Abstract
MicroRNA-466 was recently characterized as a tumor suppressor with known biological function in prostate cancer. The aim of the current study was to investigate the possible involvement of microRNA-466 in hepatocellular carcinoma (HCC). The current study demonstrated that the expression level of microRNA-466 was significantly downregulated; while the mRNA expression level of Rho-associated coiled-coil containing protein kinase 2 (ROCK2) was significantly upregulated in tumor tissue compared with adjacent healthy tissue samples obtained from patients with HCC. In addition, the relative plasma level of microRNA-466 was significantly decreased, while the relative plasma level of ROCK2 was significantly increased in patients with HCC compared with healthy controls. Expression levels of microRNA-466 and ROCK2 were inversely correlated in tumor tissue but not in adjacent healthy tissue samples obtained from patients with HCC. Plasma levels of microRNA-466 and ROCK2 were inversely correlated in patients with HCC but not in healthy controls. In addition, reduced plasma levels of microRNA-466 may have a diagnostic value in the detection of early stage HCC. MicroRNA-466 overexpression significantly suppressed ROCK2 expression in HCC cells, whereas ROCK2 overexpression did not significantly affect microRNA-466 expression. MicroRNA-466 overexpression significantly suppressed, while ROCK2 overexpression significantly enhanced HCC cell migration and invasion. In addition, ROCK2 overexpression partially reversed the inhibitory effect of microRNA-466 overexpression on HCC cell migration and invasion. Taken together, these results suggest that microRNA-466 may inhibit HCC cell migration and invasion by indirectly mediating the downregulation of ROCK2.
Keywords: hepatocellular carcinoma, microRNA-466, Rho associated coiled-coil containing protein kinase 2, migration, invasion
Introduction
Liver cancer is one of the most frequently diagnosed types of cancer that causes unacceptably high mortality rates worldwide (1). Particularly in less developed countries, such as China, the high incidence rate of liver cancer is a heavy burden on public health (2). Although increased efforts have been made regarding diagnosis and treatment of liver cancer (3,4), the survival outcome of patients is still poor due to the high prevalence of tumor metastasis at the time of diagnosis, and surgical resection no longer being a treatment option for patients with metastasis (5). Unclear pathogenesis of liver cancer is one of the major causes of treatment failure in patients with liver cancer (6,7). Therefore, understanding the molecular mechanism underlying the development of liver cancer may benefit the treatment strategy for patients with liver cancer.
Rho associated coiled-coil containing protein kinase 2 (ROCK2) is a key regulator of cell polarity and actin cytoskeleton, and may play a pivotal role in cancer (8,9). Inhibition of ROCK2 has therapeutic effects on several types of cancer, including hepatocellular carcinoma (HCC) (9). Several studies have demonstrated that ROCK2 may serve as a potential therapeutic target for cancer treatment (9,10). It has been well established that microRNAs can regulate ROCK2 expression in human diseases, including cancer (11,12). MicroRNA-466 was recently characterized as a tumor suppressor in prostate cancer (13), however, its involvement in other types of cancer, including HCC remains unknown. The present study demonstrated that microRNA-466 may inhibit cancer cell migration and invasion in HCC by indirectly mediating the downregulation of ROCK2.
Materials and methods
Patient samples
The present study analyzed tumor tissue and adjacent healthy tissue biopsies, as well as plamsa samples obtained from 62 patients with HCC (male, n=33; female, n=29; age range, 32–68 years; mean age, 48.4±4.6 years). In addition, plasma samples were also obtained from 38 healthy volunteers (male, n=20; female, n= 18; age range, 31–67 years; mean age, 48.1±4.3 years). Patient information from each group is summarized in Table I. All samples were obtained from patients and healthy volunteers admited at The Fourth Hospital of Hebei Medical University between May 2015 and May 2018. Inclusion criteria were as follows: i) Patients diagnosed with HCC confirmed by pathologcal examination; and ii) newly diagnosed HCC. Exclusion criteria were as follows: i) Patients diagnosed with other diseases; and ii) patients who received treatment ≥3 months prior to the current study. The current study was approved by the Ethics Committee of The Fourth Hospital of Hebei Medical University (Shijiangzhuang, China) and all participants provided written informed consent.
Table I.
