Abstract
Objectives:
We investigated whether patients with chronic obstructive pulmonary disease could safely receive noninvasive ventilation outside of the ICU.
Design:
Retrospective cohort study.
Setting:
Twelve states with ICU utilization flag from the State Inpatient Database from 2014.
Patients:
Patients greater than or equal to 18 years old with primary diagnosis of acute exacerbation of chronic obstructive pulmonary disease and secondary diagnosis of respiratory failure who received noninvasive ventilation.
Interventions:
None.
Measurements and Main Results:
Multilevel logistic regression models were used to obtain hospital-level ICU utilization rates. We risk-adjusted using both patient/hospital characteristics. The primary outcome was in-hospital mortality; secondary outcomes were invasive monitoring (arterial/central catheters), hospital length of stay, and cost. We examined 5,081 hospitalizations from 424 hospitals with ICU utilization ranging from 0.05 to 0.98. The overall median in-hospital mortality was 2.62% (interquartile range, 1.72–3.88%). ICU utilization was not significantly associated with in-hospital mortality (β = 0.01; p = 0.05) or length of stay (β = 0.18; p = 0.41), which was confirmed by Spearman correlation (ρ = 0.06; p = 0.20 and ρ = 0.02; p = 0.64, respectively). However, lower ICU utilization was associated with lower rates of invasive monitor placement by linear regression (β = 0.05; p < 0.001) and Spearman correlation (ρ = 0.28; p < 0.001). Lower ICU utilization was also associated with significantly lower cost by linear regression (β = 14.91; p = 0.02) but not by Spearman correlation (ρ = 0.09; p = 0.07).
Conclusions:
There is wide variability in the rate of ICU utilization for noninvasive ventilation across hospitals. Chronic obstructive pulmonary disease patients receiving noninvasive ventilation had similar in-hospital mortality across the ICU utilization spectrum but a lower rate of receiving invasive monitors and probably lower cost when treated in lower ICU-utilizing hospitals. Although the results suggest that noninvasive ventilation can be delivered safely out- side of the ICU, we advocate for hospital-specific risk assessment if a hospital were considering changing its noninvasive ventilation delivery policy. (Crit Care Med 2019; XX:00–00)
Keywords: chronic obstructive pulmonary disease, healthcare delivery, intensive care unit utilization, noninvasive ventilation, safety research
The number of adult ICU beds in the United States increased 15.9% between 2000 and 2010, whereas the occupancy remained stable at 65%, indicating that the number of ICU patients has risen (1). Annual critical care costs have doubled, now totaling $108 billion (1). Concurrently, there has been a rise in the use of noninvasive ventilation (NIV), either continuous positive airway pressure or bilevel positive airway pressure for patients with acute exacerbation of chronic obstructive pulmonary disease (COPD) (2), given its demonstrated benefits (3).
Some NIV patients are being treated in the ICU while others are being treated on the general ward. Although professional societies have developed guidelines for ICU triage (4, 5), hospital variation exists due to hospital-level resource constraints and local culture (6). For example, respiratory therapy services may not be available outside of the ICU in some hospitals, especially nighttime. Given that NIV failure is associated with higher rates of intubation-related complications (7) and mortality (2), one might argue for triaging anyone receiving NIV to the ICU.
Further research to optimize delivery of care for COPD patients is important given the high rate of COPD hospitalizations and its associated cost burden (8). We hypothesized that COPD patients receiving NIV in lower ICU-utilizing hospitals would have similar mortality but lower likelihood of receiving invasive monitors.
METHODS
Data Source
The State Inpatient Database (SID) is maintained by the Agency for Healthcare Research and Quality’s (AHRQ) Healthcare Cost and Utilization Project (HCUP) (9). SID files include nearly 100% of discharges from nonfederal hospitals in 46 states independent of payer. Any state was included if it had key variables such as an ICU utilization flag, time-to-procedure variables used to derive NIV failure, as well as an American Hospital Association (AHA) linkage file. States were AR, KY, MA, MD, NC, NJ, NV, NY, OR, UT, VT, and WA. The year 2014 was chosen because it predated enforcement of financial penalties for COPD readmissions and the transition from the ninth revision of the International Classification of Diseases.
Study Design
This is a retrospective cohort study. We included adults (≥ 18 yr old) receiving NIV with a primary diagnosis of acute exacerbation of COPD and respiratory failure (RF) as the first of the secondary diagnoses. NIV failure was defined according to previous studies (10, 11), where invasive mechanical ventilation (IMV) followed NIV using time-to-procedure variables. The administrative codes used for inclusion are listed in eTable 1 (Supplemental Digital Content 1, http://links.lww.com/CCM/E382) and are well cited in the literature (2, 3, 10–13). The exclusion criteria are described in the eMethods.
