Abstract
In this issue of Cancer Cell, Kahles et al. perform a comprehensive analysis of RNA splicing across cancer types and identify novel correlations between genetic alterations and splicing in cancer. In addition, they identify that tumor-specific splicing has the potential to generate a large new class of tumor-specific neoantigens.
RNA splicing, the process by which premature messenger RNA is processed to produce mature RNA, provides a means to produce multiple protein products from a single gene, as well as to regulate gene expression. As such, splicing has been described as altered in cancer for several decades. However, our knowledge of the contribution of altered splicing to cancer pathogenesis is still in development because the necessary means to comprehensively analyze transcript abundance have only been created relatively recently. This includes the generation of sufficient high-quality RNA sequencing (RNA-seq) data from tumor samples and counterpart normal tissues, as well as techniques to identify splicing changes between samples. Fortunately, the confluence of whole-exome sequencing (WES) paired with RNA-seq data and proteomic data from The Cancer Genome Atlas (TCGA) has now provided an opportunity to comprehensively study splicing across cancer.
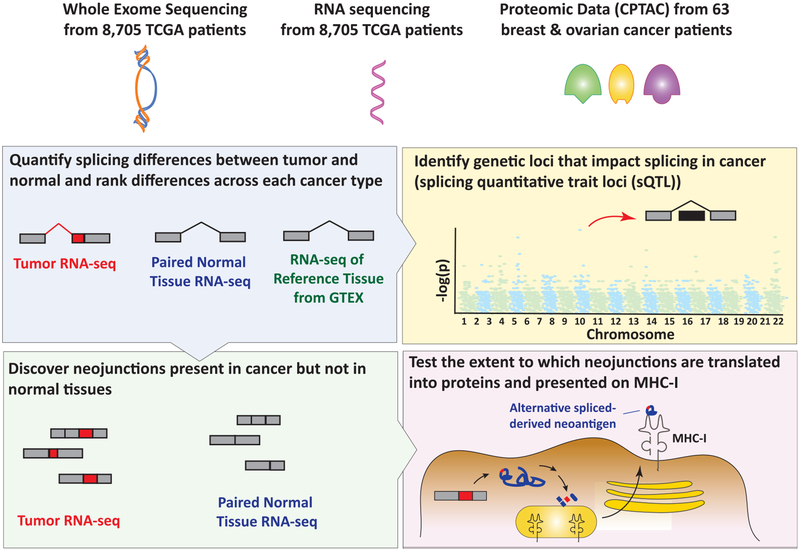
To this end, Kahles et al. present a comprehensive re-analysis of WES and RNA-seq data across 32 cancer types from the TCGA (Kahles et al., 2018). The authors utilized WES and RNA-seq data from 8,705 patients and 670 matched normal controls from TCGA along with complementary RNA-seq of normal tissues from the Genotype-Tissue Expression (GTEx) project and proteomic data from TCGA breast and ovarian tumors from the Clinical Proteomic Tumor Analysis Consortium (CPTAC). These data were integrated to address four questions (Figure 1). (1) What are the qualitative differences in splicing between tumor and normal in different cancer types? (2) What nucleotide variants are associated with alterations in splicing? (3) Can cancer-specific exon-exon junctions (EEJs) be identified? (4) To what extent are cancer-specific EEJs translated to cancer-specific antigens? To address these questions, the authors utilized a specific methodology developed by their group to systematically quantify splicing changes across the full TCGA cohort at the level of splicing events (Kahles et al., 2016). This identified several observations that have been noted in previous studies of splicing in cancer. First, they found that tumors harbor more aberrant splicing events than paired normal tissue from the same individual or reference normal tissues. Second, splicing events that are not annotated in reference databases such as GENCODE (so-called “novel” splicing events) are pervasive in cancer. Additionally, the large number of tumor types and samples analyzed here allowed the investigators to compare novel splicing events across cancer types. Interestingly, this revealed that splicing events specific to tumors are abundant but that recurrent tumor-specific splicing events are less common. Moreover, when such recurrent tumor-specific splicing events are identified, they tend to be present across multiple cancer types. One example highlighted by this study was recurrent skipping of exon 3 of PTEN, which is predicted to deleteriously reduce PTEN protein expression (Agrawal and Eng, 2006).
