Abstract
Purpose of review
There is a pressing need for effective strategies to halt the increase in both the incidence and mortality of esophageal adenocarcinoma (EAC). Screening for Barrett’s esophagus, which is the only known precursor of EAC, remains a ripe area for research, particularly, with regards to identifying the target population, screening tools, and management of screen-detected populations. This review aims to explore in depth, the rationale for screening for Barrett’s esophagus, recent biotechnological advances which may have the potential of making screening feasible, and also highlight the challenges which will have to be overcome in order make screening for BE a realistic prospect.
Recent findings
Imaging techniques such as portable transnasal endoscopy have the advantage of providing an immediate diagnosis of Barrett’s esophagus as well as other significant pathologies such as reflux esophagitis and cancer; however, larger studies in non-enriched community screening populations are required to evaluate their feasibility. The capsule sponge is a cell-sampling device coupled with a biomarker, which has been most extensively evaluated with very promising results with regards feasibility, acceptability, accuracy and cost-effectiveness. Its effectiveness in increasing the detection of Barrett’s esophagus in primary care is currently being evaluated. Several Barrett’s esophagus risk prediction scores have been developed with variable degrees of accuracy.
Summary
Several minimally- and non-invasive screening techniques have been studied including imaging and cell sampling devices. Barrett’s risk assessment models need to be further validated in independent, relevant screening populations with clear cut-offs for recommending screening to be defined.
Keywords: Screening, Barrett’s Esophagus, Esophageal adenocarcinoma, screening tools, risk stratification
Introduction:
Esophageal adenocarcinoma (EAC) is the most common histological subtype of esophageal cancer in the West. There has been an almost 6-fold increase in its incidence over the last three and a half decades in Europe and North America [1]. Up to 50% of patients with symptomatic presentation have incurable disease requiring palliative measures [2]. The 5-year survival rate remains poor at less than 20% in cases diagnosed after onset of symptoms [3]. On the other hand, cases diagnosed with early asymptomatic cancers have a substantially better than 5-year survival rates of greater than 80% [4, 5]. Barrett’s Esophagus (BE) is the only known precursor for EAC [6–8], but the majority of cases remain undiagnosed in the community [9] and 90% of patients with EAC do not have a previous diagnosis of BE, despite its presence at the time of surgery [6]. These and other data have provided an incentive to develop strategies for early detection of BE/EAC followed by curative therapy as a possible approach to reducing the incidence and mortality of this lethal cancer.
There is now renewed interest in this field following recent reports on the feasibility and safety of novel non-endoscopic (sponge based) [10, 11] and endoscopic unsedated, portable, and transnasal [12–14] techniques for BE screening in the community. Moreover, guidelines from gastroenterology societies from the United States and the United Kingdom have provided more support to screening individuals for BE and EAC, particularly those with “multiple” risk factors, such as male sex, age 50 years or older, chronic reflux symptoms, central obesity and a family history of EAC or BE [15–18]. However, the number of risk factors which may trigger a decision to screen and the tools to screen with remain unclear.
The aims of this review are to summarize: 1) the evidence for and challenges to screening; 2) advances in available tools for screening; 3) progress in identification of a target population for screening; and 3) ongoing challenges and areas for future research.
Rationale and Challenges for Screening:
Less than 10% of EACs in patients with BE are diagnosed during surveillance [19], hence the impact of surveillance on survival from EAC remains doubtful [20]. Moreover, the overall survival of patients with non-dysplastic BE appears to be similar to age and gender matched individuals with no BE [9]. In fact, only 7% of BE “surveillance” patients population die of EAC and the majority die from cardiovascular and pulmonary disease [21]. It is important to emphasize that while surveillance is aimed at detecting “incidental” cases of dysplasia and early cancer, screening is aimed at detecting the “prevalent” cases. For instance, the annual incidence of EAC in non-dysplastic BE were as low as 0.12% in one study[8], however, in the same study, approximately 66.5% of EACs occurred within a year from the initial endoscopic diagnosis of BE [8], suggesting a high rate of “prevalent” and missed cancers. Those cases are usually excluded from analysis when reporting cancer incidence, but may reflect the yield from a screening procedure [22].
Several studies have suggested that a prior diagnosis of BE, gastroesophageal reflux disease (GERD), or a prior endoscopy before the diagnosis of EAC is associated with early stage EAC an improved survival [23, 24]. While lead time and length time bias may exist, these data suggest that early detection may reduce mortality from EAC, in line with results from other studies reporting substantially improved survival rates of subjects with early stage (T1a and T1b) EAC compared to those with more advanced disease [25, 26, 4].
As a result, clinicians are faced with the challenge of a continuing rise in the incidence of a lethal cancer frequently diagnosed at a late stage, in the face of surveillance programs that target a population of low risk BE and seem to have minimal impact on overall survival.