Exposure
We used the AHRQ ICU utilization flag (14), which identifies hospitalizations with an ICU stay via validated revenue codes (15) that are listed in eTable 1 (Supplemental Digital Content 1, http://links.lww.com/CCM/E382). A hospitalization without a flag was assumed to take place on the ward. ICU admission was treated as a continuous variable to preserve power, which is consistent with previous literature (16).
Outcomes
The primary outcome was risk-adjusted, hospital-level mortality. Secondary outcomes were risk-adjusted hospital-level length of stay (LOS), cost per hospitalization, and use of invasive monitoring, including both central venous catheter and arterial catheter (eTable 1, Supplemental Digital Content 1, http://links.lww.com/CCM/E382). The codes for invasive monitoring are used frequently in the literature (16, 17). The HCUP cost-to-charge ratio report was used to calculate cost which was adjusted to 2014 dollars using the consumer price index.
Risk Adjustment
We adjusted for patient and hospital characteristics. Patient-level variables included age, sex, race/ethnicity, primary health insurance, median household income by ZIP code, and comorbid conditions. Comorbidity variables and comorbidity score were created according to Elixhauser et al (18), except for COPD because it was a part of the inclusion criteria. Additionally, the following individual organ failures by Martin et al (19) (cardiovascular, hematologic, hepatic, metabolic, neurologic, and renal) were used as binary variables in risk adjustment based on administrative codes in eTable 1 (Supplemental Digital Content 1, http://links.lww.com/CCM/E382) that have been used frequently in the literature (10, 20, 21). Respiratory-related organ failure was not used because it was part of our inclusion criteria. Codes for the presence of severe sepsis/septic shock (defined according to the Angus explicit definition [22]) and IMV (23) are also shown in eTable 1 (Supplemental Digital Content 1, http://links.lww.com/CCM/E382) and were used for risk adjustment. Characteristics of hospitals were obtained by linking to AHA files. Hospital-level variables included total number of hospital beds, teaching status, percentage of hospital beds that are ICU beds and average hospital and ICU occupancy in 2014.
Statistical Analysis
Based on previous articles (6, 16, 17, 24), we performed a mixed effects logistic regression analysis to estimate the odds of an ICU admission for our specific patient population (COPD with RF receiving NIV) for each hospital. We adjusted for patient-and hospital-level effects by including them as fixed effects, and treated individual hospitals as random effects to account for clustering of admissions within hospitals (25). We obtained the predicted risk- and reliability-adjusted ICU utilization rate and 95% CI’s for each hospital using empirical Bayesian posterior estimates from the logistic regression model in the same way that Chang et al (16) did for other conditions. Risk- and reliability-adjusted hospital-level mortality rates were calculated using empirical Bayesian posterior estimates from a separate multilevel logistic regression model with in-hospital mortality as the dependent variable. Reliability adjustment is also known as “empirical Bayes estimation” or “shrinkage adjustment” and produces more reliable estimates by shifting the predicted value of a given hospital toward the average based on that hospital’s number of patients and outcomes (26). We repeated this method for the secondary outcome of invasive monitoring using the same patient-and hospital-level factors for risk adjustment. Similarly, multilevel linear regression models were used to calculate risk-adjusted hospital-level LOS and costs. These outcomes were log transformed due to their non-normal distributions. Using the calculated estimates described above, we performed linear regression to examine the associations between ICU admission as a continuous variable and hospital mortality, use of invasive monitoring, hospital LOS, and cost per hospitalization. In addition, we created Loess (locally weighted) regression plots in order not to assume a linear association. We calculated Spearman correlations to confirm the strength and direction of these associations.
All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). All p values were two-tailed test with less than 0.05 considered statistically significant. This study was approved by the Partners Institutional Review Board.
RESULTS
Of 7,406,189 hospitalizations, 5,081 met inclusion criteria from 424 hospitals. Table 1 shows the characteristics of patients treated on the ward versus ICU. There was no significant difference in patients’ age, gender, insurance type, or income by treatment location, although the distribution of race/ethnicity differed (p < 0.001). The percent with NIV failure and percent intubated were statistically lower for patients treated on the ward (3% vs 14%; p < 0.001 and 3% vs 19%; p < 0.001, respectively). We confirmed that the majority (~75%) of patients receiving IMV had NIV failure.
TABLE 1.
