Abstract
Purpose:
This study aimed to evaluate the effects of 17% ethylenediaminetetraacetic acid (EDTA), 7% maleic acid (MA), and 10% citric acid (CA) on the push-out bond strength of ProRooT MTA and Endosequence Root Repair Material (ERRM) putty.
Materials and methods:
Eighty single-rooted extracted human teeth were instrumented to obtain a standardized immature teeth model. Based on the chelating agents tested, the specimens were randomly divided into three experimental groups: Group 1 (17% EDTA), Group 2 (7% MA), Group 3 (10% CA), and Group 4 (Positive Control) (n=20 for each group). Each group was further classified into two subgroups: Group A (ProRoot MTA) and Group B (Endosequence Root Repair Material (ERRM) putty) (n=10 for each subgroup). After irrigation and placement of cements, teeth were stored at 37°C and in 100% humidity for a week. A total of 240 dentine discs (three discs per teeth) were obtained and subjected to push-out assay. Data was analyzed using two-way analysis of variance and Tukey’s post hoc t-test.
Results:
Both types of chelating agent and calcium silicate-based cement were significantly associated with the push-out bond strength values. The push-out bond strength was significantly less for CA as compared to EDTA or MA. ERRM had higher bond strength values than ProRoot MTA (p<0.05).
Conclusion:
The use of chelating agents increased the push-out bond strength of CSC. Regardless of tested chelating agents, ERRM had higher bond strength values than ProRoot MTA.
Keywords: Calcium silicate-based cement, chelating agent, Endosequence Root Repair Material, push-out bond strength
Introduction
Calcium silicate-based cements (CSC) have a wide variety of applications in endodontic therapy (1). CSC should exhibit high bond strength and display resistance to displacement forces that may occur owing to functional consequences or placement of restorative materials (2). Displacement, leakage, and micro fractures can occur in CSC because of these forces (3). Therefore, evaluating the effect of different variables that influence the bond strength of CSC to dentin is important for clinical success.
The smear layer is a non-homogenous structure composed of microorganisms, blood cells, residual pulp, odontoblast extensions and dentin chips (4). As the smear layer can penetrate up to 40 microns into the dentin tubules, the ability of intracanal medicaments and CSC to penetrate dentin is reduced, thereby adversely affecting the bond strength (5). Chelating agents are used to remove the smear layer in root canal therapies. However, these agents cause demineralization and structural changes in root canal dentin, thus affecting the bond strength of endodontic materials to root canal dentin (6).
Ethylenediaminetetraacetic acid (EDTA) is a frequently used chelating agent in removing smear layer and stimulates cellular differentiation and tissue formation, and increases the release of growth factors throughout the root canal (7). However, EDTA can cause weakness of the dentin structure in immature teeth of young patients, as it causes erosion in the dentin tubules (8). Because EDTA interferes with the hydration of MTA, it decreases microhardness, bond strength and biocompatibility of MTA (9). As alternatives to EDTA, citric acid (CA) and maleic acid (MA) can be used in endodotic procedures (10). Both agents cause wider opening in the dentin tubules (11), and increase the bond strength, resulting in an increased contact area between root canal dentin and endodontic cement (12). MA is suggested as an alternative to EDTA. It is highly acidic, less toxic than EDTA and has a greater ability to remove the smear layer than EDTA (10). As MA is a slightly organic acid, it is recommended that MA should be used in root canal irrigation at a concentration range of 5-15% (10). CA, another chelating agent, is used in different concentrations (1-50%) to remove the smear layer (13). CA, when used at a concentration of 1%, also presents a smear layer-removal effect similar to EDTA (14). Silveiro et al. (15) reported that 10% CA was effective in removing the smear layer because its pH was close to the neutral pH and was therefore more biocompatible.
Although mineral trioxide aggregate (MTA) is frequently used with numerous applications in endodontics, it has several disadvantages such as staining of the teeth, difficulty in clinical use, and long setting time (16). Because of these disadvantages, researchers are attempting to overcome the limitations of MTA. Recently, Endosequence Root Repair Material (ERRM), a bioceramic material was produced to overcome the disadvantages of MTA (17). It has similar uses like that of MTA and is available in mix-free, ready-to-use paste or injectable pat forms.
