Abstract
MicroRNAs (miRNAs) are small non-coding RNAs that regulate gene expression by suppressing the target mRNA and inhibiting translation in order to regulate multiple biological processes. miRNAs play important roles as oncogenes or tumor suppressors in the development of various types of human cancer. The regulation of mammalian target of rapamycin (mTOR) by miRNAs has been studied in several types of cancer, including colorectal cancer (CRC). However, to the best of our knowledge, only limited information regarding the function of miRNAs in human CRC is available. In the present study, the expression of 22 miRNAs in CRC cell lines were investigated in regard to key genes in the mTOR pathway. Initially, it was revealed that mTOR, regulatory-associated protein of mTOR complex I and rapamycin-intensive companion of mTOR were overexpressed in CRC cell lines when compared with a normal colorectal cell line. Subsequently, putative miRNA-mRNA associations were identified via multiple miRNA target prediction programs. The expression levels for the candidate miRNAs were validated using quantitative real-time polymerase chain reaction. Expression analysis revealed that, among 20 miRNAs, five miRNAs (miR-496, miR-1185, miR-654, miR-3183 and miR-495) exhibited significant downregulation in association with the mTOR signaling pathway. Taken together, the results from the present study suggest that several miRNAs that are associated with CRC, with possible roles in mTOR signaling, may have potential therapeutic or diagnostic benefits in CRC treatment.
Keywords: mammalian target of rapamycin, regulatory-associated protein of mammalian target of rapamycin complex 1, rapamycin-insensitive companion of mammalian target of rapamycin, microRNA, colorectal cancer
Introduction
Colorectal cancer (CRC) accounted for ~1.4 million of the new cancer cases diagnosed in 2012 worldwide and is considered the third most common cancer (1). According to the International Agency for Research on Cancer, by 2035, the estimated number of CRC cases will reach 2.4 million cases diagnosed each year (1). Although a lot has been learned about CRC, novel therapeutic strategies are needed in order to tackle this cancer. Mammalian target of rapamycin (mTOR) is a catalytic subunit of two large signaling complexes; mTORC1 and mTORC2, along with key proteins, form these complexes. Regulatory-associated protein of mTOR (RPTOR) is the unique component of mTORC1, whereas rapamycin-insensitive companion of mTOR (RICTOR) is an exclusive component of mTORC2. The two complexes play a central role in tumorigenesis via phosphorylation of key proteins within the mTOR pathway. mTORC1 regulates mRNA translation and elongation by phosphorylating its downstream effectors, such as eukaryotic initiation factor 4E-binding protein 1 and the p70 ribosomal S6 kinase 1 (2). mTORC2 phosphorylates protein kinase B, promoting cell proliferation, apoptosis and survival (3).
MicroRNAs (miRNAs/miRs) are single-stranded, non-coding RNAs, ranging from 19–24 nucleotides in length. In order to perform their regulatory functions, mature miRNAs bind most often to the 3′untranslated region (UTR) of messenger RNAs, which inhibit the translation of mRNA and result in the downregulation of gene expression at the post-transcriptional level (4). Approximately 50% of human miRNA genes are located in genomic regions that have fragile sites, and these locations contain chromosomal abnormalities, such as deletions and amplifications. These genomic regions are vulnerable to genetic alteration in several types of human cancer (5,6). Furthermore, a single miRNA can interact and regulate more than one mRNA target (7).
In the last decade, a growing body of evidence has revealed that miRNAs are involved in tumorigenesis and cancer progression (8,9). Deregulation of miRNAs has been demonstrated in several types of cancer, including CRC (10–16). In cancer cells, alteration of miRNA expression levels can be abnormally down- or upregulated, to function as either tumor suppressors or oncogenes (17). In multiple studies, miRNAs have been demonstrated to play an important role in CRC. For example, miR-21, miR-31 and miR-223 are upregulated in CRC, whereas miR-143, miR-145 and miR-126 are downregulated (18–20).
The potential use of miRNA as diagnostic or prognostic markers in the clinical setting has been highlighted previously (21). Furthermore, miRNA mimics can be used as therapeutic agents to restore miRNA function, or miRNA inhibitors can be used to disrupt upregulated miRNA. Therefore, identifying novel cancer-associated miRNAs is important for the potential treatment of cancer.
Downregulated miRNAs are believed to act as tumor suppressors, which may greatly contribute to colorectal carcinogenesis (19,20,22,23). The aim of the present study was to identify miRNA-mRNA associations and evaluate miRNA expression in human CRC cells, by focusing on the mTOR signaling pathway.
Materials and methods
Cell lines
The human CRC cell lines, HT29, HCT116, SW620, SW480 and the FHC normal fetal human colon epithelial cell line were purchased from the American Type Culture Collection (ATCC). The CSC480 cell line was purchased from BioMedicure (San Diego, CA, USA) All cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM; 4.5 g/l D-Glucose, L-Glutamine, 110 mg/l sodium pyruvate; Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) and cultured at 37°C in a humidified incubator with 5% CO2. Cells were induced with insulin (200 nM) as previously described (24). The FHC cell line was cultured according to previously described culture conditions (25).
Bioinformatics analysis
mRNA targets were predicted for 22 miRNAs of interest using five miRNA target-prediction programs: DIANA-MICROT (diana.imis.athena-innovation.gr) (26), miRWalk (umm.uni-heidelberg.de) (27), TargetScan (targetscan.org) (7), PicTar (pictar.mdc-berlin.de) (28) and miRDB (mirdb.org) (29). These databases use a computational algorithm of miRNAs by searching for the presence of conserved sites on the 3′UTR of mRNA that match the seed region of each miRNA. The top target candidates according to the score in the database for each miRNA provided by each program were chosen for further molecular analysis.
RNA isolation and reverse transcription polymerase chain reaction (RT-PCR)
Total RNA, including miRNA, was extracted from 1×106 cultured cells using an miRNeasy Mini kit (Qiagen, Inc.), according to the manufacturer's protocol. Briefly, cells were trypsinized, collected from a 10-cm cell-culture dish and placed into a centrifuge tube, followed by the addition of 700 µl TRIzol® reagent (Qiagen, Inc., Valencia, CA, USA) then 140 µl chloroform. The aqueous phase was separated by centrifugation (5 min at room temperature) in a microfuge (Eppendorf 5430; Eppendorf, Hamburg, Germany) followed by washing sequentially with 100% ethanol. Samples were centrifuged in an RNeasy Mini spin column membrane (Qiagen, Inc.) and RNA was then eluted in RNase-free water. The concentration of RNA was determined via absorbance measurements at 260 nm using a NanoDrop spectrophotometer, and the RNA was checked by determining absorbance ratios, 260/280 and 260/230 nm, for any contamination. For mRNA expression, cDNAs were synthesized using a BluePrint 1st Strand cDNA Synthesis kit (Takara Bio, Inc.), according to the manufacturer's protocol. miRNAs were reverse-transcribed using an miScript II RT kit (Qiagen, Inc.), also according to the manufacturer's protocol. Briefly, single-stranded cDNA was synthesized from RNA in a 20 µl reaction volume. The reactions were incubated: first, at 37°C for 60 min, then the reverse transcriptase mix was inactivated by incubation at 95°C for 5 min. The cDNA generated was diluted 10-fold in RNase-free water.