Baseline Patient Characteristics for Chronic Obstructive Lung Disease Hospitalizations Using Noninvasive Ventilation by Treatment Location
| Characteristics | Overall (n = 5,081) | ||
|---|---|---|---|
| Ward (n = 2,462) | ICU (n =2,619) | P | |
| Age, median (IQR) | 66 (56–75) | 65 (56–74) | 0.05 |
| Female, n (%) | 1,474 (60) | 1,559 (60) | 0.80 |
| Race/ethnicity, n (%) | |||
| Non-Hispanic white | 1,633 (66) | 1,859 (71) | < 0.001 |
| Non-Hispanic black | 435 (18) | 492 (19) | |
| Hispanic | 201 (8) | 124 (5) | |
| Other/unknown | 193 (8) | 144 (6) | |
| Primary health insurance, n (%) | |||
| Medicare | 1,504 (61) | 1,552 (59) | 0.30 |
| Medicaid | 471 (19) | 527 (20) | |
| Private insurance | 378 (15) | 438 (17) | |
| Other/unknown | 109 (4) | 102 (4) | |
| Median income quartile ($), n (%) | |||
| 1 (1–39,999) | 701 (28) | 701 (27) | 0.45 |
| 2 (40,000–50,999) | 544 (22) | 602 (23) | |
| 3 (51,000–65,999) | 525 (21) | 589 (22) | |
| 4 (66,000+) | 692 (28) | 727 (28) | |
| Noninvasive ventilation failure, n (%) | 67 (3) | 370 (14) | < 0.001 |
| Percent intubated, n (%) | 85 (3) | 504 (19) | < 0.001 |
| Organ failures, n (%) | |||
| Cardiovascular | 47 (2) | 139 (5) | < 0.001 |
| Hematologic | 68 (3) | 106 (4) | 0.01 |
| Hepatic | < 10 | 11 (0.42) | 0.12 |
| Metabolic | 375 (15) | 542 (21) | < 0.001 |
| Neurologic | 80 (3) | 180 (7) | < 0.001 |
| Renal | 214 (9) | 327 (12) | < 0.001 |
| Elixhauser comorbidity score, median (IQR) | 4 (−1 to 11) | 7 (−1 to 13) | < 0.001 |
| Selected comorbidities, n (%) | |||
| Alcohol abuse | 128 (5) | 143 (5) | 0.68 |
| Any cancer (with or without metastases) | 91 (4) | 87 (3) | 0.47 |
| Congestive heart failure | 776 (32) | 838 (32) | 0.71 |
| Diabetes mellitus (with or without complications) | 884 (36) | 850 (32) | 0.01 |
| Liver disease | 56 (2) | 62 (2) | 0.83 |
| Obesity | 681 (28) | 710 (27) | 0.66 |
| Other neurologic disorders | 225 (9) | 286 (11) | 0.03 |
| Pulmonary circulation disorders | 16 (1) | 82 (3) | < 0.001 |
| Selected active diagnoses at admission, n (%) | |||
| Lung cancer | 32 (1) | 35 (1) | 0.91 |
| Pneumonia | 326 (13) | 504 (19) | < 0.001 |
| Pulmonary embolus | 17 (1) | 18 (1) | 0.99 |
| Severe sepsis or septic shock | < 10 | 30 (1) | < 0.001 |
| Smoking | 1,680 (68) | 1,850 (71) | 0.06 |
IQR = interquartile range.
n is the number of hospitalizations. Proportions may not equal 100% due to rounding.
As expected, patients treated on the ward had a statistically lower percent of individual organ failures, except hepatic, compared with patients in the ICU (Table 1). eTable 2 (Supplemental Digital Content 1, http://links.lww.com/CCM/ E382) directly compares characteristics of patients treated on the ward in low versus high ICU-utilizing hospitals and those treated in the ICU in low versus high ICU-utilizing hospitals, where ICU utilization is divided at the median. Beside renal failure being more common on the ward in low ICU-utilizing hospitals, there were no statistically significant differences in organ failures between ward patients treated in low versus high ICU-utilizing hospitals and ICU patients treated in low versus high ICU-utilizing hospitals. Additionally, eFigure 1A (Supplemental Digital Content 2, http://links.lww.com/ CCM/E383; legend, Supplemental Digital Content 1, http://links.lww.com/CCM/E382) shows the association between average number of organ failures by hospital and unadjusted ICU utilization. Although there was a statistically significant association, the Spearman correlation was weak (ρ = 0.17; p < 0.001) (eTable 3, Supplemental Digital Content 1, http://links.lww.com/CCM/E382), and the absolute difference in average number of organ failures between the lowest and highest utilizing hospital was only 0.15. The same information presented for individual organ failures is presented for Elixhauser score in Table 1, eFigure 1B (Supplemental Digital Content 3, http://links.lww.com/CCM/E384; legend, Supplemental Digital Content 1, http://links.lww.com/CCM/E382), and eTable 3 (Supplemental Digital Content 1, http://links.lww. com/CCM/E382).
