Abstract
A complex and highly orchestrated gene expression program chiefly establishes the properties that define the adipocyte phenotype, in which the vast majority of factors are involved in transcriptional regulation. However, the mechanisms by post-transcriptional modulation are poorly understood. Here, we showed that zinc finger protein (Zfp217) couples gene transcription to m6A mRNA modification to facilitate adipogenesis. Zfp217 modulates m6A mRNA methylation by activating the transcription of m6A demethylase FTO. Consistently, depletion of Zfp217 compromises adipogenic differentiation of 3T3L1 cells and results in a global increase of m6A modification. Moreover, the interaction of Zfp217 with YTHDF2 is critical for allowing FTO to maintain its interaction with m6A sites on various mRNAs, as loss of Zfp217 leads to FTO decrease and augmented m6A levels. These findings highlight a role for Zfp217-dependent m6A modification to coordinate transcriptional and post-transcriptional regulation and thus promote adipogenic differentiation.
INTRODUCTION
The global incidence of obesity and Type 2 diabetes has increased over the last three decades. It is well confirmed that adipose tissue greatly contributes to obesity-associated diseases. Thus, the manipulation of adipocyte differentiation and maturation could be a promising strategy for the treatment of obesity-related diseases (1). Considerable efforts have been made to elucidate the role of transcriptional and epigenetic regulation in adipogenesis and identify a vast majorly of key regulators and pathways (1,2). However, the function of post-transcriptional regulation in adipogenesis is not well understood.
N6-methyladenosine (m6A) has been identified as the most abundant modification present on eukaryotic messenger RNA (mRNA) (3–5), and plays a role in regulating cell fate and lineage transition in embryonic stem cells (5–8). The ‘writer’complex, which catalyzes m6A mRNA methylation consists of methyltransferase-like 3 (METTL3), methyltransferase-like 14 (METTL14) and Wilms tumor 1-associated protein (WTAP), was recently shown to regulate mitotic clonal expansion in adipogenesis (9,10). Notably, the first ‘eraser’ protein mediating the reversal of m6A methylation, fat mass and obesity-associated protein (FTO) has been identified in Genome-Wide Association Studies as a candidate in obesity (11,12) and also plays a critical role in maintaining adipogenesis through RNA splicing in an m6A-dependent way (13–15). Recently, it was reported that the m6A-binding protein YTH domain-containing family 2 (YTHDF2), in addition to acting as a reader of m6A modifications, can also prevent FTO from demethylating heat shock stress-induced transcripts (3–5,16). However, the regulation of m6A modification by proteins in adipogenesis is poorly understood.
Zinc finger protein 217 (Zfp217, human homolog ZNF217) is a well-known oncogenic protein upregulated in a variety of human tumors (17–19), and is also critical for embryonic stem cell differentiation (8,20,21). Noticeably, Zfp217 tightly couples gene transcription with m6A modification on the nascent RNA, suggesting a key role for Zfp217 in coordinating epigenetic and epitranscriptomic networks (8,22). While we previously identified a novel role for Zfp217 in adipogenesis, a detailed Zfp217-dependent mechanism has not been well characterized (23,24). However, these studies raise the possibility that Zfp217 may modulate the m6A modification to accelerate adipogenesis.
In this study, we find that Zfp217 deficiency impairs adipogenesis in 3T3L1 cells and leads to a global increase in m6A mRNA methylation. Furthermore, Zfp217 transcriptionally activates FTO gene expression and orchestrates m6A mRNA modification in an m6A-YTHDF2-dependent manner. Taken together, these findings illustrate that Zfp217 is an essential and multi-faceted regulator that promotes adipogenesis at both the transcriptional and post-transcriptional level.
MATERIALS AND METHODS
Cell culture and differentiation
3T3L1 and HEK293T cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco, San Diego, CA, USA) with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin/streptomycin. MEFs were prepared from 13.5-d embryos from Zfp217+/− × Zfp217+/− mice as reported elsewhere (25). For adipogenic differentiation, cells were treated with 1 μM DEX, 0.5 mM isobutyl-methylxanthine, 10 μg/ml insulin and 100 μmol/l Indomethacin. After 2 days, the cells were transferred to 10% FBS medium containing only 10 μg/ml insulin and maintained in this medium for 2 days; subsequently, cells were maintained in 10% FBS for another 2 days.
CRISPR/Cas9 knockout of Zfp217
The Zfp217 gene sequence was entered into the Zhang Lab’s online generator (http://crispr.mit.edu/), and the three CRISPR guide sequences that bind upstream and downstream with close proximity to target (TAG = 0) were chosen. Guide RNA (sgRNA) sequences were listed in Supplementary Table S1. These sequences were cloned into the pSpCas9(BB)-2A-GFP (PX458) plasmid (Addgene Plasmid # 48138). The activity of these sgRNAs was analyzed by T7E1 assay and those with the highest activity were chosen for further use. To establish Zfp217 knockout 3T3L1 cell line, PX458-sgZfp217 was transfected into 3T3L1 cells using Lipofectamine 2000. Two days after transfection, single cell-derived clone was sorted with fluorescence-activated of eGFP+ using a BD FACSAria II sorter- 488 nm (100 mw) (BD Biosciences, San Jose, CA, USA). After 7–10 days for expansion, clones were screened for CRISPR-mediated deletion with sanger sequencing. Zfp217 knockout mice got from Cyagen Biosciences Inc. (China) and animal procedures were performed under the ethical guidelines of the Institutional Animal Care and Use Committees at the Huazhong Agricultural University.
Plasmids and transfection
Zfp217, YTHDF2 and FTO cDNA was obtained from a mouse cDNA library and cloned into a pCMV-N-FLAG vector (Clontech, Pato Alto, CA, USA) at the BamH1 sites. About 2000 bp of FTO promoter fragments were cloned into a pGL3-basic vector (Promega, Madison, USA) at the KpnI and XhoI sites. Deletion fragments of Zfp217-binding site (ATTCTG) were generated using this plasmid DNA as a template.The primers used for PCR amplification are listed in Supplementary Table S2. For transfection, cells were seed into a 12-well plate and transfected with plasmids using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), following the manufacturer’s instructions.
Oil red O (ORO) staining
The cells were washed twice with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde for 30 min at room temperature. Cells were then washed again with PBS and stained with freshly diluted Oil red O (Sigma-Aldrich, St. Louis, MO, USA) for 15 min. After staining, the cells were washed with PBS and photographed using a microscope (Nikon, Japan).
RNA isolation, real-time quantitative PCR (QPCR) and m6A dot blot
Total RNA was extracted using the Trizol reagent (Invitrogen, USA) and transcribed into cDNA using a first-strand cDNA synthesis kit (TOYOBO, Japan). Quantitation of the mRNA level by QPCR was performed on a real-time PCR system using iTaq Universal SYBR Green Supermix (Bio-Rad, Richmond, CA, USA). The cycle thresholds (CT) of the target gene was normalized to the CT of the internal reference β-actin gene using the formula ‘2−ΔΔCt’, which yielded relative gene expression level values. The primers used are listed in Supplementary Table S3. For m6A dot blots, RNA samples were denatured at 65°C for 5 min. An equal volume of chilled 20× SSC buffer (Sigma-Aldrich) was then added before samples were spotted on the Amersham Hybond-N+ membrane (GE Healthcare). After UV crosslinking, the membrane was washed with PBST buffer, blocked with 5% non-fat milk and incubated with anti-m6A antibody (Abcam, USA, #151230) overnight at 4°C. Then, the secondary antibody was incubated at room temperature for 1 h. The membrane was exposed by using WesternBright™ Peroxide (Advansta, California, USA) in the imaging system (Carestream, New York, USA).
Western blot and immunoprecipitation (IP)
The cells were extracted with protein lysis buffer (Beyotime, China) supplemented with protease inhibitor cocktail. Protein concentration was determined using the BCA Kit (Beyotime, China). Proteins (25–35 μg) were separated on a 10% polyacrylamide precast SDS gel (Bio-Rad) followed by blotting on PVDF membranes (Millipore Billerica, MA, USA). The membranes were probed with the following antibodies against: Zfp217 (Abcam, USA, #48133), METTL3 (Abcam, #195352), ALKBH5 (Abcam, #69325), PPARγ (Cell Signaling Technology, USA, #C26H12), aP2 (Cell Signaling Technology, #D25B3), FTO (Santa cruz, USA, #sc-271713), YTHDF2 (Proteintech, China, #24744-1-AP), β-actin (Abclonal, China, #AC026), LMNB1 (Abclonal, #A1910) and Tublin (Abclonal, #AC021). Secondary antibodies and detection were according to description previously. For IP experiments, cells were lysed in IP buffer (Beyotime, China) and incubated with antibodies followed by the pull-down with protein A/G beads for subsequent western blot analysis.