Basic information for each group of participants.
| Variable | Patients with hepatocellular carcinoma (n=62) | Healthy controls (n=38) |
|---|---|---|
| Sex (male/female) | 33/29 | 20/18 |
| Age range (years) | 32–68 | 31–67 |
| Mean age (years) | 48.4±4.6 | 48.1±4.3 |
| Clinical stage | ||
| I | 12 | N/A |
| II | 14 | N/A |
| III | 12 | N/A |
| IV | 24 | N/A |
N/A, not applicable.
Cell culture and transfection
Human HCC cell lines SNU-398 (ATCC® CRL-2233™) and SNU-182 (ATCC® CRL-2235™) were purchased from the American Type Culture Collection (ATCC). Cells were cultured in RPMI-1640 medium (ATCC) supplemented with heat-inactivated 10% fetal bovine serum (FBS; ATCC) and maintained at 37°C in 5% CO2-humidified incubator.
Cells were transfected with 15 nM ROCK2 expression pcDNA3 vectors or empty pcDNA3 vectors, purchased from GeneCopoeia, using Lipofectamine® 3000 reagent (Thermo Fisher Scientific, Inc.). Cells were transfected with 50 nM miRNA-466 mimics (5′-AUACACAUACACGCAACACACAU-3′) or negative control miRNA (5′-UUCUCCGAACGUGUCACGUdTdT-3′), purchased from Applied Biosystems (Thermo Fisher Scientific, Inc.), using Lipofectamine® 3000 reagent. Cells transfected with empty vector or control miRNA were used as the negative control, while untransfected cells were used as the control. Subsequent experiments were performed 24 h post-transfections.
Target site analysis
TargetScan bioinformatics analysis (www.targetscan.org) was used to predict potential targets of has-miRNA-466 on ROCK2 with default parameters (human species).
Reverse transcription-quantitative PCR (RT-q) PCR
Total RNA and miRNA was extracted from tissues as well as SNU-398 and SNU-182 cells using the MPure™ Total RNA Extraction kit (cat. no. 117022160; MP Biomedicals, LLC) and miRNeasy Mini kit (cat. no. 217004; Qiagen, Inc.), repectively. To detect the mRNA expression level of ROCK2, qPCR was subseqeuntly performed using SYBR Green Master mix (Bio-Rad Laboratories, Inc.). The following primer pairs were used for qPCR: ROCK2 forward, 5′-TCCCAACCAACTGTGAGGCATGT-3′ and reverse, 5′-TGTGGCACCTACGGCACTCT-3′; GAPDH forward, 5′-GCATCTTCTTTTGCGTCG-3′ and reverse, 5′-TGTAAACCATGTAGTTGAGGT-3′. To detect the expression level of miRNA-466, qPCR was performed using a TaqMan Real-Time PCR assay (Thermo Fisher Scientific, Inc.). The following primer pairs were used for qPCR: miRNA-466 forward, 5′-GTCGTATCCAGTGCAGGGTCC-3′ and reverse, 5′-TTGTAGTCACTAGGGCAC-3′; U6 small nuclear RNA (U6) forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′. qPCR reactions were performed on a 7500 Fast Real Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.) using the following thermocycling conditions: Initial denaturation at 95°C for 1 min; followed by 40 cycles of 95°C for 10 sec and 58.5°C for 35 sec. ROCK2 and miRNA-466 expression levels were quantified using the 2−ΔΔCq method (14) and normalized to GAPDH and U6, respectively.
Transwell migration and invasion assays
Following transfection with ROCK2 and/or microRNA-466, serum-free cell suspensions (3×104 cells/ml) were prepared and 0.1 ml cell suspension/well was added to the upper chamber (Transwell membranes were pre-coated with Matrigel prior to the invasion assay). Corning® HTS Transwell-96 well plate (pore size, 8.0 µm; Corning, Inc.) was used. The lower chamber was filled with RPMI-1640 culture medium containing 20% FBS. Following 24-h incubation at 37°C, the cells were stained with 0.5% crystal violet (Sigma-Aldrich; Merck KGaA) for 15 min at room temperature. Stained cells were counted under a light microscope (magnification, ×40; Olympus Corporation).