Figure 1. Integrating WES and RNA-Seq with Proteomics Data from The Cancer Genome Atlas to Identify the Full Complement of Aberrant Splicing Changes in Cancer and the Extent to which These Changes Generate Splicing-Derived Neoantigens.
Kahles et al. utilized WES and RNA-seq data from 8,705 patients and 670 matched normal controls from TCGA, along with complementary RNA-seq of normal tissues from the GTEx project and proteomic data from TCGA breast and ovarian tumors from CPTAC. These data were integrated to determine qualitative differences in splicing between tumor and normal tissue in different cancer types (top left). In addition, the authors performed a splicing quantitative trait loci (sQTL) study to associate genomic variants with splicing changes in tumor versus normal (top right). Data from the first aim were then used to identify cancer-specific exon-exon junctions (bottom left). Finally, through in silico analyses, the authors evaluated the extent to which these exon-exon junctions are translated to cancer-specific antigens that could be presented on MHC-I (bottom right).
Although alterations in splicing have been known to play a role in cancer for some time, the discovery of recurrent change-of-function mutations in RNA splicing proteins in a variety of cancers in 2011 further underscored the importance of altered splicing in cancer. Here, the authors attempted to systematically identify associations between genetic variants in tumors to splicing changes in tumors. This was done using a splicing quantitative trait loci study whereby RNA-seq-derived splicing quantifications were associated with WES-derived genetic variants. Although genome-wide association studies have been performed previously to identify germline genetic variants that impact splicing in normal tissues (Zhang et al., 2015), such an association study between genetic variants and splicing has not previously been performed in cancer. This effort confirmed known trans associations with mutations in the core RNA splicing factors SF3B1 and U2AF1 with alterations in splicing but also identified new and unexpected associations. For example, the authors identified that variants in TADA1, PPP2R1A, and EGFR impact splicing of other genes in trans. The magnitude of the effect of these variants on splicing was not so clear, however, nor is the mechanistic basis for this association. In addition, recent re-analyses of TCGA data evaluating mutations in genes known to be centrally involved in splicing highlighted far more genetic alterations expected to impact splicing than identified here (Seiler et al., 2018).
Based on the observation that tumors harbor more splicing changes than normal cells and that numerous novel splicing events are present in cancer, the authors next sought out to quantify novel EEJs (so-called “neojunctions”) across cancers. This resulted in several fascinating observations. First, for reasons that are not clear, certain cancer types tend to harbor more neojunctions than others, and this is independent from mutational load. In fact, some tumors harbored massive numbers of novel splicing events, far out of proportion to their burden of mutations in genomic DNA, a situation the authors refer to as “syndeothripsis” (syndeéo being the Greek word for “connect” and a term analogous to chromothripsis at the level of DNA). One key question for the role of splicing changes in cancer is whether aberrant splicing changes are selected for in cancer cells or provide any benefit to cancer. Although the authors could not identify any neojunctions selectively enriched in cancer, future functional studies to dissect the importance of novel splicing events in cancer cells could be very enlightening.
The discovery of large numbers of cancer-specific neojunctions brings to light the possibility for splicing-derived cancer-specific proteins to be utilized for cancer diagnosis, prognosis, and therapy. Thus, the final goal of this study was to determine the potential for cancer-specific neojunction to result in neoepitopes. Data here suggest that tumor-specific alternative splicing events are far more abundant than the somatic single-nucleotide variants, which have typically been studied for their potential to generate neoepitopes. It is important to note, however, that the true contribution of alterations in splicing to proteome diversity is complicated. Aberrant splicing often involves low-abundance isoforms (Pickrell et al., 2010), and the sensitivity of proteogenomic detection of neomorphic and non-canonical proteins created by such splicing events is not clear. Moreover, it has also been noted that the enzymes most commonly used to generate peptides from full-length proteins for mass spectrometry have a tendency to cleave proteins at EEJs, thereby obscuring detection of neopep-tides translated from neojunctions (Wang et al., 2018).