Cell cycle and apoptosis analysis
For cell cycle analysis, cells were trypsinized and fixed with 70% ethanol. and then incubated with 50 μg/ml propidium iodide (PI). DNA content of single cell was quantified by flow cytometry analysis. For cell apoptosis analysis, cells were trypsinized and incubated with Annexin V-PE/7-AAD. Dead cells were quantified by flow cytometry analysis.
Luciferase reporter assays
Luciferase reporter assays were performed as described in a previous study (26). The FTO-promoter (or Zfp217 binding site-deleted fragments) luciferase vector and renilla luciferase-expressing plasmid (pTK) were co-transfected with pCMV-N-Flag-Zfp217 or empty vector. After 24 h transfection, cells were lysed using Dual-Glo luciferase reagent (Promega, Madison, USA). The luciferase activity was determined using a dual-luciferase reporter assay system and luminometer (Dynex Technologies, UK). The luciferase values were normalized to the renilla values.
Chromatin immunoprecipitation (ChIP) assay
ChIP was performed using the ChIP Assay Kit according to the manufacturer’s instructions (Beyotime, China). Briefly, after crosslinking (1% formaldehyde), cells were quenched by addition of 0.125 M glycine and harvested in SDS lysis buffer for sonication digestion, the DNA–protein complexes were immunoprecipitated with ChIP grade antibodies against Zfp217 or IgG. DNA obtained from the immunoprecipitation were analyzed by quantitative PCR and normalized to inputs. The primers used are listed in Supplementary Table S4.
Cytoplasmic and nuclear extracts extraction
Cytoplasmic and nuclear extracts were prepared using nuclear and cytoplasmic extraction kit (BestBio, China) according to the manufacturer’s instructions. The protein expression level of Zfp217 and YTHDF2 in nucleus or cytoplasm were detected by western blot. Lamin B1 was used as a nuclear envelope marker. Tubulin was used as a cytoplasmic protein marker.
Immunofluorescence and confocal imaging
Cells were washed twice with PBS and fixed in 4% paraformaldehyde for 30 min at room temperature. After blocked with 1% BSA for 1 h. Cells were stained with indicated primary antibody Zfp217 (Thermo fisher, #MA5-27088) and YTHDF2 (Proteintech, #24744-1-AP) 2 h, followed by incubation with Alexa Fluor 555 goat anti-mouse secondary antibody or Alexa Fluor 488 goat anti-rabbit secondary antibody for 1 h. DAPI (4,6-diamidino-2-phenylindole; Molecular Probes) was used to label DNA. Confocal imaging performed using a confocal laser scanning microscope (Carl Zeiss, Germany) equipped with an incubation chamber and a motorized table.
RNA interference
Synthetic siRNA oligonucleotides specific for regions in the mouse Zfp217 and YTHDF2 mRNA were designed and synthesized by GenePharma (Shanghai, China). The sequences that gave successful knockdown were Zfp217-1, 5′-CACACTTCCACGGAATCATAC-3′; Zfp217-2, 5′-TCACATCAGCACCTATCTAAC-3′ (8); METTL3, 5′-CGTCAGTATCTTGGGCAAATT-3′ (8) YTHDF2, 5′- GCTCCAGGCATG AATACTATA -3′(16); Ccdc141, 5′-GCGCAUCACUUUCA GACAATT-3′; Hspa1a-1, 5′- GCGACCUGAACAAGAGCAUTT -3′; Hspa1a-2, 5′- GACUUCUA CACAUCCAUCATT -3′; Efcab11, 5′- CCCAACACUUCUGGUGU UUTT -3′. Negative control (NC) siRNA was 5′-TTCTCCGAACGTGTCACGT-3′. Cells were transfected at 50–70% confluence with siRNA duplexes using Lipofectamine RNAiMAX (Invitrogen) in accordance with the manufacturer’s instructions.
Quantitative analysis of m6A level using LC-MS/MS
Quantification of the m6A and cap m6Am in polyadenylated RNA using LC-MS/MS as described previously (27–29). Polyadenylated RNA was purified from total RNA using Dynabeads® mRNA DIRECT kit (Thermofisher, #61006) according to the manufacturer’s instructions, and further treated with RppH (NEB, M0356S) to remove the cap. About 200 ng polyadenylated RNA was digested with 2U nuclease P1 (Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 2 h in 25 μl of buffer containing 25 mM NaCl and 2.5 mM of ZnCl2, followed by the addition of NH4HCO3(1 M, 3 μl) and alkaline phosphatase (0.5 U) for 2 h at 37°C. The digestion mixture was diluted to 1 ml using deionized water and centrifuged at 10 000 rpm for 5 min to remove any solid material. The digestion mixture was then filtered with 0.22 μM Millipore membrane and 5 μl of this solution was injected into the LC-MS/MS system. A, m6A and m6Am were later separated by UPLC-ESI-QQQ on a C18 column (Rapid Resolution HT, 50 mm × 2.1 mm I.D, 1.8 μm, Agilent, Santa Clara, California, USA). Formic acid in water (0.1%, v/v, solvent A) and formic acid in methanol (0.1%, v/v, solvent B) were employed as the mobile phase. The mass spectrometry detection was performed under positive electrospray ionization mode, by which the product ion scans of the protonated A at m/z (268 → 136), m6A at m/z (282 → 150) and m6Am m/z (296 → 150) were acquired. The quantification was carried out using a standard curve generated from A, m6A and m6Am standards (0.1–10 nM for m6A and m6Am, 50–2000 nM for A) ran during the same batch of the samples. The m6A and m6Am levels were calculated as the ratio of m6A and m6Am to A.
MeRIP QPCR of target genes
m6A immunoprecipitation (MeRIP) was performed as described previously (27,30,31). A 1 μg aliquot of m6A antibody (Abcam, #ab151230) and Normal rabbit IgG (ABclonal, #AC005) were respectively conjugated to 20 μl SureBeads protein A/G mixed magnetic beads (Bio-rad, #161-4013; #161-4023) overnight at 4°C. A 100 μg aliquot of fragmented total RNA was incubated with the antibody in immunoprecipitation buffer (50 mM Tris-HCl, 750 mM NaCl and 0.5% NP40) supplemented with 40U RNase inhibitor overnight at 4°C. RNA was eluted from the beads by incubation in 300 μl of elution buffer (5 mM Tris-HCl, 1 mM EDTA and 0.05% SDS) with 8.4 μg of proteinase K for 1.5 h at 50°C. Following phenol extraction and ethanol precipitation, the input and m6A-enriched RNA were reversely transcribed with random hexamers, and the enrichment was determined by QPCR. The primers used for detection for m6A-enriched gene mRNA were shown in Supplementary Table S5.
In vitro RNA pull-down and competition assay
Synthesized mRNA with a single m6A at A103 was label by biotin (Takara, Japan). Binding of the labeled RNA to streptavidin magnetic beads was performed in RNA capture buffer (20 mM Tris, pH 7.5, 1 M NaCl, 1 mM EDTA) for 30 min at room temperature with rotation. The RNA-protein binding reaction was conducted in protein–RNA binding buffer (20 mM Tris (pH 7.5), 50 M NaCl, 2 mM MgCl2, 0.1% Tween-20 Detergent) at 4°C for 60 min with rotation. After washing three times with the wash buffer (20 mM Tris (pH 7.5), 10 mM NaCl, 0.1% Tween-20 Detergent), protein was eluted by Biotin Elution Buffer (Pierce) and detected by western blot (16,32). In vitro competition assay was performed as previous report (29). Briefly, 1 μg polyadenylated RNA purified from 3T3L1 cells was incubated with 2 μg FTO, Zfp217 and YTHDF2 in 100 μl of reaction buffer containing KCl (100 mM), MgCl2 (2 mM), SUPERNase In (0.2 U/ml), L-ascorbic acid (2 mM), a-ketoglutarate (300 mM), (NH4)2Fe(SO4)2·6H2O (150 mM) and 50 mM of HEPES buffer (pH 6.5). The reaction buffer was incubated for 3 h at room temperature, and quenched by adding 5 mM EDTA. RNA was isolated by TRIzol and followed by dot blot.
Localized surface plasmon resonance (LSPR)
The protein–protein and protein–RNA interactions were measured using an OpenSPR localized surface plasmon resonance (LSPR) biosensor (Nicoya Life Science, Inc., Kitchener, Canada), as described previously (33,34). In brief, 100 μl (1 μg/μl) of Flag-YTHDF2 was immobilized on a COOH sensor chip (Nicoya # SEN-AU-100-12-COOH) at a flow rate of 20 μl/min in 1× PBS buffer (pH = 7.4, RNase-free) and 0.1% v/v Tween 20. Free activated carboxyl groups were deactivated with the addition of 100 μl blocking buffer (Nicoya). The immobilized protein was washed with running buffer (1× PBS, pH 7.4; 0.1% v/v Tween 20; 0.1% w/v BSA, RNase-free) until a stable baseline was achieved. Buffer-matched recombinant Flag-Zfp217 (20 μl; 325–650 nM) or m6A-modificated mRNA were injected into the flow cell at a rate of 20 μl/min. Following a 5-min interaction time, the dissociation was recorded for an additional 7 min. Kinetic binding analysis was performed with the TraceDrawer software package (Ridgeview Instruments, Uppsala, Sweden).