Western blot analysis
Total protein was extracted from SNU-398 and SNU-182 cells using a CelLytic™ MEM Protein Extraction kit (Sigma-Aldrich; Merck KGaA). A BCA kit (Sangon Biotech Co., Ltd.) was used to measure protein concentrations. Electrophoresis was performed using SDS-PAGE (10% gel) with 30 µg protein per lane. The proteins were transferred to a PVDF membrane followed by blocking in 5% non-fat milk for 2 h at room temperature. The membranes were incubated with primary antibodies against ROCK2 (1:1,500; cat. no. ABS436; EMD Millipore) and GAPDH (1:1,200; cat. no. ab9485; Abcam) for 12 h at 4°C prior to incubation with horseradish peroxidase-labeled secondary antibody (1:1,200; cat. no. MBS435036; MyBioSource, Inc.) for 2 h at room temperature. Protein bands were visualized using Pierce™ ECL Western Blotting Substrate (Pierce; Thermo Fisher Scientific, Inc.). Protein expression was quantified using ImageJ software (v1.46; National Institutes of Health).
Statistical analysis
Data were presented as the mean ± standard deviation from three independent experiments. All statistical analyses were perfromed using GraphPad Prism software (version 6.0; GraphPad Software, Inc.). Pearson correlation coefficient was used to examine the correlation between the expression of ROCK2 and miRNA-466. Student's t-test was used to analyze differences between two groups. Paired t-test was used to analyze differences between tumor tissue and adjacent healthy tissue samples. One-way analysis of variance followed by Tukey's post hoc test was used to analyze differences among multiple groups. Receiver operating characteristic analysis was used to evaluate the diagnostic value of serum miRNA-466. P<0.05 was considered to indicate a statistically significant difference.
Results
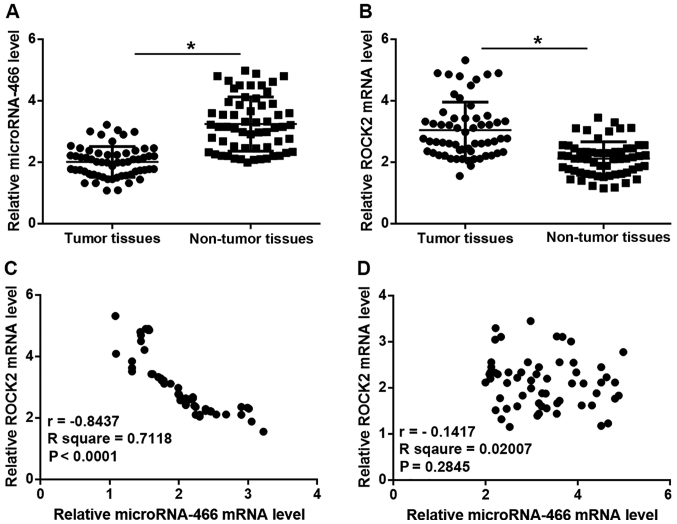
Expression levels of microRNA-466 and ROCK2 are inversely correlated in tumor tissues but not in adjacent healthy tissues in patients with HCC
The relative expression level of microRNA-466 was significantly reduced, while the relative mRNA expression level of ROCK2 was significantly increased in tumor tissue compared with adjacent healthy tissue samples in patients with HCC (P<0.05; Fig. 1A and B). In addition, Pearson correlation coefficient analyses demonstrated that the expression levels of microRNA-466 and ROCK2 were inversely correlated in tumor tissue samples (P<0.0001; Fig. 1C), however, there was no correlation observed between the expression levels of microRNA-466 and ROCK2 in adjacent healthy tissue samples (Fig. 1D).
Figure 1.
Expression levels of microRNA-466 and ROCK2 are inversely correlated in tumor tissues but not in adjacent healthy tissues in patients with HCC. (A) The relative expression level of miRNA-466 was determined by RT-qPCR in tumor tissue and adjacent healthy tissue samples from patients with HCC. (B) The relative mRNA expression level of ROCK2 was determined by RT-qPCR in tumor tissue and adjacent healthy tissue samples from patients with HCC. Pearson correlation coefficient was used to examine the correlation between the relative expression levels of microRNA-466 and ROCK2 in (C) tumor tissue and (D) adjacent healthy tissue samples. *P<0.05 as indicated. ROCK2, rho associated coiled-coil containing protein kinase 2; HCC, hepatocellular carcinoma; RT-qPCR, reverse transcription-quantitative PCR.
