Abstract
Endovascular repair has become the standard of care for treatment of abdominal aortic aneurysms. The endografts and delivery systems for endovascular aneurysm repair have undergone multiple generations of technologic advancements. However, a significant remaining challenge for a satisfactory long-term outcome is to improve the performance of these devices in nonideal proximal sealing zones. In particular, short (<15 mm) and highly angulated (>60 degrees) necks can threaten long-term exclusion of the aneurysm even with the current generation of endografts. One of the main reasons for proximal infrarenal neck failure is the inability to accurately position the endograft precisely below both renal arteries. This is a report of the first-in-human implantations of the GORE EXCLUDER Conformable AAA Endoprosthesis (W. L. Gore & Associates, Flagstaff, Ariz), an investigational device, in anatomies with standard neck lengths and angulation. This device has been designed to provide repositionability, conformability, and, for the first time, optional angulation control. This device is commercially available in Europe.
Keywords: Gore, Excluder, Conformable, High neck angle
The endografts and delivery systems for endovascular repair of abdominal aortic aneurysms (AAAs) have undergone multiple generations of technologic advancements. One of the few remaining challenges that prevents ubiquitous use of this technology is an unfavorable infrarenal aortic neck anatomy.1, 2 In particular, short (<15 mm), highly angulated (>60 degrees) necks can threaten long-term exclusion of the aortic aneurysm, even with the current generation of endografts. However, with the integration of active control technology, namely, enhanced device positioning, conformability, and angulation control, the boundaries of treating an aneurysm with a “hostile” neck are continually being pushed.3
Most current AAA endografts were designed for use in a relatively straight, uniform proximal neck. In a highly angulated neck, complete wall apposition is often unattainable with many current devices simply because the device cannot be delivered to conform to this hostile region. Most current devices are approved for relatively low-angled necks (≤60 degrees), with only a small fraction of the devices Food and Drug Administration approved for an aortic neck angle up to 90 degrees.1 Highly angulated necks may translate to potentially high rates of type I endoleaks and secondary interventions.
Current management of these highly angulated necks includes additional ballooning, proximal cuffs, balloon-expandable stents, endoanchors, chimney or snorkels, and use of complex fenestrated or branched endografts. Whereas many of these techniques involve off-label use of devices, none of these techniques is optimal, and all have individual challenges.
The next generation of endovascular endografts to specifically address these issues should ideally be engineered with the capability of actively angulating and “conforming” the proximal end of the graft to the native aortic anatomy before final deployment. This provides the physician with the ability to align the leading edge of the graft perpendicular to the flow lumen, thereby taking advantage of every millimeter of the available proximal sealing zone. With accurate orthogonal placement, wall apposition is maximized and sealing zone extended to the level of the renal arteries bilaterally.
This report presents the first-in-human experiences of the new investigational device, GORE EXCLUDER Conformable AAA Endoprosthesis (caution—investigational device, limited by federal law to investigational use; W. L. Gore & Associates, Flagstaff, Ariz), which is currently undergoing an investigational clinical trial in the United States. This device is commercially available in Europe. The clinical study will assess the safety and effectiveness of the device in treating infrarenal AAA in patients with challenging proximal aortic anatomy. The clinical study consists of two substudies, each assessing the device for a different range of patient anatomies. This first implantation was part of the short neck substudy to assess the device in aortic neck angles ≤60 degrees and aortic neck lengths ≥10 mm. The high neck angulation substudy will evaluate proximal aortic neck angles >60 degrees and ≤90 degrees and aortic neck lengths ≥10 mm. This report describes selected case experiences from 5 of the first 10 patients, all from different study sites and selected to highlight variation in anatomies treated, implanted in the investigational device exemption study. The Institutional Review Board at each participating institution approved the study protocol and the informed consent form. All patients gave written informed consent to participate in the study.