RNA-seq and relative data analysis
RNA-Seq was performed at Novogene Bioinformatics Institute (China). In brief, RNA samples from WT and Zfp217 knockout with MDI inducing 0 and 2 d were extracted using the Trizol reagent (Invitrogen). RNA purity was checked using the NanoPhotometer® spectrophotometer (IMPLEN, CA, USA). Sequencing libraries were generated using NEBNext® Ultra™ RNA Library Prep Kit for Illumina® (NEB, USA). Sequencing was then performed on an Illumina Hiseq platform and 150 bp paired-end reads were generated. Reads of each sample were aligned to the mouse genome (NCBI build 38/mm10) using Bowtie v2.2.3. HTSeq v0.6.1 was used to count the reads numbers mapped to each gene and then FPKM of each gene was calculated. Differential expression genes was identified by the DESeq R package (1.18.0) using a false discovery rate (FDR) < 0.05 and fold-change >1.5 or < 0.7. Gene Ontology (GO) enrichment analysis of differentially expressed genes was implemented by the GOseq R package.
m6A-seq assays and data analysis
RNA samples were fragmented by RNA fragmentation buffer (10 mM Tris-HCl (pH 7.0), 10 mM ZnCl2). Fragmented RNA was incubated for 2 h at 4°C with 0.5 mg/ml anti-m6A antibody in IP buffer (150 mM NaCl, 0.1% Igepal CA-630, 10 mM Tris-HCl (pH 7.4)) with RNasin (40 U/μl). Library preparation and sequencing were performed at Novogene Bioinformatics Institute (China) as previously description. For data analysis, read sequences were filtered by the FASTQC and then aligned to the mouse genome (NCBI build 38/mm10) using BWA mem v 0.7.12. MACS version 2.1.0 was used for peak detection. A q-value threshold of enrichment of 0.05 was used for all data sets. MEME and DREME were used to detect the sequence motif, after which, Tomtom software was performed to annotate the motifs. Integrative Genomics Viewer (IGV) were used to visualize the distributions of the m6A peaks.
Statistical analysis
Data were analyzed using GLM procedures followed by Tukey’s post-hoc tests using the SAS statistical package (v 8.2, SAS Inst., Inc., Cary, NC). All values are presented as mean ± standard deviation. P value <0.05 indicated significant difference.
RESULTS
Loss of Zfp217 retards adipose differentiation
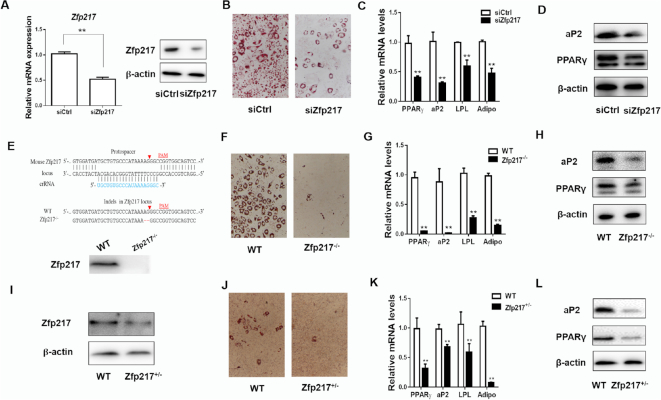
To explore the role of Zfp217 in adipogenic differentiation, we conducted loss-of-function assays by using siRNA that exhibited significant reduction of Zfp217 mRNA and protein (Figure 1A and Supplementary Figure S1). Zfp217 knockdown significantly blocked adipogenesis as indicated by decreased level of Oil Red O staining as well as lower expression of key adipogenic genes PPARγ, aP2, LPL and Adiponectin (Figure 1B–D). Furthermore, Zfp217 gene was totally deleted using CRISPR/Cas9 system (Figure 1E), which abolished adipogenesis in 3T3L1 cells (Figure 1F–H). We also examined the effects of Zfp217 decrease on adipogenesis using the embryonic fibroblasts from mice with haploinsufficiency of Zfp217 (MEF- Zfp217+/−, Figure 1I–L). Overall, our findings suggested that Zfp217 has a profound impact on adipose differentiation.
Figure 1.
Zfp217 depletion impairs adipogenesis of 3T3L1 cells. (A) The mRNA and protein expression levels of Zfp217 were detected in 3T3L1 cells treated with siRNA for 36 h (n = 3). (B) The adipogenic phenotypes of 3T3L1 cells transfected with siZfp217 or siCtrl after MDI induction for 6 days were assessed by ORO staining; magnification: 200×. 3T3L1 cells were differentiated into adipocytes using MDI cocktail medium. (C) mRNA levels of adipogenic key genes PPARγ, aP2, LPL and Adiponectin at day 6 were detected by QPCR (n = 3). (D) Protein levels of PPARγ and aP2 at day 6 were detected by western blot (n = 3). (E) Schematic representation of knockout of Zfp217. (F) The adipogenic phenotypes of WT and Zfp217−/- cells with the treatment of MDI for 6 days. (G) mRNA levels of PPARγ, aP2, LPL and Adiponectin from WT and Zfp217−/- cells with the treatment of MDI for 6 days (n = 3). (H) Protein levels of PPARγ and aP2 from WT and Zfp217−/- cells with the treatment of MDI for 6 days (n = 3). (I) Protein expression of Zfp217 in MEF-Zfp217+/- cells. (J) The adipogenic phenotypes of MEF-Zfp217+/- cells with the treatment of MDI for 6 days. (K) mRNA expression of adipogenic key genes in MEF-Zfp217+/- cells with the treatment of MDI for 6 days (n = 3). (l) protein expression of adipogenic key genes in MEF-Zfp217+/- cells with the treatment of MDI for 6 days (n = 3). Presented as means ± SD ( **P < 0.01).
Zfp217 inhibition enhances m6A modification during adipogenesis
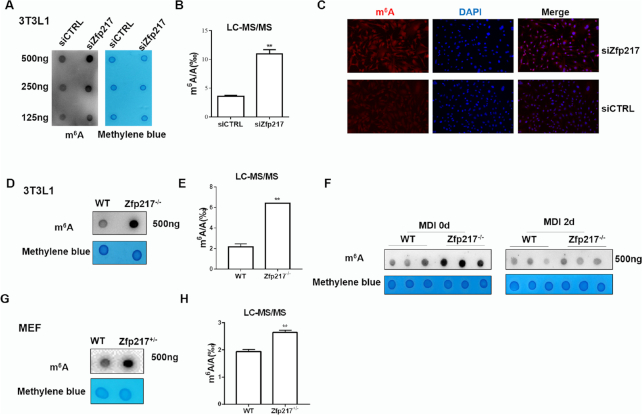
Previously, Zfp217 was reported to influence m6A modification in embryo stem cells (ESCs) maintenance (8). Here, to screen function of Zfp217 in 3T3L1 cells, we detected m6A modification in mRNAs by using dot blot and liquid chromatography-tandem mass spectrometry (LC-MS/MS) quantification. It showed a global increase of m6A level in Zfp217-depleted cells by using siRNA and CRISPR/Cas9 system, compared with the control group (Figure 2A, B, D and E). m6A immunostaining method also revealed higher m6A modification in Zfp217 knockdown cells (Figure 2C). Recently, some papers reported that FTO is a multifunctional demethylase, not only for m6A, but also for m6Am of mRNA (29). Performed by LC-MS/MS, we also found that m6Am level increased significantly in Zfp217-deficient cells (Supplementary Figure S2). It may be noted that the total amount of m6A is around 10-fold higher than that of m6Am in mRNA, which is consistent with the data mentioned in this paper. Therefore, m6A rather than m6Am may be main point to focus on here. Moreover, to identify the role of Zfp217 in regulating m6A modification during adipogenesis, m6A levels were detected by dot blot after 2-day MDI induction. It showed no difference between the control and Zfp217-depleted cells, which may imply the early function of Zfp217-m6A way in adipogenesis (Figure 2F). m6A modification was also dramatically increased in the MEF-Zfp217+/- comparing with wild-type (Figure 2G and H). Taken together, these results advised that Zfp217 is critical for cellular m6A RNA methylation.
Figure 2.