Exposure to irrigation solutions during chemomechanical irrigation changes the chemical and mechanical properties of the root canal dentin surface and so evaluating the effect of chelating agents on the bond strength of CSC should be investigated. Therefore, the purpose of this study was to examine the effects of 17% EDTA, 7% MA, and 10% CA on the push-out bond strength of ProRooT MTA and ERRM. The null hypotheses tested were as follows: (1) the chelating agent has no effect on the push-out bond strength of ProRoot MTA and ERRM; and (2) there is no difference between the push-out bond strength values of ProRoot MTA and ERRM.
Material and methods
Teeth Selection
Ethical approval was obtained from the Health Ethics Committee of the University of Cumhuriyet, Sivas, Turkey (ID: 2016-12/08). Based on the data from a pilot study, the values used in the power analysis were based on the following: α = 0.05, β = 0.10, 1- β = 0.90. It was decided to take a sample of 80 teeth.
The present study was conducted on 80 single-rooted human teeth freshly extracted due to periodontal problems. The teeth were immersed in NaOCI (Wizard, Ankara, Turkey) for 3 hours and root surfaces were cleaned using a curette. Teeth were stored in 0.1% thymol solution at 4°C under the laboratory procedures. Multidimensional preoperative radiographs were taken to confirm the root curvature as less than 20° and also to confirm the presence of a single, noncomplicated root canal.
Specimen Preparation
Each tooth was decoronated below the cementoenamel junction using diamond burs and the root lengths were standardized to 15±1 mm. Working length (wl) was determined by inserting a no. 10 K file (Dentsply Maillefer, Ballaigues, Switzerland) into each root canal until apically visible and then subtracting one mm from this point. Each root canal was instrumented with nickel titanium rotary Protaper Next files (Dentsply Maillefer, Ballaigues, Switzerland) up to size F5. During each file change, 1 mL of 2.5% NaOCl was applied with a side vented 27-gauge needle (Monoject, Tyco Healthcare, Mettawa, IL, USA) for 1 min. To provide an immature tooth model with a standard intracanal diameter, Peeso reamers (Mani Inc, Tochigi, Japan) from #1 to #6 were used sequentially. Finally, a #6 Peeso reamer protruded one mm beyond the apical foramen (3). Each root canal was irrigated with 5 ml of 5.25% NaOCl for 5 min. Finally, all roots were irrigated with 15 mL of bidistilled water. The root canals were then dried with paper cones (Dentsply, Maillefer, Switzerland).
The specimens were randomly divided into three experimental groups according to the chelating agents tested: Group 1 (17% EDTA (Wizard, Rehber Chemistry, Istanbul, Turkey)), Group 2 (7% MA (Merck Schuchardt, OHG, Hohenburn, Germany)), Group 3 (10% CA (Cumhuriyet University, Faculty of Medicine,Sivas, Turkey) and Group 4 (Positive Control) (n=20 for each group). Each group was further classified into two subgroups with regard to the type of CSC tested: Group A (ProRoot MTA) and Group B (ERRM) (n=10 for each group).
Irrigation Procedure and Placement of Cements
Each tooth was irrigated for 4 min and the total chelating agent volume delivered was 20 mL for each canal (18). Continuous irrigation was applied using a special irrigation device (VATEA, ReDent-Nova, Israel) that pumped the irrigants at the rate of 5 mL/min. Further, the chelating agents were removed by rinsing with 10 mL bidistilled water for 2 min. Approximately 4 mm of each type of cement tested (ProRoot MTA and ERRM) was placed in the coronal third of the root canals by using a MTA gun (MAP System, Dentsply Tulsa Dental, OK, USA) and compressed with hand plugs (Dentsply, Maillefer, Switzerland). ProRoot MTA was manually mixed using a metal spatula with a water to powder ratio of 0.33 following the manufacturer’s recommendations. ERRM is premixed by the manufacturer. Each type of cement was gently applied to the dentinal walls with a moistened cotton pellet. The teeth were wrapped with a wet gauze and stored at 37°C and in 100% humidity for a week (2).
Push- Out Test
The teeth were embedded in acrylic blocks prepared as apical thirds in acrylic. Parallel transverse sections were obtained with a water-cooled low-speed IsoMet diamond saw (Buehler, Lake Bluff, NY, USA) from the coronal to the apical direction (three slices per tooth) (3). A total of 240 dentin slices (approximately 1 mm-thickness) were obtained. The thickness of each slice was measured using digital calipers (Teknikel, Istanbul, Turkey) with an accuracy of 0.001 mm. The homogeneity of the groups in terms of slice thickness was confirmed through analysis of variance (ANOVA) (p>0.05).
