Abstract
Introduction
Chronic pain and reduced function are significant problems for Military Service members and Veterans following amputation. Peripheral nerve stimulation (PNS) is a promising therapy, but PNS systems have traditionally been limited by invasiveness and complications. Recently, a novel percutaneous PNS system was developed to reduce the risk of complications and enable delivery of stimulation without surgery.
Materials and Methods
Percutaneous PNS was evaluated to determine if stimulation provides relief from residual and phantom limb pain following lower-extremity amputation. PNS leads were implanted percutaneously to deliver stimulation to the femoral and/or sciatic nerves. Patients received stimulation for up to 60 days followed by withdrawal of the leads.
Results
A review of recent studies and clinical reports found that a majority of patients (18/24, 75%) reported substantial (≥50%) clinically relevant relief of chronic post-amputation pain following up to 60 days of percutaneous PNS. Reductions in pain were frequently associated with reductions in disability and pain interference.
Conclusions
Percutaneous PNS can durably reduce pain, thereby enabling improvements in quality of life, function, and rehabilitation in individuals with residual or phantom limb pain following amputation. Percutaneous PNS may have additional benefit for Military Service members and Veterans with post-surgical or post-traumatic pain.
Keywords: peripheral nerve stimulation, post-amputation pain, phantom pain, amputation
INTRODUCTION
Post-amputation pain (PAP) is a substantial military health problem. Over 50,000 US Service members have been injured in military conflicts since 2001 (e.g., Operation Enduring Freedom [OEF], Operation Iraqi Freedom [OIF]), and as a result, over 1,600 major limb amputations have occurred.1,2 Due to advances in military medicine including improved body armor, faster access to advanced life support and theater-based medical facilities, and effective technological care in the field, the mortality rate of severely injured Service members is decreasing.3,4 As a result, there is an increasing prevalence of warriors surviving traumatic injuries and a strong emphasis on alleviating pain and improving quality of life in these individuals.5
Recent studies indicate that more than 85% of US Service members with combat-related traumatic amputations from OEF/OIF suffer from moderate to severe PAP.6,7 Pain is a leading cause of disability, and PAP in particular can be extremely debilitating. Only 3–18% of Veterans from Vietnam and OEF/OIF with amputations are able to perform high-impact aerobics.8 Post-amputation pain significantly decreases quality of life, increases the risk of depression, discouragement, and anger, and negatively affects inter-personal relationships and the ability to return to work (amputees have a low probability [2–7%] of returning to active duty).7,9–12 Pain also negatively affects a Service member’s ability to comfortably wear a prosthetic device. Over 40% of Vietnam and OIF/OEF Service members and Veterans with multiple lower limb loss suffer from pain while wearing their prosthetic devices, and some ultimately abandon their prosthetics, which negatively impacts their ability to perform physical activities and maximize their level of fitness.8 Poorly controlled pain is an important barrier that must be overcome in order to promote functional recovery and effective rehabilitative therapy.
Chronic PAP includes residual limb pain (RLP) and phantom limb pain (PLP). RLP can be caused by multiple sources after amputation, including tissue trauma associated with surgery, wound infection or poor healing, heterotopic ossification, and direct or referred mechanical pain from joint degeneration or prosthetic fit.13–15 In most cases, residual limb pain is nociceptive in nature. Although neuroplasticity can accompany any pain condition, PLP may be more commonly associated with peripheral and central sensitization than RLP, including functional reorganization of nociceptive pathways in the spinal cord and brain, sensory remapping, expansion of receptive fields, and altered cortical representation of the limb.13–16 Pain may also be referred into the phantom limb from neuromas, radiculopathy, a proximal neural lesion, or even biomechanical non-neuropathic causes such as bursitis.14
The various potential mechanisms underlying PAP were traditionally categorized as nociceptive pain (due to actual or threatened tissue damage) or neuropathic pain (due to a disease or lesion affecting the somatosensory nervous system) (Table I).13 For example, prosthogenic pain and heterotopic ossification are nociceptive in nature, whereas hyperalgesia and allodynia from neuroma growth are neuropathic mechanisms. Recently, the International Association for the Study of Pain introduced a third mechanistic category, nociplastic pain, that includes pain not otherwise related to tissue insult or a somatosensory neural lesion.17 Some of the changes that are observed after amputation, such as maladaptive cortical plasticity, central sensitization, decreases in the activity of descending inhibitory systems, and abnormal activity in the sympathetic nervous system,16 may be considered nociplastic mechanisms that promote maladaptive pain in the absence of overt pathology and persist beyond tissue healing timelines (Table I). Table II illustrates differences in clinical characteristics among the three pain classifications.
Table I.
Potential Sources of Chronic Post-Amputation Pain, by Pain Classification
| Nociceptive | Neuropathic | Nociplastic |
|---|---|---|
| Post-surgical pain | Neuroma growth | Peripheral and central sensitization*,† |
| Poor-fitting prosthesis | Spontaneous discharge from transected nerves | Decrease in descending inhibitory systems*,† |
| Ulcers or poor wound healing | Referred pain from spinal pathology (e.g., radiculopathy) | Sympathetic sprouting and enhanced activity*,† |
| Referred pain from mechanical structures (e.g., bursitis, spinal degeneration) | Complex regional pain syndrome type II | Complex regional pain syndrome type I |
| Heterotopic ossification | Spinal and cortical reorganization | Post-traumatic fibromyalgia |
| Ischemia |
*Often also present in neuropathic pain.