Zfp217 depletion increases m6A modification in 3T3L1 cells. (A) Dot blot was used to detect the m6A modification after knockdown of Zfp217. Methylene blue staining was used as a loading control (n = 3). (B) m6A/A ratio in polyadenylated RNA was measured from control and Zfp217 knockdown 3T3L1 cells using LC-MS/MS (n = 2). (C) m6A immunostaining of 3T3L1 cells transfected with siCtrl and siZfp217. Nucleus was stained with DAPI (magnification: 200×). (D) Dot blot was used to detect the m6A modification after knockout of Zfp217 (n = 3). (E) m6A modification levels were measured after knockout of Zfp217 using LC-MS/MS (n = 2). (F) m6A level during adipogenesis of 3T3L1 (n = 3). (G) Dot blot was used to detect the m6A modification in MEF-Zfp217+/- cells (n = 3). (H) m6A modification level was measured in MEF-Zfp217+/− cells using LC-MS/MS (n = 2). Presented as means ± SD (**P < 0.01).
Zfp217 directly activates the transcription of FTO
Although Zfp217 inactivation in cells influenced m6A modification, Zfp217 is not a methyltransferase or demethylase per se. How does Zfp217 inhibit m6A mRNA methylation? To clarify the role of Zfp217 in regulating m6A modification, we detected the expression of key proteins involved in m6A methylation. Interestingly, knockdown of Zfp217 markedly increased the protein expression of METTL3 and decreased the expression of FTO (Figure 3A). We also found that knockdown of METTL3 increased the protein expression of Zfp217, and METTL3 knockdown rescued the m6A modification level in Zfp217 knockdown cells (Supplementary Figure S3A and B). These findings suggested that both FTO and METTL3 may have functions in Zfp217-related m6A modification. Subsequently, mRNA level of FTO was coordinately regulated by Zfp217 overexpression or knockdown in 3T3L1 cells (Figure 3B), whereas METTL3 level was not changed (Supplementary Figure S3C and D). Henceforth, we refer to verify whether Zfp217 could regulate the expression of FTO as a transcriptional factor.
Figure 3.
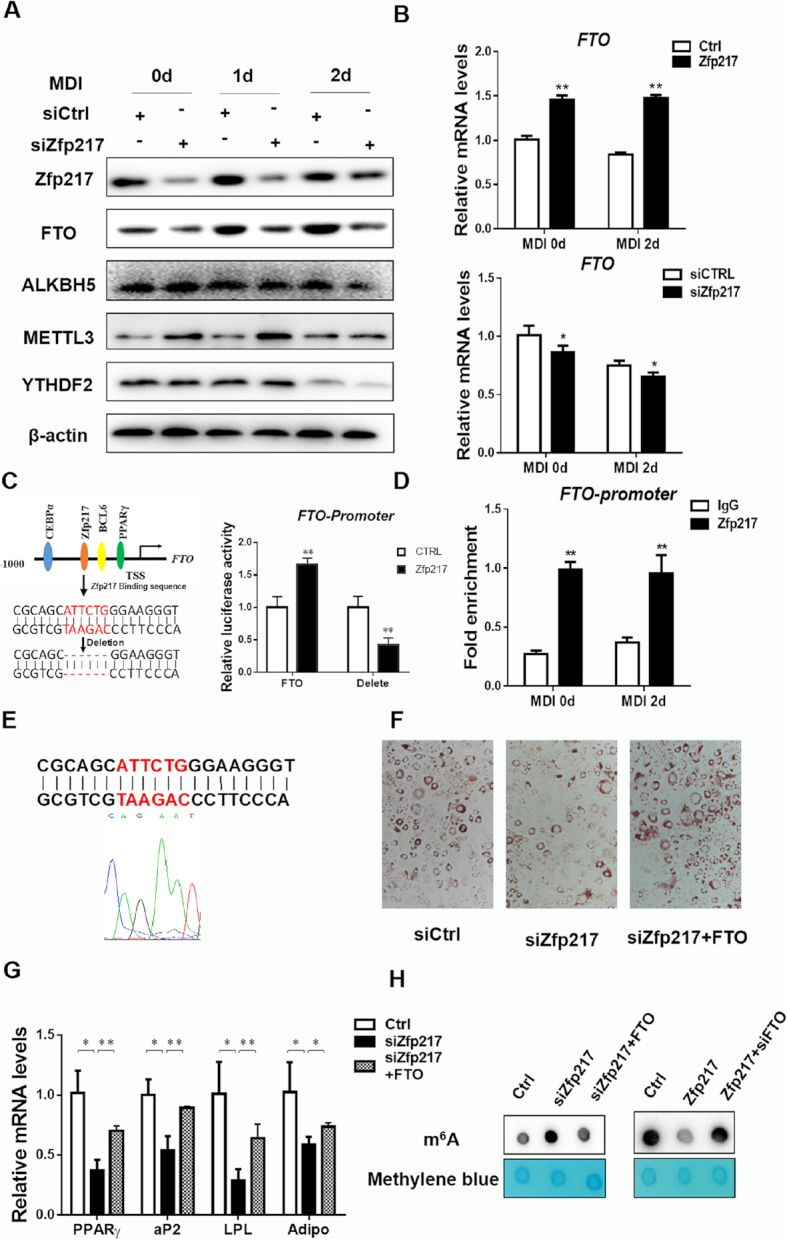
Activation of FTO transcription by Zfp217 for adipogenesis. (A) Protein expression levels of m6A-related proteins during MDI treatment in Zfp217 knockdown cells were detected by western blot (n = 3). β-Actin was used as a loading control. (B) mRNA levels of FTO in 3T3L1 cells with overexpression and knockdown of Zfp217 were detected by QPCR (n = 3). (C) Left panel: schematic representation of transcription factor binding promoter of FTO; right panel: dual-luciferase activity of normal and binding site-deleted FTO promoter by overexpressing Zfp217 in HEK293T cells (n = 3). (D) ChIP-QPCR assay was used to measure the binding of Zfp217 on FTO promoter in 3T3L1 cells with or without treatment of MDI (n = 3). Total chromatin was indicated as input and IgG was used as a negative control. (E) The position of the amplified regions of Zfp217 binding sequence in FTO promoter was indicated. (F) The effect of Zfp217 knockdown or together with overexpression of FTO on adipogenesis of 3T3L1 cells (n = 3). (G) mRNA expression levels of adipogenic genes were measured in 3T3L1 cells with Zfp217 knockdown or together with overexpression of FTO and treated with MDI for 6 days (n = 3). (H) The effect of overexpression (or knockdown) of Zfp217 or together with depletion (or overexpression) of FTO in 3T3L1 cells on m6A modification (n = 3). Presented as means ± SD (*P < 0.05, **P < 0.01).
We reasoned that FTO acts as a target gene of Zfp217 using bioinformatics method (Figure 3C). Next, dual luciferase reporter system was employed to validate that Zfp217 enhanced the promoter activity of FTO, while deletion of the Zfp217 binding sequence in FTO promoter inhibited it (Figure 3C), suggesting a direct regulation of Zfp217 on FTO. Accordingly, localization of Zfp217 at the promoter region of FTO was also confirmed by ChIP-QPCR assays using the Zfp217 specific antibody with or without MDI treatment (Figure 3D). By gene sequence analysis, the accurate binding DNA sequence is completely consistent with genomic sequence of FTO (Figure 3E). In agreement with these observation, we found that FTO rescues the inhibition of adipogenesis with the lack of Zfp217 and increases the gene expression of PPARγ, aP2, LPL and Adiponection consistently (Figure 3F and G). Given the rescue of m6A modification level by FTO overexpression (or knockdown) in Zfp217 knockdown (or overexpressed) 3T3L1 cells, m6A may be the main target of Zfp217-FTO-dependent way to regulate adipogenesis (Figure 3H). Collectively, our data demonstrated that Zfp217, act as a transcriptional factor, binds to promoter of FTO gene to augment adipogenesis, which may be one way to modulate m6A modification during adipogenesis.
Zfp217 positively regulates the epitranscriptome involved in adipogenesis
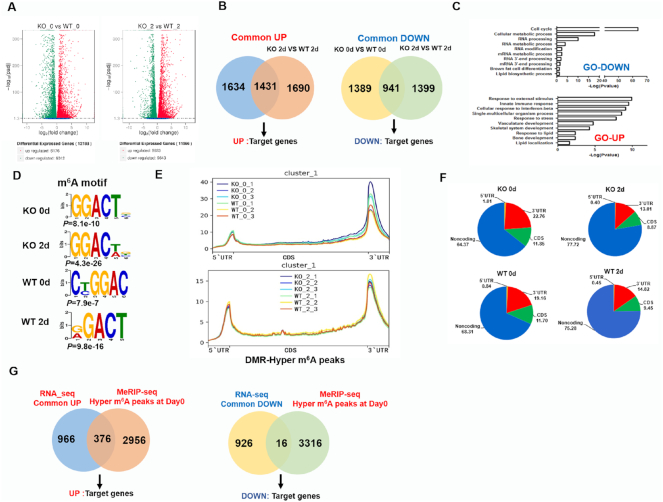
To gain an overview of the global role of Zfp217 in adipogenesis, we performed RNA sequencing (RNA-seq) in control and Zfp217 knockout 3T3L1 cells with MDI treatment for 0 and 2 d and identified 12 188 and 11 566 different expressed genes (DEG) for 0 and 2d, respectively (Figure 4A; Supplementary Figure S4A and Table S6). Compared with the wild-type, the vast majority (1431) of upregulated genes in Zfp217 depleted cells with MDI treatment for 0 d notably overlapped with that of the group with MDI for 2 d, while 941 downregulated genes were also identified here (Figure 4B and Supplementary Table S7). By Gene Ontology (GO) analysis, the downregulated genes were found to be mostly associated with cell cycle, RNA processing and lipid biosynthetic progress (Figure 4C; Supplementary Figure S4B; Tables S8 and S9), suggesting that Zfp217 is involved in proper regulation of RNA processing and lipid metabolism.
