Abstract
Trisomy 16q is a rare disorder with severe abnormalities, which always leads to early postnatal mortality. It usually results from a parental translocation, exhibiting 16q duplication associated with another chromosomal deletion. The present study reports on the clinical presentation and molecular cytogenetic results of a small-for-gestational-age infant, consisting of partial trisomy 16q21→qter and monosomy 2p25.3→pter. The proband presented with moderately low birthweight, small anterior fontanelles, prominent forehead, low hairline, telecanthus, flat nasal bridge, choanal atresia, clinodactyly of the fifth fingers, urogenital anomalies, congenital muscular torticollis and congenital laryngomalacia. The last two traits have not previously been reported in any trisomy 16q and monosomy 2p cases. The proband was trisomic for the 16q21→qter chromosomal region with the karyotype 46,XY,der(2)t(2;16)(p25;q21)pat. The chromosomal anomaly was the result of unbalanced segregation of a paternal balanced translocation, 46,XY,t(2;16)(p25;q21). In this case, molecular cytogenetic analysis had a critical role in delineating the proband's clinical phenotype. Although this patient had a 16q21→qter duplication and a 2p25.3→pter deletion, the latter may have had mild phenotypic effects when associated with trisomy 16q. The literature was also reviewed, focusing on cases with the same breakpoints, localizations and clinical features reported in recent years.
Keywords: 16q trisomy, 2p monosomy, unbalanced segregation of a paternal balanced translocation, molecular cytogenetic diagnosis, case report
Introduction
Trisomy 16 is recognized as the most common type of trisomy in first-trimester spontaneous abortions, occurring in 1% of all clinically recognized pregnancies, while being rarer in the second and third trimesters (1–3). Early lethality and incompatibility with life have been described as its major outcomes (1). Trisomy 16 may be classified into three major types: Full trisomy, mosaics and partial trisomy of 16p or 16q. Since Schmickel (4) reported the first case of 16q trisomy as identified using a chromosome banding technique in 1975, >30 cases of partial trisomy 16q have been described. The common clinical features of trisomy 16q include low birthweight, hypotonia, failure to thrive, psychomotor retardation, periorbital edema, high prominent forehead, microcephaly, low-set ears, flat nasal bridge, small and down-slanting palpebral fissures, micrognathia, hypertelorism, long philtrum and posterior cleft palate (3). In the literature, few cases of 16q trisomy among liveborn infants have been reported. The unbalanced segregation of a parental balanced translocation frequently leads to partial trisomy 16, which is common in newborns and is associated with a wide range of clinical congenital abnormalities (5).
Pure terminal deletions of 2p are rare in a clinical context. To date, ~30 such patients have been reported, who share the following common clinical features: Early-onset obesity/overweight associated with intellectual disabilities and behavioral difficulties (6–8).
The present case study reports on a male newborn with multi-organ malformations exhibiting partial trisomy 16q21→qter and monosomy 2p25.3→pter. The proband exhibited the clinical manifestations of congenital muscular torticollis and congenital laryngomalacia, which have not been reported previously in either trisomy 16q or monosomy 2p. The clinical features of cases reported in the published literature were also described.
Case report
The proband was admitted to the neonatal department at The First Hospital of Jilin University (Changchun, Jilin) due to wet lung and small stature for his gestational age directly after birth in March 2017. The patient was born at 40 weeks 3 days of gestation, with a body weight of 2,350 g, body height of 46 cm and Apgar scores of 6 at 1 min and 8 at 5 min. The infant was the first child of the non-consanguineous and healthy couple: A 32-year-old father and a 28-year-old mother, who had taken a tocolytic agent due to suspected risk of spontaneous abortion in the first trimester. Physical examination indicated that the proband had small anterior fontanelles, prominent forehead, low hairline, telecanthus, flat nasal bridge, choanal atresia, clinodactyly of the fifth fingers, small penis and right cryptorchidism. Brain magnetic resonance imaging indicated no obvious brain abnormalities, but regular follow-up to dynamically observe the proband's neurological condition remained necessary. At the age of 2 days, abnormal findings from chest X-rays revealed pneumonia in the patient, with the ultrasonic cardiogram exhibiting patent ductus arteriosus. Given an intolerance for normal feeding of the patient, feeding was accomplished via a nasogastric tube. The patient also presented with poor psychomotor development. Considering that the subject failed a hearing screening test and was positive for cytomegalovirus pp65 antigen, ganciclovir therapy was performed. Subsequently, congenital muscular torticollis (at 19 days) and congenital laryngomalacia (at 24 days) were diagnosed. Finally, the proband developed stable vital signs and was discharged at the age of one month old. At 19 months after the proband's birth, a follow-up was performed, during which the presence of low-set ears, torticollis, hypotonia and an inability to walk were noted, and laryngeal wheezing during sleep was reported. The parents refused surgical treatments for the right cryptorchidism and laryngomalacia. The protocol of the present study was approved by the Ethics Committee of the First Hospital of Jilin University (Changchun, China) and written informed consent was obtained from the parents of the patient.