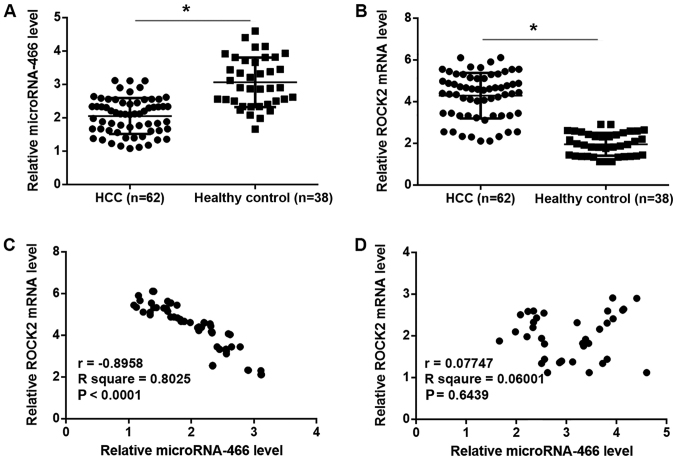
Plasma levels of microRNA-466 and ROCK2 are inversely correlated in patients with HCC but not in healthy controls
The relative expression level of microRNA-466 was significantly reduced, while the relative mRNA expression level of ROCK2 was significantly increased in plasma samples from patients with HCC compared with healthy controls (P<0.05; Fig. 2A and B). Pearson correlation coefficient analyses demonstrated that plasma levels of microRNA-466 and ROCK2 were inversely correlated in patients with HCC patients (P<0.0001; Fig. 2C), however, there was no correlation observed between the plasma expression levels of microRNA-466 and ROCK2 in the healthy control group (Fig. 2D).
Figure 2.
Plasma levels of microRNA-466 and ROCK2 are inversely correlated in patients with HCC but not in healthy controls. (A) The relative plasma expression level of miRNA-466 was determined by RT-qPCR in patients with HCC and healthy controls. (B) The relative plasma expression level of ROCK2 was determined by RT-qPCR in patients with HCC and healthy controls. Pearson correlation coefficient was used to examine the correlation between the relative expression levels of microRNA-466 and ROCK2 in (C) patients with HCC and (D) healthy controls. *P<0.05 as indicated. ROCK2, rho associated coiled-coil containing protein kinase 2; HCC, hepatocellular carcinoma; RT-qPCR, reverse transcription-quantitative PCR.
Diagnostic value of the reduced plasma expression level of microRNA-466 in the detection of early stage HCC
Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic value of serum microRNA-466 to distinguish between patients with early stage HCC (n=26) and healthy controls (n=38). The area under the curve was 0.9074 (95% confidence interval: 0.8381–0.9767) with a standard error of 0.03534 (P<0.0001; Fig. 3).
Figure 3.
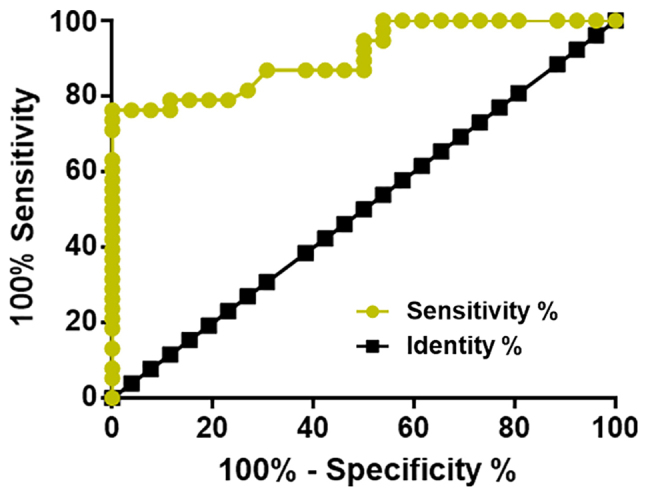
Reduced plasma expression level of microRNA-466 may have a diagnostic value in the detection of early stage HCC. ROC analysis was used to examine the diagnostic value of serum miRNA-466 to distinguish patients with early stage HCC from healthy controls.