RT-quantitative (q)PCR analysis of mRNA
Quantitative, RT-qPCR was used to measure mRNA expression levels for all three genes (mTOR, RPTOR and RICTOR). qPCR was performed using a LightCycler 480 thermal cycling system (Roche Diagnostics) with SYBR Premix Ex Taq II (Takara Bio, Inc.) in a 20 µl reaction mixture. The sequences of primers used for qPCR analysis are listed in Table I. Thermal cycling conditions were stage 1: Initial denaturation (1 cycle) 95°C for 5 min; stage 2: PCR (40 cycles) 95°C for 10 sec followed by 56°C for 10 sec and 72°C for 20 sec; and stage 3: Melting curve analysis 95°C for 5 sec, 65°C for 60 sec and 97°C. A non-template control (nuclease-free water) was included for each primer set and each sample was analyzed in triplicate. Relative expression was calculated using the 2−ΔΔCq method (30).
Table I.
mTOR, RPTOR and RICTOR primer sequences.
| Primer | Sequence | Amplicon size |
|---|---|---|
| mTOR | 146 (bp) | |
| F | 5′-AGCATCGGATGCTTAGGAGTGG-3′ | |
| R | 5′-CAGCCAGTCATCTTTGGAGACC-3′ | |
| RPTOR | 148 (bp) | |
| F | 5′-GATCGTCAACAGCTATCACACGG-3′ | |
| R | 5′-CGAGTCGAAGTTCTGCCAGATC-3′ | |
| RICTOR | 125 (bp) | |
| F | 5′-GCCAAACAGCTCACGGTTGTAG-3′ | |
| R | 5′-CCAGATGAAGCATTGAGCCACTG-3′ | |
| GAPDH | 131 (bp) | |
| F | GTCTCCTCTGACTTCAACAGCG | |
| R | ACCACCCTGTTGCTGTAGCCAA |
mTOR, mammalian target of rapamycin; RPTOR, regulatory-associated protein of mTOR complex I; RICTOR, rapamycin-insensitive companion of mTOR; F, forward; R, reverse.
RT-qPCR analysis of miRNAs
RT-qPCR was performed on selected miRNAs using the LightCycler 480 thermal cycling system (Roche Diagnostics). Expression levels of mature miRNAs were quantified using an miScript SYBR Green PCR kit (Qiagen), according to the manufacturer's instructions. The small nucleolar RNA, C/D box 68 (SNORD68), and the U6 small nuclear 2 RNA (RNU6-2) (Qiagen, Inc.) were used as endogenous controls, due to their relatively stable expression. Forward primers (Table II) were designed by first converting the miRNA sequences to DNA and adjusting the melting temperature of the primer to 60°C, either by adding a thymine to the 3′ of the primer or removing a nucleotide from the 5′ end of the primer. Primers were purchased from Integrated DNA Technologies, Inc., while the universal reverse primer was included in the miScript SYBR Green kit. The amplification reaction (10 µl) included 2X SYBR Green PCR master mix (5 µl), universal primer (250 nM), miRNA-specific primer (250 nM), RNase-free water (1.75 µl) and cDNA (2 µl). Pipetting was performed using the epMotion 5075 automated pipetting system (Eppendorf). The reaction conditions were as follows: Initial activation step (1 cycle), 95°C for 15 min followed by 3-step cycling (40 cycles), (denaturation) 94°C for 15 sec, (annealing) 55°C for 30 sec and (extension) 70°C for 30 sec, with melting curve: 95°C for 5 sec, 65°C for 60 sec and 97°C. All reactions were performed in triplicate and the average values were used in subsequent analysis. Expression was normalized to SNORD68 and RNU6-2. The fold change was calculated using the 2−ΔΔCq method (30) to acquire relative expression levels.
Table II.
Designed miRNA forward sequences.
| miRNA | Forward sequence primer |
|---|---|
| hsa-miR-1271-5p | 5′-TTGGCACCTAGCAAGCACTC-3′ |
| hsa-miR-496 | 5′-GCTGAGTATTACATGGCCAATCTC-3′ |
| hsa-miR-581 | 5′-GCGTCTTGTGTTCTCTAGATCAGTAAA-3′ |
| hsa-miR-1185-3p | 5′-GCATATACAGGGGGAGACTCTTATAAA-3′ |
| hsa-miR-767-3pa | 5′-CTGCTCATACCCCATGGTTTC-3′ |
| hsa-miR-96-5p | 5′-TTGGCACTAGCACATTTTTGC-3′ |
| hsa-miR-335-3p | 5′-GTTTTTCATTATTGCTCCTGACCA-3′ |
| hsa-miR-3182 | 5′-GCCGCTTCTGTAGTGTAGTCAAA-3′ |
| hsa-miR-1294 | 5′-TGTGAGGTTGGCATTGTTGTCT-3′ |
| hsa-miR-2114-3p | 5′-GAGCCTCAAGCAAGGGACTT-3′ |
| hsa-miR-3121-3p | 5′-GCTAAATAGAGTAGGCAAAGGACAAA-3′ |
| hsa-miR-3183 | 5′-CTCTCTCGGAGTCGCTCG-3′ |
| hsa-miR-340-3p | 5′-GCTCCGTCTCAGTTACTTTATAGCAA-3′ |
| hsa-miR-4802-3p | 5′-TACATGGATGGAAACCTTCAAGC-3′ |
| hsa-miR-548o-3p | 5′-CCAAAACTGCAGTTACTTTTGCA-3′ |
| hsa-miR-654-5p | 5′-GTGGGCCGCAGAACATG-3′ |
| hsa-miR-1323 | 5′-TCAAAACTGAGGGGCATTTTC-3′ |
| hsa-miR-142-3p | 5′-GCTGTAGTGTTTCCTACTTTATGGAAAA-3′ |
| hsa-miR-194-5p | 5′-TGTAACAGCAACTCCATGTGGA-3′ |
| hsa-miR-495 | 5′-GAAACAAACATGGTGCACTTCTTAA-3′ |
| hsa-miR-659-3p | 5′-TTGGTTCAGGGAGGGTCC-3′ |
| hsa-miR-98-5P | 5′-GCCTGAGGTAGTAAGTTGTATTGTTAAAA-3′ |
miRNA, microRNA; miR, microRNA.
Statistical analysis
Data analysis was conducted using Prism software (version 6.0; GraphPad Software, Inc.). One-way analysis of variance (ANOVA) was used to determine statistical significance, with the Dunnett's multiple comparisons test used as a post hoc test. P<0.05 was considered to indicate a statistically significant difference. In the ANOVA, each CRC line was compared to the control FHC cell line, and the multiple group graphs represent significance in regard to FHC. Data are presented as the mean of three replicates with standard error.