Figure 4.
Zfp217 positively regulates the epitranscriptome of key genes involved in adipogenesis. (A) Overview of different expression genes (DEG) by RNA-seq. (B) Overlapped transcripts of control and Zfp217 knockout in 3T3L1 cells at 0 or 2 d after MDI treatment by RNA-seq. (C) GO analysis of up- and down-regulated genes by RNA seq. The P value for the enrichment of biological process GO-term is shown. (D) Sequence logo representing consensus motif of m6A sites in Zfp217-dependent peaks by MeRIP-seq. (E and F) Metagene profiles of m6A distribution across the transcriptome of 3T3L1 cells at 0 or 2 d after MDI treatment by MeRIP-seq. (G) Overlapped the DEG of RNA-seq with DMR from MDI 0 d of MeRIP -seq respectively to identify the key target genes.
Next, to elucidate the mechanism by which Zfp217 regulates m6A modification, m6A methylated RNA immunoprecipitation sequencing (MeRIP-seq) was employed to analyze the m6A mRNA methylation in control and Zfp217 knockout 3T3L1 cells with MDI treatment for 0 and 2 d. In agreement with previous reports in mammals, it was found that GGACU is indeed highly enriched and consistently represents as the best sequence motif (Figure 4D). We next sought to investigate whether critical m6A sites are affected by Zfp217 depletion. It revealed that the relative density of m6A sites in the 5′UTR and 3′UTR are higher than other regions (Figure 4E and F; Supplementary Figure S5A; Table S10). In particularly, Zfp217 depletion increased the m6A enrichment after MDI for 0 d, not 2 d, which means these transcripts are in Zfp217 knockout-dependent manner (Figure 4G). Based on the above results, we assumed that DEG from RNA-seq may be more likely regulated when the m6A peaks are enriched in these corresponding transcripts of DEG. Thus, we further overlapped the DEG from UP and DOWN groups with 3332 differentially methylated regions (DMRs) from MDI for 0 d group, respectively, to identify the key target genes (Figure 4G; Supplementary Tables S11–S13). Based on RNA-seq data, it was found that cell cycle may be one of the important biological events in Zfp217 knockout cells. Results of cell cycle and apoptosis, presented by flow cytometry analysis as well as mRNA expression of key genes, suggested that knockout of Zfp217 may suppress proliferation and promote apoptosis of 3T3L1 cell with or without MDI treatment (Supplementary Figure S6). However, cell cycle related key genes cannot be found in MeRIP-seq data overlapped with RNA-seq data, which might suggest that cell cycle is not the main related biological event in Zfp217-m6A mediated adipogenesis.
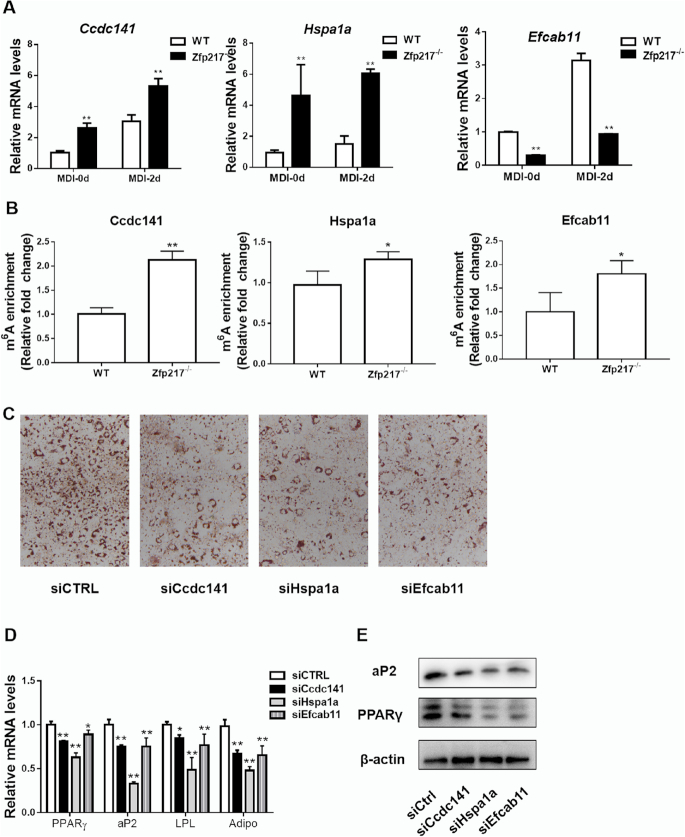
Some of other key targets were selected for further experiments, Ccdc141 from UP group, Efcab11 from DOWN group, Hspa1a from references and observed significant changes in RNA-seq data (Supplementary Figure S4C). Next, to validate the results of RNA-seq and MeRIP-seq, these genes were chosen for QPCR and MeRIP-QPCR analysis. They showed similar gene expression profiles with RNA-seq (Figure 5A and Supplementary Figure S4C), and MeRIP-QPCR revealed an increase of m6A modification at these transcripts after Zfp217 depletion (Figure 5B; Supplementary Figure S5B and C). Moreover, to validate the roles of these target genes, loss-of-function assays by siRNAs for Ccdc141, Hspa1a and Efcab11 exhibited decreased levels of adipogenesis (Figure 5C–E). Taken together, our data revealed the positive relationship between mRNA expression and m6A mRNA methylation upon Zfp217 knockout during adipogenesis.
Figure 5.
Effect of Zfp217 deletion on the expression of target genes. (A) Validation of Ccdc141, Hspa1a and Efcab11 mRNA levels from RNA-seq data by QPCR (n = 3). (B) Validation of m6A modification in Ccdc141, Hspa1a and Efcab11 mRNA by MeRIP-QPCR (n = 3). (C) The adipogenic phenotypes of 3T3L1 cells transfected with siCcdc141, siHspa1a, siEfcab11 or siCtrl after MDI induction for 6 days were assessed by ORO staining. (D) mRNA expression levels of adipogenic key genes PPARγ, aP2, LPL and Adiponectin were detected by QPCR (n = 3). (E) Protein levels of PPARγ and aP2 were detected by western blot (n = 3).Presented as means ± SD (*P < 0.05, **P < 0.01).
Zfp217 interacts with YTHDF2 to maintain m6A demethylation activity of FTO
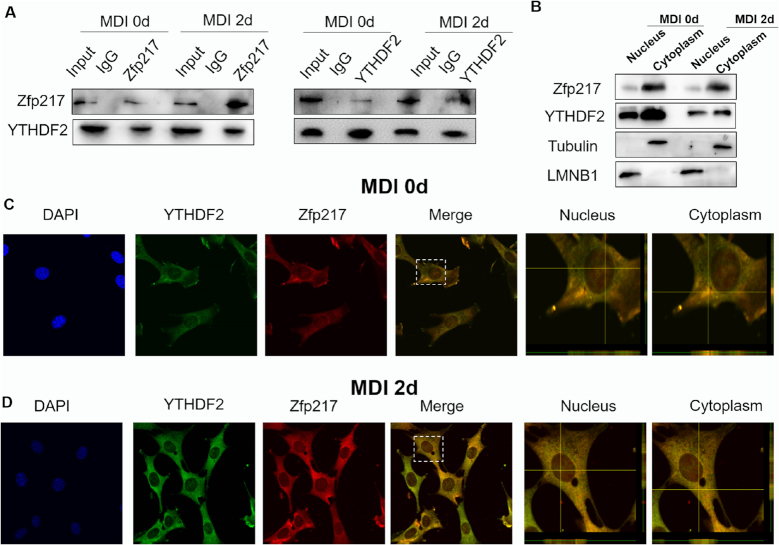
Previous study reported Zfp217 exerts its function through interacting with other proteins in embryonic development and human tumors (22). To further explore the global molecular mechanism by which Zfp217 promotes adipogenesis, we performed immunoprecipitation experiments with overexpression of Flag-N-Zfp217 in HEK293T cells followed by immunoblotting proteins related to m6A mRNA modification (Supplementary Figure S7A). Unexpectedly, FTO, ALKBH5 or METTL3/14 were not detected by Co-IP with Flag antibody (Supplementary Figure S7A). Using antibodies against METTL3 and Zfp217, endogenous METTL3 and Zfp217 interaction may be found in HEK293T cells (Supplementary Figure S7B), which is similar to the results in ESCs (8). Strikingly, we verified a specific interaction between Zfp217 and YTHDF2 by Co-IP and reverse Co-IP experiments of the endogenous proteins as well as Flag-tagged proteins both in 3T3L1 (with or without MDI treatment) and HEK293T cells (Figure 6A and Supplementary Figure S7C). Notably, Zfp217-YTHDF2 interaction was RNA-independent (Supplementary Figure S7D) as treating the cellular extracts with RNase A does not affect the precipitation of YTHDF2. To further confirm the subcellular interaction, fractions isolated from nucleus and cytoplasm were used to measure the expression of Zfp217 and YTHDF2. Consequently, both of these two proteins were whole cell distributed both in 3T3L1 and HEK293T. Intriguingly, Zfp217 has more expression in cytoplasm than nucleus (Figure 6B and Supplementary Figure S7E). Moreover, we also performed confocal microscopy for monitoring the cellular location of endogenous Zfp217 and YTHDF2. Interestingly, co-localization of YTHDF2 and Zfp217 was observed in nucleus and cytoplasm using confocal Z-analysis, which may indicate the specific function of YTHDF2 in Zfp217-dependent adipogenesis (Figure 6C and D; Supplementary Figure S7F).