†May also be present in chronic nociceptive pain.
Table II.
Clinical Characteristics of Nociceptive, Neuropathic, and Nociplastic Post-Amputation Pain
| Nociceptive Post-Amputation Pain | Neuropathic Post-Amputation Pain | Nociplastic Pain in Amputees | |
|---|---|---|---|
| Etiology | Actual or potential tissue damage, referred pain from mechanical structures | Severing of nerve, neuroplastic changes in the peripheral and central nervous systems | Altered nociception despite no evidence of actual or threatened tissue damage, or evidence for a lesion affecting the somatosensory system. Trauma is a common antecedent to CRPS type I, uncommon for other types of nociplastic pain. |
| Frequency | Most common cause of residual limb pain | Most common cause of phantom limb pain | Infrequent stand-alone cause of post-amputation pain, though altered pain processing may accompany nociceptive and neuropathic postamputation pain |
| Descriptors | Throbbing, aching, pressure-like | Lancinating, shooting, electrical-like | Highly variable |
| Accompanying Sensory Changes | Infrequent, outside of a nerve or nerve root distribution | Phantom sensations very common | Common, but often outside the distribution of nerve or tissue injury |
| Hypersensitivity | Uncommon except for hypersensitivity in the immediate area after trauma or amputation, often elicited by palpation of pain generator | Allodynia and hyperalgesia may be present in residual limb | Hallmark of the condition |
| Location | Proximal radiation frequent | Distal radiation common, telescoping often observed | Diffuse, outside the distribution of an injured nerve(s) or amputated body part |
| Time course | Acute postsurgical pain decreases over several weeks. Pain from other sources stabilizes or slightly diminishes over time, though referred pain from degenerative diseases may persist or worsen | Often experienced within 1 week of amputation, prevalence peaks within 2 years and remains stable or declines in intensity | Pain post-injury disproportionate to inciting event. Delays in diagnosis common. |
| Paroxysms | Exacerbations less common and often associated with specific activities (putting on prostheses, ambulation) | Exacerbations common and unpredictable | May be superimposed on low-grade continuous pain |
| Autonomic signs | Uncommon | Can occur in 1/3 to 1/2 of patients | Frequent in CRPS type I and other types of nociplastic pain |
| Associated symptoms | Psychiatric co-morbidities common | Psychiatric co-morbidities common | High co-prevalence rate of other nociplastic pain conditions. Cognitive deficits, psychiatric co-morbidities, fatigue, poor sleep and sensitivity to light and other stimuli common |
PERIPHERAL NERVE STIMULATION (PNS) IS A THERAPEUTIC OPTION FOR CHRONIC PAIN FOLLOWING AMPUTATION, BUT HAS TRADITIONALLY BEEN LIMITED BY COMPLICATIONS AND INVASIVENESS
Likely because of its complex, multifaceted nature, PAP has historically been a challenging condition to treat, and amputees often progress through a battery of management techniques and procedures without finding adequate relief. Many approaches have been used, including opioid and non-opioid oral analgesics, nerve blocks and radiofrequency therapies, spinal cord stimulation (SCS), and physical and psychological therapies, but clinical experience and controlled trials have not demonstrated consistent and effective pain management using these traditional methods.13,14,18–21 For example, opioid analgesics have shown some success in a few trials, but data are limited and many amputees either fail to achieve consistent long-term pain relief or suffer from side effects common to opioid medications, such as nausea, drowsiness, headache, insomnia, loss of libido, and addiction.18,21 Rising levels of opioid addiction and associated socioeconomic burdens have also prompted a reexamination of prescribing practices and highlight the need for non-opioid pain management options.