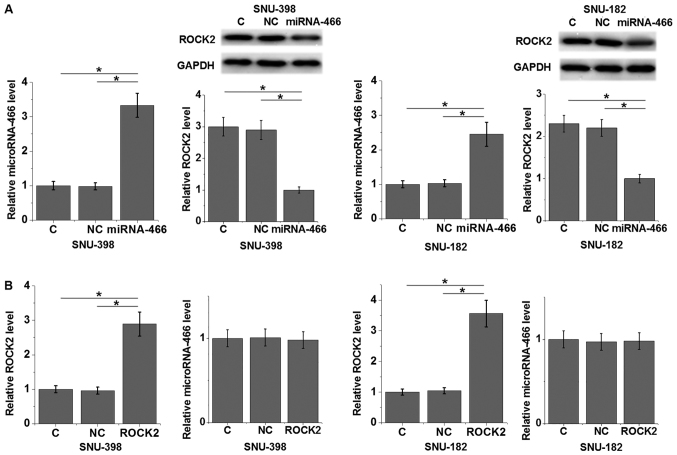
MicroRNA-466 overexpression suppresses ROCK2 expression in HCC cell lines
The relative expression level of microRNA-466 was significantly increased in both HCC cell lines following transfection with microRNA-466 mimic compared with control and negative control groups (P<0.05; Fig. 4A). In addition, transfection with microRNA-466 mimic significantly decreased the relative protein expression level of ROCK2 in SNU-398 and SNU-182 HCC cell lines compared with control and negative control groups (P<0.05; Fig. 4A). The relative mRNA expression level of ROCK2 was significantly increased in both HCC cell lines following transfection with ROCK2 expression vector compared with control and negative control groups (P<0.05; Fig. 4B). Furthermore, overexpression of ROCK2 did not significantly alter the expression level of microRNA-466 (Fig. 4B). Taken together, these results suggest that miRNA-466 may be an upstream inhibitor of ROCK2 in HCC. However, bioinformatics analysis did not predict a target site of microRNA-466 in ROCK2 (data not shown).
Figure 4.
MicroRNA-466 overexpression suppresses ROCK2 expression in HCC cell lines SNU-398 and SNU-182. (A) The relative expression levels of miRNA-466 and ROCK2 were determined by RT-qPCR and western blotting, respectively, in HCC cells following transfection with miRNA-466 mimic. (B) The relative expression levels of miRNA-466 and ROCK2 were determined by RT-qPCR and western blotting, respectively, in HCC cell lines following transfection with ROCK2 expression vector. *P<0.05 as indicated. HCC. ROCK2, rho associated coiled-coil containing protein kinase 2; HCC, hepatocellular carcinoma; RT-qPCR, reverse transcription-quantitative PCR.
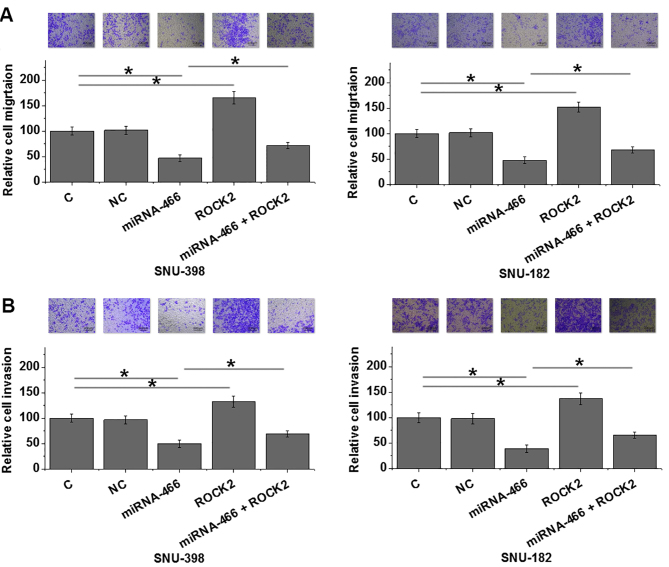
MicroRNA-466 overexpression inhibits HCC cell migration and invasion via ROCK2
MicroRNA-466 overexpression significantly suppressed HCC cell migration and invasion, while ROCK2 overexpression significantly enhanced HCC cell migration and invasion in both HCC cell lines SNU-398 and SNU-182 compared with control (P<0.05; Fig. 5A and B). Furthermore, ROCK2 overexpression partially reversed the inhibitory effect of microRNA-466 overexpression on HCC cell migration and invasion (Fig. 5A and B).
Figure 5.
MicroRNA-466 overexpression inhibits HCC cell migration and invasion via ROCK2. (A) HCC cell migration was examined in HCC cell lines, SNU-398 and SNU-18, following transfection with miRNA-466 mimic and/or ROCK2 expression vector. (B) HCC cell invasion was examined in HCC cell lines, SNU-398 and SNU-18, following transfection with miRNA-466 mimic and/or ROCK2 expression vector. *P<0.05 as indicated. HCC, hepatocellular carcinoma; ROCK2, rho associated coiled-coil containing protein kinase 2.