Results
miRNA target prediction analysis of mTOR pathway genes
In order to determine potential target genes and signaling pathways implicated in mTOR, RPTOR and RICTOR, five programs were used for miRNA target prediction analysis, namely DIANA-MICROT, miRWalk, TargetScan, PicTar and miRDB. Using the combination of five programs provided a more reliable model of target prediction, due to a different computer-aided algorithm for each target prediction program. From the miRNAs predicted for each gene, the highest miRNAs predicted by at least 2 prediction programs were chosen. A total of 22 miRNAs for all three genes were selected for further analysis (Tables III–V).
Table III.
miRNA candidates targeting the mTOR gene predicted by bioinformatics analysis.
| Database algorithm | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| miRNA ID | Proposed target gene | Ensembl gene ID | miRNA sequence | Diana | miRWalk | TargetScan | PicTar | miRDB | |
| hsa-miR-581 | mTOR | ENSG00000198793 | UCUUGUGUUCUCUAGAUCAGU | Score | 0.95 | – | – | 3.42 | 63 |
| P-value | 0.04 | 0.014 | – | – | Rank 20 | ||||
| Binding | 8mer | 8mer | – | – | 7mer | ||||
| Region | UTR3 | UTR3 | – | UTR3 | UTR3 | ||||
| hsa-miR-96-5p | mTOR | ENSG00000198793 | UUUGGCACUAGCACAUUUUUGCU | Score | 0.85 | – | 95 | – | 60 |
| P-value | 0.04 | – | 0.79 | – | Rank 24 | ||||
| Binding | 7mer | – | 7mer-m8 | – | 7mer | ||||
| Region | UTR3 | – | UTR3 | – | UTR3 | ||||
| hsa-miR-767-3pa | mTOR | ENSG00000198793 | UCUGCUCAUACCCCAUGGUUUCU | Score | 0.99 | – | – | – | 88 |
| P-value | 0.004 | 0.001 | – | – | Rank 4 | ||||
| Binding | 6mer | 10mer | – | – | 7mer | ||||
| Region | UTR3 | UTR3 | – | – | UTR3 | ||||
| hsa-miR-1185-3p | mTOR | ENSG00000198793 | AUAUACAGGGGGAGACUCUUAU | Score | 0.88 | – | – | – | 65 |
| P-value | 0.04 | – | – | – | Rank 15 | ||||
| Binding | 8mer | – | – | – | 7mer | ||||
| Region | UTR3 | – | – | – | UTR3 | ||||
| hsa-miR-1271-5p | mTOR | ENSG00000198793 | CUUGGCACCUAGCAAGCACUCA | Score | 0.84 | – | 95 | – | 61 |
| P-value | 0.03 | – | 0.79 | – | Rank 22 | ||||
| Binding | 7mer | – | 7mer | – | 7mer | ||||
| Region | UTR3 | – | UTR3 | – | UTR3 | ||||
| hsa-miR-496 | mTOR | ENSG00000198793 | UGAGUAUUACAUGGCCAAUCUC | Score | 0.86 | – | – | – | 72 |
| P-value | 0.03 | 0.014 | – | – | Rank 9 | ||||
| Binding | 8mer | 8mer | – | – | 7mer | ||||
| Region | UTR3 | UTR3 | – | – | UTR3 | ||||
| hsa-miR-335-3p | mTOR | ENSG00000198793 | UUUUUCAUUAUUGCUCCUGACC | Score | 0.85 | – | – | – | 67 |
| P-value | 0.03 | 0.014 | – | – | Rank 13 | ||||
| Binding | 8mer | 8mer | – | – | 7mer | ||||
| Region | UTR3 | UTR3 | – | – | UTR3 | ||||
| hsa-miR-3182 | mTOR | ENSG00000198793 | GCUUCUGUAGUGUAGUC | Score | 0.85 | – | – | – | 79 |
| P-value | 0.028 | – | – | – | Rank 6 | ||||
| Binding | 7mer | – | – | – | 7mer | ||||
| Region | UTR3 | – | – | – | UTR3 | ||||
miRNA, microRNA; miR, microRNA; UTR3, 3 untranslated region; mTOR, mammalian target of rapamycin.
Table V.
miRNA candidates targeting the RICTOR gene predicted by bioinformatics analysis.
| Database algorithm | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| miRNA ID | Proposed target gene | Ensembl gene ID | miRNA sequence | Diana | miRWalk | TargetScan | PicTar | miRDB | |
| hsa-miR-495 | RICTOR | ENSG00000164327 | AAACAAACAUGGUGCACUUCUU | Score | 0.871 | – | −0.17 | – | – |
| P-value | 0.002 | 0.0166 | N/A | – | – | ||||
| Binding | 8mer | 9mer | 8mer | – | – | ||||
| Region | UTR3 | UTR3 | UTR3 | – | – | ||||
| hsa-miR-194-5p | RICTOR | ENSG00000164327 | UGUAACAGCAACUCCAUGUGGA | Score | 0.79 | – | – | – | 83 |
| P-value | 0.003 | – | – | – | Rank 28 | ||||
| Binding | 6mer | – | – | – | 7mer | ||||
| Region | UTR3 | – | – | – | UTR3 | ||||
| hsa-miR-659-3p | RICTOR | ENSG00000164327 | CUUGUUCAGGGAGGGUCCCCA | Score | 0.986 | – | – | – | 75 |
| P-value | 0.008 | – | – | – | Rank 48 | ||||
| Binding | 7mer | – | – | – | 7mer | ||||
| Region | UTR3 | – | – | – | UTR3 | ||||
| hsa-miR-142-3p | RICTOR | ENSG00000164327 | UGUAGUGUUUCCUACUUUAUGGA | Score | 0.999 | – | – | – | 100 |
| P-value | 0.03 | 0.0166 | 0.99 | – | Rank 61 | ||||
| Binding | 8mer | 9mer | 8mer | – | 7mer | ||||
| Region | UTR3 | UTR3 | UTR3 | – | UTR3 | ||||
| hsa-miR-98-5P | RICTOR | ENSG00000164327 | UGAGGUAGUAAGUUGUAUU | Score | 0.999 | – | −0.14 | – | |
| P-value | 0.007 | 0.0166 | 0.98 | – | Rank | ||||
| Binding | 8mer | 9mer | 8mer | – | 7mer | ||||
| Region | UTR3 | UTR3 | UTR3 | – | UTR3 | ||||
| hsa-miR-1323 | RICTOR | ENSG00000164327 | UCAAAACUGAGGGGCAUUUUCU | Score | 0.75 | – | – | – | 80 |
| P-value | 0.006 | 0.004 | – | – | Rank 37 | ||||
| Binding | 8mer | 10mer | – | – | 7mer | ||||
| Region | UTR3 | UTR3 | – | – | UTR3 | ||||
miRNA, microRNA; miR, microRNA; UTR3, 3 untranslated region; RICTOR; rapamycin-insensitive companion of mammalian target of rapamycin.