From the proband, 5 ml of peripheral blood was collected using a standard vacuum extraction blood-collecting system containing EDTA and heparin. Genomic DNA was isolated from whole blood using a QIAamp DNA Mini kit (Qiagen GmbH) in accordance with the manufacturer's protocol.
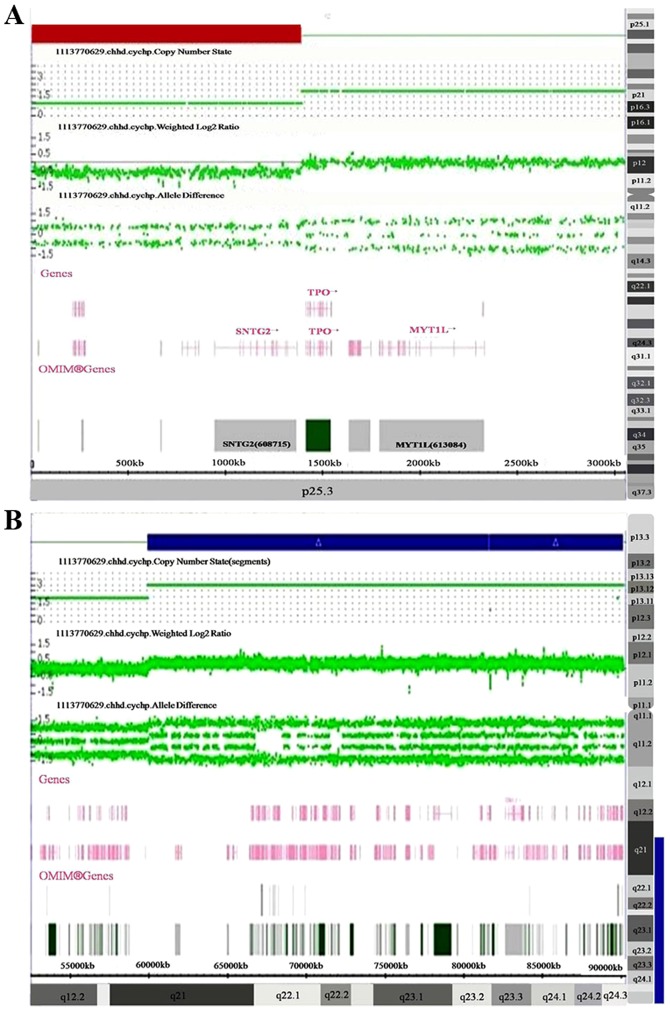
Chromosome microarray (CMA) was performed using Affymetrix CytoScan HD arrays, in accordance with the manufacturer's protocol. The procedure included genomic DNA extraction, digestion and ligation, PCR amplification, PCR product purification, quantification and fragmentation, labeling, array hybridization, washing and scanning. The array was designed specifically for cytogenetic research, including ~1,950,000 copy number variation markers and 750,000 single-nucleotide polymorphism markers. Data were analyzed using Affymetrix Chromosome Analysis Suite v3.3 Software. Thresholds for genome-wide screening were set at ≥100 kb for gains and ≥50 kb for losses. The detected copy number gains or losses were systematically evaluated for clinical significance by comparing them with values reported in the scientific literature and the following databases: i) Database of Genomic Variants (http://projects.tcag.ca/variation/), ii) DECIPHER (http://decipher.sanger.ac.uk/), iii) ISCA (https://www.iscaconsortium.org/), iv) ECARUCA (http://www.ecaruca.net), v) Online Mendelian Inheritance in Man (OMIM; http://www.ncbi.nlm.nih.gov/omim) and vi) Clinical Genome Resource (https://www.clinicalgenome.org/; (9). The CMA results for the proband revealed a 1.4 Mb deletion of 2p25.3 (12,770-1,393,107; Fig. 1A) and a 30.2 Mb duplication of 16q21q24.3 (59,939,853-90,155,062; Fig. 1B).
Figure 1.
The Affymetrix CytoScan HD arrays depicted (A) a1.4 Mb deletion in the short arm of chromosome 2 located at 2p25.3 to 2pter, presented in red, and (B) a 30.2 Mb duplication in the long arm of chromosome 16 located at 16q21 to 16qter, presented in blue.