Case report
Patient 1
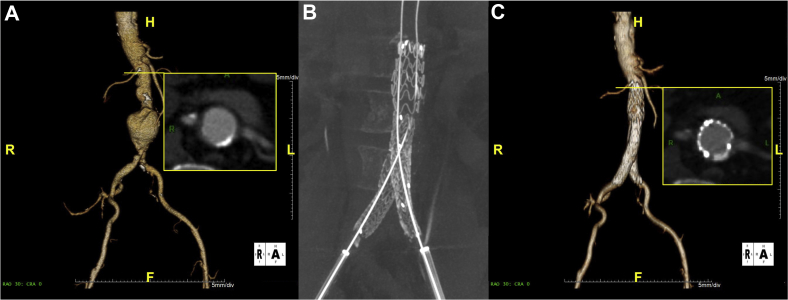
The first patient was implanted with the new device on December 19, 2017. This patient, a 78-year-old man, had an expanding AAA (>1 cm in 1 year) with a site-reported angle of 10 degrees and a site-reported neck length of 30 mm (Fig 1). The only area of concern was a small calcified region in the proximal neck. The procedure was performed per the instructions for use while repositioning the device twice during the case using the optional angulation feature. Procedure time was 68 minutes, with 46 mL of contrast material used and a total of 18 minutes of fluoroscopy time. Device apposition was achieved at the proximal seal zone, and no clinical or technical issues were encountered. Postprocedure aortography demonstrated absence of any endoleaks and appropriate endograft placement. The calcified region in the proximal neck was easily sealed with the new stent structure.
Fig 1.
Case images from selected patient 1. A, Preoperative three-dimensional image highlighting axial view of calcified region in the proximal aortic neck. B, Immediate postoperative noncontrast-enhanced cone beam computed tomography scan demonstrating stent patency. C, The 30-day follow-up image with axial view of calcified region in proximal aortic neck.
Patient 2
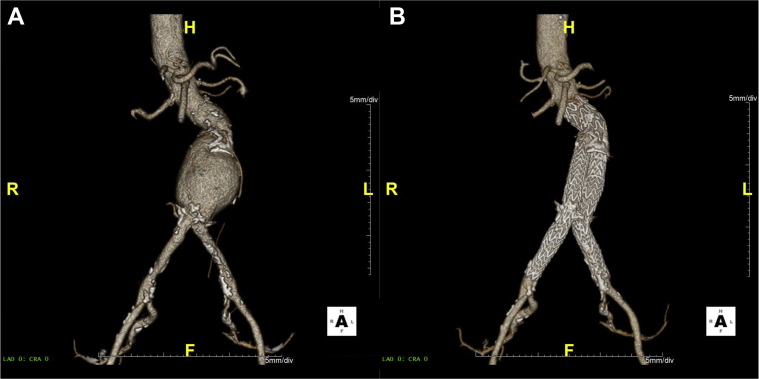
Selected patient 2, a 76-year-old woman, had a 60-mm AAA with a site-reported neck angle of 48 degrees and site-reported neck length of 34 mm (Fig 2). The primary area of interest was slightly offset renal arteries in the proximal neck. The device was repositioned, and the optional angulation feature was used to position the device orthogonal with the offset renal arteries. Device apposition and positioning were achieved with no clinical or technical issues. Procedure time was 55 minutes, with 95 mL of contrast material used and a total of 12 minutes of fluoroscopy time. Postprocedure aortography demonstrated absence of any endoleaks and appropriate device placement. The optional angulation control aided in placing the device orthogonal to the offset renal arteries.
Fig 2.
Case images from selected patient 2. Preoperative (A) and 30-day follow-up images (B) for selected patient 2.
Patient 3
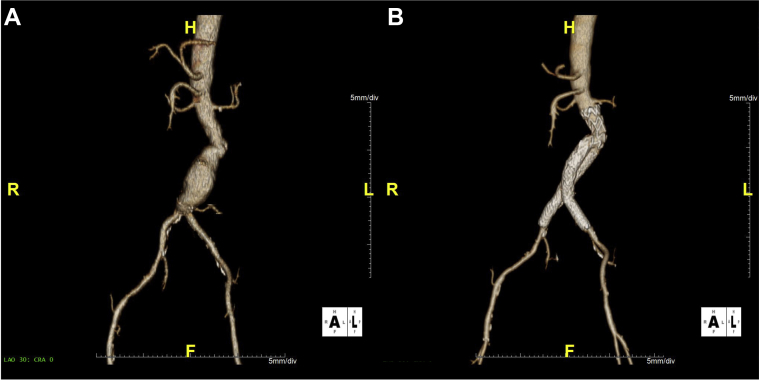
Selected patient 3, a 72-year-old man, had a 55-mm AAA with a site-reported angle of 60 degrees and a site-reported neck length of 10 mm (Fig 3). The device was deployed as intended, and postprocedure aortography demonstrated absence of any endoleaks. Procedure time was 100 minutes, with 106 mL of contrast material used and a total of 9 minutes of fluoroscopy time. The device was able to accommodate the distal aortic neck angle with no clinical or technical issues without use of the optional delivery system features.