Figure 6.
Zfp217 interacts with YTHDF2. (A) CoIP and reverse CoIP analysis of interaction between Zfp217 and YTHDF2 in 3T3L1 cells with or without MDI treatment for 2 days using antibodies against the endogenous proteins (n = 3). IgG was used as a negative control. (B) Protein expression level of Zfp217 and YTHDF2 in cytoplasmic and nuclear fractions was analyzed by western blot (n = 3). (C and D) Subcellular localization of Zfp217 and YTHDF2 in 3T3L1 cells with or without MDI treatment for 2 days. 3T3L1 cells were immunostained with antibodies against Zfp217 and YTHDF2. DAPI was used for nuclear staining. Cells analyzed with a confocal laser scanning microscope (63× magnification) with Z-scan analysis.
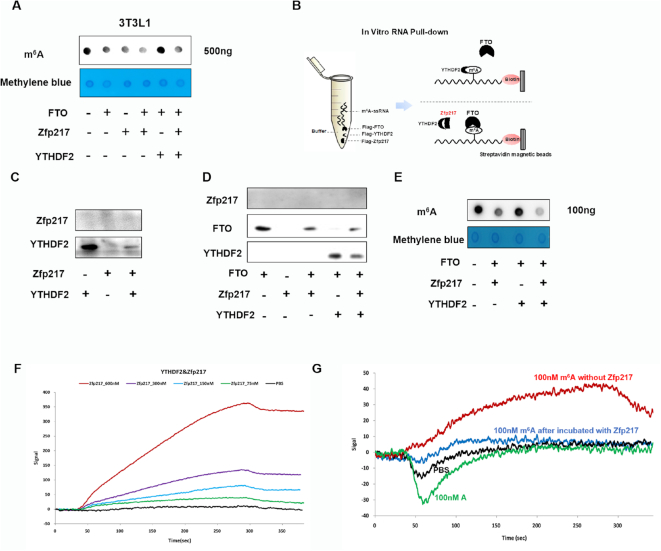
As reported that YTHDF2 could maintain m6A level by inhibiting FTO activity under heat stress (16), we further comprehensively analyzed biological function of interaction between YTHDF2 and Zfp217 to FTO. As expected, overexpression of FTO and Zfp217 showed lower m6A level and YTHDF2 blocked the demethylase activity of FTO to increase m6A levels, whereas Zfp217 functioned as a regulator to tilt the equilibrium toward demethylation (Figure 7A and Supplementary Figure S8A). Furthermore, we confirmed direct competition between FTO and YTHDF2 to target m6A ssRNA from Hspa1a (16) by in vitro m6A RNA pull-down assay (Figure 7B and D). Obviously, Zfp217, not binding to RNA, interfered with location of YTHDF2 (Figure 7C) and rescued FTO to bind RNA (Figure 7D). Combining in vitro RNA pull-down and dot blot assay to assess the m6A demethylation activity of FTO, it was also confirmed that Zfp217 sequestered YTHDF2 to maintain m6A demethylation activity of FTO (Figure 7E). To determine whether Zfp217 directly interacts with YTHDF2 in vitro, we performed LSPR. The results clearly showed that Zfp217 directly interacts with YTHDF2 in a dose-dependent manner (Figure 7C). After combining YTHDF2, Zfp217 prohibited m6A-containing ssRNA from binding with YTHDF2 (Figure 7D). Overall, our findings indicated that Zfp217 interacts with YTHDF2 to keep the accessibility of FTO to m6A sites.
Figure 7.
Zfp217 influences competition between YTHDF2 and FTO in m6A-binding sites. (A) Dot blot was used to measure the effect of overexpression of FTO, Zfp217 or YTHDF2 in 3T3L1 cells on m6A modification (n = 3). (B) Schematic representation of RNA pull down. (C) Synthesized mRNA with m6A was incubated with YTHDF2 in the absence or presence of Zfp217, followed by RNA pull-down and western blot (n = 3). (D) Synthesized mRNA with m6A was incubated with FTO in the absence or presence of YTHDF2 and Zfp217, followed by RNA pull-down and western blot (n = 3). (E) mRNA of 3T3L1 cells was incubated with FTO, Zfp217 and YTHDF2, followed by m6A dot blot (n = 3). (F) Zfp217 directly interacts with YTHDF2 in a dose-dependent manner by LSPR. (G) Zfp217 blocked the binding capacity between YTHDF2 and m6A ssRNA by LSPR.
Knockdown of YTHDF2 rescues inhibition of Zfp217 depletion-mediated adipogenesis
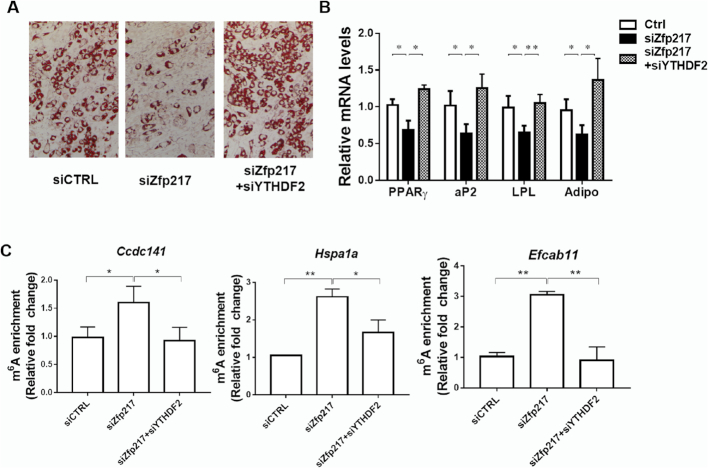
To further investigate the underlying molecular mechanism how Zfp217 regulates the target genes by m6A modification, we focused on YTHDF2 and verified its role in Zfp217-mediated adipogenesis. Intriguingly, knockdown of YTHDF2 drastically rescued adipogenesis in Zfp217-deficient cells (Figure 8A and B), while the m6A modification of genes was lower after YTHDF2 knockdown (Figure 8C), suggesting the critical role of YTHDF2 in Zfp217-mediated adipogenesis.
Figure 8.
Knockdown of YTHDF2 rescues inhibition of Zfp217 depletion-mediated adipogenesis. (A) Knockdown of YTHDF2 rescues adipogenesis in Zfp217-deficient 3T3L1 cells. The adipogenic phenotypes were assessed by ORO staining; magnification: 200×. (B) mRNA levels of adipogenic key genes were detected by QPCR (n = 3). (C) MeRIP-QPCR was performed to detect the m6A abundance of target genes in YTHDF2 knockdown cells (n = 3).
Collectively, these results identified that Zfp217 balances the expression of key genes in a YTHDF2-dependent way, ultimately leading to the promotion of adipogenesis. We proposed a model in which Zfp217 regulates adipogenesis in Figure 9.
Figure 9.
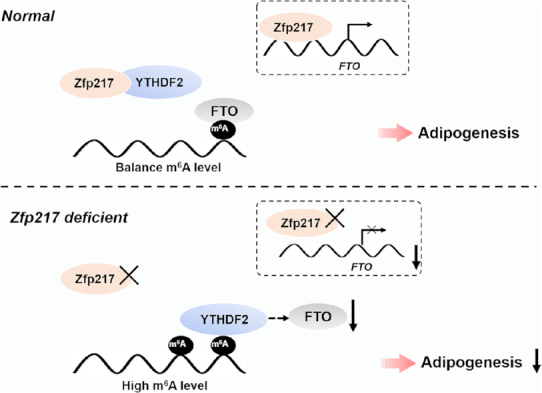
A diagram demonstrates the role of Zfp217-dependent m6A modification in adipogenesis. In our model, Zfp217 orchestrates FTO expression at transcriptional level and interaction with YTHDF2 to regulate m6A mRNA methylation and facilitates adipogenic differentiation in a YTHDF2-dependent manner.