PNS has been used effectively for immediate and long-lasting relief of chronic pain, including in individuals with chronic pain following amputation.22–24 PNS has also been shown to improve sleep, increase quality of life and activity levels, allow a significant percentage (up to 50%) of patients to return to work, and reduce or eliminate dependence on opioid analgesia.25,26 In one review of 117 patients receiving PNS who were followed up to 53 months, 65% reported an increase in their activities of daily living and more than 75% were satisfied with therapy.27
PNS can have a high success rate and positively impact function and quality of life through pain relief, but traditional methods of surgically placing the lead near or in direct contact with the nerve have historically been limited by invasiveness, complexity of surgical implantation, and risk of complications such as nerve damage, lead migration (24–33%), infection (1–5%), pain at the site of implantable pulse generator (IPG) (0.9–5.8%), and hardware or battery failure (1.6–2%).28 In one retrospective review of traditional PNS techniques, Ishizuka and colleagues found that 64% of patients required reoperation because initial pain relief following surgical implantation was lost due to lead migration, infection, or poor initial lead placement.29 Collectively, these complications contribute to an overall revision rate of 27% among patients undergoing surgical implantation of traditional PNS systems, nearly half of which (15%) require explantation.28
PERCUTANEOUS PNS IS DESIGNED TO ADDRESS MANY OF THE CHALLENGES PREVIOUSLY ASSOCIATED WITH PNS
It was theorized that a system designed specifically for the non-surgical, reversible implantation of leads remote from the target nerve in the peripheral nervous system would facilitate effective pain management while reducing or eliminating many of the common complications previously associated with PNS. In particular, analysis of stimulation thresholds suggested that implantation of leads remote from the target nerve (0.5–3 cm) would enable preferential activation of targeted (pain-relieving) fibers while avoiding activation of nontarget fibers that can cause discomfort and limit the utility or therapeutic window of PNS therapy.30
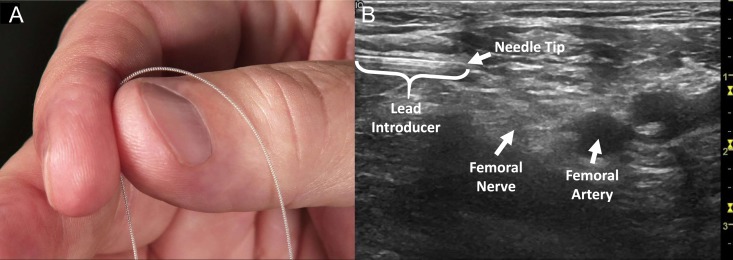
An FDA-cleared percutaneous PNS system was developed with the intent of overcoming the challenges of lead migration and invasiveness that limited previous approaches to PNS by employing flexible, fine-wire, helically-coiled leads designed to resist migration (Fig. 1). Open-coil percutaneous leads have a history of over 40 years of effective use in the periphery and are designed to resist migration in part by promoting healthy tissue ingrowth between the coils to anchor the lead in tissue.31–37 The leads are pre-loaded within a needle-based introducer and percutaneously implanted, typically under ultrasound guidance.30,38 The leads are implanted remote from the target nerve (0.5–3 cm), minimizing the risk of nerve injury (Fig. 1). PNS therapy is delivered for up to 60 days, after which the leads are withdrawn.
FIGURE 1.
(A) The percutaneous PNS therapy uses fine-wire, coiled leads typically implanted under ultrasound guidance. (B) Ultrasound image showing the implantation of a lead approximately 0.5–1 cm remote from the femoral nerve for the treatment of chronic pain in a lower extremity amputee.
PERCUTANEOUS PNS REDUCES PAIN AND PAIN INTERFERENCE IN INDIVIDUALS WITH CHRONIC PAIN FOLLOWING AMPUTATION
The feasibility of using percutaneous PNS for relieving chronic pain after amputation was first demonstrated by Rauck and colleagues in an NIH-funded case series study.30 Fourteen of 16 patients (88%) with RLP and/or PLP following lower-limb amputation reported immediate clinically-relevant pain relief following implantation of leads remote from the femoral and/or sciatic nerves.30 Leads were implanted percutaneously under ultrasound guidance distal to the inguinal crease approximately 0.5–1 cm superficial or lateral to the femoral nerve (Fig. 1B), and transgluteally approximately 1–1.5 cm superficial or lateral to the sciatic nerve between the greater trochanter and ischial tuberosity.30 Nine patients continued use of the PNS system for two weeks and reported a 72% mean reduction of RLP and 81% mean reduction of PLP (Fig. 2).30 Reductions in post-amputation pain were sustained following lead withdrawal throughout the 4-week follow-up period.30 In addition to pain relief, patients reported 81–83% mean reductions in RLP and PLP interference, which measures the degree to which pain interferes with mood and daily activities such as mobility (Fig. 2), 70% mean reduction in disability as measured by the Pain Disability Index, and 43% mean reduction in depression as measured by the Beck Depression Inventory II (BDI-II).30
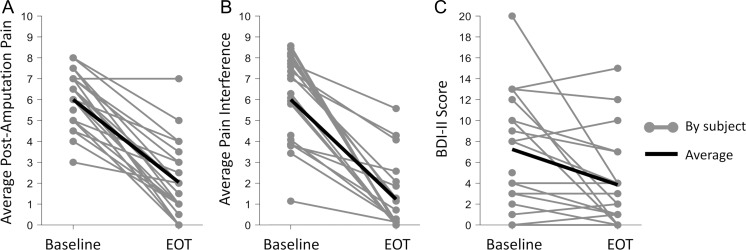
FIGURE 2.