Table 2 shows hospital characteristics. The overall median ICU admission rate was 15% (interquartile range [IQR], 10–27%). The median ICU occupancy was 19% (IQR, 13–30%), whereas median hospital occupancy was 12% (10–14%). Sixty-two percent of hospitals had 0–10% of their beds designated as ICU beds, and the other 38% had greater than 10%. The most frequent type of hospital was a metropolitan teaching hospital in the northeast with 100–399 beds. eFigure 2 (Supplemental Digital Content 2, http://links.lww. com/CCM/E384; legend, Supplemental Digital Content 1, http://links.lww.com/CCM/E382) shows that the range of ICU utilization was 0.05–0.98 with the median denoted by the dotted line. eTable 4 (Supplemental Digital Content 1, http://links.lww.com/CCM/E382) lists the condition-specific ICU utilization rate for each hospital.
TABLE 2.
Baseline Hospital Characteristics
| Characteristics | Overall (n = 424) |
|---|---|
| ICU admission rate, median % (IQR) | 15 (10–27) |
| Region, n (%) | |
| Northeast | 208 (49) |
| South | 142 (33) |
| West | 74 (17) |
| Hospital type, n (%) | |
| Metropolitan nonteaching | 136 (32) |
| Metropolitan teaching | 255 (60) |
| Nonmetropolitan | 33 (8) |
| Hospital size, number of beds, n (%) | |
| 1–99 | 72 (17) |
| 100–399 | 251 (59) |
| 400–699 | 70 (17) |
| ≥ 700 | 31 (7) |
| Percent ICU beds, n (%) | |
| > 0–10 | 261 (62) |
| > 10 | 163 (38) |
| Hospital occupancy, median % (IQR) | 12 (10–14) |
| ICU occupancy, median % (IQR) | 19 (13–30) |
IQR = interquartile range.
n is the number of hospitals. Proportions may not equal 100% due to rounding.
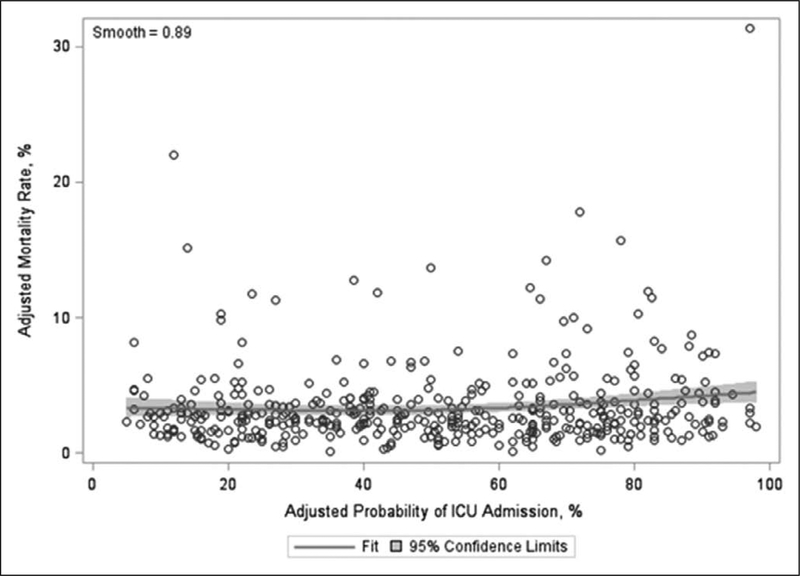
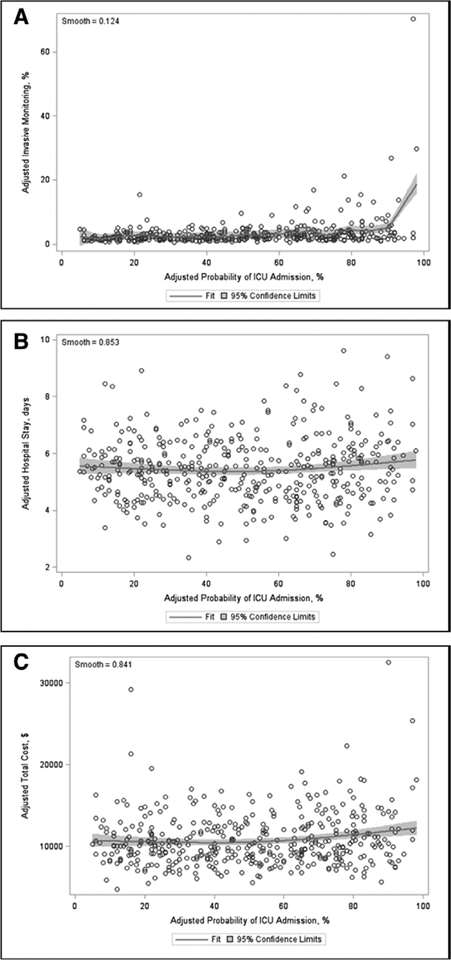
The overall median in-hospital mortality was 2.62% (IQR, 1.72–3.88%). We did not find a statistically significant association between ICU utilization and either in-hospital mortality (β estimate 0.01; p = 0.05) (Table 3) or LOS (β estimate 0.18; p = 0.41). However, hospitals with lower utilization rates were less likely to place invasive monitors (β estimate 0.05; p < 0.001) and had lower cost per hospitalization (β estimate 14.91; p = 0.02). The Spearman correlation was only statistically significant for invasive monitoring (ρ = 0.28; p < 0.001) but not for in-hospital mortality, LOS, or cost (eTable 5, Supplemental Digital Content 1, http://links.lww.com/CCM/ E382). Finally, Figure 1 shows a Loess plot of the relationship between adjusted hospital-level ICU utilization and mortality with 95% CI (smooth = 0.89). The corresponding Loess plots for the secondary outcomes of invasive monitoring, LOS, and cost are shown in Figure 2A–C, respectively.
TABLE 3.
Associations Between Risk-Adjusted Hospital-Level ICU Utilization Rate and Hospital Mortality, Use of Invasive Monitoring, Length of Stay, and Cost
| Adjusted Outcomes | β (95% CI) | P |