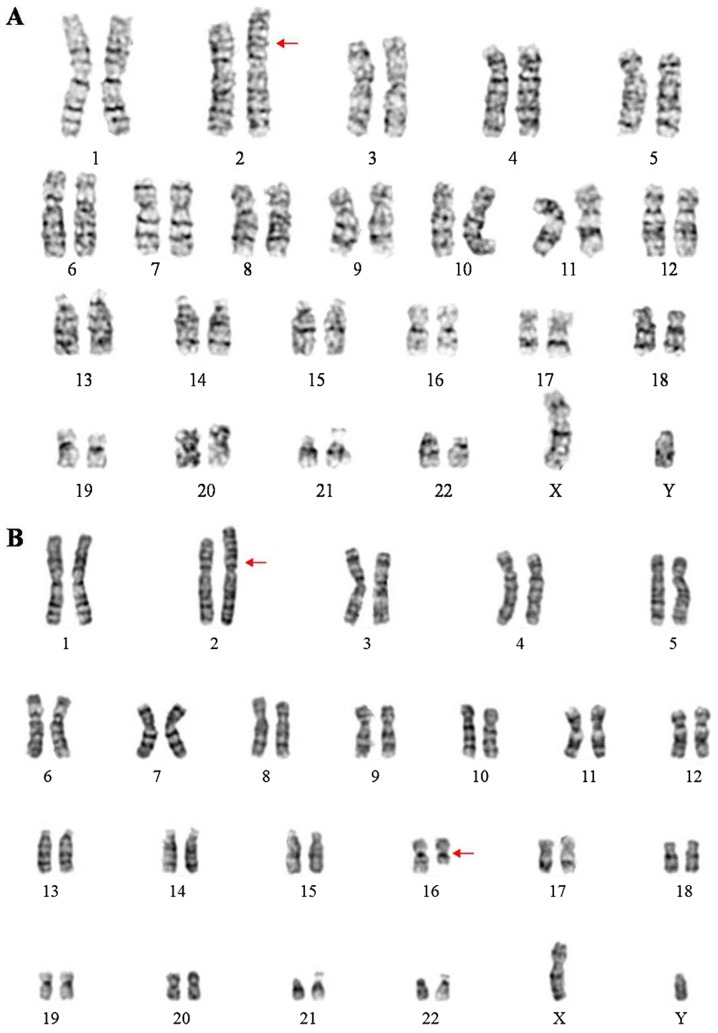
To further identify the chromosomal anomalies, blood from the proband and the proband's parents was obtained for karyotyping after obtaining written informed consent, in accordance with conventional G-banding techniques. The ISCN 2013 nomenclature was used to describe the karyotype (10). The proband's karyotype was 46,XY,der(2)t(2;16)(p25;q21)pat (Fig. 2A). The proband's father's karyotype was 46,XY,t(2;16)(p25;q21) (Fig. 2B) and the proband's mother's karyotype was 46,XX. Thus, the proband was trisomic for 16q21→qter due to unbalanced segregation of a paternally inherited balanced translocation. According to the follow-up, the patient remained alive and stable although he did not accept any further surgical treatments.
Figure 2.
Karyograms of the proband and the proband's father. (A) Karyogram of the proband with the karyotype 46,XY,der(2)t(2;16)(p25;q21)pat. (B) Karyogram of the proband's father with the karyotype 46,XY,t(2;16)(p25;q21).
Discussion
The present study reports on a male infant with an unbalanced der(2)t(2;16)(p25;q21) chromosome resulting in a 2p25.3 deletion and 16q24.3 duplication, presenting with psychomotor retardation, small anterior fontanelles, prominent forehead, low hairline, telecanthus, flat nasal bridge, choanal atresia, finger and genital deformities and multiple other malformations.
Trisomy 16q21→qter is a rare chromosomal abnormality among the different types of trisomy 16q. Brisset et al (1) established a series of phenotype-genotype correlations for trisomy16q: High/prominent forehead, bitemporal narrowing, periorbital edema in the neonatal period; severe mental retardation; vertebral, genital and anal abnormalities associated with 16q24; distal joint contractures and camptodactyly with 16q23; cleft palate and renal anomalies with 16q22; beaked nose and gall bladder agenesis with 16q21; gut malrotation, and lung and liver anomalies with 16q13; and behavioral abnormalities with band 16q11-q13. Barber et al (11) concluded that duplications of proximal 16q were not associated with dysmorphic features, but were linked to speech delay, learning difficulties and behavioral problems. Laus et al (5) suggested that the involved region ranging from 16q11 to 16q22 was necessary for the characteristic phenotypes of trisomy 16q.
To establish phenotype-karyotype correlations for trisomy16q21→qter, the present study mainly compared the clinical features of the present case with those from the literature on partial trisomy 16q21→qter cases, as reviewed in Table I (3,12–16). The rates of the different clinical characteristics are as follows: Psychomotor retardation (7/7), prominent forehead (6/7), hypotonia (6/7), low-set ears (6/7), respiratory distress (5/7), urogenital anomalies (5/7), flat nasal bridge (5/7), hypertelorism (4/7), weak sucking (4/7), clinodactyly of the fifth fingers (3/7) and small anterior fontanelles (2/7). To the best of our knowledge, the present study was the first to report on congenital muscular torticollis and congenital laryngomalacia in a case of partial trisomy 16q21→qter. It is generally accepted that unbalanced segregation of a parental balanced translocation usually leads to chromosome partial trisomy and monosomy (16). All cases of trisomy 16q21→qter in the reviewed literature were derived from a balanced translocation of parental origin, involving monosomy of the other chromosomes (2p25.3, 4q35.2, 9p24, 10q26.3, 15p13, 18p11.2 and 22p12) at the same time. In the present case, the proband's karyotype was 46,XY,der(2)t(2;16)(p25;q21)pat. The proband's mother had a normal karyotype, while the proband's father's karyotype was 46,XY,t(2;16)(p25;q21). The proband had thus clearly inherited a paternal unbalanced translocation between chromosomes 2 and 16. It is difficult to distinguish whether the clinical phenotypes are associated with trisomy 16q and monosomy 2p separately or are a consequence of the genome rearrangements. The major limitation of the present study is that published studies on partial trisomy 16q21→qter are limited, most of which attempt to establish the phenotype- karyotype correlation through the clinical manifestations. Part of the characteristics of the present case are different from those of cases reported in the previous literature, while partial phenotypes overlap with them, which enhances the current knowledge on the phenotype-karyotype correlation to a certain extent. Furthermore, as presented in Table I, poor postnatal survival has been reported, ranging from 18 days to 7 years. Although it is impossible to make an accurate prediction regarding the proband's survival, it is difficult to be optimistic about his longevity.