(A) Reductions of ≥50% in average post-amputation pain were reported in 18/24 subjects (75%), (B) reductions of ≥50% in average pain interference were reported in 17/21 subjects (81%), and (C) Beck Depression Inventory II (BDI-II) scores were reduced from baseline in 13/21 subjects (62%) at the end of up to 60 days of percutaneous PNS therapy (EOT) in recent studies and clinical reports.30,39,42
Following the initial feasibility study, a DoD-funded, multicenter, randomized, double-blind, placebo-controlled trial further evaluated percutaneous PNS for the treatment of chronic pain following amputation in 28 traumatic lower extremity amputees.39 Leads were placed 0.5–1 cm remote from the femoral nerve and 1–1.5 cm remote from the sciatic nerve using approaches similar to those described by Rauck et al.30 Gilmore and colleagues found that a significantly greater proportion of patients who received PNS for up to 60 days experienced substantial (≥ 50%) reductions in pain compared to patients who received placebo therapy (Fig. 2). These reductions in pain were achieved with concurrent reductions in opioid usage in some patients; the subset of subjects taking moderate-to-high opioid doses at baseline reported 71% average reductions in daily opioid consumption after up to 60 days of PNS.39 According to Gilmore et al., prospective follow-up in this study was ongoing and 5 patients had completed the 12-month follow-up at the time of writing (with statistical analysis pending completion of follow-up in additional patients). Substantial (≥ 50%) reductions in pain were sustained in 4 of 5 patients (80%) who had completed the 12-month follow-up visit to-date.39
Gilmore and colleagues also found significant improvements in function, as measured by pain interference, Patient Global Impression of Change (PGIC), and BDI-II. Patients in the PNS therapy group reported average reductions in pain interference of ≥ 4 points on a 0–10 rating scale (Fig. 2),39 which was more than four-times higher than the one-point threshold for a minimally important change.40 Pain interference was reduced by 75% compared to baseline, and 80% of patients reported ≥ 50% reductions. Patients receiving PNS also reported being “Much Improved” to “Very Much Improved” on the PGIC survey and had statistically significantly greater global improvement and greater decreases in depression as measured by BDI-II compared to patients receiving placebo.39 Disability and quality of life are significant issues for amputees including injured military Service members, and pain relief from percutaneous PNS may provide additional benefits by enabling greater function and return to activities of daily living previously affected by chronic pain after amputation.
In addition to studies that focused predominantly on individuals with traumatic amputations such as those that occur secondary to combat-related injuries, percutaneous PNS therapy has also been used to treat Veterans with non-traumatic amputations outside of the clinical research setting. The Hunter Holmes McGuire VA Medical Center (HHMVAMC) is one of seven Regional Amputation Centers in the Veteran’s Health Administration Amputation System of Care (ASoC).41 The ASoC was implemented in 2008 to help provide Veterans with limb loss with state-of-the-art care delivered with an interdisciplinary focus on pain management, residual limb care, and other aspects of rehabilitation.41 Three Veterans at HHMVAMC who experienced chronic pain following dysvascular or infection-related lower extremity amputation have undergone implantation of percutaneous leads targeting the femoral and/or sciatic nerves and received PNS for up to 60 days.42 Each patient reported 50% or greater reductions in post-amputation pain during the stimulation period (Fig. 2). Two patients were followed for an additional 8 and 16 weeks after lead withdrawal and continued to report 57% and 75% reductions, respectively, in post-amputation pain relative to their pre-PNS baselines.
Collectively, the studies and clinical reports reviewed here suggest that percutaneous PNS can effectively treat multiple aspects of a complex pain state like PAP. Overall, 18/24 (75%) of patients reported substantial (≥ 50%) clinically significant relief of both RLP and PLP during the stimulation period, suggesting that the therapy decreased both nociceptive and neuropathic sources of pain. This is consistent with evidence from traditional implanted PNS systems that PNS can attenuate transmission of nociceptive signals from the periphery,43 and attenuate neuropathic pain of various etiologies.22,24,44 In both the nociceptive and neuropathic cases, it is theorized that percutaneous PNS activates spinal segmental inhibitory mechanisms to attenuate pain, such as the gate control mechanism originally proposed by Wall and Melzack,45 to reduce pain during the stimulation period.
Of additional interest are the reports that pain relief endured for significant periods of time after the end of the percutaneous PNS stimulation period (pain relief up to one year in 4 of 5, 80%39). These results suggest a modification of the neuropathic and nociplastic mechanisms associated with centralized chronic PAP, especially the maladaptive cortical plasticity believed to underlie PLP.16 Percutaneous PNS may enable reversal of aberrant plasticity by modulating painful signals from the periphery, as suggested by review of recent results following nerve blocks for PLP.46 Analysis of studies using cutaneous EMG to provide sensory feedback to the cortex suggests that PNS may, over the course of the therapy period, enable beneficial cortical plasticity by generating non-nociceptive sensory input from the periphery.47 By promoting functional plasticity in response to non-painful sensory input from stimulation, percutaneous PNS therapy may “unwind” nociplastic mechanisms, thereby altering the chronic pain state. This may manifest as a decrease or absence of pain after withdrawal of the stimulating leads, even in the presence of ongoing nociceptive or neuropathic input.