|---|---|---|
| In-hospital mortality | 0.01 (−0.9 × 10−4 to 0.02) | 0.05 |
| Invasive monitors | 0.05 (0.03–0.06) | < 0.001 |
| Hospital length of stay | 0.18 (−0.25 to 0.61) | 0.41 |
| Total cost | 14.91 (2.62–27.20) | 0.02 |
Invasive monitoring group contains both arterial and central venous catheters. Cost is per hospitalization.
Figure 1.
Loess plot of adjusted hospital-level ICU utilization and hospital mortality.
Figure 2.
Loess plots of adjusted hospital-level ICU utilization and (A) invasive monitoring, (B) length of stay, and (C) cost. Invasive monitoring includes both central and arterial catheters. Cost is per hospitalization.
DISCUSSION
Our primary results show that COPD patients with RF receiving NIV have the same in-hospital mortality rate and LOS regardless of the hospital’s tendency to treat them on the ward versus the ICU. However, patients in lower ICU-utilizing hospitals were less likely to receive invasive monitors after adjusting for both patient/hospital factors. One explanation for this observation is lower severity of illness in the lower ICU-utilizing hospitals, that is, invasive monitors placed in high ICU-utilizing hospitals provided necessary information to tailor therapy for sicker patients. However, we demonstrate that there is only weak correlation between average organ failures and ICU utilization with the absolute difference being only 0.15 organ failures between the lowest and highest ICU-utilizing hospital. We also demonstrate that patients on the ward or in the ICU in hospitals with ICU utilization below the median compared with hospitals above the median have equal number of individual organ failures (except for renal failure being more common in ward patients in low ICU-utilizing hospitals). Additionally, a previous article using a different data source showed that hospitals have varying thresholds for placing invasive monitors (27), so our finding could represent true overutilization.
Because a randomized trial of ICU triage is unlikely to occur in the United States, we must use observational data to test hypotheses. As in any observational study, unmeasured confounders, such as increased work of breathing or frailty, could influence bed triage and patient outcome. Although a major disadvantage in working with large administrative databases is not having access to physiologic variables or nurse/physician assessments, the SID offers the unique opportunity to examine ICU utilization rates from hundreds of hospitals in diverse states. The wide range of ICU utilization that we found raises the fundamental issue of why care differs so much between institutions. At the very least, this article provides the rationale for conducting network-based studies that 1) contain more granular physiologic data and 2) involve hospitals with differing policies on NIV delivery.