DISCUSSION
Adipogenesis involves different levels of regulatory mechanism, with the majority of known control factors are involved in transcriptional regulation (35). In the present study, we delineated a role for multifunctional factor Zfp217 in adipocyte differentiation. Complementary in vitro and ex vivo analyses demonstrated that depletion of Zfp217 inhibits adipogenic protein PPARγ and other key adipogenic-related protein expression and in turn adipogenesis (Figure 1). Interestingly, deletion of Zfp217 increases global m6A RNA modification, which indicates the role of Zfp217 at post-transcriptional level (Figures 2 and 4). Mechanistically, we showed that Zfp217 binds directly to the promoter of m6A demethylase FTO (Figure 3C–E), and interacts with m6A reader YTHDF2 (Figure 6), thereby enhances the location and demethylase activity of FTO to m6A target RNAs (Figure 7). Ultimately, Zfp217 promotes adipogenesis in an m6A-YTHDF2-dependent manner (Figure 8). These findings for the first time demonstrated the multifaceted and complex function of Zfp217 in adipogenesis.
Zfp217 acts as a classical transcription factor to promote expression of FTO
Zfp217 is a classical transcriptional factor from zinc finger protein family, with eight conserved C2H2 zinc finger motifs and a proline-rich transactivation domain (18). Conceptually, after binding to a specific DNA sequence of target gene, Zfp217 exerts activation of specific gene expression programs, and also cooperates in transcriptional silencing programs by recruiting chromatin modifiers in embryonic development and human tumors (17,21,22). Thus, depletion of Zfp217 may directly affect the expression of downstream target genes. As reported by our group previously, expression of Zfp217 was found to be significantly upregulated during adipogenesis (24). Accordingly, in current study, we showed that deletion of Zfp217 in 3T3L1 cells significantly blocks the expression of PPARγ, aP2 and other adipogenic genes by performing RNA-seq together with the adipogenic phenotype (Figures 1 and 4A–C), which indicates that Zfp217 might take part in transcriptional cascade regulation of key genes in adipogenesis.
Using bioinformatics of promoter binding analysis, we predicted that FTO is the potential target gene of Zfp217 (Figure 3C–E). Interestingly, FTO is well known for its role in associated with obesity and related diseases (11,36). The numbers of studies have been reported the positive function of FTO in adipogenesis (9,15). Here, the multiple methods for transcriptional regulation validated that Zfp217 directly binds to the promoter of FTO, and overexpression of FTO reversed inhibition of adipogenesis with Zfp217 knockdown (Figure 3F and G), thereby providing a reliable cue for clarifying the facilitative role of Zfp217 in adipogenesis.
Zfp217 mediates m6A mRNA methylation through FTO and YTHDF2
Previous studies showed that Zfp217 sustains undifferentiated state of ESCs and supports its pluripotent state by epigenetically regulating m6A methylation (8). In this study, we found that Zfp217 depletion significantly increases the m6A modification level in 3T3L1 cells and MEF-Zfp217+/−(Figure 2), but it needs to be unraveled how could a transcription factor regulate m6A modification.
FTO was reported as the first m6A demethylase of mRNA, and the function of FTO in adipogenesis has been linked to its m6A demethylase activity (13,36). Here, we showed knockdown of FTO retrieved m6A modification level in Zfp217 overexpressed cells (Figure 3H). The finding indicated that Zfp217 reduces m6A modification through increasing the expression of FTO in a transcriptional regulatory way. Consistently, other studies have also reported that with the treatment of R-2HG, CEBPα suppresses the expression of FTO by directly binding to its promoter, resulting to the high m6A modification (32). Interestingly, it was observed high m6A modification of FTO mRNA itself after Zfp217 knockout through MeRIP-seq as well, implying a feedback mechanism of which high m6A modification of FTO mRNA inhibits FTO expression itself (32). We also found a higher m6A modification in FTO mRNA in Zfp217 deficiency cells (Supplementary Figure S5C). And, strikingly, we have previously reported that higher m6A modification in FTO mRNA leads to its low protein expression in placenta (27). Together, it may be certain to infer that the transcriptional activation of FTO by Zfp217 in m6A modification acts as an inherent property of this transcriptional factor.
Notably, Zfp217 is also known as a core component of protein complexes with histone deacetylase (HDAC), CoREST and CTBPs (37–39), which suggests its multifunctional roles in biology events. Therefore, clarifying the interacted proteins of Zfp217 could gain insight into the molecular mechanism of Zfp217-dependent adipogenesis. Here, we focused on the m6A-related proteins that may be interacted with Zfp217. Applied Co-IP with antibodies against endogenous proteins, we found the interaction between METTL3 and Zfp217 (Supplementary Figure S7B), which corroborated the previous study in HEK293T cells (8). Interestingly, there may be mutual inhibition between METTL3 and Zfp217 and METTL3 knockdown rescued the m6A modification level in Zfp217 knockdown cells (Figure 3A; Supplementary Figure S3A and B), suggesting it may be one of the well-known ways to regulate m6A modification (8). We also validated the actual interaction between Zfp217 and m6A ‘reader’ protein YTHDF2 (Figure 6). Monitored by confocal microscope, the interaction between Zfp217 and YTHDF2 occurred almost in whole cell and mainly in cytoplasm after MDI for 2 days. As in earlier study, it has elucidated that YTHDF2 preserves m6A methylation by limiting FTO from demethylation under heat shock stress (16). Here, we found that Zfp217 may act as a regulator for YTHDF2–FTO competition, which sequesters YTHDF2 from limiting the accessibility of FTO to m6A sites (Figure 7). Overall, besides transcriptional activation of FTO, it seems that Zfp217 could sustain the demethylase activity of FTO by interaction with m6A ‘reader’ YTHDF2. Of particular note, a recent seminal study revealed a crosstalk between histone modification and RNA methylation and uncovered another layer of gene expression regulation (40). Here, Zfp217 may also function across transcriptional and post-transcriptional level, which sheds new light on the role of this transcription factor in adipogenesis (22).
In summary, we illustrated that Zfp217 is essential for adipogenesis by connecting gene transcription with m6A mRNA modification. The regulatory role of Zfp217 that keeps low m6A modification of the target mRNAs provides fundamental insight into the post-transcriptional gene regulation network in adipocyte differentiation, revealing potential novel ways to regulate adipogenesis and other vital events at this new frontier of transcriptional factors. These findings offer a new approach to dissect the molecular mechanism of adipogenesis, and, ultimately, counteract obesity and its associated risks of metabolic syndromes.
DATA AVAILABILITY
The accession number for the next-generation sequencing data reported in this paper is NCBI GEO: GSE119564.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Guifang Jia (Peking University, China) and Dr Chuan He (The University of Chicago, USA) for pure m6Am nucleoside; Dr Bifeng Yuan (Wuhan University, China) for detailed advices about LC-MS/MS; Dr Shengqing Li and Qiongyao Gu for helping with LC-MS/MS analysis.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Natural Science Foundation of China [31472075]; China Agriculture Research System [CARS-36]; Hubei provincial Creative TeamProject of Agricultural Science and Technology (2007-620); Fundamental Research Funds for the Central Universities of China [2662017PY017]. Funding for open access charge: National Natural Science Foundation of China [31472075].
Conflict of interest statement. None declared.