THE PERCUTANEOUS PNS SYSTEM AND FINE-WIRE COILED LEADS HAVE DEMONSTRATED A STRONG SAFETY PROFILE
The percutaneous PNS system has demonstrated a strong safety profile in numerous studies in chronic and acute pain indications.33–37,48–51 Consistent with previous studies using percutaneous PNS for shoulder pain, low back pain, and acute post-surgical pain, the most common adverse events reported during the use of percutaneous PNS for post-amputation pain were mild discomfort due to the lead implantation procedure or lead withdrawal, and irritation at the lead exit site or related to adhesive tapes and bandages.30,39 The rate of suspected lead fracture during treatment of chronic pain following amputation was also consistent with previous reports from a variety of indications (7.5% across 267 leads).31–37,49,52,53 MR-Conditional lead fragments54 most commonly occurred during lead withdrawal at or near the tip of the lead, leaving a relatively short length of the 100-micron wire lead. Fragments were observed in situ and no fragment-related sequelae were subsequently reported during follow-up,30,39 similar to previous reports.33,35,37,38
PNS and percutaneous systems were historically associated with risk of infection, but a recent review found that an open-coil lead design significantly reduces this risk. Across 46 studies with over 7,000 coiled and non-coiled leads, the number of infections per lead indwelling time was 25-times lower in coiled leads compared to traditional non-coiled leads used with previous methods of PNS.55
PERCUTANEOUS PNS HAS POTENTIAL APPLICATIONS IN POST-SURGICAL AMPUTATION PAIN AND NON-AMPUTEE MILITARY SERVICE MEMBERS AND VETERANS WITH POST-TRAUMATIC PAIN
Multiple studies have evaluated the efficacy and safety of percutaneous PNS in the back and upper and lower extremities for the treatment of acute and chronic pain conditions in addition to post-amputation pain, including for the treatment of chronic neuropathic pain, musculoskeletal pain and post-surgical pain.30–37,48 A recent review of these data found that approximately 89% of patients treated with percutaneous PNS experienced clinically-meaningful (≥ 30%40) pain relief and/or reductions in the interference of pain on daily activities, and 74% experienced substantial (≥ 50%40) benefit, suggesting the therapy has the potential to be employed in patients with a wide range of pain indications.30,35–37,39,48,49,51,53,56–58
Amputation surgeries cause significant post-surgical pain in addition to the potential development of chronic nociceptive, neuropathic, and nociplastic pain. Acute post-amputation pain and post-surgical opioid consumption are key predictors of the development of chronic RLP and PLP,59 and as such post-surgical pain management is an important part of post-amputation care. Pain management strategies have been designed to reduce the risk of chronic post-surgical pain, but current strategies can be ineffective and are often limited by risk of complications (e.g., motor deficit or infection from peripheral nerve catheters) and undesirable side effects (e.g., nausea and sedation from opioid medications).60,61 Recent data suggest that percutaneous PNS can provide pain relief, functional benefit, and reduced opioid consumption in the post-surgical period following total knee replacement and a variety of ambulatory surgeries.31,33,34,38 One ongoing study is evaluating outcomes following amputation (including the incidence of chronic RLP and PLP) in patients treated with percutaneous PNS post-surgically compared to those treated with conventional pain management (Clinicaltrials.gov NCT03484429). Results from an initial case suggest that percutaneous PNS can help manage acute post-amputation pain and may have some impact on post-surgical opioid use.62 Future results from this study will be important to help determine the potential impact of PNS in the acute post-amputation setting.
Amputation is highly visible and debilitating, but traumatic combat injuries can also cause chronic pain without requiring amputation. Nerve injuries are more prevalent than amputation in trauma patients, and a large majority of nerve injuries result in chronic pain.63 Whereas amputations represented just over 3% of the 52,351 reported casualties across all services from 2001–2015,2 up to 24–53% of Service members injured in OIF/OEF had extremity pain.64,65 Because trauma and subsequent amputation result in nerve injuries similar to those in non-amputee nerve trauma patients, data from the successful use of percutaneous PNS in amputees suggest this therapy has the potential to provide significant pain relief and restoration of function following other types of injuries or trauma (both combat- and noncombat-related) that have a high prevalence among military Service members and Veterans.
CONCLUSIONS
Chronic pain in amputees is an important barrier that must be overcome in order to promote functional recovery and effective rehabilitative therapy among injured military Service members and Veterans. Recent evidence suggests that percutaneous PNS may effectively reduce chronic pain following amputation. This therapy fills an unmet need and has the potential to become a standard option to relieve post-amputation pain, and through such pain relief, optimize recovery and restoration of function for Service members and Veterans with amputations. By reducing pain and thereby restoring function in active duty amputees, percutaneous PNS may offer a viable new rehabilitative strategy that could ultimately enable return to duty. Percutaneous PNS may also provide additional benefit for military Service members and Veterans with chronic post-surgical, post-traumatic, or musculoskeletal pain.
ACKNOWLEDGMENTS
The authors would like to acknowledge Srinivasa Raja, MD, for his feedback on Tables I and II.
PRESENTATIONS
This work was presented as a poster at the Military Health System Research Symposium (MHSRS-18–1390), August 20–23, 2018, Kissimmee, FL.
FUNDING
A portion of the work was funded by the National Institute of Neuroloical Disorders and Stroke (R43NS066523) and the Department of Defense (W81XWH-12-2-0132).
REFERENCES
- 1. Fischer H: A guide to U.S. Military casualty statistics: Operation Freedom’s Sentinel, Operation Inherent Resolve, Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom 2015. Available at https://fas.org/sgp/crs/natsec/RS22452.pdf. Accessed January 31, 2019.
