypes of SNPs with the significance < 0.001 in Saudi female with low VO2max
| CHR | Block | Haplotype | Case, Control Frequencies | Chi Square | P Value | Haplotypes | Risk/Protective |
|---|---|---|---|---|---|---|---|
| 1 | Block 1 | GAT | 0.468, 0.052 | 13.512 | 2.00E-04 | rs10800201G; rs4657537A; rs4657583T** | Risk |
| 1 | GAC | 0.236, 0.226 | 0.009 | 0.9244 | rs10800201G; rs4657537A; rs4657583C | ||
| 1 | AGT | 0.156, 0.271 | 1.302 | 0.2538 | rs10800201A; rs4657537G; rs4657583T | ||
| 1 | AGC | 0.048, 0.284 | 7.097 | 0.0077 | rs10800201A; rs4657537G; rs4657583C | Protective | |
| 1 | GGT | 0.056, 0.034 | 0.173 | 0.6777 | rs10800201G; rs4657537G; rs4657583T | ||
| 1 | GGC | 0.004, 0.091 | 3.148 | 0.076 | rs10800201G; rs4657537G; rs4657583C | ||
| 1 | AAC | 0.002, 0.043 | 1.425 | 0.2326 | rs10800201A; rs4657537A; rs4657583C | ||
| 1 | AAT | 0.031, 0.001 | 0.831 | 0.3619 | rs10800201A; rs4657537A; rs4657583T | ||
| 1 | Block 2 | CG | 0.866, 0.497 | 10.664 | 0.0011 | rs859667C; rs10218696G | Risk |
| 1 | TA | 0.024, 0.247 | 7.654 | 0.0057 | rs859667T; rs10218696A | Protective | |
| 1 | TG | 0.055, 0.146 | 1.577 | 0.2092 | rs859667T; rs10218696G | ||
| 1 | CA | 0.055, 0.110 | 0.683 | 0.4085 | rs859667C; rs10218696A | ||
| 3 | Block 1 | GATG | 0.605, 0.179 | 12.016 | 5.00E-04 | rs978979G; rs7637449A; rs111934125T; rs9835332G** | Risk |
| 3 | AGCC | 0.105, 0.449 | 10.144 | 0.0014 | rs978979A; rs7637449G; rs111934125C; rs9835332C | Protective | |
| 3 | AGTC | 0.158, 0.229 | 0.538 | 0.4634 | rs978979A; rs7637449G; rs111934125T; rs9835332C | ||
| 3 | GGTC | 0.079, 0.056 | 0.126 | 0.7222 | rs978979G; rs7637449G; rs111934125T; rs9835332C | ||
| 3 | GGCC | 0.053, 0.086 | 0.294 | 0.5874 | rs978979G; rs7637449G; rs111934125C; rs9835332C | ||
| 6 | Block 1 | AC | 0.447, 0.821 | 9.448 | 0.0021 | rs1936797A; rs9491696C | Protective |
| 6 | GG | 0.447, 0.107 | 8.836 | 0.003 | rs1936797G; rs9491696G | Risk | |
| 6 | AG | 0.105, 0.071 | 0.223 | 0.6365 | rs1936797A; rs9491696G | ||
| 7 | Block 1 | TGTC | 0.814, 0.428 | 10.542 | 0.0012 | rs11761888T; rs17854248G; rs17164867T; rs968404C | Risk |
| 7 | CTCT | 0.131, 0.428 | 7.432 | 0.0064 | rs11761888C; rs17854248T; rs17164867C; rs968404T | Protective | |
| 7 | CGTC | 0.054, 0.036 | 0.127 | 0.7217 | rs11761888C; rs17854248G; rs17164867T; rs968404C | ||