REFERENCES
- 1. Rosen E.D., Spiegelman B.M.. What we talk about when we talk about fat. Cell. 2014; 156:20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siersbæk R., Nielsen R., Mandrup S.. Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends Endocrinol. Metab. Tem. 2012; 23:56–64. [DOI] [PubMed] [Google Scholar]
- 3. Roundtree I.A., Evans M.E., Pan T., He C.. Dynamic RNA modifications in gene expression regulation. Cell. 2017; 169:1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meyer K.D., Jaffrey S.R.. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat. Rev. Mol. Cell Biol. 2014; 15:313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao B.S., Roundtree I.A., He C.. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 2017; 18:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang X., Lu Z., Gomez A., Hon G.C., Yue Y., Han D., Fu Y., Parisien M., Dai Q., Jia G.. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014; 505:117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Geula S., Moshitchmoshkovitz S., Dan D., Mansour A.F., Kol N., Salmondivon M., Hershkovitz V., Peer E., Mor N., Manor Y.S.. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science. 2015; 6225:1002–1006. [DOI] [PubMed] [Google Scholar]
- 8. Aguilo F., Zhang F., Sancho A., Fidalgo M., Di Cecilia S., Vashisht A., Lee D.F., Chen C.H., Rengasamy M., Andino B. et al.. Coordination of m(6)A mRNA methylation and gene transcription by ZFP217 regulates pluripotency and reprogramming. Cell Stem Cell. 2015; 17:689–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang X., Zhu L., Chen J., Wang Y.. mRNA m 6 A methylation downregulates adipogenesis in porcine adipocytes. Biochem. Biophys. Res. Commun. 2015; 459:201–207. [DOI] [PubMed] [Google Scholar]
- 10. Kobayashi M., Ohsugi M., Sasako T., Awazawa M., Umehara T., Iwane A., Kobayashi N., Okazaki Y., Kubota N., Suzuki R. et al.. The RNA methyltransferase complex of WTAP, METTL3, and METTL14 regulates mitotic clonal expansion in adipogenesis. Mol. Cell. Biol. 2018; 38:e00116-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dina C., Meyre D., Gallina S., Durand E., Körner A., Jacobson P., Carlsson L.M., Kiess W., Vatin V., Lecoeur C.. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat. Genet. 2007; 39:724–726. [DOI] [PubMed] [Google Scholar]
- 12. Loos R.J., Yeo G.S.. The bigger picture of FTO: the first GWAS-identified obesity gene. Nat. Rev. Endocrinol. 2014; 10:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao X., Yang Y., Sun B.F., Shi Y., Yang X., Xiao W., Hao Y.J., Ping X.L., Chen Y.S., Wang W.J.. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014; 24:1403–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu R., Yao Y., Jiang Q., Cai M., Liu Q., Wang Y., Wang X.. Epigallocatechin gallate targets FTO and inhibits adipogenesis in an mRNA m 6 A-YTHDF2-dependent manner. Int. J. Obes. 2018; 42:1378–1388. [DOI] [PubMed] [Google Scholar]
- 15. Merkestein M., Laber S., Mcmurray F., Andrew D., Sachse G., Sanderson J., Li M., Usher S., Sellayah D., Ashcroft F.M.. FTO influences adipogenesis by regulating mitotic clonal expansion. Nat. Commun. 2015; 6:6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou J., Wan J., Gao X., Zhang X., Jaffrey S.R., Qian S.B.. Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature. 2015; 526:591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rahman M.T., Nakayama K., Rahman M., Nakayama N., Ishikawa M., Katagiri A., Iida K., Nakayama S., Otsuki Y., Shih Ie M. et al.. Prognostic and therapeutic impact of the chromosome 20q13.2 ZNF217 locus amplification in ovarian clear cell carcinoma. Cancer. 2012; 118:2846–2857. [DOI] [PubMed] [Google Scholar]
- 18. Quinlan K.G., Verger A., Yaswen P., Crossley M.. Amplification of zinc finger gene 217 (ZNF217) and cancer: when good fingers go bad. Biochim. Biophys. Acta. 2007; 1775:333–340. [DOI] [PubMed] [Google Scholar]
- 19. Rooney P.H., Boonsong A., Mcfadyen M.C., Mcleod H.L., Cassidy J., Curran S., Murray G.I.. The candidate oncogene ZNF217 is frequently amplified in colon cancer. J. Pathol. 2004; 204:282–288. [DOI] [PubMed] [Google Scholar]
- 20. Lin Y., Li X.Y., Willis A.L., Liu C., Chen G., Weiss S.J.. Snail1-dependent control of embryonic stem cell pluripotency and lineage commitment. Nat. Commun. 2014; 5:3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kwak S., Kim T.W., Kang B.H., Kim J.H., Lee J.S., Lee H.T., Hwang I.Y., Shin J., Lee J.H., Cho E.J.. Zinc finger proteins orchestrate active gene silencing during embryonic stem cell differentiation. Nucleic Acids Res. 2018; 46:6592–6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee D.F., Walsh M.J., Aguiló F.. ZNF217/ZFP217 meets chromatin and RNA. Trends Biochem. Sci. 2016; 41:986–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xiang H., Zhong Z.X., Peng Y.D., Jiang S.W.. The emerging role of Zfp217 in adipogenesis. Int. J. Mol. Sci. 2017; 18:1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jiang S., Wei H., Song T., Yang Y., Peng J., Jiang S.. Transcriptome comparison between porcine subcutaneous and intramuscular stromal vascular cells during adipogenic differentiation. PLoS One. 2013; 8:e77094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu F., Gao Z., Zhang J., Rivera C.A., Yin J., Weng J., Ye J.. Lack of SIRT1 (Mammalian Sirtuin 1) activity leads to liver steatosis in the SIRT1+/- mice: a role of lipid mobilization and inflammation. Endocrinology. 2010; 151:2504–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Song T., Zhou Y., Peng J., Tao Y.X., Yang Y., Xu T., Peng J., Ren J., Xiang Q., Wei H.. GPR120 promotes adipogenesis through intracellular calcium and extracellular signal-regulated kinase 1/2 signal pathway. Mol. Cell. Endocrinol. 2016; 434:1–13. [DOI] [PubMed] [Google Scholar]
- 27. Song T., Lu J., Deng Z., Xu T., Yang Y., Wei H., Li S., Jiang S., Peng J.. Maternal obesity aggravates the abnormality of porcine placenta by increasing N(6)-methyladenosine. Int. J. Obes. 2018; 42:1812–1820. [DOI] [PubMed] [Google Scholar]
- 28. Jia G., Fu Y., Zhao X., Dai Q., Zheng G., Yang Y., Yi C., Lindahl T., Pan T., Yang Y.G.. N6-Methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011; 7:885–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wei J., Liu F., Lu Z., Fei Q., Ai Y., He P.C., Shi H., Cui X., Su R., Klungland A. et al.. Differential m(6)A, m(6)Am, and m(1)A demethylation mediated by FTO in the cell nucleus and cytoplasm. Mol. Cell. 2018; 71:973–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang C., Samanta D., Lu H., Bullen J.W., Zhang H., Chen I., He X., Semenza G.L.. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m6A-demethylation of NANOG mRNA. Proc. Natl. Acad. Sci. U.S.A. 2016; 113:E2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dominissini D., Moshitch-Moshkovitz S., Salmon-Divon M., Amariglio N., Rechavi G.. Transcriptome-wide mapping of N(6)-methyladenosine by m(6)A-seq based on immunocapturing and massively parallel sequencing. Nat. Protoc. 2013; 8:176–189. [DOI] [PubMed] [Google Scholar]
- 32. Su R., Dong L., Li C., Nachtergaele S., Wunderlich M., Qing Y., Deng X., Wang Y., Weng X., Hu C.. R-2HG exhibits Anti-tumor activity by targeting FTO/m6A/MYC/CEBPA signaling. Cell. 2017; 172:90–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Panneer S.S., Roth B.M., Nganga R., Kim J., Cooley M.A., Helke K., Smith C.D., Ogretmen B.. Balance between senescence and apoptosis is regulated by telomere damage-induced association between p16 and caspase-3. J. Biol. Chem. 2018; 293:9784–9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McGurn L.D., Moazami-Goudarzi M., White S.A., Suwal T., Brar B., Tang J.Q., Espie G.S., Kimber M.S.. The structure, kinetics and interactions of the β-carboxysomal β-carbonic anhydrase, CcaA. Biochem. J. 2016; 473:4559–4572. [DOI] [PubMed] [Google Scholar]
- 35. Rosen E.D., Macdougald O.A.. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006; 7:885–896. [DOI] [PubMed] [Google Scholar]
- 36. Deng X., Su R., Stanford S., Chen J.. Critical enzymatic functions of FTO in obesity and cancer. Front. Endocrinol. 2018; 9:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cowger J.J.M., Zhao Q., Isovic M., Torchia J.. Biochemical characterization of the zinc-finger protein 217 transcriptional repressor complex: identification of a ZNF217 consensus recognition sequence. Oncogene. 2007; 26:3378–3386. [DOI] [PubMed] [Google Scholar]
- 38. Quinlan K.G.R., Nardini M., Verger A., Francescato P., Yaswen P., Corda D., Bolognesi M., Crossley M.. Specific recognition of ZNF217 and other zinc finger proteins at a surface groove of C-Terminal binding proteins. Mol. Cell. Biol. 2006; 26:8159–8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Banck M.S., Li S., Nishio H., Cheng W., Beutler A.S., Walsh M.J.. The ZNF217 oncogene is a candidate organizer of repressive histone modifiers. Epigenetics. 2009; 4:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang H., Weng H., Zhou K., Wu T., Zhao B.S., Sun M., Chen Z., Deng X., Xiao G., Auer F.. Histone H3 trimethylation at lysine 36 guides m 6 A RNA modification co-transcriptionally. Nature. 2019; 567:414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession number for the next-generation sequencing data reported in this paper is NCBI GEO: GSE119564.