Gene expression of mTOR, RICTOR, RPTOR and validation of specific miRNAs
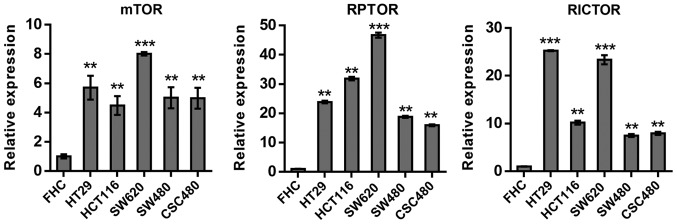
To assess alterations of the mTOR pathway in CRC cells, mTOR, RPTOR and RICTOR expression levels were investigated in five human CRC cell lines, HT29, HCT116, SW620, SW480 and CSC480, using RT-qPCR. The gene GAPDH was used as a reference. The comparison of results from mTOR, RPTOR and RICTOR expression revealed significantly (P<0.01) elevated mRNA expression levels in all cell lines compared with normal FHC cells (Fig. 1).
Figure 1.
Reverse transcription-quantitative PCR analysis of differentially expressed mRNAs in three genes, mTOR, RPTOR and RICTOR, in 5 human colorectal cancer cell lines. The expression levels of all genes were elevated in all cell lines (stimulated with 200 nM insulin) compared with normal FHC cells. In the RICTOR bar graph, error bars are included but are small. **P<0.01; ***P≤0.001. Number of repeats, 3. mTOR, mammalian target of rapamycin; RPTOR, regulatory-associated protein of mTOR complex I; RICTOR, rapamycin-insensitive companion of mTOR.
mTOR pathway-targets miRNAs differentially regulated
Next, the most significant candidates of miRNAs were selected, as predicted by the bioinformatics analysis, to target the genes of interest for further confirmatory studies. A selection of CRC cell lines, as well as the control (FHC), were used to examine whether miRNAs were associated with transcriptional expression of their relevant target gene and to identify miRNAs involved in CRC cells. Based on the results from the bioinformatics analysis, candidate miRNAs that were identified to be associated with each gene were assessed using qPCR.
mTOR
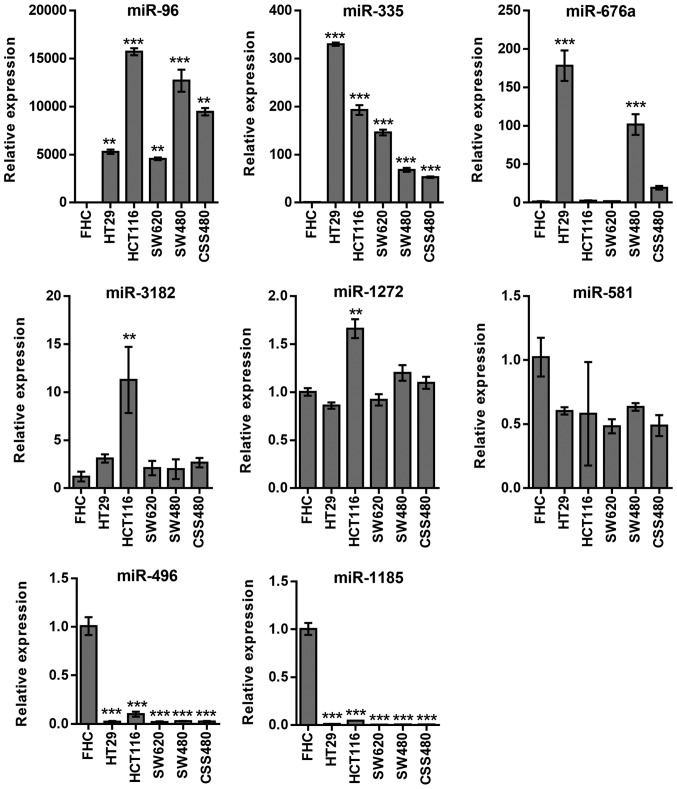
A statistically significant upregulation in the expression levels of miR-96 (P<0.01) and miR-335 (P<0.001), which was associated with mTOR, was observed in all cell lines (Fig. 2). miRNAs, such as miR-676a (HT29 and SW480, P<0.001) and miR-3182 (HCT116 P<0.05), were upregulated in certain cell lines, while miR-1272 and miR-581 expression remained unchanged. miR-496 and miR-1185 expression levels were significantly downregulated (P<0.001) in all cancer cell lines (Fig. 2).
Figure 2.
Quantitative-PCR analysis of differentially expressed miRNAs in association with mTOR expression in 5 human colorectal cancer cell lines, stimulated with insulin (200 nM). The data presented revealed that miRNAs, miR-96, miR-335 and miR-767 were upregulated, whereas miR-1272 and miR-581 were unchanged compared to FHC cells. Number of repeats, 3. **P≤0.01; ***P≤0.001. miRNA, microRNA; mTOR, mammalian target of rapamycin.
RPTOR
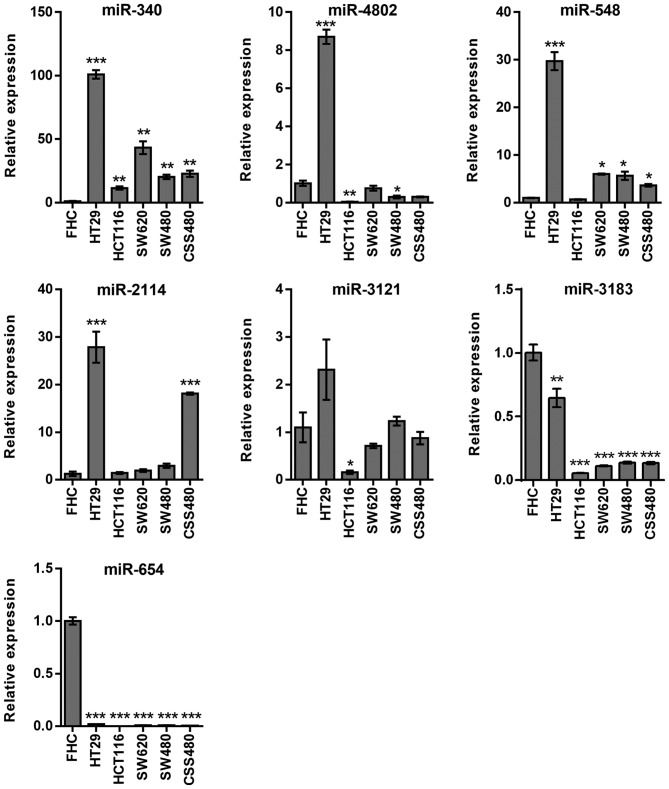
In miRNAs associated with RPTOR, miR-340 exhibited high expression levels in all cell lines (P<0.01), whereas miR-4802 was upregulated (P<0.001) in HT29 cells only (Fig. 3). Furthermore, miR-548 was upregulated in all cell lines (P<0.05) except HCT116. HT29 and CSC480 were the only cell lines that exhibited upregulated miR-2114 (P<0.001). There was no statistically significant difference in the expression levels of miR-3121, except for the downregulation in HCT116 cells (P<0.05). However, miR-654 (P<0.01) and miR-3183 (P<0.001) expression levels were downregulated in all cell lines (Fig. 3).