It remains plausible that current strategies are not identifying a majority of subjects with prevalent BE in the community, leading to limiting clinical attention (by surveillance) to the minority of clinically diagnosed BE in the population. Despite the significant increase in the use of endoscopy, it is estimated that only one third of patients with BE in the population are clinically diagnosed, while the remainder are undiagnosed [9]. Indeed, more than 90% of patients who present with EAC do not have a previous diagnosis of BE [6], an indication of the underlying challenge.
Until recently, the lack of minimally invasive cost-effective therapies (to both prevent progression of dysplasia to EAC and treat early EAC) other than esophagectomy, were lacking. Endoscopic mucosal resection and ablation have now become the standard treatments for dysplastic BE, hence, this has put further impetus to develop and study techniques which can detect precursors to EAC in an acceptable, accurate and cost effective manner [27, 28]. In order to establish a strategy of population screening for BE, several hurdles have to be overcome, namely: 1) the lack of an acceptable, accurate and cost effective tool for screening; 2) the lack of a well characterized screening population; 3) the potential downstream costs of performing universal surveillance on screen detected BE patients as currently recommended (regardless of an individual’s risk of progression to EAC); and 4) lack of prospective randomized trial evidence that screening will lead to improved outcomes. In order to evaluate the latter, the former 3 hurdles need to be addressed. In the next few sections, we will review some recent advances, which may partially mitigate and address some of these limitations.
Recent developments: How to screen?
Sedated endoscopy may not be a cost-effective tool for BE population screening [29] due to both direct (sedation, monitoring, staff, recovery) and indirect (time off work and reduced productivity) costs. Other considerations include the need for an endoscopy suite, the small but significant risk of complications from conscious sedation and the procedure itself. Screening tests should ideally be simple, safe to perform, and acceptable to the population [30]. As a result, several alternatives to sedated endoscopy have been evaluated.
Non-endoscopic techniques:
Cell sampling devices
Initial prototypes yielded limited success. A cytology balloon collected samples following swallowing and withdrawal of a 30 mm balloon with a mesh cover. When compared with standard endoscopy and brush cytology, adequate samples were obtained in only 83% of patients with the former compared to 97% with the latter. Sensitivity of the balloon cytology for HGD and EAC was 80% but only 25% for LGD [31]. A non-endoscopic flexible mesh balloon catheter was also evaluated but adequate specimens were obtained in only 73% of patients. The sensitivity of identifying goblet cells was 87.5% [32]. These techniques were limited by the inadequacy of sampling and poor accuracy.
Over the last decade, a novel cell collection device coupled with a biomarker was developed by researchers at Cambridge University [10]. The Cytosponge is a gelatin capsule attached to a string. Once swallowed, the capsule dissolves in the stomach and releases a mildly abrasive reticulated foam sphere (approximately 30 mm in diameter) which can be pulled orally, with the attached string to collect cytology specimens from the esophagus. The samples are then analyzed for the presence of Trefoil Factor 3 (TFF3), a biomarker of specialized columnar epithelium. This device has been evaluated in 2 large studies so far with promising results [10, 11]. In one large primary care study, patients with gastroesophageal reflux disease (GERD) symptoms were invited to undergo the capsule sponge test in the primary care office followed by endoscopy with biopsies at the hospital. 504 patients (18%) agreed to take part. The capsule sponge test was technically successful in 99% of patients and was well tolerated. The sensitivity and specificity of the test for the detection of BE (circumferential length ≥1 cm) was 73.3% and 93.8%, respectively [10]. The second study was a case control study of 463 controls with dyspepsia and 647 BE cases [11]. The overall sensitivity in this population was 79.9% for circumferential BE ≥1 cm. This strategy has been found to be cost-effective in two economic modeling studies [33, 29]. A similar approach with a slightly smaller 25 mm sponge (EsophaCap, CapNostics, NJ, USA) combined with a panel of two methylated DNA markers has been reported to be highly accurate (with sensitivity and specificity of 100% and area under the curve of 1.0) in the detection of BE in a smaller pilot study conducted in the United States [34].
While, this approach is very promising with regards to applicability for community use, acceptability to patients and safety, it is a non-endoscopic technique, needing a confirmatory endoscopy with biopsies. Moreover, further evaluation of this approach to detect prevalent dysplasia and early EAC (an important aim of a BE screening program) is needed and ongoing.
Circulating and Exhaled molecular markers
A non-invasive screening test in the form of blood, breath, saliva, or other samples is a very attractive prospect for screening in view of safety, applicability for use in primary care, and no need for skilled operators to perform the test. .