Discussion
MicroRNA-466 was recently characterized as a tumor suppressor with a known biological function in prostate cancer (13). The present study demonstrated that microRNA-466 may function as a tumor suppressor in HCC, the most common type of primary liver cancer, which accounts for 90% of all cases of primary liver cancer (1–4). The current study demonstrated that microRNA-466 may be involved in the regulation of HCC cancer cell migration and invasion by downregulating ROCK2 expression.
The development and progression of liver cancer is associated with changes in a large set of human genes, including miRNAs (15,16). Differential expression of specific microRNAs can predict the survival and treatment outcome of patients with liver cancer (15,16). The present study revealed that microRNA-466 was significantly downregulated in tumor tissue compared with adjacent healthy control tissue samples obtained from patients with HCC. In addition, plasma microRNA-466 was significantly downregulated in patients with HCC compared with healthy controls. These results suggest that downregulation of microRNA-466 may be involved in the pathogenesis of HCC.
The treatment outcome of patients with early stage HCC is generally satisfactory, while the outcome and survival of patients with late-stage HCC is poor due to the existence of tumor metastasis (17). Therefore, improved diagnosis and treatment strategy following early diagnosis is important for the treatment of HCC. In the current study, ROC curve analysis demonstrated that reduced plasma expression level of microRNA-466 may be used to effectively distinguish patients with HCC from healthy controls, indicating the potential application of plasma microRNA-466 to detect early stage HCC. However, multiple biomarkers should be used to improve overall diagnostic specificity due to the unknown expression pattern of microRNA-466 in other diseases.
ROCK2 serves an oncogenic role and is overexpressed in several types of human cancer, such as oral cancer and renal cancer (18,19). Therefore, inhibition of ROCK2 may serve as a potential therapeutic target for cancer treatment. The present study demonstrated that microRNA-466 suppressed ROCK2 expression in HCC cells, which suggests that miRNA-466 may be an upstream inhibitor of ROCK2 in HCC. However, the lack of reverse correlation between expression levels of microRNA-466 and ROCK2 in adjacent healthy tissue samples from patients with HCC patients, as well as in the plasma samples from healthy controls, suggests the inhibitory effect of microRNA-466 on ROCK2 expression may be achieved indirectly. Therefore, other pathological factors, which mediate the interaction between microRNA-466 and ROCK2, may exist in patients with HCC. For example, microRNA-466 may directly target specific genes involved in HCC, and those genes may interact with ROCK2. However, studies are required to further understand the interaction between microRNA-466 and ROCK2 in HCC.
The present study revealed that microRNA-466 may be involved in the regulation of HCC cell migration and invasion via ROCK2; however, microRNA-466 is not involved in HCC cell proliferation (data not shown). By contrast, a recent study demonstrated that microRNA-466 is involved in tumor growth in prostate cancer (13), which suggests that microRNA-466 may have different functions in different types of cancer. In addition, bioinformatics analysis did not identify a target binding site for microRNA-466 on ROCK2, indicating an indirect interaction between microRNA-466 and ROCK2. However, further studies are required to understand the underlying molecular mechanism of microRNA-466 in regulating cell migration and invasion in HCC.
In conclusion, the relative expression level of microRNA-466 was downregulated, while the relative expression level of ROCK2 was upregulated in HCC. The present findings suggest that microRNA-466 may be involved in the regulation of cancer cell migration and invasion in HCC by downregulating ROCK2 expression.
Acknowledgements
Not applicable.
Funding
The current study was supported by a grant from the Key Project of Medical Research in Hebei Province (grant no. 20150335).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
NA and FY designed experiments. NA, BL and LL performed experiments. ZL, HJ and GY assisted in the experiments and analyzed data. FY drafted the manuscript and all authors approved the final version to be published.
Ethics approval and consent to participate
The current study was approved by the Ethics Committee of The Fourth Hospital of Hebei Medical University (Shijiangzhuang, China) and all participants provided written informed consent.