Table I.
Clinical features of patients with partial trisomy 16q21→qter.
| Phenotype/presentation | Balestrazzi et al, 1979 (12) | Garau et al, 1980 (13) | Lessick et al, 1989 (14) | Maher et al, 1991 (3) | De Carvalho et al, 2010 (15) | Mishra et al, 2018 (16) | Present case |
|---|---|---|---|---|---|---|---|
| Parental translocation | t(16;22) | t(16;18) | t(9;16) | t(10;16) | t(4;16) | t(15;16) | t(2;16) |
| (q21;p12) mat | (q21;p11.2) pat | (p24;q21)mat | (q26.3;q21)pat | (q35.2;q21)mat | (p13;q21) mat | (p25;q21)pat | |
| Sex/gestational age | M/40 w | F/at term | F/at term | M/32 w | F/41 w | M/33 w | M/40+3 d |
| Birth weight (g) | 2,600 | 2,470 | 2,325 | IGR(<3rd centile) | 2,400 | 1,230 | 2,350 |
| Birth head circumference (cm) | N.R. | N.R. | 32.5 | 29 | 35 | 28 | N.R. |
| Body length (cm) | N.R. | 40 | 46 | 42 | 48 | 42 | 46 |
| Psychomotor retardation | + | + | + | + | + | + | + |
| Hypotonia | + | + | + | N.R. | + | + | + |
| High/prominent forehead | + | + | + | + | + | N.R. | + |
| Small anterior fontanelles | + | + | N.R. | N.R. | + | N.R. | + |
| Small palpebral fissures | N.R. | N.R. | + | N.R. | + | + | N.R. |
| Epicanthus | + | + | + | N.R. | + | N.R. | N.R. |
| Hypertelorism | + | − | N.R. | N.R. | + | N.R. | + |
| Strabismus | + | + | + | N.R. | − | N.R. | N.R. |
| Broad flat nasal bridge | + | N.R. | − | + | + | N.R. | + |
| Long philtrum | + | + | N.R. | + | + | N.R. | N.R. |
| Thin upper lip | + | N.R. | + | N.R. | + | N.R. | N.R. |
| Micrognathia | + | N.R. | + | + | + | N.R. | N.R. |
| Dysplastic/low-set ears | + | N.R. | + | + | + | + | + |
| Abnormal palmar creases | + | + | + | + | + | N.R. | N.R. |
| Abnormalities in palate | + | N.R. | High palate | N.R. | High palate | Cleft palate | High palate |
| Disease | − | − | − | Congenital heart disease | Congenital heart disease | Congenital heart disease | Congenital muscular torticollis and congenital laryngomalacia |
| Urogenital anomalies | + | + | − | + | + | N.R. | + |
| Weaksucking | + | + | N.R. | N.R. | + | N.R. | + |
| Clinodactyly of the fifth fingers | N.R. | N.R. | + | N.R. | + | N.R. | + |
| Respiratory distress | + | + | + | N.R. | + | N.R. | + |
| Survival time | 3 y6 m | 22 d | 6 m | 18 d | 7 y | 10 m | 19 ma |
Proband is in a stable state and alive. M, male; F, female; +, feature present; -, feature absent; N.R., not reported; m, months; d, days; y, years.
The selection of plausible candidate genes exhibiting a ‘dose effect’ may partly explain the clinically observed phenotypes. The 2p25.3 region involved in the present case contains the genes family with sequence similarity 110 member C (FAM110C), SH3 and SYLF domain containing 1(SH3YL1), acid phosphatase 1 (ACP1), FAM150B, transmembrane protein 18 (TMEM18) and syntrophin gamma 2(SNTG2). Doco-Fenzy et al (7) reported on five patients with a 2p25 deletion presenting with early-onset obesity, hyperphagia, intellectual deficiency and behavioral difficulties. They speculated that the ACP1 and TMEM18 genes may be involved in early-onset obesity. Heterozygous loss of the SNTG2 gene has been reported to be associated with intellectual deficiency and behavioral difficulties. Zou et al (17) reported on a 7-year-old female patient with a de novo unbalanced der(2)t(2;16)(p25.3;q24.3) chromosome resulting in 2p25.3 deletion and 16q24.3 duplication. However, the distinct trisomy 16q causes various clinical manifestations, which explains that monosomy 2p may have mild effects when associated with trisomy 16q. However, the haploinsufficiency of genes in the region of 2p25.3 has yet to be evaluated.