Circulating microRNAs (miRNAs) are ~21–25 nucleotides in length, stable, and can be detected in plasma. They regulate several cellular processes and dysregulation in their function is associated with the pathogenesis of many diseases including cancer. A recent systematic review identified five miRNA biomarkers with the potential for diagnosing BE [35]. A subsequent pilot study reported a significant increase in 2 miRNAs (miR-194–5p and miR-451) and a decrease in one (miR136) in BE patients compared to controls [36]. The latter 3 were combined with another 3 miRNAs in a larger validation study and the authors reported that a panel combination of 4 of these miRNAs was the most accurate in differentiating BE (41 patients) from healthy controls (15 patients) with a sensitivity and specificity of 78.4% (95% CI, 61.8%–90.2%) and 85.7% (95% CI, 57.2%–98.2%), respectively [36]. However, the controls in this study had no reflux symptoms so the changes in miRNA levels could be due to GERD rather than BE. Salivary miRNAs have also been studies with promising results [37]. Further validation of these findings in larger cohorts is required.
Biomarker detection in breath is another attractive tool for screening, but studies are limited. One study identified distinct exhaled breath volatile organic compounds (VOCs) profiles that can distinguish patients with esophageal and gastric adenocarcinoma from non-cancer controls [38]. A proof of concept study evaluated 122 patients with and without BE using the commercially available portable e-nose device (Aeonose, The eNose Company, Zutphen, Netherlands). VOC profiles were introduced into an artificial neural network in a supervised fashion to identify data classifiers to discriminate differences in subjects stratified by the presence or absence of BE by biopsies. The sensitivity, specificity and area under the curve for the detection of BE was 82%, 80%, and 0.79, respectively [39]. The investigators postulated that the VOC differences detected may reflect metabolic activity of the BE mucosa itself or may be that of permissive microbiota in the upper gastrointestinal tract.
Imaging-based techniques:
Esophageal Capsule Endoscopy (ECE) allows visualization of the esophagus, but does not have the capability of biopsy sampling. While initial studies reported good accuracy for ECE in diagnosing BE [40], subsequent studies revealed suboptimal and conflicting results [41]. In a meta-analysis of 9 studies comprising a total of 618 patients [42], the pooled sensitivity and specificity of ECE was 77% and 86%, respectively. While ECE is safe, non-invasive and does not require sedation[43], it was not cost-effective when compared to standard endoscopy [44], and hence it is not currently recommended for BE screening. A modified ECE with a string attached to allow for controlled movement up and down the esophagus has been evaluated. While this device is less costly because it is re-usable, its sensitivity and specificity was 78.3% and 82.8%, respectively [45]. Therefore, its accuracy remains suboptimal for use in screening.
Unsedated transnasal endoscopy (TNE) has been shown to be an accurate alternative to standard endoscopy for the diagnosis of BE [46–48]. Moreover, a recent systematic review and meta-analysis reported equivalent technical success rates to SE [49]. The pooled difference in proportion of patients who preferred TNE over EGD was 63% (95% confidence interval [CI], 49.0–76.0, 10 studies), and acceptability was high for TNE with 85.2% (95% CI, 79.1–89.9; 16 studies) of patients willing to undergo the procedure again in the future if needed[49]. However, the currently available TNE devices require dedicated endoscopy suites with specialized equipment and reprocessing facilities. This could hamper their use for widespread screening. More recently, EndoSheath®technology (Vision-Sciences Inc., Orangeburg, New York) has been developed. It utilizes a disposable silicone sheath that covers the scope obviating the need for disinfection and utilizes a more compact processing system allowing for easy portability. This technology was utilized for mobile community and hospital based screening in a recent prospective randomized population based study[14]. The authors reported comparable clinical effectiveness, safety and participation rates to SE using the EndoSheath®. This approach was associated with substantially reduced direct and indirect costs when used for community screening of BE [50]. Another promising device is the EG Scan®(Intromedic Ltd., Seoul, South Korea), which incorporates a disposable probe omitting the need for disinfection. It is highly compact and portable and therefore can also potentially be used in the community. The feasibility, accuracy and performance characteristics of the EG Scan was recently evaluated in an international multicenter case control study using SE as the reference standard [51]. Sensitivity and specificity of EG Scan for BE diagnosis was 89.4% (95% CI 83.3– 95.6) and 90.3% (95% CI 84 – 96.7) respectively. Sensitivity was superior for long segment BE (93%). The test was feasible, safe and well tolerated[51]. TNE can also be performed successfully by physician extenders reducing operator costs [52]. These technologies are promising but further studies to evaluate their feasibility and cost-effectiveness for BE screening in the community.