| 7 | CTCC | 0.000, 0.036 | 1.371 | 0.2416 | rs11761888C; rs17854248T; rs17164867C; rs968404C | ||
| 7 | TGTT | 0.000, 0.036 | 1.371 | 0.2416 | rs11761888T; rs17854248G; rs17164867T; rs968404T | ||
| 7 | CTTC | 0.000, 0.036 | 1.378 | 0.2404 | rs11761888C; rs17854248T; rs17164867T; rs968404C | ||
| 8 | Block 1 | GT | 0.947, 0.679 | 8.386 | 0.0038 | rs13277113G; rs2618476T | Risk |
| 8 | AC | 0.053, 0.321 | 8.386 | 0.0038 | rs13277113A; rs2618476C | Protective | |
| 11 | Block 1 | CGCA | 0.868, 0.500 | 10.674 | 0.0011 | rs1030726C; rs1030723G; rs11033800C; rs11033801A | Risk |
| 11 | AGAG | 0.105, 0.214 | 1.49 | 0.2221 | rs1030726A; rs1030723G; rs11033800A; rs11033801G | ||
| 11 | AAAG | 0.026, 0.286 | 9.211 | 0.0024 | rs1030726A; rs1030723A; rs11033800A; rs11033801G | Protective | |
| 14 | Block 1 | TCGTT | 0.737, 0.357 | 9.515 | 0.002 | rs12891553T; rs34693535C; rs17211285G; rs17277221T; rs17277228T | Risk |
| 14 | CTTCC | 0.263, 0.643 | 9.515 | 0.002 | rs12891553C; rs34693535T; rs17211285T; rs17277221C; rs17277228C | Protective | |
| 16 | Block 1 | TG | 0.842, 0.500 | 8.933 | 0.0028 | rs1134760T; rs5923G | Risk |
| 16 | CA | 0.079, 0.357 | 7.888 | 0.005 | rs1134760C; rs5923A | Protective | |
| 16 | CG | 0.079, 0.143 | 0.694 | 0.4046 | rs1134760C; rs5923G | ||
| 17 | Block 1 | CC | 0.763, 0.295 | 14.384 | 1.00E-04 | rs13696C; rs1901187C** | Risk |
| 17 | CT | 0.184, 0.241 | 0.314 | 0.575 | rs13696C; rs1901187T | ||
| 17 | TT | 0.000, 0.331 | 14.608 | 1.00E-04 | rs13696T; rs1901187T* | Protective | |
| 17 | TC | 0.053, 0.134 | 1.335 | 0.2479 | rs13696T; rs1901187C | ||
| 19 | Block 1 | TA | 0.342, 0.750 | 10.739 | 0.001 | rs4801433T; rs4801200A | Protective |
| 19 | CT | 0.658, 0.250 | 10.739 | 0.001 | rs4801433C; rs4801200T | Risk |
CHR: Chromosome number;
Risk haplotypes (P < 1.0 × 10-4);
Protective haplotypes (P < 1.0 × 10-4).
Functional Enrichment Analysis revealed that the genes with SNPs P < 0.001 have significantly involved in the heart rate (6 Genes; P = 0.00442; HS3ST4, CYFIP2, CNTNAP2, MYH6, ROS1, FNDC9), body weight (7 Genes; P = 0.00629; PTPRD, MYO18B, MCTP2, C1ORF220, ZMIZ1, CYFIP2, CNTNAP2), breath tests (4 Genes; P = 0.0147; PTPRD, ZMIZ1, CNTNAP2, LDLRAD3), proteolysis (7 Genes; P = 0.00623; CPA5, MMP26, C1R, KLK1, CPA1, CAPN3, CTRL) and cardiac muscle fiber development (2 Genes; P = 0.0263; MYO18B, MYH6).