A continuous load was applied to the center of the cements tested using a stainless steel cylindrical plunger of one mm in diameter, mounted onto a Lloyd LRX universal testing machine (Lloyd Instruments, Ltd., Fareham, UK). Loading was applied with a speed of 1 mm min−1 from the apical to coronal direction until dislodgement of the cement occurred. All three slices of each teeth were tested using the push-out test and the mean was taken. The push-out bond strength was calculated in megapascals (MPa) by dividing the maximum load at failure (N) by the area of surface adhesion using the formula (19), area = 2πr × h (where π = 3.14, a constant value, r = radius of intraradicular space, and h = slice thickness in mm) (20).
Evaluation of Failure Patterns
After the push-out test, the fracture surfaces of all specimens were examined with a stereomicroscope (Zeiss) under 25× magnification. Photographs of different fracture types of the specimens were obtained with a stereomicroscope-based photographic machine (Canon EOS 1000D, Canon, Inc., Tokyo, Japan). Each sample was classified into one of the following categories: (i) adhesive failure at cement/dentin interface; (ii) cohesive failure within cement, and (iii) mixed failure in both cement and dentin. One representative specimen for each group was examined for scanning electron microscopy (SEM) analysis. Each specimen was dehydrated in graded ethanol series 25%, 50%, 75%, 90% for 25 min and finally in 100% ethanol for 1 h. The specimens were critically point‑dried, mounted on aluminum stubs, sputter‑coated with gold/palladium and examined with a scanning electron microscope (SEM) (Leo 440 CCD, Leica, Bensheim, Germany).
Statistical Analysis
Data was processed by SPSS for Windows, Version 22.0 (SPSS Inc., Chicago, IL, USA). The mean and standard deviation values of the push-out bond strength were calculated for each group. The effects of the type of chelating agents and endodontic cements on push-out bond strength were analyzed through two-way ANOVA and multiple comparisons were performed by Tukey’s post- hoc test. A p-value less than 0.05 was considered statistically significant.
Results
Table 1 shows the mean values and standard deviations of the push-out bond strength (MPa). The use of chelating agents increased the push-out bond strength of endodontic cements. Both types of chelating agent and endodontic cement were significantly associated with the push-out bond strength values (p<0.05). Regardless of the endodontic cements used, the push-out bond strength was significantly less for CA as compared to EDTA or MA (p<0.05). There was no statistically significant difference between EDTA and MA (p>0.05). Regardless of the chelating agents tested, ERRM had higher bond strength values than ProRoot MTA (p<0.05).
Table 1.
Push-out bond strength values (MPa), and distribution of failure modes for each group
| Chelating Agents | ProRoot MTA | Endosequence RRM | Number of failure modes (A/C/M) |
|---|---|---|---|
| 17% EDTA | 4.71±0.84a | 5.17±0.90a,* | 11/34/15 |
| 7% Maleic Acid | 4.75±1.13a | 5.23±1.20a,* | 11/13/36 |
| 10% Citric Acid | 3.99±0.89b | 4.50±0.86b,* | 36/12/12 |
| Control (no agent) | 3.97±0.70b | 4.23±0.81b | 15 /19/ 26 |
Table 1 shows the distribution of the failure patterns. Adhesive failure was the failure pattern mostly observed in the CA group, whereas, cohesive and mixed failures were the failure pattern mostly observed in the EDTA and MA groups, respectively. Figure 1 shows the representative stereomicroscope and SEM images of failure modes: (a) adhesive failure, (b) cohesive failure, and (c) mixed failure.
Figure 1.