Previous articles have suggested that hospitals are self-regulating care delivery within the context of their institution’s local culture and resources. Admon et al (28) observed that hospitals’ ICU utilization tended to be consistent across conditions. They reported a Spearman correlation to be 0.38 (p < 0.001) for likelihood of admitting patients with conditions as different as stroke and hip fracture (28). Notably, only ~3% of patients in the cohort received NIV but for those who received it, even surgical patients, the authors reported a Spearman correlation for likelihood of admitting a patient getting NIV with pneumonia or COPD to be greater than 0.4 (28). These analyses employed standard risk adjustment techniques for administrative data, and the results demonstrate that hospital factors, in addition to patient factors, may be contributing to the way patients are triaged.
Our results raise the question about whether NIV can be delivered safely outside of the ICU. Although hospitals are incentivized monetarily to keep their ICU beds full, many institutions operate at maximum capacity and are looking for creative ways to offload the ICU. To explain the wide variability in ICU utilization that we found, we believe that hospitals have developed local policies to ensure patient safety while delivering respiratory care, or other advanced/specialty care, outside of the ICU setting. For example, there has been an increase in step-down units in the United States (29), so some NIV care could be clustered on specialty floors that have respiratory-specific expertise. Wards could be staffed with higher ratios of respiratory therapists or employ nurses who are trained to do deep suctioning or blood gases. Some hospitals may have developed standardized protocols for nurse/physician monitoring during NIV use (30), multidisciplinary team huddles after initiation of NIV (31), or pulmonary consultation if a patient does not improve on NIV. For hospitals considering adopting a policy where NIV can be delivered outside of the ICU, we would recommend full risk/resource assessments, as there may be important local factors that influence care.
The approach we took using mixed effects models has been used in previous articles (6, 16, 17, 24). Using two states from SID, Chang et al (16) found no association between mortality or cost and ICU utilization for four conditions (ketoacidosis, pulmonary embolism, gastrointestinal bleed, and heart failure) but found higher rates of invasive procedures for those more likely to be treated in the ICU (IMV, thrombolytics, endoscopy, and pulmonary artery catheterization, respectively). We focused on a different patient population and did not detect a mortality difference but did find lower cost. To explain this difference, it’s possible that: 1) there is greater cost reduction in delivering NIV outside of the ICU compared with other interventions, 2) analyzing 424 hospitals instead of 94 hospitals allowed for greater power to detect small differences in cost, and/or 3) our result was spurious given that the p value for the Spearman correlation was not consistent with the linear regression result.
Valley et al (12, 32) have published several articles assessing ICU utilization. In one article, they reported that patients admitted to the ward versus the ICU with COPD, heart failure, or myocardial infarction, who did not have overt critical care needs, had equivalent 30-day mortality and cost (12). They divided hospitals into low and high ICU-utilizing hospitals and advocated that the ICU may be overused for elderly COPD patients with low/moderate risk of death. Our primary results for mortality corroborate their findings, but again we found a lower cost associated with lower ICU utilization. There are several methodologic differences between the two studies that could explain the difference. Valley et al (12) used instrumental variable analysis to examine a larger sample of 2,600 hospitals, but it was limited to elderly patients. Additionally, less than 25% of patients in their study received NIV.
There are several advantages to our study design. First, we used all states with ICU utilization flags that contained the necessary variables to conduct the study. Our sample of 424 hospitals from 12 states was indeed diverse in hospital characteristics, which favors generalizability. The four previous articles using SID to study ICU utilization contained data from less than or equal to three states (6, 16, 17, 24). Second, we used narrow inclusion criteria to ensure a pure sample. COPD was the primary diagnosis to avoid including patients getting NIV for less evidence-based diagnoses, such as pneumonia (10). RF was the first of the secondary diagnoses to avoid including patients getting NIV for obstructive sleep apnea, for example. All patients must have received NIV because hospitals are known to vary in their utilization of NIV for COPD with more utilization leading to lower rates of IMV and mortality (13). Third, we adjusted for comorbidities as well as several severity indicators, including intubation and six widely used organ failures that are derived from administrative codes (19). These were not restricted to the present-on-admission timeframe so could have occurred any time during the hospitalization.
Beside the limitation mentioned above of not containing physiologic data for risk adjustment, our study has other potential limitations. We classified 3% of patients as receiving mechanical ventilation while being on the ward (i.e., no ICU utilization flag). This could represent care in step-down units (29), or errors in the utilization flag, despite there being less than 2% variability between the development/validation cohorts (14) and favorable external validation studies (15). Also, 5% of ICU patients were intubated but who had not failed NIV, which could have been patients receiving NIV for a different indication, such as post-extubation, or errors in the time-to-procedure variables. We also could not detect which patients were transferred to the ICU after failing NIV on the ward, as they may be misclassified and could be at higher risk of mortality (2). However, these patients are presumably more common in lower ICU-utilizing hospitals, so finding equivalent outcomes regardless of ICU utilization is reassuring. Last, although widely used in the literature, administrative codes for central venous catheter (33) and IMV (34) have been reported to be less than 50% sensitive in some settings, so the significant result associating invasive monitors could be spurious if we did not detect all events. Fortunately, outcome variables like mortality undergo robust quality control through HCUP (35).