Discussion
The rapid development of socioeconomic status in the gulf regions impose a harmful sedentary lifestyle which burden society with health concerns. According to the WHO, physical inactivity considered one of the leading causes to cardiovascular diseases (CVD) that account for 54% of deaths from non-communicable disease in the Eastern Mediterranean regions [https://www.who.int/nmh/about/chp/en/]. Studies on the sedentary lifestyle with obesity and Physical activity (PA) in Saudi population revealed that physical inactivity level is 66.6% [24] and account for 71% in youth and 52% in children [25]. Different factors such as sex, geographical location and age appears to impact the PA [26]. Moreover, males generally are physically more active than females [27] and this tend to decrease with age [26]. Therefore, many studies were focusing on females as a high-risk group for CVD as per social and environmental factors may contributed to poor PA among Saudi females [28]. The PA and cardiovascular risk factors have been investigated heavily among adolescents and adults of both gender in different regions of Saudi Arabia [29-31]. However, less towards cardiorespiratory fitness which linked to CVD. Cardiorespiratory fitness has been known as a predictor of mortality caused by CVD, and further used to evaluate the cardiovascular health by measuring maximum oxygen consumption (VO2max) during sustained exercise [32]. Different correlation studies of cardiorespiratory fitness have been conducted within Saudi population tackling different aspects. Some indicated a positive correlation between 25(OH) D/body weight and VO2max (as VO2 peak) in young Saudi females [28]. However, less stringent association was conveyed in other study, improve VO2max after vitamin D supplementation was reported though [33]. Saudi females showed inverse correlation of VO2max and body fat composition with BMI [28,34]. Researches were aiming to characterize the aerobic fitness of Saudi athletes and soccer players [35]. The promotion towards physical activities have been initiated [25]. Recent findings noticed an association of a high VO2max with decrease incidence of CVD and improve the quality of life [36]. Studies are scanty on the investigation of VO2max in association with type 2 diabetes mellitus, dementia and environment, metabolic and genetic factors to ensure a better understanding of the cardiovascular health and quality life of Saudis. The present study is a pilot model for the genetic association and functional molecular mechanism of low VO2max in young females in Saudi Arabia. The genes (CYFIP2, FNDC9, C1R, TOP2A, LOC112268276, LOC112268276, CCDC66, FAM208A, STX2, TNS4, MMP26, OR51F1, OR11H6) identified with the most significant SNPs, risk and protective haplotypes and multi SNPs can be considered for the detailed study on the impact of lowering VO2max in young females. Limitation of the study: Number samples are relatively low. None of SNPs were satisfied the Bonferroni corrections (corrected α = 0.05/243345 = 2.05 × 10-07) among the 24,3345 variants. The expression of TOP2A has been shown to be significant response marker to therapy and prognosis in the patients of breast cancer [37], however a study reported insignificant association with breast cancer risk or clinical outcome [38]. A recent study has described the significant association of C/C genotype in rs13695 for lowering the risk of neutropenia in small cell lung cancer patients during chemotherapy [39], the SNP rs13695 has been reported in breast cancer patients [40], hence detailed studies on female with rs13695C are needed to confirm the possibilities to correlate the breast cancer and low VO2max.
Conclusion
The low VO2max in young Saudi females are associated with genetic variants that are significantly involved in the heart rate, body weight, breath tests, proteolysis and cardiac muscle fiber development. Furthermore, the identified genetic predispositions and gene-annotation enrichment in low VO2max in young Saudi females revealed that they are at high risk for developing cardiovascular complications.
Acknowledgements