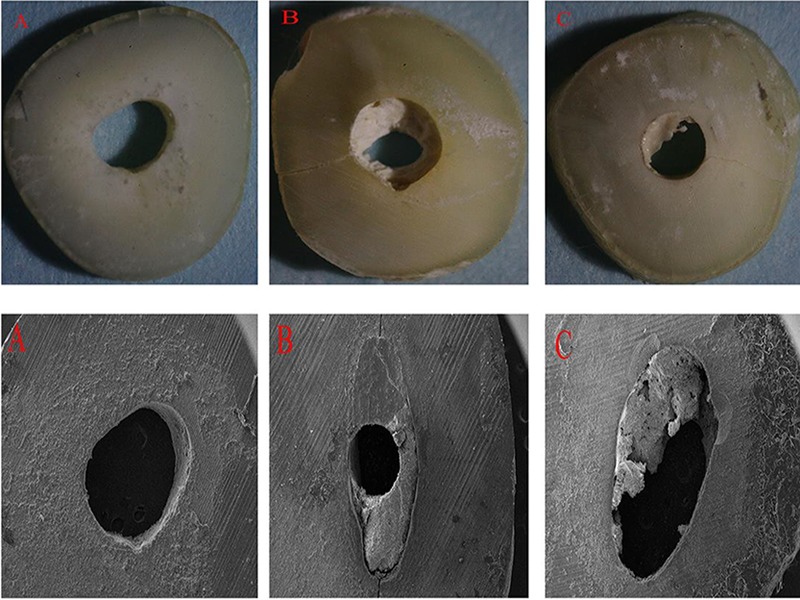
Representative stereomicroscope (25×) and scanning electron microscope (SEM) (54×) images of failure modes; (A) adhesive failure at cement/dentin interface; (B) cohesive failure within cement, and (C) mixed failure in both cement and dentin.
Discussion
Previous studies examined the effects of various variables on the bond strength of different CSC (2, 3). These studies reported that the success of endodontic treatments was due to the well-adapted coronal restoration as well as the resistance of the repair agents to displacement forces generated during the condensation of permanent restorative materials. CSC are desired to show dislocation resistance to mechanical forces such as occlusion or condensation of restorative materials (2). It has been reported that the physical properties of endodontic cements change after root canal irrigation (21). Also, the removal of the smear layer causes a closer contact between the cement and root canal dentin which is required for optimal adhesion, as a result this allows chemical bonding or micromechanical interlocking.
There are several studies on the effects of various variables, such as the different types of cement, intracanal medicaments (2, 3), placement techniques of cement, and irrigation regimens (21) on the bond strength of CSC. However, there has been limited research focus on the effect of chelating agents on the bond strength of CSC. Based on this information, the effects of 17% EDTA, 7% MA, and 10% CA on bond strength of ProRooT MTA and ERRM were examined in the present study. Both null hypotheses of the study were rejected because both EDTA and MA increased the bond strength values of endodontic cements as compared to CA. In addition, ERRM was found to have higher bond strength values than ProRooT MTA.
There are several methods to test the bond strength (22). In this study, push-out bond strength test was used. This is a commonly used test to measure the bond strength in the root canal (22). Goracci et al. reported that the push-out test better reflects the clinical conditions of the fracture pattern than microshear or microtensile methods, and is more reliable than other tests (22). Not only were there numerous failures in the preparation of the samples in the microtensile test, the observed data in such tests were distributed over a wide range. On the contrary, the method used in our study allows testing of regional differences and reduces premature failure rates as compared to other tests (19).
Irrigation of root canals with chelating agents such as EDTA, MA or CA is recommended to effectively remove the smear layer, (1, 10, 13, 15). However, MA has been shown to be more biocompatible than EDTA(23), with a better smear layer-removal ability in sclerotic root canals (10). MA at a concentration of 7% was used in this study since higher concentrations may cause damage to intertubular dentin as reported previously (24). CA at a recommended concentration of 10% was used in this study. The decalcifying action of 10% CA was found to be double or more than that of 1% CA (15).
The results of the present study can be attributed to various factors. The first is the region where the discs were obtained. In the present study, dentin discs were obtained from the coronal third of the root canal. This is consistent with the study by Ballal et al. (10), in which the authors reported that one minute application of 7% MA was more effective than 17% EDTA in removing the smear layer in the apical third of the root canal system, but not in the middle and coronal third. In addition, no significant difference was found to exist between MA and EDTA with respect to the degree of microhardness as reported by Ballal et al. (25). In contrast, Ulusoy and Gorgul reported that MA had a higher reduction in dentine microhardness as compared to EDTA (26). In our study, the push-out bond strength was found to be significantly less for CA than for EDTA and MA (p<0.05). This is consistent with a previous finding that CA was less effective than EDTA in removal of the smear layer (27).