The genes in the region of 16q21-16q24.3 and the associated diseases are summarized in Table II. The region contains >250 genes including 61 morbidity-associated genes. The gene chromatin licensing and DNA replication factor 1(CDT1), located at 16q24.3, which is required for DNA replication at multiple stages of development and mitosis, is expressed only in the G1 and S phases (18). The homozygous or compound heterozygous mutation of the gene CDT1 has been indicated to be associated with Meier-Gorlin syndrome 4. Patients with this syndrome usually have short stature, distinctive facial features, low-set/rotated ears, micrognathia, full lips, a narrow nose with a high nasal bridge, patellar aplasia/hypoplasia and abnormalities in sexual development. Small testes and cryptorchidism are also involved in this condition, along with difficulty in feeding and breathing problems (19). Ankyrin repeat domain 11(ANKRD11), located in 16q24.3, is a member of a family of ankyrin repeat-containing cofactors that interacts with p160 nuclear receptor coactivators and inhibits ligand-dependent transcriptional activation (20). Heterozygous mutation in the ANKRD11 gene has been reported to lead to KBG syndrome, characterized by short stature, global developmental delay, intellectual disability, distinctive craniofacial features, seizures, hypertelorism and skeletal abnormality (brachydactyly or clinodactyly) (21,22). However, the triplosensitivity of these genes caused by partial trisomy 16q has not been clearly described.
Table II.
Genes in the region of 16q21-16q24.3 and the associated diseases.
| Gene | Location | OMIM | Description | Disease |
|---|---|---|---|---|
| BEAN1 | 16q21 | 612051 | Brain expressed associated with NEDD4 1 | Spinocerebellar ataxia 31 |
| TK2 | 16q21 | 188250 | Thymidine kinase 2 | Mitochondrial DNA depletion syndrome 2 (myopathic type); progressive external ophthalmoplegia with mitochondrial DNA deletions, autosomal recessive 3 |
| CBFB | 16q22.1 | 121360 | Core-binding factor subunit beta | Leukemia, Acute Myeloid; AML |
| HSF4 | 16q22.1 | 602438 | Heat shock transcription factor 4 | Cataract 5, multiple types |
| NOL3 | 16q22.1 | 605235 | Nucleolar protein 3 | Myoclonus, familial cortical |
| HSD11B2 | 16q22.1 | 614232 | Hydroxysteroid 11-beta dehydrogenase 2 | Apparent mineralocorticoid excess |
| CTCF | 16q22.1 | 604167 | CCCTC-binding factor | Mental retardation, autosomal dominant 21 |
| ACD | 16q22.1 | 609377 | ACD, shelterin complex subunit and telomerase recruitment factor | Dyskeratosis Congenita, Autosomal Dominant 6; DKCA6 |
| LCAT | 16q22.1 | 606967 | Lecithin-cholesterol acyltransferase | Fish-eye disease; Norum disease |
| AGRP | 16q22.1 | 602311 | Agouti-related neuropeptide | Obesity |
| CDH3 | 16q22.1 | 114021 | Cadherin 3 | Ectodermal dysplasia, ectrodactyly and macular dystrophy; hypotrichosis, congenital, with juvenile macular dystrophy |
| PRMT7 | 16q22.1 | 610087 | Proteinargininemethyltransferase 7 | Short stature, brachydactyly, intellectual developmental disability and seizures |
| CDH1 | 16q22.1 | 192090 | Cadherin 1 | Breast cancer; Blepharocheilodontic syndrome 1; Gastric Cancer, Hereditary Diffuse; HDGC; Ovarian Cancer; Prostate cancer; Endometrial Cancer |
| COG8 | 16q22.1 | 606979 | Component of oligomericgolgi complex 8 | Congenital disorder of glycosylation, type IIh |
| COG4 | 16q22.1 | 606976 | Component of oligomericgolgi complex 4 | Congenital disorder of glycosylation, type IIj |
| AARS | 16q22.1 | 601065 | Alanyl-tRNAsynthetase | Charcot-Marie-tooth disease, axonal, type 2N; epileptic encephalopathy, early infantile, 29 |
| NQO1 | 16q22.1 | 125860 | NAD(P)H quinone dehydrogenase 1 | Nad(P)H Dehydrogenase, Quinone 1; NQO1 |
| VAC14 | 16q22.1-q22.2 | 604632 | Vac14, PIKFYVE complex component | Striatonigral degeneration, childhood-onset |
| HYDIN | 16q22.2 | 610812 | HYDIN, axonemal central pair apparatus protein | Ciliary dyskinesia, primary, 5 |
| DHODH | 16q22.2 | 126064 | Dihydroorotate dehydrogenase (quinone) | Miller syndrome |
| TAT | 16q22.2 | 613018 | Tyrosine aminotransferase | Tyrosinemia, type II |
| HP | 16q22.2 | 140100 | Haptoglobin | Anhaptoglobinemia |
| ZFHX3 | 16q22.2-q22.3 | 104155 | Zinc finger homeobox 3 | Prostate cancer |