Figure 3.
Confirmation of miRNAs associated with RPTOR expression in colorectal cancer cell lines (stimulated with 200 nM insulin) by reverse transcription PCR. Number of repeats, 3. *P≤0.05; **P≤0.01; ***P≤0.001. miRNA, microRNA; RPTOR, regulatory-associated protein of mammalian target of rapamycin complex I; miR, microRNA.
RICTOR
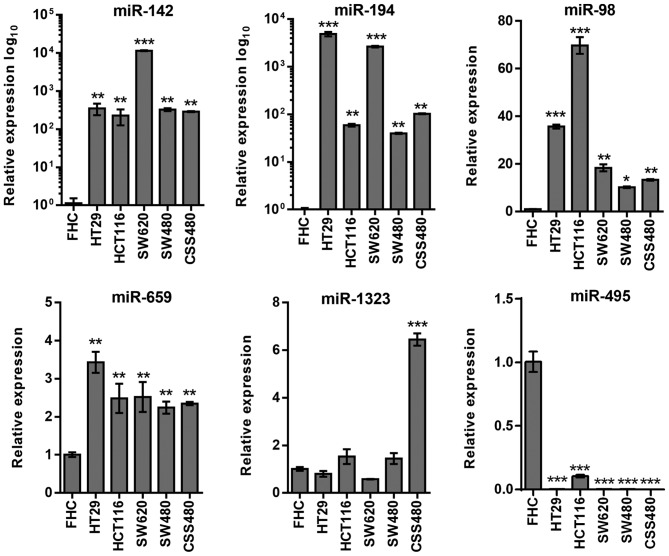
In miRNAs associated with RICTOR, miR-142, miR-194, miR-98 and miR-659 demonstrated high expression levels in all cells (P<0.01), while miR-1323 was upregulated (P<0.001) in CSC480 cells only (Fig. 4). The expression levels of miR-495 were downregulated in all cell lines (P<0.001).
Figure 4.
Expression levels of miRNAs in regard to RICTOR in 5 colorectal cancer cell lines. miRNAs were analyzed using quantitative PCR to confirm miRNA target prediction results. Cells were stimulated with insulin, 200 nM. Number of repeats, 3. *P≤0.05; **P≤0.01; ***P≤0.001. miRNA, microRNA; RICTOR, rapamycin-insensitive companion of mammalian target of rapamycin; miR, microRNA.
Discussion
Deregulation of the mTOR pathway occurs frequently in human cancer types (3,24,31,32). Regulation of mTOR by miRNAs has been investigated in a number of different types of cancer, including CRC (33,34). In the present study, it was revealed that 6 miRNAs (miR-96, miR-335, miR-340, miR-142, miR-194, miR-659 and miR-98) were upregulated in the 5 investigated human CRC cell lines when comparing with FHC. Furthermore, among 20 miRNAs, 5 (miR-496, miR-1185, miR-3183, miR-654 and miR-495) revealed significant downregulation in association with the 3 key mTOR signaling pathway genes, mTOR, RPTOR and RICTOR.
Several software programs are available to aid in identifying miRNA target prediction. The majority of these approaches use the seed region, which is approximately 6–8 nucleotides in length within the miRNA, as methods to target mRNA and bind at the 3′UTR of the target gene when searching for complementary strands (35). The present study used five algorithmic tools, Diana-MicroT, miRWalk, TargetScan, PicTar and miRDB, for miRNA target prediction analysis, which also considered seed-based methods, and miRDB analyses identified those genes affected by miRNAs, using a support vector machine trained on multiple microarray datasets (29). Despite the recent advances in miRNA target prediction databases (26–29), the results of miRNA-mRNA association analyses were varied depending on the database. A possible explanation is that each program has its methodological variances with respect to detecting miRNA-binding regions. To minimize the differences in miRNA target predictions, miRNAs that were identified by at least three prediction programs were selected for analysis.
Consistent with the results from the previous study, miR-335 has been demonstrated to be overexpressed in gastric cancer and involved in the oncogenic mTOR signaling pathway (36). In addition, miR-335 has been revealed as upregulated in a number of different types of cancer, including CRC (37–39). In numerous studies, the miRNAs miR-96 (40–45), miR-340 (46), miR-142 (47–49), miR-194 (50–52) and miR-98 (53–55), have demonstrated overexpression in a number of different types of cancer, including CRC.
Numerous studies have demonstrated downregulation of miRNAs in different types of cancer and their capacity to play a role in the mTOR signaling pathway (56–59). In the present study, the expression analysis was focused on the most highly downregulated miRNAs that were associated with upregulated mTOR, RPTOR and RICTOR gene expression in CRC cell lines. Among all five downregulated miRNAs investigated in the present study, miR-495, miR-1185, miR-654 and miR-496 have previously been reported in various types of cancer (56–59).
When regarding the role of miR-495 in cancer, several studies have revealed decreased miR-495 expression levels in a number of different types of cancer, such as CRC, prostate cancer, glioma, leukemia and lung cancer (58,60–63); in addition, miR-495 has been identified as a tumor suppressor in these cancer types. However, in a study conducted on breast cancer stem cells, miR-495 overexpression promoted oncogenesis (64).
Xu et al (65) demonstrated downregulation of miR-1185 in stage IV colorectal carcinoma. Tan et al (66) revealed that miR-654 acts as a tumor suppressor in breast cancer, by modulation of its target EPSTI1. Furthermore, another study indicated that miR-654 has tumor suppressor properties in papillary thyroid cancer (67). miR-495 was revealed to be downregulated in malignant cells and tissues of the breast (68), while its overexpression acts as a critical tumor suppressor in CRC cells, through targeting FAM83D (69).
Previous in vitro findings have identified an inverse correlation between miR-496 and miR-1185 expression and mTOR (1), miR-654 and miR-3183 expression and RPTOR (2), miR-495 expression and RICTOR (3) in human CRC cells. These downregulated miRNAs highlight the importance of miRNAs for use as potential tumor suppressors via targeting the mTOR signaling pathway. Further studies using 3′ luciferase reporters are needed to confirm the targets of these miRNAs in the mTOR pathway. Increasing miRNA expression levels using mimics to examine mTOR signaling and cancer progression is an important approach in CRC research. Furthermore, the role of these miRNAs requires confirmation by applying more functional in vitro and in vivo studies, such as miRNA inhibitor studies.