As a healthcare system, we strive to deliver safe and equitable care across hospitals (36), yet the use of critical care resources to deliver NIV varies widely. Although there may be an opportunity to modestly reduce cost and increase ICU bed availability by delivering NIV on the ward, this large-scale, observational study merely confirms variability in NIV delivery, as it cannot adjust for severity of illness using physiologic data and cannot address hospital-level policies about delivery of NIV. In conclusion, it may be safe to deliver NIV outside of the ICU, but we urge hospital leaders, safety specialists and respiratory clinicians to do a multistakeholder risk assessment in their institutions before implementing a new ward-based policy.
Supplementary Material
ACKNOWLEDGMENTS
We thank Joshua Metlay, MD, PhD at the Massachusetts General Hospital (MGH) for critically reviewing the first draft of the article. We thank Hang Lee, PhD from the MGH Biostatistics Core for statistical advice and Andrew Admon, MD, MPH from the Institute for Policy and Innovation at the University of Michigan for content advice. We also thank Janice Espinola, MPH at MGH for assisting with revisions.
The statistical analysis was paid for by an internal hospital grant.
Dr. Myers received funding from Massachusetts General Hospital (an internal grant to pay for the statistical analysis). Dr. Currier received support for article research from Massachusetts General Hospital Clinical Innovation Award. Dr. Camargo’s institution received funding from Agency for Healthcare Research and Quality (AHRQ) R01 grant and an internal grant (Division of Pulmonary & Critical Care Medicine), and he received support for article research from AHRQ. Dr. Faridi disclosed that he does not have any potential conflicts of interest.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ ccmjournal).
REFERENCES
- 1.Halpern NA, Goldman DA, Tan KS, et al. : Trends in critical care beds and use among population groups and Medicare and Medicaid beneficiaries in the United States: 2000–2010. Crit Care Med 2016; 44:1490–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walkey AJ, Wiener RS: Use of noninvasive ventilation in patients with acute respiratory failure, 2000–2009: A population-based study. Ann Am Thorac Soc 2013; 10:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindenauer PK, Stefan MS, Shieh MS, et al. : Outcomes associated with invasive and noninvasive ventilation among patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med 2014; 174:1982–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egol A, Fromm R, Guntupalli KK, et al. : Guidelines for intensive care unit admission, discharge, and triage. Task Force of the American College of Critical Care Medicine and Society of Critical Care Medicine. Crit Care Med 1999; 27:633–638 [PubMed] [Google Scholar]
- 5.Dawson JA: Admission, discharge, and triage in critical care. Principles and practice. Crit Care Clin 1993; 9:555–574 [PubMed] [Google Scholar]
- 6.Seymour CW, Iwashyna TJ, Ehlenbach WJ, et al. : Hospital-level variation in the use of intensive care. Health Serv Res 2012; 47: 2060–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosier JM, Sakles JC, Whitmore SP, et al. : Failed noninvasive positive-pressure ventilation is associated with an increased risk of intubation-related complications. Ann Intensive Care 2015; 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perera PN, Armstrong EP, Sherrill DL, et al. : Acute exacerbations of COPD in the United States: Inpatient burden and predictors of costs and mortality. COPD 2012; 9:131–141 [DOI] [PubMed] [Google Scholar]
- 9.Agency for Healthcare Quality and Research Healthcare Cost and Utilization Project: Overview of State Inpatient Database. 2018. Available at: https://www.hcup-us.ahrq.gov/sidoverview.jsp. Accessed March 31, 2018 [Google Scholar]
- 10.Mehta AB, Douglas IS, Walkey AJ: Evidence-based utilization of non-invasive ventilation and patient outcomes. Ann Am Thorac Soc 2017; 14:1667–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta AB, Douglas IS, Walkey AJ: Hospital noninvasive ventilation case volume and outcomes of acute exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc 2016; 13:1752–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valley TS, Sjoding MW, Ryan AM, et al. : Intensive care unit admission and survival among older patients with chronic obstructive pulmonary disease, heart failure, or myocardial infarction. Ann Am Thorac Soc 2017; 14:943–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindenauer PK, Stefan MS, Shieh MS, et al. : Hospital patterns of mechanical ventilation for patients with exacerbations of COPD. Ann Am Thorac Soc 2015; 12:402–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elixhauser A, Barrett M, Nisbet J: Development of Utilization Flags for Use With UB-92 Administrative Data, Methods Series Report #2006–04. 2018. Available at: https://www.hcup-us.ahrq.gov/reports/meth-ods/methods.jsp. Accessed August 10, 2017 [Google Scholar]