Despite the above technical challenges, data derived from this study and another recent analysis of splice-site-creating mutations from the TCGA (Jayasinghe et al., 2018) identify that polypep-tides generated from cancer-specific EEJs have the potential to bind MHC-I and potentially serve as a neoantigens. In fact, 68% of the breast cancer and ovarian cancer samples studied here had at least one alternative splicing-derived neoepitope detected in proteomic data and predicted to bind MHC-I. Nonetheless, it is important to note that experimental validation of the immunogenicity of neoantigens derived from altered splicing is still needed. Moreover, data evaluating the immunogenicity of tumors based on RNA expression of immune markers have differed in their conclusions of whether cancer-associated splicing changes are associated with reduced or increased immune cell infiltration (Jayasinghe et al., 2018; Seiler et al., 2018). Nonetheless, this study, along with three other recent analyses of specific aspects of RNA splicing from TCGA (Climente-González et al., 2017; Jayasinghe et al., 2018; Seiler et al., 2018), greatly increase our understanding of the contribution of altered splicing to cancer development and the potential therapeutic opportunities created by further analysis of aberrant RNA processing in cancer.
ACKNOWLEDGMENTS
L.E.H. is supported by grants from the Pancreatic Cancer Action Network-AACR Pathway to Leadership Program and the National Pancreas Foundation. O.A.-W. is supported by grants from NIH/NHLBI (R01 HL128239), the Department of Defense Bone Marrow Failure Research Program (W81XWH-16-1-0059), The Starr Foundation (I8-A8-075), The Henry & Marilyn Taub Foundation, The Edward P. Evans Foundation, the Leukemia and Lymphoma Society, and the Pershing Square Sohn Cancer Research Alliance.
REFERENCES
- Agrawal S, and Eng C (2006). Differential expression of novel naturally occurring splice variants of PTEN and their functional consequences in Cowden syndrome and sporadic breast cancer. Hum. Mol. Genet 15, 777–787. [DOI] [PubMed] [Google Scholar]
- Climente-González H, Porta-Pardo E, Godzik A, and Eyras E (2017). The functional impact of alternative splicing in cancer. Cell Rep. 20, 2215–2226. [DOI] [PubMed] [Google Scholar]
- Jayasinghe RG, Cao S, Gao Q, Wendl MC, Vo NS, Reynolds SM, Zhao Y, Climente-González H, Chai S, Wang F, et al. ; Cancer Genome Atlas Research Network (2018). Systematic analysis of splice-site-creating mutations in cancer. Cell Rep. 23, 270–281.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahles A, Ong CS, Zhong Y, and Rätsch G (2016). SplAdder: identification, quantification and testing of alternative splicing events from RNA-Seq data. Bioinformatics 32, 1840–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahles A, Lehmann KV, Toussaint NC, Huser M, Stark S, Sachsenberg T, Stegle O, Kohlbacher O, Sander C, and Rätsch G; Cancer Genome Atlas Research Network (2018). Comprehensive analysis of alternative splicing across tumors from 8,705 patients. Cancer Cell 34, this issue, 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell JK, Pai AA, Gilad Y, and Pritchard JK (2010). Noisy splicing drives mRNA isoform diversity in human cells. PLoS Genet. 6, e1001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler M, Peng S, Agrawal AA, Palacino J, Teng T, Zhu P, Smith PG, Buonamici S, and Yu L; Cancer Genome Atlas Research Network (2018). Somatic mutational landscape of splicing factor genes and their functional consequences across 33 cancer types. Cell Rep. 23, 282–296.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Codreanu SG, Wen B, Li K, Chambers MC, Liebler DC, and Zhang B (2018). Detection of proteome diversity resulted from alternative splicing is limited by trypsin cleavage specificity. Mol. Cell. Proteomics 17, 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Joehanes R, Chen BH, Huan T, Ying S, Munson PJ, Johnson AD, Levy D, and O’Donnell CJ (2015). Identification of common genetic variants controlling transcript isoform variation in human whole blood. Nat. Genet 47, 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]