Table IV.
miRNA candidates targeting the RPTOR gene predicted by bioinformatics analysis.
| Database algorithm | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| miRNA ID | Proposed target gene | Ensembl gene ID | miRNA sequence | Diana | miRWalk | TargetScan | PicTar | miRDB | |
| hsa-miR-340-3p | RPTOR | ENSG00000141564 | UCCGUCUCAGUUACUUUAUAGC | Score | 0.79 | – | – | – | 63 |
| P-value | 0.03 | 0.008 | – | – | Rank 9 | ||||
| Binding | 9mer | 9mer | – | – | 9mer | ||||
| Region | UTR3 | UTR3 | – | – | UTR3 | ||||
| hsa-miR-548o-3p | RPTOR | ENSG00000141564 | CCAAAACUGCAGUUACUUUUGC | Score | 0.71 | – | – | – | – |
| P-value | 0.002 | 0.008 | – | – | – | ||||
| Binding | 9mer | 9mer | – | – | – | ||||
| Region | UTR3 | UTR3 | – | – | – | ||||
| hsa-miR-3121-3p | RPTOR | ENSG00000141564 | UAAAUAGAGUAGGCAAAGGACA | Score | 0.997 | – | – | – | 64 |
| P-value | 0.10 | – | – | – | Rank 6 | ||||
| Binding | 8mer | – | – | – | 7mer | ||||
| Region | UTR3 | – | – | – | UTR3 | ||||
| hsa-miR-4802-3p | RPTOR | ENSG00000141564 | UACAUGGAUGGAAACCUUCAAGC | Score | 0.83 | – | – | – | 66 |
| P-value | 0.04 | – | – | – | Rank 4 | ||||
| Binding | 8mer | – | – | – | 7mer | ||||
| Region | UTR3 | – | – | – | UTR3 | ||||
| hsa-miR-2114-3p | RPTOR | ENSG00000141564 | CGAGCCUCAAGCAAGGGACUU | Score | 0.73 | – | – | – | 67 |
| P-value | 0.02 | – | – | – | Rank 3 | ||||
| Binding | 8mer | – | – | – | 7mer | ||||
| Region | UTR3 | – | – | – | UTR3 | ||||
| hsa-miR-3183 | RPTOR | ENSG00000141564 | GCCUCUCUCGGAGUCGCUCGGA | Score | 0.71 | – | – | – | 77 |
| P-value | 0.003 | – | – | – | Rank 1 | ||||
| Binding | 7mer | – | – | – | 7mer | ||||
| Region | UTR3 | – | – | – | UTR3 | ||||
| hsa-miR-654-5p | RPTOR | ENSG00000141564 | UGGUGGGCCGCAGAACAUGUGC | Score | – | – | – | – | 59 |
| P-value | – | 0.008 | – | – | Rank 13 | ||||
| Binding | – | 9mer | – | – | 7mer | ||||
| Region | – | UTR3 | – | – | UTR3 | ||||
miRNA, microRNA; miR, microRNA; UTR3, 3 untranslated region; RPTOR, regulatory-associated protein of mTOR complex I.
Acknowledgements
Not applicable.
Funding
This present study was supported by a PhD scholarship awarded to Naif Alqurashi by the Imam Abdulrahman Bin Faisal University. Funding at Griffith University was provided to Ming Wei through the higher degree research office.
Availability of data and materials
The data and materials including within the present study are available from the corresponding author upon reasonable request.
Authors contributions
SMH conceptualized the design of the present study. NA curated the data, while SMH and AF analyzed the data. FA and MQW acquired funding for the present study. NA, SMH and FA performed the experiments. MQW and SI supervised and provided administrative support for the study. NA wrote the manuscript, and SMH, FA, SI, AF and MQW critically reviewed the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Zoncu R, Efeyan A, Sabatini DM. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 4.Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli AE. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–563. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 5.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitade Y, Akao Y. MicroRNAs and their therapeutic potential for human diseases: microRNAs, miR-143 and −145, function as anti-oncomirs and the application of chemically modified miR-143 as an anti-cancer drug. J Pharmacol Sci. 2010;114:276–280. doi: 10.1254/jphs.10R12FM. [DOI] [PubMed] [Google Scholar]
- 7.Krek A, Grün D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 9.Farazi TA, Spitzer JI, Morozov P, Tuschl T. miRNAs in human cancer. J Pathol. 2011;223:102–115. doi: 10.1002/path.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciafrè SA, Galardi S, Mangiola A, Ferracin M, Liu CG, Sabatino G, Negrini M, Maira G, Croce CM, Farace MG. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun. 2005;334:1351–1358. doi: 10.1016/j.bbrc.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 11.Kim YK, Yu J, Han TS, Park SY, Namkoong B, Kim DH, Hur K, Yoo MW, Lee HJ, Yang HK, Kim VN. Functional links between clustered microRNAs: Suppression of cell-cycle inhibitors by microRNA clusters in gastric cancer. Nucleic Acids Res. 2009;37:1672–1681. doi: 10.1093/nar/gkp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 13.Wu WK, Law PT, Lee CW, Cho CH, Fan D, Wu K, Yu J, Sung JJ. MicroRNA in colorectal cancer: From benchtop to bedside. Carcinogenesis. 2011;32:247–253. doi: 10.1093/carcin/bgq243. [DOI] [PubMed] [Google Scholar]
- 14.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 15.Michael MZ, SM OC, van Holst Pellekaan NG, Young GP, James RJ. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res. 2003;1:882–891. [PubMed] [Google Scholar]
- 16.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 17.Pradhan AK, Emdad L, Das SK, Sarkar D, Fisher PB. The enigma of miRNA regulation in cancer. Adv Cancer Res. 2017;135:25–52. doi: 10.1016/bs.acr.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Slaby O, Svoboda M, Fabian P, Smerdova T, Knoflickova D, Bednarikova M, Nenutil R, Vyzula R. Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology. 2007;72:397–402. doi: 10.1159/000113489. [DOI] [PubMed] [Google Scholar]
- 19.Guo C, Sah JF, Beard L, Willson JK, Markowitz SD, Guda K. The noncoding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signaling and is frequently lost in colon cancers. Genes Chromosomes Cancer. 2008;47:939–946. doi: 10.1002/gcc.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaedcke J, Grade M, Camps J, Søkilde R, Kaczkowski B, Schetter AJ, Difilippantonio MJ, Harris CC, Ghadimi BM, Møller S, et al. The rectal cancer microRNAome-microRNA expression in rectal cancer and matched normal mucosa. Clin Cancer Res. 2012;18:4919–4930. doi: 10.1158/1078-0432.CCR-12-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah MY, Ferrajoli A, Sood AK, Lopez-Berestein G, Calin GA. microRNA therapeutics in cancer-an emerging concept. EBioMedicine. 2016;12:34–42. doi: 10.1016/j.ebiom.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grady WM, Parkin RK, Mitchell PS, Lee JH, Kim YH, Tsuchiya KD, Washington MK, Paraskeva C, Willson JK, Kaz AM, et al. Epigenetic silencing of the intronic microRNA hsa-miR-342 and its host gene EVL in colorectal cancer. Oncogene. 2008;27:3880–3888. doi: 10.1038/onc.2008.10. [DOI] [PubMed] [Google Scholar]