- 15.Weissman GE, Hubbard RA, Kohn R, et al. : Validation of an administrative definition of ICU admission using revenue center codes. Crit Care Med 2017; 45:e758–e762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang DW, Shapiro MF: Association between intensive care unit utilization during hospitalization and costs, use of invasive procedures, and mortality. JAMA Intern Med 2016; 176:1492–1499 [DOI] [PubMed] [Google Scholar]
- 17.Admon AJ, Seymour CW, Gershengorn HB, et al. : Hospital-level variation in ICU admission and critical care procedures for patients hospitalized for pulmonary embolism. Chest 2014; 146:1452–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elixhauser A, Steiner C, Harris DR, et al. : Comorbidity measures for use with administrative data. Med Care 1998; 36:8–27 [DOI] [PubMed] [Google Scholar]
- 19.Martin GS, Mannino DM, Eaton S, et al. : The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348:1546–1554 [DOI] [PubMed] [Google Scholar]
- 20.Elias KM, Moromizato T, Gibbons FK, et al. : Derivation and validation of the acute organ failure score to predict outcome in critically ill patients: A cohort study. Crit Care Med 2015; 43:856–864 [DOI] [PubMed] [Google Scholar]
- 21.Courtright KR, Halpern SD, Bayes B, et al. : Adaptation of the acute organ failure score for use in a medicare population. Crit Care Med 2017; 45:1863–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwashyna TJ, Odden A, Rohde J, et al. : Identifying patients with severe sepsis using administrative claims: Patient-level validation of the angus implementation of the international consensus conference definition of severe sepsis. Med Care 2014; 52:e39–e43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wunsch H, Linde-Zwirble WT, Angus DC, et al. : The epidemiology of mechanical ventilation use in the United States. Crit Care Med 2010; 38:1947–1953 [DOI] [PubMed] [Google Scholar]
- 24.Gershengorn HB, Iwashyna TJ, Cooke CR, et al. : Variation in use of intensive care for adults with diabetic ketoacidosis*. Crit Care Med 2012; 40:2009–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitzmaurice G, Laird N, Ware J: Applied Longitudinal Analysis. Hoboken, NJ, John Wiley & Sons, 2011 [Google Scholar]
- 26.Wakeam E, Hyder JA: Reliability of reliability adjustment for quality improvement and value-based payment. Anesthesiology 2016; 124:16–18 [DOI] [PubMed] [Google Scholar]
- 27.Gershengorn HB, Garland A, Kramer A, et al. : Variation of arterial and central venous catheter use in United States intensive care units. Anesthesiology 2014; 120:650–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Admon AJ, Wunsch H, Iwashyna TJ, et al. : Hospital contributions to variability in the use of ICUs among elderly medicare recipients. Crit Care Med 2017; 45:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sjoding MW, Valley TS, Prescott HC, et al. : Rising billing for intermediate intensive care among hospitalized medicare beneficiaries between 1996 and 2010. Am J Respir Crit Care Med 2016; 193:163–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ergan B, Nasilowski J, Winck JC: How should we monitor patients with acute respiratory failure treated with noninvasive ventilation? Eur Respir Rev. 2018; 27:170107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hess DR: Noninvasive ventilation for acute respiratory failure. Respir Care 2013; 58:950–972 [DOI] [PubMed] [Google Scholar]
- 32.Valley TS, Sjoding MW, Ryan AM, et al. : Association of intensive care unit admission with mortality among older patients with pneumonia. JAMA 2015; 314:1272–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walkey AJ, Wiener RS, Lindenauer PK: Utilization patterns and outcomes associated with central venous catheter in septic shock: A population-based study. Crit Care Med 2013; 41:1450–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerlin MP, Weissman GE, Wonneberger KA, et al. : Validation of administrative definitions of invasive mechanical ventilation across 30 intensive care units. Am J Respir Crit Care Med 2016; 194:1548–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrett ML, Ross D: Healthcare Cost and Utilization Project Quality Control Procedures, Deliverable #1707.05. 2018. Available at: https://www.hcup-us.ahrq.gov/db/quality.pdf. Accessed April 6, 2018 [Google Scholar]
- 36.Institute of Medicine Committee on Quality of Healthcare in America: Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC, National Academy Press, 2001 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.