| RFWD3 | 16q23.1 | 614151 | Ring finger and WD repeat domain 3 | Fanconi anemia; complementationgroup W |
| FA2H | 16q23.1 | 611026 | Fatty acid 2-hydroxylase | Spastic paraplegia 35; autosomal recessive |
| CHST6 | 16q23.1 | 605294 | Carbohydrate sulfotransferase 6 | Macular corneal dystrophy |
| TMEM231 | 16q23.1 | 614949 | Transmembrane protein 231 | Joubert syndrome 20; Meckel syndrome 11 |
| KARS | 16q23.1 | 601421 | Lysyl-tRNAsynthetase | Charcot-Marie-Tooth disease; recessive intermediate; B; deafness, autosomal recessive 89 |
| ADAMTS18 | 16q23.1 | 607512 | ADAM metallopeptidase with thrombospondin type 1 motif 18 | Microcornea; myopic chorioretinal atrophy; telecanthus |
| WWOX | 16q23.1-q23.2 | 605131 | WW domain containing oxidoreductase | Esophageal cancer; spinocerebellar ataxia, autosomal recessive 12; epileptic encephalopathy, early infantile, 28 |
| MAF | 16q23.2 | 177075 | MAF bZIP transcription factor types | Ayme-Gripp syndrome; cataract 21, multiple |
| GCSH | 16q23.2 | 238330 | Glycine cleavage system protein H | Glycine encephalopathy |
| BCMO1 | 16q23.2 | 605748 | Beta-carotene oxygenase 1 | Hypercarotenemia and vitamin A deficiency, autosomal dominant |
| GAN | 16q23.2 | 605379 | Gigaxonin | Giant axonal neuropathy-1 |
| PLCG2 | 16q24.1 | 600220 | Phospholipase C gamma 2 | Familial cold autoinflammatory syndrome 3; autoinflammation, antibody deficiency and immune dysregulation syndrome |
| MLYCD | 16q23.3 | 606761 | Malonyl-CoA decarboxylase | Malonyl-CoA decarboxylase deficiency |
| SLC38A8 | 16q23.3 | 615585 | Solute carrier family 38 member 8 | Foveal hypoplasia 2, with or without optic nerve misrouting and/or anterior segment dysgenesis |
| DNAAF1 | 16q24.1 | 613190 | Dynein axonemal assembly factor 1 | Ciliary dyskinesia, primary, 13 |
| RF8 | 16q24.1 | 601565 | Interferon regulatory factor 8 | Immunodeficiency 32A, mycobacteriosis, autosomal dominant; immunodeficiency 32B, monocyte and dendritic cell deficiency, autosomal recessive |
| FOXF1 | 16q24.1 | 601089 | Forkhead box F1 | Alveolar capillary dysplasia with misalignment of pulmonary veins |
| FOXC2 | 16q24.1 | 602402 | Forkhead box C2 | Lymphedema-distichiasis syndrome |
| FBXO31 | 16q24.2 | 609102 | F-box protein 31 | Mental retardation, autosomal recessive 45 |
| JPH3 | 16q24.2 | 605268 | Junctophilin 3 | Huntington disease-like 2 |
| CA5A | 16q24.2 | 114761 | Carbonic anhydrase 5A | Hyperammonemia due to carbonicanhydrase VA deficiency |
| ZNF469 | 16q24.2 | 612078 | Zinc finger protein 469 | Brittle cornea syndrome 1 |
| CDT1 | 16q24.3 | 605525 | Chromatin licensing and DNA replication factor 1 | Meier-Gorlin syndrome 4 |
| CYBA | 16q24.2 | 608508 | Cytochrome b-245 alpha chain | Chronic granulomatous disease, autosomal, due to deficiency of CYBA |
| MVD | 16q24.2 | 603236 | Mevalonatediphosphate decarboxylase | Porokeratosis 7, multiple types |
| GALNS | 16q24.3 | 612222 | Galactosamine (N-acetyl)-6-sulfatase | Mucopolysaccharidosis IVA |
| ACSF3 | 16q24.3 | 614245 | Acyl-CoA synthetase family member 3 | Combined malonic and methylmalonicaciduria |
| CDH15 | 16q24.3 | 114019 | Cadherin 15 | Mental retardation, autosomal dominant 3 |
| ANKRD11 | 16q24.3 | 611192 | Ankyrin repeat domain 11 | KBG syndrome |
| SPG7 | 16q24.3 | 602783 | SPG7, paraplegin matrix AAA peptidase subunit | Spastic paraplegia 7, autosomal recessive |
| CHMP1A | 16q24.3 | 164010 | Charged multivesicular body protein 1A | Pontocerebellar hypoplasia, type 8 |
| CDK10 | 16q24.3 | 603464 | Cyclin-dependent kinase 10 | Al Kaissi syndrome |
| FANCA | 16q24.3 | 607139 | FA complementation group A | Fanconianemia, complementation group A |
| MC1R | 16q24.3 | 155555 | Melanocortin 1 receptor | Albinism, oculocutaneous, type II; Skin/hair/eye pigmentation, variation in, 2; analgesia from kappa-opioid receptor agonist, female-specific; melanoma, cutaneous malignant, 5 |
| TUBB3 | 16q24.3 | 602661 | Tubulin beta 3 class III | Fibrosis of extraocular muscles, congenital, 3A; cortical dysplasia, complex, with other brain malformations 1 |
| GAS8 | 16q24.3 | 605178 | Growth arrest-specific 8 | Ciliary dyskinesia, primary, 33 |
| PIEZO1 | 16q24.3 | 611184 | Piezo type mechanosensitive ion channel component 1 | Dehydrated hereditary stomatocytosis with or without pseudohyperkalemia and/or perinatal edema; Lymphatic malformation 6 |