- 2. Butowicz CM, Dearth CL, Hendershot BD: Impact of traumatic lower extremity injuries beyond acute care: Movement-based considerations for resultant longer term secondary health conditions. Adv Wound Care 2017; 6(8): 269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gawande A: Casualties of war-military care for the wounded from Iraq and Afghanistan. N Engl J Med 2004; 351(24): 2471–5. [DOI] [PubMed] [Google Scholar]
- 4. Clark ME, Bair MJ, Buckenmaier CC III, Gironda RJ, Walker RL: Pain and combat injuries in soldiers returning from operations enduring freedom and Iraqi freedom: Implications for research and practice. J Rehabil Res Dev 2007; 44(2): 179–94. [DOI] [PubMed] [Google Scholar]
- 5. Amputee Coalition of America : U.S. Military builds on rich history of amputee care. Military in-Step 18 Sep 2008. Available at: https://www.amputee-coalition.org/military-instep/rich-history.html; Accessed January 31, 2019
- 6. Ketz AK: The experience of phantom limb pain in patients with combat-related traumatic amputations. Arch Phys Med Rehabil 2008; 89(6): 1127–32. [DOI] [PubMed] [Google Scholar]
- 7. Reiber GE, McFarland LV, Hubbard S, et al. : Servicemembers and veterans with major traumatic limb loss from Vietnam war and OIF/OEF conflicts: survey methods, participants, and summary findings. J Rehabil Res Dev 2010; 47(4): 275–97. [DOI] [PubMed] [Google Scholar]
- 8. Dougherty PJ, McFarland LV, Smith DG, Esquenazi A, Blake DJ, Reiber GE: Multiple traumatic limb loss: a comparison of vietnam veterans to OIF/OEF servicemembers. J Rehabil Res Dev 2010; 47(4): 333–48. [DOI] [PubMed] [Google Scholar]
- 9. Bonica JJ, Loeser JD: History of pain concepts and therapies In: History of pain concepts and therapies, pp 3–16. Edited by Loeser JD.New York, Lippincott Williams & Wilkins, 2001. [Google Scholar]
- 10. Kashani JH, Frank RG, Kashani SR, Wonderlich SA, Reid JC: Depression among amputees. J Clin Psychiatry 1983; 44(7): 256–8. [PubMed] [Google Scholar]
- 11. Cansever A, Uzun O, Yildiz C, Ates A, Atesalp AS: Depression in men with traumatic lower part amputation: a comparison to men with surgical lower part amputation. Mil Med 2003; 168(2): 106–9. [PubMed] [Google Scholar]
- 12. Kishbaugh D, Dillingham TR, Howard RS, Sinnott MW, Belandres PV: Amputee soldiers and their return to active duty. Mil Med 1995; 160(2): 82–4. [PubMed] [Google Scholar]
- 13. Neil MJE: Pain after amputation. BJA Educ 2016; 16(3): 107–12. [Google Scholar]
- 14. Hsu E, Cohen SP: Postamputation pain: epidemiology, mechanisms, and treatment. J Pain Res 2013; 6: 121–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nikolajsen L: Postamputation pain: studies on mechanisms. Danish Med J 2012; 59(10): B4527. [PubMed] [Google Scholar]
- 16. Flor H, Nikolajsen L, Staehelin Jensen T: Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci 2006; 7(11): 873–81. [DOI] [PubMed] [Google Scholar]
- 17. Kosek E, Cohen M, Baron R, et al. : Do we need a third mechanistic descriptor for chronic pain states? Pain 2016; 157(7): 1382–6. [DOI] [PubMed] [Google Scholar]
- 18. Alviar MJ, Hale T, Dungca M: Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev 2016; 10: CD006380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nikolajsen L, Christensen KF: Phantom limb pain In: Phantom limb pain, Vol. 2: pp 23–34. Edited by Tubbs RS, Shoja MM, Barbaro N, Rizk E, Loukas M, Spinner RJ. London, UK, Academic Press, 2015. [Google Scholar]
- 20. Aiyer R, Barkin RL, Bhatia A, Gungor S: A systematic review on the treatment of phantom limb pain with spinal cord stimulation. Pain Manag 2017; 7(1): 59–69. [DOI] [PubMed] [Google Scholar]
- 21. Hall N, Eldabe S: Phantom limb pain: a review of pharmacological management. Br J Pain 2018; 12(4): 202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Campbell JN, Long DM: Peripheral nerve stimulation in the treatment of intractable pain. J Neurosurg 1976; 45(6): 692–9. [DOI] [PubMed] [Google Scholar]
- 23. Long DM: Electrical stimulation for relief of pain from chronic nerve injury. J Neurosurg 1973; 39(6): 718–22. [DOI] [PubMed] [Google Scholar]
- 24. Long DM, Erickson D, Campbell J, North R: Electrical stimulation of the spinal cord and peripheral nerves for pain control. A 10-year experience. Appl Neurophysiol 1981; 44(4): 207–17. [DOI] [PubMed] [Google Scholar]