Secondly, the results of the present study can also be attributed to the irrigation procedure employed in our study. There is no definite protocol of the type or concentration of chelating agents. However, different irrigation solutions have been shown to affect the adhesion of materials to dentine surfaces as a result of the effect on dentinal walls which includes alteration of surface energy or wetting ability of dentinal walls. Consistent with this study, Ballal et al. (10) have reported the decreased surface tension of 17% EDTA compared to 7% MA, which may be a possible explanation for the higher bond strength of tested CSC in our study. In addition, while EDTA has been shown to cause complete demineralization of the root canal dentine, MA and CA generate mineral gradients (10). One reason for the lower bond strength values of CA groups may be because the decalcifying capacity of CA is time-dependent. Lopez et al. (28) reported that the amount of Ca2 extracted in the CA and EDTA solutions increased with longer immersion time. Consistent with the findings of the present study, Ballal et al. reported that MA is highly acidic and has a better demineralizing effect (25).
In this study, irrespective of the chelating agents tested, ERRM was found to have higher bond strength values than ProRooT MTA (p <0.05). One of the reasons may be due to the physical and chemical properties of cements that were tested. The presence of zirconium oxide improved certain physical properties of bioceramics. The composition and particle size of existing cements affecte the interaction between cement and root canal dentin (21). ERRM has a smaller particle size than MTA. In addition, ERRM can form chemical bonds with root canal dentin walls, thus creating a robust connection. It was argued that the bioceramic cements when reacted with moisture, form hydroxyapatite that may chemically bond to the tooth structure (29). This may result in a 2% expansion because of the setting reaction, thus adapting better to the root canals. Because of crystal growth in dentin tubules, the effect of dentinal bridge formation can be strengthened, thereby increasing micromechanical involvement. Furthermore, the bond strength of ERRM may be higher than that of ProRooT MTA owing to the particle structure and hydrophilic properties of ERRM (30). Inconsistent with the present study, Shokohijenad et al. (31) reported that bond strength of MTA and ERRM paste was significantly lower in samples stored in conditions with an acidic pH; however, the push-out bond strength of the ERRM putty was not influenced by acidity. However, while the samples were kept in an acidic medium for 4 days in their study, the total contact with the root surface of the chelating agents tested was limited to five minutes in our study, consistent with the recommended clinical use.
One limitation of the present study was that it was an in vitro study. Thus, it was not possible to fully reflect the oral environment (occlusal stresses, blood-saliva contamination, etc.). Therefore, further in vivo studies are needed to investigate the actual bond strength of the tested materials.
Conclusion
Within the limitations, it may be concluded that the use of chelating agents increased the push-out bond strength of CSC. Both of EDTA and MA increased the bond strength of CSC when compared to CA. ERRM had higher bond strength values than ProRooT MTA.
Footnotes
Ethics committee approval:Ethical approval was obtained from the Health Ethics Committee of the University of Cumhuriyet, Sivas, Turkey (ID: 2016-12/08).
Informed consent:Informed consent was obtained from the participants.
Peer review: Externally peer-reviewed.
Author contributions:BB, FO and AK participated in designing the study. BB, FO and AK participated in generating the data for the study. BB, FO and AK participated in gathering the data for the study. BB participated in the analysis of the data. BB wrote the majority of the original draft of the paper. BB, FO and AK participated in writing the paper. All authors approved the final version of this paper.
Conflict of interest:The authors have no conflicts of interest to declare.
Financial disclosure:This research project received no financial support.