| APRT | 16q24.3 | 102600 | Adenine phosphoribosyltransferase | Adenine phosphoribosyltransferase deficiency |
In conclusion, the present case study reports on a newborn suffering from multiple organ malformations, with 2p25.3 deletion and 16q21q24.3 duplication. Concomitant partial monosomy 2p and partial trisomy 16q is unusual in a clinical context. The present results not only underline the importance of trisomy 16q21→qter, but also uncover certain unprecedented clinical features, which expand on the current knowledge of the clinical phenotypes of trisomy 16q21→qter syndrome. The limited number of relevant previous studies put a restriction on the clear delineation of the phenotype-karyotype correlation of trisomy 16q21→qter. Considering the observed clinical manifestations, the SNTG2, CDT1 and ANKRD11 genes are plausible candidates contributing to the proband's phenotype. As more and more clinical and molecular cytogenetic evaluations of cases with partial trisomy 16q emerge, it is anticipated that more accurate information on genotype-phenotype correlations will be established. Taking all of the information obtained into consideration, pre-implantation genetic diagnosis and prenatal diagnosis would be the most suitable choice if the proband's parents intend to conceive again.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Special Funds for Projects of Independent Innovation and New and Hi-tech Industry Development of Jilin Province (grant no. 2017C025).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
FY obtained the clinical information, collected data from the literature and wrote the manuscript. YJ and YP critically reviewed the manuscript. LeL and LiL performed the cytogenetic study and CMA analysis on the proband and the proband's parents. RL acquired funding and designed the study. RW conceived and designed the study, and performed the final review and editing of the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of the First Hospital of Jilin University (Changchun, China). The parents of the patient provided written informed consent for their child to participate in this study, and they also consented to participate themselves.
Patient consent for publication
The present case report was published with the informed consent of the patient's parents, whose anonymities were preserved.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Brisset S, Joly G, Ozilou C, Lapierre JM, Gosset P, LeLorc'h M, Raoul O, Turleau C, Vekemans M, Romana SP. Molecular characterization of partial trisomy 16q24.1-qter: Clinical report and review of the literature. Am J Med Genet. 2002;113:339–345. doi: 10.1002/ajmg.b.10740. [DOI] [PubMed] [Google Scholar]
- 2.Hassold T, Merrill M, Adkins K, Freeman S, Sherman S. Recombination and maternal age-dependent nondisjunction: Molecular studies of trisomy 16. Am J Hum Genet. 1995;57:867–874. [PMC free article] [PubMed] [Google Scholar]
- 3.Maher ER, Willatt L, Cuthbert G, Chapman C, Hodgson SV. Three cases of 16q duplication. J Med Genet. 1991;28:801–802. doi: 10.1136/jmg.28.11.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmickel R, Poznanski A, Himebaugh J. 16q trisomy in a family with a balanced 15/16 translocation. Birth Defects Orig Artic Ser. 1975;11:229–236. [PubMed] [Google Scholar]
- 5.Laus AC, Baratela WA, Laureano LA, Santos SA, Huber J, Ramos ES, Rebelo CC, Squire JA, Martelli L. Karyotype/phenotype correlation in partial trisomies of the long arm of chromosome 16: Case report and review of literature. Am J Med Genet A 158A. 2012:821–827. doi: 10.1002/ajmg.a.32988. [DOI] [PubMed] [Google Scholar]
- 6.De Rocker N, Vergult S, Koolen D, Jacobs E, Hoischen A, Zeesman S, Bang B, Béna F, Bockaert N, Bongers EM, et al. Refinement of the critical 2p25.3 deletion region: The role of MYT1L in intellectual disability and obesity. Genet Med. 2015;17:460–466. doi: 10.1038/gim.2014.124. [DOI] [PubMed] [Google Scholar]