- 25. Hassenbusch SJ, Stanton-Hicks M, Schoppa D, Walsh JG, Covington EC: Long-term results of peripheral nerve stimulation for reflex sympathetic dystrophy. J Neurosurg 1996; 84(3): 415–23. [DOI] [PubMed] [Google Scholar]
- 26. Novak CB, Mackinnon SE: Outcome following implantation of a peripheral nerve stimulator in patients with chronic nerve pain. Plast Reconstr Surg 2000; 105(6): 1967–72. [DOI] [PubMed] [Google Scholar]
- 27. Shetter AG, Racz GB, Lewis R, et al. : Peripheral nerve stimulation In: Neurosurgical management of pain, pp 261–70. Edited by North RB, Levy RM. New york, Springer, 1997. [Google Scholar]
- 28. McJunkin TL, Lynch PJ, Srejic E: Complications of peripheral nerve stimulation: open technique, percutaneous technique, and peripheral nerve field stimulation In: Reducing Risks and Complications of Interventional Pain Procedures, pp. 11–18. Edited by Ranson M, Pope J, Deer T. Philadelphia, Elsevier Saunders, 2012. [Google Scholar]
- 29. Ishizuka K, Oaklander AL, Chiocca EA: A retrospective analysis of reasons for reoperation following initially successful peripheral nerve stimulation. J Neurosurg 2007; 106(3): 388–90. [DOI] [PubMed] [Google Scholar]
- 30. Rauck RL, Cohen SP, Gilmore CA, et al. : Treatment of post-amputation pain with peripheral nerve stimulation. Neuromodulation 2014; 17(2): 188–97. [DOI] [PubMed] [Google Scholar]
- 31. Ilfeld BM, Ball ST, Gabriel RA, et al. : A feasibility study of percutaneous peripheral nerve stimulation for the treatment of postoperative pain following total knee arthroplasty. Neuromodulation 2018 10.1111/ner.12790; (E-pub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kapural L, Gilmore CA, Chae J, et al. : Percutaneous peripheral nerve stimulation for the treatment of chronic low back pain: two clinical case reports of sustained pain relief. Pain Pract 2018; 18(1): 94–103. [DOI] [PubMed] [Google Scholar]
- 33. Ilfeld BM, Gabriel RA, Said ET, et al. : Ultrasound-guided percutaneous peripheral nerve stimulation: neuromodulation of the sciatic nerve for postoperative analgesia following ambulatory foot surgery, a proof-of-concept study. Reg Anesth Pain Med 2018; 43(6): 580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ilfeld BM, Said ET, Finneran JJ, et al. : Ultrasound-guided percutaneous peripheral nerve stimulation: neuromodulation of the femoral nerve for postoperative analgesia following ambulatory anterior cruciate ligament reconstruction: A proof of concept study. Neuromodulation 2018 10.1111/ner.12851; (E-pub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chae J, Yu DT, Walker ME, et al. : Intramuscular electrical stimulation for hemiplegic shoulder pain: a 12-month follow-up of a multiple-center, randomized clinical trial. Am J Phys Med Rehabil 2005; 84(11): 832–42. [DOI] [PubMed] [Google Scholar]
- 36. Wilson RD, Gunzler DD, Bennett ME, Chae J: Peripheral nerve stimulation compared with usual care for pain relief of hemiplegic shoulder pain: a randomized controlled trial. Am J Phys Med Rehabil 2014; 93(1): 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu DT, Chae J, Walker ME, et al. : Intramuscular neuromuscular electric stimulation for poststroke shoulder pain: a multicenter randomized clinical trial. Arch Phys Med Rehabil 2004; 85(5): 695–704. [DOI] [PubMed] [Google Scholar]
- 38. Ilfeld BM, Grant SA: Ultrasound-guided percutaneous peripheral nerve stimulation for postoperative analgesia: could neurostimulation replace continuous peripheral nerve blocks? Reg Anesth Pain Med 2016; 41(6): 720–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gilmore CA, Ilfeld BM, Rosenow JM, et al. : Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic post-amputation pain: a multi-center randomized placebo-controlled trial. Reg Anesth Pain Med. 10.1136/rapm-2018-100109. [DOI] [PubMed] [Google Scholar]
- 40. Dworkin RH, Turk DC, Wyrwich KW, et al. : Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008; 9(2): 105–21. [DOI] [PubMed] [Google Scholar]
- 41. Meier RH 3rd, Heckman JT: Principles of contemporary amputation rehabilitation in the united states, 2013. Phys Med Rehabil Clin N Am 2014; 25(1): 29–33. [DOI] [PubMed] [Google Scholar]
- 42. Phan T, Murphy D, Lester D: Effects of percutaneous peripheral nerve stimulation on ambulation in an amputee with refractory pain. AAPM&R Conference. 2018. Orlando, FL.