- 23.Tazawa H, Tsuchiya N, Izumiya M, Nakagama H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci USA. 2007;104:15472–15477. doi: 10.1073/pnas.0707351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alqurashi N, Hashimi SM, Alowaidi F, Ivanovski S, Wei MQ. Dual mTOR/PI3K inhibitor NVP-BEZ235 arrests colorectal cancer cell growth and displays differential inhibition of 4E-BP1. Oncol Rep. 2018;40:1083–1092. doi: 10.3892/or.2018.6457. [DOI] [PubMed] [Google Scholar]
- 25.Alowaidi F, Hashimi SM, Alqurashi N, Alhulais R, Ivanovski S, Bellette B, Meedenyia A, Lam A, Wood S. Assessing stemness and proliferation properties of the newly established colon cancer ‘stem’ cell line, CSC480 and novel approaches to identify dormant cancer cells. Oncol Rep. 2018;39:2881–2891. doi: 10.3892/or.2018.6392. [DOI] [PubMed] [Google Scholar]
- 26.Kiriakidou M, Nelson PT, Kouranov A, Fitziev P, Bouyioukos C, Mourelatos Z, Hatzigeorgiou A. A combined computational-experimental approach predicts human microRNA targets. Genes Dev. 2004;18:1165–1178. doi: 10.1101/gad.1184704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dweep H, Sticht C, Pandey P, Gretz N. miRWalk-database: Prediction of possible miRNA binding sites by ‘walking’ the genes of three genomes. J Biomed Inform. 2011;44:839–847. doi: 10.1016/j.jbi.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, El Naqa IM. Prediction of both conserved and nonconserved microRNA targets in animals. Bioinformatics. 2008;24:325–332. doi: 10.1093/bioinformatics/btm595. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, Wang X, Larsson O, Selvaraj A, Liu Y, et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science. 2010;328:1172–1176. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melo SA, Esteller M. Dysregulation of microRNAs in cancer: Playing with fire. FEBS Lett. 2011;585:2087–2099. doi: 10.1016/j.febslet.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 34.AlQurashi N, Hashimi S, Wei M. Chemical inhibitors and microRNAs (miRNA) targeting the mammalian target of rapamycin (mTOR) pathway: Potential for novel anticancer therapeutics. Int J Mol Sci. 2013;14:3874–3900. doi: 10.3390/ijms14023874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oğul H. Computational prediction of microRNA function and activity. Methods Mol Biol. 2014;1107:243–256. doi: 10.1007/978-1-62703-748-8_15. [DOI] [PubMed] [Google Scholar]
- 36.Yan Z, Xiong Y, Xu W, Gao J, Cheng Y, Wang Z, Chen F, Zheng G. Identification of hsa-miR-335 as a prognostic signature in gastric cancer. PLoS One. 2012;7:e40037. doi: 10.1371/journal.pone.0040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng HH, Zhang YD, Gong LS, Liu WD, Zhang Y. Increased expression of microRNA-335 predicts a favorable prognosis in primary gallbladder carcinoma. Onco Targets Ther. 2013;6:1625–1630. doi: 10.2147/OTT.S53030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang YX, Zhang XY, Zhang BF, Yang CQ, Chen XM, Gao HJ. Initial study of microRNA expression profiles of colonic cancer without lymph node metastasis. J Dig Dis. 2010;11:50–54. doi: 10.1111/j.1751-2980.2009.00413.x. [DOI] [PubMed] [Google Scholar]
- 39.Vickers MM, Bar J, Gorn-Hondermann I, Yarom N, Daneshmand M, Hanson JE, Addison CL, Asmis TR, Jonker DJ, Maroun J, et al. Stage-dependent differential expression of microRNAs in colorectal cancer: Potential role as markers of metastatic disease. Clin Exp Metastasis. 2012;29:123–132. doi: 10.1007/s10585-011-9435-3. [DOI] [PubMed] [Google Scholar]
- 40.Sarver AL, French AJ, Borralho PM, Thayanithy V, Oberg AL, Silverstein KA, Morlan BW, Riska SM, Boardman LA, Cunningham JM, et al. Human colon cancer profiles show differential microRNA expression depending on mismatch repair status and are characteristic of undifferentiated proliferative states. BMC Cancer. 2009;9:401. doi: 10.1186/1471-2407-9-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bandrés E, Cubedo E, Agirre X, Malumbres R, Zárate R, Ramirez N, Abajo A, Navarro A, Moreno I, Monzó M, García-Foncillas J. Identification by Real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues. Mol Cancer. 2006;5:29. doi: 10.1186/1476-4598-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Kong X, Li J, Luo Q, Li X, Shen L, Chen L, Fang L. miR-96 promotes tumor proliferation and invasion by targeting RECK in breast cancer. Oncol Rep. 2014;31:1357–1363. doi: 10.3892/or.2013.2934. [DOI] [PubMed] [Google Scholar]
- 43.Pineau P, Volinia S, McJunkin K, Marchio A, Battiston C, Terris B, Mazzaferro V, Lowe SW, Croce CM, Dejean A. miR-221 overexpression contributes to liver tumorigenesis. Proc Natl Acad Sci USA. 2010;107:264–269. doi: 10.1073/pnas.0907904107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Navon R, Wang H, Steinfeld I, Tsalenko A, Ben-Dor A, Yakhini Z. Novel rank-based statistical methods reveal microRNAs with differential expression in multiple cancer types. PLoS One. 2009;4:e8003. doi: 10.1371/journal.pone.0008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, Zhu MJ, Ren AM, Wu HF, Han WM, Tan RY, Tu RQ. A ten-microRNA signature identified from a genome-wide microRNA expression profiling in human epithelial ovarian cancer. PLoS One. 2014;9:e96472. doi: 10.1371/journal.pone.0096472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao Y, Suo AL, Li ZF, Liu LY, Tian T, Ni L, Zhang WG, Nan KJ, Song TS, Huang C. MicroRNA profiling of human gastric cancer. Mol Med Rep. 2009;2:963–970. doi: 10.3892/mmr_00000199. [DOI] [PubMed] [Google Scholar]
- 47.Wang XY, Wu MH, Liu F, Li Y, Li N, Li GY, Shen SR. Differential miRNA expression and their target genes between NGX6-positive and negative colon cancer cells. Mol Cell Biochem. 2010;345:283–290. doi: 10.1007/s11010-010-0582-7. [DOI] [PubMed] [Google Scholar]