- 7.Doco-Fenzy M, Leroy C, Schneider A, Petit F, Delrue MA, Andrieux J, Perrin-Sabourin L, Landais E, Aboura A, Puechberty J, et al. Early-onset obesity and paternal 2pter deletion encompassing the ACP1, TMEM18, and MYT1L genes. Eur J Hum Genet. 2014;22:471–479. doi: 10.1038/ejhg.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens SJ, van Ravenswaaij-Arts CM, Janssen JW, Klein Wassink-Ruiter JS, van Essen AJ, Dijkhuizen T, van Rheenen J, Heuts-Vijgen R, Stegmann AP, Smeets EE, Engelen JJ. MYT1L is a candidate gene for intellectual disability in patients with 2p25.3 (2pter) deletions. Am J Med Genet A 155A. 2011:2739–2745. doi: 10.1002/ajmg.a.34274. [DOI] [PubMed] [Google Scholar]
- 9.Wu XL, Li R, Fu F, Pan M, Han J, Yang X, Zhang YL, Li FT, Liao C. Chromosome microarray analysis in the investigation of children with congenital heart disease. BMC Pediatr. 2017;17:117. doi: 10.1186/s12887-017-0863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaffer LG, Slovak ML, Campbell LJ, editors. Basel, Switzerland: Karger S.; 2013. ISCN 2013: An international system for human cytogenetic nomenclature; p. 138. [Google Scholar]
- 11.Barber JC, Zhang S, Friend N, Collins AL, Maloney VK, Hastings R, Farren B, Barnicoat A, Polityko AD, Rumyantseva NV, et al. Duplications of proximal 16q flanked by heterochromatin are not euchromatic variants and show no evidence of heterochromatic position effect. Cytogenet Genome Res. 2006;114:351–358. doi: 10.1159/000094225. [DOI] [PubMed] [Google Scholar]
- 12.Balestrazzi P, Giovannelli G, Landucci Rubini L, Dallapiccola B. Partial trisomy 16q resulting from maternal translocation. Hum Genet. 1979;49:229–235. doi: 10.1007/BF00277648. [DOI] [PubMed] [Google Scholar]
- 13.Garau A, Crisponi G, Peretti D, Peretti D, Vanni R, Zuffardi O. Trisomy 16q21=to qter. Hum Genet. 1980;53:165–167. doi: 10.1007/BF00273489. [DOI] [PubMed] [Google Scholar]
- 14.Lessick ML, Israel J, Wong PW, Szego K. Partialtrisomy 16q secondary to a maternal 9;16 translocation. J Med Genet. 1989;26:63–64. doi: 10.1136/jmg.26.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Carvalho AF, da Silva Bellucco FT, dos Santos NP, Pellegrino R, de Azevedo Moreira LM, Toralles MB, Kulikowski LD, Melaragno MI. Trisomy 16q21→qter: Seven-year follow-up of a girl with unusually long survival. Am J Med Genet A 152A. 2010:2074–2078. doi: 10.1002/ajmg.a.33524. [DOI] [PubMed] [Google Scholar]
- 16.Mishra R, Paththinige CS, Sirisena ND, Nanayakkara S, Kariyawasam UGIU, Dissanayake VHW. Partial trisomy 16q21→qter due to an unbalanced segregation of a maternally inherited balanced translocation 46,XX,t(15;16)(p13;q21): A case report and review of literature. BMC Pediatr. 2018;18:4. doi: 10.1186/s12887-017-0980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou YS, Van Dyke DL, Ellison JW. Microarray comparative genomic hybridization and FISH studies of an unbalanced cryptic telomeric 2p deletion/16q duplication in a patient with mental retardation and behavioral problems. Am J Med Genet A 143A. 2007:746–751. doi: 10.1002/ajmg.a.31645. [DOI] [PubMed] [Google Scholar]
- 18.Wohlschlegel JA, Dwyer BT, Dhar SK, Cvetic C, Walter JC, Dutta A. Inhibition of eukaryotic DNA replication by geminin binding to Cdt1. Science. 2000;290:2309–2312. doi: 10.1126/science.290.5500.2309. [DOI] [PubMed] [Google Scholar]
- 19.de Munnik SA, Bicknell LS, Aftimos S, Al-Aama JY, van Bever Y, Bober MB, Clayton-Smith J, Edrees AY, Feingold M, Fryer A, et al. Meier-Gorlin syndrome genotype-phenotype studies: 35 individuals with pre-replication complex gene mutations and 10 without molecular diagnosis. Eur J Hum Genet. 2012;20:598–606. doi: 10.1038/ejhg.2011.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang A, Yeung PL, Li CW, Tsai SC, Dinh GK, Wu X, Li H, Chen JD. Identification of a novel family of ankyrin repeats containing cofactors for p160 nuclear receptor coactivators. J Biol Chem. 2004;279:33799–33805. doi: 10.1074/jbc.M403997200. [DOI] [PubMed] [Google Scholar]
- 21.Sirmaci A, Spiliopoulos M, Brancati F, Powell E, Duman D, Abrams A, Bademci G, Agolini E, Guo S, Konuk B, et al. Mutations in ANKRD11 cause KBG syndrome, characterized by intellectual disability, skeletal malformations, and macrodontia. Am J Hum Genet. 2011;89:289–294. doi: 10.1016/j.ajhg.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleyner R, Malcolmson J, Tegay D, Ward K, Maughan A, Maughan G, Nelson L, Wang K, Robison R, Lyon GJ. KBG syndrome involving a single-nucleotide duplication in ANKRD11. Cold Spring Harb Mol Case Stud. 2016;2:a001131. doi: 10.1101/mcs.a001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.