The authors thank the Dean of Institute for Research and Medical Consultations (IRMC), Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia for her continuous support and encouragement. Authors thank the administrative staffs and facilities provided at IRMC, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia. This study was supported by The Deanship of Scientific Research, Imam Abdulrahman Bin Faisal University (To Dr LIA Grant No: 2015292). We also appreciate the technical assistance from Mr. Ranilo M. Tumbaga, Mr. Horace T. Pacifico, and Ms. Jee E. Aquino.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Williams CJ, Williams MG, Eynon N, Ashton KJ, Little JP, Wisloff U, Coombes JS. Genes to predict VO2max trainability: a systematic review. BMC Genomics. 2017;18(Suppl 8):831. doi: 10.1186/s12864-017-4192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortega R, Grandes G, Sanchez A, Montoya I, Torcal J. Cardiorespiratory fitness and development of abdominal obesity. Prev Med. 2019;118:232–7. doi: 10.1016/j.ypmed.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Karter AJ, D’Agostino RB Jr, Mayer-Davis EJ, Wagenknecht LE, Hanley AJ, Hamman RF, Bergman R, Saad MF, Haffner SM IRAS investigators. Abdominal obesity predicts declining insulin sensitivity in non-obese normoglycaemics: the insulin resistance atherosclerosis study (IRAS) Diabetes Obes Metab. 2005;7:230–8. doi: 10.1111/j.1463-1326.2004.00441.x. [DOI] [PubMed] [Google Scholar]
- 4.Ko G, Davidson LE, Brennan AM, Lam M, Ross R. Abdominal adiposity, not cardiorespiratory fitness, mediates the exercise-induced change in insulin sensitivity in older adults. PLoS One. 2016;11:e0167734. doi: 10.1371/journal.pone.0167734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacon AP, Carter RE, Ogle EA, Joyner MJ. VO2max trainability and high intensity interval training in humans: a meta-analysis. PLoS One. 2013;8:e73182. doi: 10.1371/journal.pone.0073182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denham J, Marques FZ, O’Brien BJ, Charchar FJ. Exercise: putting action into our epigenome. Sports Medn. 2014;44:189–209. doi: 10.1007/s40279-013-0114-1. [DOI] [PubMed] [Google Scholar]
- 7.Mann TN, Lamberts RP, Lambert MI. High responders and low responders: factors associated with individual variation in response to standardized training. Sports Med. 2014;44:1113–24. doi: 10.1007/s40279-014-0197-3. [DOI] [PubMed] [Google Scholar]
- 8.Wilson MG, Ellison GM, Cable NT. Basic science behind the cardiovascular benefits of exercise. Br J Sports Med. 2016;50:93–9. doi: 10.1136/bjsports-2014-306596rep. [DOI] [PubMed] [Google Scholar]
- 9.Bouchard C, Antunes-Correa LM, Ashley EA, Franklin N, Hwang PM, Mattsson CM, Negrao CE, Phillips SA, Sarzynski MA, Wang PY, Wheeler MT. Personalized preventive medicine: genetics and the response to regular exercise in preventive interventions. Prog Cardiovasc Dis. 2015;57:337–46. doi: 10.1016/j.pcad.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rankinen T, Bouchard C. Genetic predictors of exercise training response. Current Cardiovascular Risk Reports. 2011;5:368–72. [Google Scholar]
- 11.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G* power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–60. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 12.Al Asoom LI. Is cardiopulmonary fitness level a risk factor in young Saudi females? Journal of King Abdulaziz University-Medical Sciences. 2015;22:19–28. [Google Scholar]
- 13.Ferguson B. ACSM’s guidelines for exercise testing and prescription 9th Ed. 2014. J Can Chiropr Assoc. 2014;58:328. [Google Scholar]
- 14.Shazia SM, Badaam KM, Deore DN. Assessment of aerobic capacity in overweight young females: a cross-sectional study. Int J Appl Basic Med Res. 2015;5:18. doi: 10.4103/2229-516X.149224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glusman G, Caballero J, Mauldin DE, Hood L, Roach JC. Kaviar: an accessible system for testing SNV novelty. Bioinformatics. 2011;27:3216–3217. doi: 10.1093/bioinformatics/btr540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dayem Ullah AZ, Lemoine NR, Chelala C. SNPnexus: a web server for functional annotation of novel and publicly known genetic variants (2012 update) Nucleic Acids Res. 2012;40:W65–70. doi: 10.1093/nar/gks364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett JC, Fry B, Maller JD, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2004;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 18.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, De Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 20.Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, Jensen LJ, von Mering C. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papatheodorou I, Fonseca NA, Keays M, Tang YA, Barrera E, Bazant W, Burke M, Füllgrabe A, Fuentes AM, George N, Huerta L. Expression Atlas: gene and protein expression across multiple studies and organisms. Nucleic Acids Res. 2017;46:D246–51. doi: 10.1093/nar/gkx1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabregat A, Jupe S, Matthews L, Sidiropoulos K, Gillespie M, Garapati P, Haw R, Jassal B, Korninger F, May B, Milacic M. The reactome pathway knowledgebase. Nucleic Acids Res. 2017;46:D649–55. doi: 10.1093/nar/gkx1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heyward V. Advance fitness assessment and exercise prescription, the cooper institute for aerobics research. Dallas TX USA. 1998 [Google Scholar]