Patient consent for publication
All patients provided informed consent for publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Kudo M. Surveillance, diagnosis, treatment, and outcome of liver cancer in Japan. Liver Cancer. 2015;4:39–50. doi: 10.1159/000367727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao YY, Chen H, Zhou YY, Wang LT, Hou Y, Xia XH, Ding Y. Intraorgan targeting of gold conjugates for precise liver cancer treatment. ACS Appl Mater Interfaces. 2017;9:31458–31468. doi: 10.1021/acsami.7b08969. [DOI] [PubMed] [Google Scholar]
- 5.Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: Worldwide incidence and trends. Gastroenterology 127 (5 Suppl 1) 2004:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57:69–76. doi: 10.1016/j.jhep.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vigil D, Kim TY, Plachco A, Garton AJ, Castaldo L, Pachter JA, Dong H, Chen X, Tokar B, Campbell SL, Der CJ. ROCK1 and ROCK2 are required for non-small cell lung cancer anchorage-independent growth and invasion. Cancer Res. 2012;72:5338–5347. doi: 10.1158/0008-5472.CAN-11-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M, Ke J, Wang Q, Qian H, Yang L, Zhang X, Xiao J, Ding H, Shan X, Liu Q, et al. Upregulation of ROCK2 in gastric cancer cell promotes tumor cell proliferation, metastasis and invasion. Clin Exp Med. 2017;17:519–529. doi: 10.1007/s10238-016-0444-z. [DOI] [PubMed] [Google Scholar]
- 9.Zheng F, Liao YJ, Cai MY, Liu YH, Liu TH, Chen SP, Bian XW, Guan XY, Lin MC, Zeng YX, et al. The putative tumour suppressor microRNA-124 modulates hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2J. Gut. 2012;61:278–289. doi: 10.1136/gut.2011.239145. [DOI] [PubMed] [Google Scholar]
- 10.Rath N, Olson MF. Rho-associated kinases in tumorigenesis: Re-considering ROCK inhibition for cancer therapy. EMBO Rep. 2012;13:900–908. doi: 10.1038/embor.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroiss A, Vincent S, Decaussin-Petrucci M, Meugnier E, Viallet J, Ruffion A, Chalmel F, Samarut J, Allioli N. Androgen-regulated microRNA-135a decreases prostate cancer cell migration and invasion through downregulating ROCK1 and ROCK2. Oncogene. 2015;34:2846–2855. doi: 10.1038/onc.2014.222. [DOI] [PubMed] [Google Scholar]
- 12.Peng F, Jiang J, Yu Y, Tian R, Guo X, Li X, Shen M, Xu M, Zhu F, Shi C, et al. Direct targeting of SUZ12/ROCK2 by miR-200b/c inhibits cholangiocarcinoma tumourigenesis and metastasis. Br J Cancer. 2013;109:3092–3104. doi: 10.1038/bjc.2013.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colden M, Dar AA, Saini S, Dahiya PV, Shahryari V, Yamamura S, Tanaka Y, Stein G, Dahiya R, Majid S. MicroRNA-466 inhibits tumor growth and bone metastasis in prostate cancer by direct regulation of osteogenic transcription factor RUNX2. Cell Death Dis. 2017;8:e2572. doi: 10.1038/cddis.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Ji J, Shi J, Budhu A, Yu Z, Forgues M, Roessler S, Ambs S, Chen Y, Meltzer PS, Croce CM, et al. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med. 2009;361:1437–1447. doi: 10.1056/NEJMoa0901282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang S, He X. The role of microRNAs in liver cancer progression. Br J Cancer. 2011;104:235–240. doi: 10.1038/sj.bjc.6606010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lepage C, Capocaccia R, Hackl M, Lemmens V, Molina E, Pierannunzio D, Sant M, Trama A, Faivre J, EUROCARE-5 Working Group Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999–2007: Results of EUROCARE-5. Eur J Cancer. 2015;51:2169–2178. doi: 10.1016/j.ejca.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 18.Dourado MR, de Oliveira CE, Sawazaki-Calone I, Sundquist E, Coletta RD, Salo T. Clinicopathologic significance of ROCK2 expression in oral squamous cell carcinomas. J Oral Pathol Med. 2018;47:121–127. doi: 10.1111/jop.12651. [DOI] [PubMed] [Google Scholar]
- 19.Xu Z, Hong Z, Ma M, Liu X, Chen L, Zheng C, Xi X, Shao J. Rock2 promotes RCC proliferation by decreasing SCARA5 expression through β-catenin/TCF4 signaling. Biochem Biophys Res Commun. 2016;480:586–593. doi: 10.1016/j.bbrc.2016.10.097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.