Fig 3.
Case images from selected patient 3. Preoperative (A) and 30-day follow-up images (B) for selected patient 3.
Patient 4
Selected patient 4, a 71-year-old man, had a 61-mm AAA with a site-reported neck angle of 42 degrees and a site-reported neck length of 50 mm. The case presented with an uneventful AAA anatomy. The device was successfully deployed, and apposition was achieved without use of the optional delivery system features. Procedure time was 58 minutes, with 75 mL of contrast material used and a total of 14 minutes of fluoroscopy time. The limbs were successfully crossed with the new main body design.
Patient 5
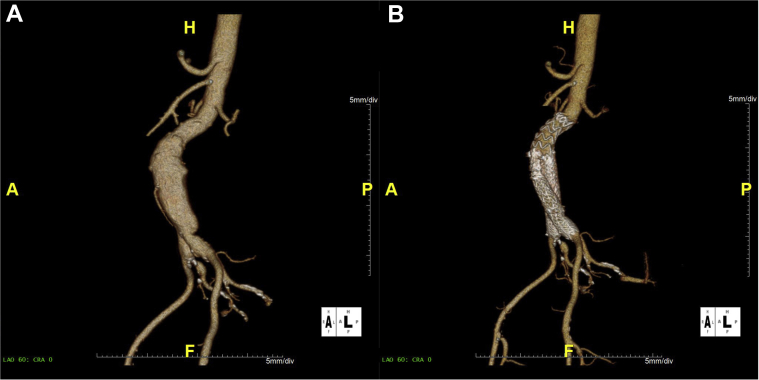
Selected patient 5, a 63-year-old man, had a 58-mm expanding AAA with a site-reported neck angle of 48 degrees and a site-reported neck length of 27 mm (Fig 4). The device was planned to be deployed distal to an accessory renal artery. The constraining system was used to reposition the device during the case without use of the optional angulation control. Procedure time was 96 minutes, with 91 mL of contrast material used and a total of 16 minutes of fluoroscopy time. The device was deployed as intended distal to the accessory renal artery, and postprocedure aortography demonstrated absence of any endoleaks, with no clinical or technical issues.
Fig 4.
Case images from selected patient 5. Preoperative (A) and 30-day follow-up images (B) for selected patient 5. The device was deployed distal to an accessory renal artery.
Conclusions
This is the first report of human implantation of the GORE EXCLUDER Conformable AAA Endoprosthesis. The reconstraining and optional angulation features performed as intended and allowed the operators a high degree of control during endovascular aneurysm repair in patients with standard neck length and angulation. The short neck and high neck angulation substudies in the investigational device exemption trial are under way to provide valuable clinical data regarding the safety and efficacy of the new investigational device in the short and long term.
Footnotes
This Food and Drug Administration-approved clinical investigational device exemption trial is funded by W. L. Gore.
ClinicalTrials.gov identifier: NCT02489539.
Author conflict of interest: R.R. is the national principle investigator for the study trial and a consultant for W. L. Gore. G.O. is a consultant for and receives grants from W. L. Gore.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Antoniou G.A., Georgiadis G.S., Antoniou S.A., Kuhan G., Murray D. A meta-analysis of outcomes of endovascular abdominal aortic aneurysm repair in patients with hostile and friendly neck anatomy. J Vasc Surg. 2013;57:527–538. doi: 10.1016/j.jvs.2012.09.050. [DOI] [PubMed] [Google Scholar]
- 2.Hager E.S., Cho J.S., Makaroun M.S., Park S.C., Chaer R., Marone L. Endografts with suprarenal fixation do not perform better than those with infrarenal fixation in the treatment of patients with short straight proximal aortic necks. J Vasc Surg. 2012;55:1242–1246. doi: 10.1016/j.jvs.2011.11.088. [DOI] [PubMed] [Google Scholar]
- 3.Verhoeven E.L., Katsargyris A., Bachoo P., Larzon T., Risher R., Ettles D., GREAT European C3 Module Investigators Real-world performance of the new C3 Gore Excluder stent-graft: 1-year results from the European C3 module of the Global Registry for Endovascular Aortic Treatment (GREAT) Eur J Vasc Endovasc Surg. 2014;48:131–137. doi: 10.1016/j.ejvs.2014.04.009. [DOI] [PubMed] [Google Scholar]