- 24.Al-Zalabani AH, Al-Hamdan NA, Saeed AA. The prevalence of physical activity and its socioeconomic correlates in kingdom of Saudi Arabia: a cross-sectional population-based national survey. J Taibah Univ Sci. 2015;10:208–15. [Google Scholar]
- 25.Alahmed Z, Lobelo F. Physical activity promotion in Saudi Arabia: a critical role for clinicians and the health care system. J Epidemiol Glob Health. 2018;7:S7–15. doi: 10.1016/j.jegh.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Nuaim AA, Al-Nakeeb Y, Lyons M, Al-Hazzaa HM, Nevill A, Collins P, Duncan MJ. The prevalence of physical activity and sedentary behaviours relative to obesity among adolescents from Al-Ahsa, Saudi Arabia: rural versus urban variations. J Nutr Metab. 2012;2012:417589. doi: 10.1155/2012/417589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Nakeeb Y, Lyons M, Collins P, Al-Nuaim A, Al-Hazzaa H, Duncan MJ, Nevill A. Obesity, physical activity and sedentary behavior amongst British and Saudi youth: a cross-cultural study. Int J Environ Res Public Health. 2012;9:1490–506. doi: 10.3390/ijerph9041490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al Asoom LI. Assessment of plasma level of 25(OH) D and its correlation with cardiorespiratory fitness in young females of Dammam city, KSA. J Taibah Univ Sci. 2016;11:456–63. [Google Scholar]
- 29.Banday AH, Want FA, Alris FF, Alrayes MF, Alenzi MJ. A cross-sectional study on the prevalence of physical activity among primary health care physicians in Aljouf region of Saudi Arabia. Mater Sociomed. 2015;27:263. doi: 10.5455/msm.2015.27.263-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Hazzaa HM. Physical inactivity in Saudi Arabia revisited: a systematic review of inactivity prevalence and perceived barriers to active living. Int J Med Health Sci. 2018;12:50. [PMC free article] [PubMed] [Google Scholar]
- 31.Mahfouz AA, Shatoor AS, Khan MY, Daffalla AA, Mostafa OA, Hassanein MA. Nutrition, physical activity, and gender risks for adolescent obesity in Southwestern Saudi Arabia. Saudi J Gastroenterol. 2011;17:318–22. doi: 10.4103/1319-3767.84486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antunes-Correa LM. Maximal oxygen uptake: new and more accurate predictive equation. Eur J Prev Cardiol. 2018;25:1075–1076. doi: 10.1177/2047487318780442. [DOI] [PubMed] [Google Scholar]
- 33.Eslami O, Shidfar F, Akbari-Fakhrabadi M. Vitamin D and cardiorespiratory fitness in the general population: a systematic review. Int J Vitam Nutr Res. 2017;1:12. doi: 10.1024/0300-9831/a000490. [DOI] [PubMed] [Google Scholar]
- 34.Al-Asiri ZA, Shaheen AA. Body mass index and health related physical fitness in Saudi girls and adolescents aged 8-15 years. Open Journal of Therapy and Rehabilitation. 2015;3:116. [Google Scholar]
- 35.Badawy MM, Muaidi QI. Aerobic profile during high-intensity performance in professional Saudi athletes. Pak J Biol Sci. 2018;21:24–8. doi: 10.3923/pjbs.2018.24.28. [DOI] [PubMed] [Google Scholar]
- 36.Al-Mallah MH, Sakr S, Al-Qunaibet A. Cardiorespiratory fitness and cardiovascular disease prevention: an update. Curr Atheroscler Rep. 2018;20:1. doi: 10.1007/s11883-018-0711-4. [DOI] [PubMed] [Google Scholar]
- 37.Rody A, Karn T, Ruckhaberl E, Muller V, Gehrmann M. Gene expression of topoisomerase II alpha (TOP2A) by microarray analysis is highly prognostic in estrogen receptor (ER) positive breast cancer. Breast Cancer Res Treat. 2009;113:457–466. doi: 10.1007/s10549-008-9964-x. [DOI] [PubMed] [Google Scholar]
- 38.Shi H, Bevier M, Johansson R, Enquist-Olsson K, Henriksson R, Hemminki K, Lenner P, Försti A. Prognostic impact of polymorphisms in the MYBL2 interacting genes in breast cancer. Breast Cancer Res Treat. 2012;131:1039–47. doi: 10.1007/s10549-011-1826-2. [DOI] [PubMed] [Google Scholar]
- 39.Nicoś M Krawczyk P, Rolska-Kopińska A, Grenda A, Bożyk A, Szczyrek M, Milanowski J. Effect of TOP2A and ERCC1 genes polymorphism on the efficacy and toxicity of cisplatin and etoposide therapy in SCLC patients. J Thorac Oncol. 2017;12:S1765–S1766. doi: 10.5114/aoms.2020.92572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fasching PA, Weihbrecht S, Haeberle L, Gasparyan A, Villalobos IE, Ma Y, Ekici AB, Wachter DL, Hartmann A, Beckmann MW, Slamon DJ. HER2 and TOP2A amplification in a hospital-based cohort of breast cancer patients: associations with patient and tumor characteristics. Breast Cancer Res Treat. 2014;145:193–203. doi: 10.1007/s10549-014-2922-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.