- 48.Chen WC, Lin MS, Ye YL, Gao HJ, Song ZY, Shen XY. microRNA expression pattern and its alteration following celecoxib intervention in human colorectal cancer. Exp Ther Med. 2012;3:1039–1048. doi: 10.3892/etm.2012.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang B, Li J, Sun M, Sun L, Zhang X. miRNA expression in breast cancer varies with lymph node metastasis and other clinicopathologic features. IUBMB Life. 2014;66:371–377. doi: 10.1002/iub.1273. [DOI] [PubMed] [Google Scholar]
- 50.Gu J, Wang Y, Wu X. MicroRNA in the pathogenesis and prognosis of esophageal cancer. Curr Pharm Des. 2013;19:1292–1300. doi: 10.2174/138161213804805775. [DOI] [PubMed] [Google Scholar]
- 51.Sundaram P, Hultine S, Smith LM, Dews M, Fox JL, Biyashev D, Schelter JM, Huang Q, Cleary MA, Volpert OV, Thomas-Tikhonenko A. p53-responsive miR-194 inhibits thrombospondin-1 and promotes angiogenesis in colon cancers. Cancer Res. 2011;71:7490–7501. doi: 10.1158/0008-5472.CAN-11-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J, Zhao CY, Zhang SH, Yu DH, Chen Y, Liu QH, Shi M, Ni CR, Zhu MH. Upregulation of miR-194 contributes to tumor growth and progression in pancreatic ductal adenocarcinoma. Oncol Rep. 2014;31:1157–1164. doi: 10.3892/or.2013.2960. [DOI] [PubMed] [Google Scholar]
- 53.Sampson VB, Rong NH, Han J, Yang Q, Aris V, Soteropoulos P, Petrelli NJ, Dunn SP, Krueger LJ. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res. 2007;67:9762–9770. doi: 10.1158/0008-5472.CAN-07-2462. [DOI] [PubMed] [Google Scholar]
- 54.Du L, Schageman JJ, Subauste MC, Saber B, Hammond SM, Prudkin L, Wistuba II, Ji L, Roth JA, Minna JD, Pertsemlidis A. miR-93, miR-98, and miR-197 regulate expression of tumor suppressor gene FUS1. Mol Cancer Res. 2009;7:1234–1243. doi: 10.1158/1541-7786.MCR-08-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deng ZQ, Yin JY, Tang Q, Liu FQ, Qian J, Lin J, Shao R, Zhang M, He L. Over-expression of miR-98 in FFPE tissues might serve as a valuable source for biomarker discovery in breast cancer patients. Int J Clin Exp Pathol. 2014;7:1166–1171. [PMC free article] [PubMed] [Google Scholar]
- 56.Nagaraja AK, Creighton CJ, Yu Z, Zhu H, Gunaratne PH, Reid JG, Olokpa E, Itamochi H, Ueno NT, Hawkins SM, et al. A link between mir-100 and FRAP1/mTOR in clear cell ovarian cancer. Mol Endocrinol. 2010;24:447–463. doi: 10.1210/me.2009-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duan Z, Choy E, Harmon D, Liu X, Susa M, Mankin H, Hornicek F. MicroRNA-199a-3p is downregulated in human osteosarcoma and regulates cell proliferation and migration. Mol Cancer Ther. 2011;10:1337–1345. doi: 10.1158/1535-7163.MCT-11-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen SM, Chen HC, Chen SJ, Huang CY, Chen PY, Wu TW, Feng LY, Tsai HC, Lui TN, Hsueh C, Wei KC. MicroRNA-495 inhibits proliferation of glioblastoma multiforme cells by downregulating cyclin-dependent kinase 6. World J Surg Oncol. 2013;11:87. doi: 10.1186/1477-7819-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fornari F, Milazzo M, Chieco P, Negrini M, Calin GA, Grazi GL, Pollutri D, Croce CM, Bolondi L, Gramantieri L. miR-199a-3p regulates mTOR and c-Met to influence the doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2010;70:5184–5193. doi: 10.1158/0008-5472.CAN-10-0145. [DOI] [PubMed] [Google Scholar]
- 60.Formosa A, Markert EK, Lena AM, Italiano D, Finazzi-Agro' E, Levine AJ, Bernardini S, Garabadgiu AV, Melino G, Candi E. MicroRNAs, miR-154, miR-299-5p, miR-376a, miR-376c, miR-377, miR-381, miR-487b, miR-485-3p, miR-495 and miR-654-3p, mapped to the 14q32.31 locus, regulate proliferation, apoptosis, migration and invasion in metastatic prostate cancer cells. Oncogene. 2014;33:5173–5182. doi: 10.1038/onc.2013.451. [DOI] [PubMed] [Google Scholar]
- 61.Jiang X, Huang H, Li Z, He C, Li Y, Chen P, Gurbuxani S, Arnovitz S, Hong GM, Price C, et al. miR-495 is a tumor-suppressor microRNA down-regulated in MLL-rearranged leukemia. Proc Natl Acad Sci USA. 2012;109:19397–19402. doi: 10.1073/pnas.1217519109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chu H, Chen X, Wang H, Du Y, Wang Y, Zang W, Li P, Li J, Chang J, Zhao G, Zhang G. miR-495 regulates proliferation and migration in NSCLC by targeting MTA3. Tumour Biol. 2014;35:3487–3494. doi: 10.1007/s13277-013-1460-1. [DOI] [PubMed] [Google Scholar]
- 63.Chuang AY, Chuang JC, Zhai Z, Wu F, Kwon JH. NOD2 expression is regulated by microRNAs in colonic epithelial HCT116 cells. Inflamm Bowel Dis. 2014;20:126–135. doi: 10.1097/01.MIB.0000436954.70596.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hwang-Verslues WW, Chang PH, Wei PC, Yang CY, Huang CK, Kuo WH, Shew JY, Chang KJ, Lee EY, Lee WH. miR-495 is upregulated by E12/E47 in breast cancer stem cells, and promotes oncogenesis and hypoxia resistance via downregulation of E-cadherin and REDD1. Oncogene. 2011;30:2463–2474. doi: 10.1038/onc.2010.618. [DOI] [PubMed] [Google Scholar]
- 65.Xu XH, Wu XB, Wu SB, Liu HB, Chen R, Li Y. Identification of miRNAs differentially expressed in clinical stages of human colorectal carcinoma-an investigation in Guangzhou, China. PLoS One. 2014;9:e94060. doi: 10.1371/journal.pone.0094060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan YY, Xu XY, Wang JF, Zhang CW, Zhang SC. miR-654-5p attenuates breast cancer progression by targeting EPSTI1. Am J Cancer Res. 2016;6:522–532. [PMC free article] [PubMed] [Google Scholar]
- 67.Geraldo MV, Nakaya HI, Kimura ET. Down-regulation of 14q32-encoded miRNAs and tumor suppressor role for miR-654-3p in papillary thyroid cancer. Oncotarget. 2017;8:9597–9607. doi: 10.18632/oncotarget.14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang L, Liu JL, Yu L, Liu XX, Wu HM, Lei FY, Wu S, Wang X. Downregulated miR-495 [Corrected] inhibits the G1-S phase transition by targeting Bmi-1 in breast cancer. Medicine (Baltimore) 2015;94:e718. doi: 10.1097/MD.0000000000000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yan L, Yao J, Qiu J. miRNA-495 suppresses proliferation and migration of colorectal cancer cells by targeting FAM83D. Biomed Pharmacother. 2017;96:974–981. doi: 10.1016/j.biopha.2017.11.138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials including within the present study are available from the corresponding author upon reasonable request.