- 43. Chung JM, Lee KH, Hori Y, Endo K, Willis WD: Factors influencing peripheral nerve stimulation produced inhibition of primate spinothalamic tract cells. Pain 1984; 19(3): 277–93. [DOI] [PubMed] [Google Scholar]
- 44. Slavin KV: Peripheral nerve stimulation for neuropathic pain. Neurotherapeutics 2008; 5(1): 100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Melzack R, Wall PD: Pain mechanisms: a new theory. Science 1965; 150(699): 971–9. [DOI] [PubMed] [Google Scholar]
- 46. Birbaumer N, Lutzenberger W, Montoya P, et al. : Effects of regional anesthesia on phantom limb pain are mirrored in changes in cortical reorganization. J Neurosci 1997; 17(14): 5503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Flor H: The modification of cortical reorganization and chronic pain by sensory feedback. Appl Psychophysiol Biofeedback 2002; 27(3): 215–27. [DOI] [PubMed] [Google Scholar]
- 48. Gilmore CA, Kapural L, McGee MJ, Boggs JW: Percutaneous peripheral nerve stimulation (PNS) for the treatment of chronic low back pain provides sustained relief. Neuromodulation 2018 10.1111/ner.12854; (E-pub ahead of print). [DOI] [PubMed] [Google Scholar]
- 49. Chae J, Wilson RD, Bennett ME, Lechman TE, Stager KW: Single-lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case series. Pain Pract 2013; 13(1): 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Renzenbrink GJ, IJzerman MJ: Percutaneous neuromuscular electrical stimulation (P-NMES) for treating shoulder pain in chronic hemiplegia. Effects on shoulder pain and quality of life. Clin Rehabil 2004; 18(4): 359–65. [DOI] [PubMed] [Google Scholar]
- 51. Ilfeld BM, Gilmore CA, Grant SA, et al. : Ultrasound-guided percutaneous peripheral nerve stimulation for analgesia following total knee arthroplasty: a prospective feasibility study. J Orthop Surg Res 2017; 12(4): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wilson RD, Bennett ME, Lechman TE, Stager KW, Chae J: Single-lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case report. Arch Phys Med Rehabil 2011; 92(5): 837–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yu DT, Chae J, Walker ME, Fang ZP: Percutaneous intramuscular neuromuscular electric stimulation for the treatment of shoulder subluxation and pain in patients with chronic hemiplegia: a pilot study. Arch Phys Med Rehabil 2001; 82(1): 20–5. [DOI] [PubMed] [Google Scholar]
- 54. Shellock FG, Zare A, Ilfeld BM, Chae J, Strother RB: In vitro magnetic resonance imaging evaluation of fragmented, open-coil, percutaneous peripheral nerve stimulation leads. Neuromodulation 2018; 21(3): 276–83. [DOI] [PubMed] [Google Scholar]
- 55. Ilfeld BM, Gabriel RA, Saulino MF, et al. : Infection rates of electrical leads used for percutaneous neurostimulation of the peripheral nervous system. Pain Pract 2017; 17(6): 753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wilson RD, Harris MA, Gunzler DD, Bennett ME, Chae J: Percutaneous peripheral nerve stimulation for chronic pain in subacromial impingement syndrome: a case series. Neuromodulation 2014; 11(10): 12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nguyen VQ, Bock WC, Groves CC, et al. : Fully implantable peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case report. Am J Phys Med Rehabil 2015; 94(2): 146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ilfeld BM, Grant SA, Gilmore CA, et al. : Neurostimulation for postsurgical analgesia: a novel system enabling ultrasound-guided percutaneous peripheral nerve stimulation. Pain Pract 2017; 17(7): 892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hanley MA, Jensen MP, Smith DG, Ehde DM, Edwards WT, Robinson LR: Preamputation pain and acute pain predict chronic pain after lower extremity amputation. J Pain 2007; 8(2): 102–9. [DOI] [PubMed] [Google Scholar]
- 60. Apfelbaum JL, Chen C, Mehta SS, Gan TJ: Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg 2003; 97(2): 534–40. [DOI] [PubMed] [Google Scholar]
- 61. Kent ML, Hsia HJ, Van de Ven TJ, Buchheit TE: Perioperative pain management strategies for amputation: a topical review. Pain Med 2017; 18(3): 504–19. [DOI] [PubMed] [Google Scholar]
- 62. Phan T, Trainer B, Lester D: Postoperative management of transtibial amputation in an opioid-tolerant patient using peripheral nerve stimulation. 2018 World Congress on Regional Anesthesia and Pain Medicine. New York, NY. Available at https://epostersonline.com/ASRAWORLD18/node/805. Accessed January 31, 2019.
- 63. Ciaramitaro P, Mondelli M, Logullo F, et al. : Traumatic peripheral nerve injuries: epidemiological findings, neuropathic pain and quality of life in 158 patients. J Peripher Nerv Syst 2010; 15(2): 120–7. [DOI] [PubMed] [Google Scholar]
- 64. Cohen SP, Griffith S, Larkin TM, Villena F, Larkin R: Presentation, diagnoses, mechanisms of injury, and treatment of soldiers injured in operation Iraqi freedom: an epidemiological study conducted at two military pain management centers. Anesth Analg 2005; 101(4): 1098–1103. [DOI] [PubMed] [Google Scholar]
- 65. Clark ME, Scholten JD, Walker RL, Gironda RJ: Assessment and treatment of pain associated with combat-related polytrauma. Pain Med 2009; 10(3): 456–69. [DOI] [PubMed] [Google Scholar]