References
- 1.Kishen A, Peters OA, Zehnder M, Diogenes AR, Nair MK. Advances in endodontics: potential applications in clinical practice. J Conserv Dent. 2016. May-Jun;19(3):199–206. 10.4103/0972-0707.181925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagas E, Cehreli ZC, Uyanik MO, Vallittu PK, Lassila LV. Effect of several intracanal medicaments on the push-out bond strength of ProRoot MTA and Biodentine. Int Endod J. 2016. February;49(2):184–8. 10.1111/iej.12433 [DOI] [PubMed] [Google Scholar]
- 3.Topçuoğlu HS, Arslan H, Akçay M, Saygili G, Çakici F, Topçuoğlu G. The effect of medicaments used in endodontic regeneration technique on the dislocation resistance of mineral trioxide aggregate to root canal dentin. J Endod. 2014. December;40(12):2041–4. 10.1016/j.joen.2014.08.018 [DOI] [PubMed] [Google Scholar]
- 4.Şen BH, Wesselink PR, Türkün M. The smear layer: a phenomenon in root canal therapy. Int Endod J. 1995. May;28(3):141–8. 10.1111/j.1365-2591.1995.tb00289.x [DOI] [PubMed] [Google Scholar]
- 5.Torabinejad M, Handysides R, Khademi AA, Bakland LK. Clinical implications of the smear layer in endodontics: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002. December;94(6):658–66. 10.1067/moe.2002.128962 [DOI] [PubMed] [Google Scholar]
- 6.Serper A, Çalt S. The demineralizing effects of EDTA at different concentrations and pH. J Endod. 2002. July;28(7):501–2. 10.1097/00004770-200207000-00002 [DOI] [PubMed] [Google Scholar]
- 7.Galler KM, Widbiller M, Buchalla W, Eidt A, Hiller KA, Hoffer PC, et al. EDTA conditioning of dentine promotes adhesion, migration and differentiation of dental pulp stem cells. Int Endod J. 2016. June;49(6):581–90. 10.1111/iej.12492 [DOI] [PubMed] [Google Scholar]
- 8.Calt S, Serper A. Time-dependent effects of EDTA on dentin structures. J Endod. 2002. January;28(1):17–9. 10.1097/00004770-200201000-00004 [DOI] [PubMed] [Google Scholar]
- 9.Lee YL, Lin FH, Wang WH, Ritchie HH, Lan WH, Lin CP. Effects of EDTA on the hydration mechanism of mineral trioxide aggregate. J Dent Res. 2007. June;86(6):534–8. 10.1177/154405910708600609 [DOI] [PubMed] [Google Scholar]
- 10.Ballal NV, Kandian S, Mala K, Bhat KS, Acharya S. Comparison of the efficacy of maleic acid and ethylenediaminetetraacetic acid in smear layer removal from instrumented human root canal: a scanning electron microscopic study. J Endod. 2009. November;35(11):1573–6. 10.1016/j.joen.2009.07.021 [DOI] [PubMed] [Google Scholar]
- 11.Verdelis K, Eliades G, Oviir T, Margelos J. Effect of chelating agents on the molecular composition and extent of decalcification at cervical, middle and apical root dentin locations. Endod Dent Traumatol. 1999. August;15(4):164–70. 10.1111/j.1600-9657.1999.tb00795.x [DOI] [PubMed] [Google Scholar]
- 12.Elnaghy AM. Effect of QMix irrigant on bond strength of glass fibre posts to root dentine. Int Endod J. 2014. March;47(3):280–9. 10.1111/iej.12145 [DOI] [PubMed] [Google Scholar]
- 13.Hülsmann M, Heckendorff M, Lennon A. Chelating agents in root canal treatment: mode of action and indications for their use. Int Endod J. 2003. December;36(12):810–30. 10.1111/j.1365-2591.2003.00754.x [DOI] [PubMed] [Google Scholar]
- 14.Di Lenarda R, Cadenaro M, Sbaizero O. Effectiveness of 1 mol L-1 citric acid and 15% EDTA irrigation on smear layer removal. Int Endod J. 2000. January;33(1):46–52. 10.1046/j.1365-2591.2000.00273.x [DOI] [PubMed] [Google Scholar]
- 15.Machado-Silveiro LF, González-López S, González-Rodríguez MP. Decalcification of root canal dentine by citric acid, EDTA and sodium citrate. Int Endod J. 2004. June;37(6):365–9. 10.1111/j.1365-2591.2004.00813.x [DOI] [PubMed] [Google Scholar]
- 16.Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review—part II: leakage and biocompatibility investigations. J Endod. 2010. February;36(2):190–202. 10.1016/j.joen.2009.09.010 [DOI] [PubMed] [Google Scholar]
- 17.Alanezi AZ, Jiang J, Safavi KE, Spangberg LS, Zhu Q. Cytotoxicity evaluation of endosequence root repair material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010. March;109(3):e122–5. 10.1016/j.tripleo.2009.11.028 [DOI] [PubMed] [Google Scholar]
- 18.Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004. April;30(4):196–200. 10.1097/00004770-200404000-00003 [DOI] [PubMed] [Google Scholar]
- 19.Nagas E, Cehreli ZC, Durmaz V, Vallittu PK, Lassila LV. Regional push-out bond strength and coronal microleakage of Resilon after different light-curing methods. J Endod. 2007. December;33(12):1464–8. 10.1016/j.joen.2007.07.028 [DOI] [PubMed] [Google Scholar]
- 20.Saghiri MA, Garcia-Godoy F, Gutmann JL, Lotfi M, Asatourian A, Ahmadi H. Push-out bond strength of a nano-modified mineral trioxide aggregate. Dent Traumatol. 2013. August;29(4):323–7. 10.1111/j.1600-9657.2012.01176.x [DOI] [PubMed] [Google Scholar]
- 21.Guneser MB, Akbulut MB, Eldeniz AU. Effect of various endodontic irrigants on the push-out bond strength of biodentine and conventional root perforation repair materials. J Endod. 2013. March;39(3):380–4. 10.1016/j.joen.2012.11.033 [DOI] [PubMed] [Google Scholar]
- 22.Goracci C, Tavares AU, Fabianelli A, Monticelli F, Raffaelli O, Cardoso PC, et al. The adhesion between fiber posts and root canal walls: comparison between microtensile and push-out bond strength measurements. Eur J Oral Sci. 2004. August;112(4):353–61. 10.1111/j.1600-0722.2004.00146.x [DOI] [PubMed] [Google Scholar]
- 23.Ballal NV, Kundabala M, Bhat S, Rao N, Rao BS. A comparative in vitro evaluation of cytotoxic effects of EDTA and maleic acid: root canal irrigants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009. October;108(4):633–8. 10.1016/j.tripleo.2009.05.039 [DOI] [PubMed] [Google Scholar]
- 24.Prabhu S, Rahim N, Bhat K, Mathew J. Comparison of removal of endodontic smear layer using NaOCl, EDTA, and different concentrations of maleic acid–A SEM study. Endodontology. 2003;15(1):20–5. [Google Scholar]
- 25.Ballal NV, Mala K, Bhat KS. Evaluation of the effect of maleic acid and ethylenediaminetetraacetic acid on the microhardness and surface roughness of human root canal dentin. J Endod. 2010. August;36(8):1385–8. 10.1016/j.joen.2010.04.002 [DOI] [PubMed] [Google Scholar]
- 26.Ulusoy Öİ, Görgül G. Effects of different irrigation solutions on root dentine microhardness, smear layer removal and erosion. Aust Endod J. 2013. August;39(2):66–72. 10.1111/j.1747-4477.2010.00291.x [DOI] [PubMed] [Google Scholar]
- 27.Khedmat S, Shokouhinejad N. Comparison of the efficacy of three chelating agents in smear layer removal. J Endod. 2008. May;34(5):599–602. 10.1016/j.joen.2008.02.023 [DOI] [PubMed] [Google Scholar]
- 28.González-López S, Camejo-Aguilar D, Sanchez-Sanchez P, Bolaños-Carmona V. Effect of CHX on the decalcifying effect of 10% citric acid, 20% citric acid, or 17% EDTA. J Endod. 2006. August;32(8):781–4. 10.1016/j.joen.2006.02.006 [DOI] [PubMed] [Google Scholar]
- 29.Shokouhinejad N, Hoseini A, Gorjestani H, Shamshiri AR. The effect of different irrigation protocols for smear layer removal on bond strength of a new bioceramic sealer. Iran Endod J. 2013;8(1):10–3. [PMC free article] [PubMed] [Google Scholar]
- 30.Shokouhinejad N, Razmi H, Nekoofar MH, Sajadi S, Dummer PM, Khoshkhounejad M. Push-out bond strength of bioceramic materials in a synthetic tissue fluid. J Dent (Tehran). 2013. November;10(6):540–7. [PMC free article] [PubMed] [Google Scholar]
- 31.Shokouhinejad N, Yazdi KA, Nekoofar MH, Matmir S, Khoshkhounejad M. Effect of acidic environment on dislocation resistance of endosequence root repair material and mineral trioxide aggregate. J Dent (Tehran). 2014. March;11(2):161–6. [PMC free article] [PubMed] [Google Scholar]