Volumetric laser endomicroscopy (VLE) is a new generation optical coherence tomography that produces high resolution cross-sectional images of the esophagus. A tethered capsule endomicroscopy device has been recently developed [53]. It enables the comprehensive assessment of subsurface microstructures that cannot be visualized by endoscopy. In a small, proof-of-principle study in 7 healthy and 6 BE patients, endomicroscopic images of the esophageal mucosa, could distinguish between patients with and without BE [53]. The training required to conduct the procedure is minimal. It can be performed in a primary care physician’s office. It can be retrieved and disinfected, therefore reducing the cost, making it potentially feasible for large population screening, but data on its performance in this setting are still awaited.
Hence, in the last few years, several new and promising techniques for BE screening which are potentially acceptable, reasonably accurate, widely applicable and potentially cost effective, have emerged (figure 1). Further refinement and comparative evaluation of these technologies is required before the issue of the most optimal screening tool can be addressed. .
Figure 1:

Tools currently undergoing evaluation for use in BE screening. A, Tethered capsule endomicroscopy; B, Cytosponge; C, Transnasal EG Scan system; D, E-Nose device; E and F, Transnasal EndoSheath system. (Modified with permission from Sami SS, et al [76]. Copyright Elsevier).
Recent Developments: who to screen?
The absence of a well-defined target population remains one of the major challenges to screening. While GERD symptoms are the strongest risk factor for BE and EAC, with a 5 and 8 fold increase in risk, respectively [54, 55], prevalence rates of 14.9%, 25% and 45% for BE have been reported in patients who were asymptomatic for GERD [56–58]. This indicates that one cannot rely solely on the presence of GERD as a selection criterion. Moreover, the target population will be very large due to the high prevalence of GERD in Western populations (15–20%) [59].
Additional risk factors for developing BE include: male sex; Caucasian race; age greater than 50 years; central obesity, smoking and family history [60]. The median age of diagnosis is 60 years and prevalence increases with age [61]. In a recent modeling study the authors estimated the symptom-, age-, and sex-specific incidences of EAC, and compared these figures to other cancers for which screening is endorsed, namely, colorectal and breast cancer [62]. The projected incidence of EAC in women with GERD was estimated to be comparable to that of breast cancer in men, and hence the value of screening in women was questioned. Indeed recent recommendations do not suggest screening for BE in women [18].
Central adiposity (measured as visceral adipose tissue area, increased waist-hip ratio, or waist/abdominal circumference) rather than BMI has also been implicated in the pathogenesis of BE [63]. The association between central adiposity and BE has been consistently demonstrated in several studies (OR, 1.98; 95% CI, 1.52–2.57) independent of BMI and symptomatic GERD [64]. Central obesity is also strongly associated with EAC and the effects may be mediated by reflux-independent and dependent mechanisms [64].
Family history of BE or EAC is also linked with an increased risk of BE and up to 28% of first degree relatives of patients with EAC or dysplastic BE have BE [65]. A recent large genome-wide association study (GWAS) study identified genetic variants associated with BE risk at two loci; one at chromosome 16q24 (OR 1.14, 95%CI 1.10–1.19), and another on chromosome 6p21 (OR 1.21, 95%CI 1.13–1.28). The closest protein-coding gene to this chromosome was FOXF1, which is a transcription factor involved in esophageal development and structure [66]. MSR1, ASCC1, and CTHRC1 genes germline mutations have also been connected with BE and EAC [67]. Patients with BE are significantly more likely to have ever smoked compared to population-based controls (OR: 1.67; 95% CI: 1.04–2.67) or GERD controls (OR: 1.61; 95% CI: 1.33–1.96).
In an attempt to increase the yield from screening, BE risk prediction scores have been developed for use an inexpensive pre-selection tool for screening. So far, 6 models with different set of predictors have been published [68–73]. Thrift et al [68], initially evaluated a large number of putative risk factors in a case-control study with external validation. The area under the receiver operating characteristic curve (AUROC) was 0.70 and 0.61 in the derivation and validation groups, respectively. The accuracy was further improved in a subsequent model with the addition of serum biomarkers [69]. The combination of the multi-biomarker risk score model with a demographic and clinical features model (based on GERD frequency and duration, age, sex, race, waist-to-hip ratio, and H. Pylori status) was significantly more accurate at predicting the presence of BE compared to a combination of the risk score with a model based on GERD frequency and duration alone (AUROC 0.85 vs. 0.74; p = 0.01).
Another modestly accurate model is the online Michigan Barrett’s Esophagus pREdiction Tool (M-BERET) (http://mberet.umms.med.umich.edu/). This model was based on a population of colonoscopy screenees who consented to a study endoscopy and included four variables (weekly GERD, age, waist-to-hip ratio and pack-years of cigarette smoking). It performed better (AUROC = 0.72) than a model with reflux parameters alone (AUROC = 0.61, p<0.001) [70]. This model has recently been validated in four other populations [74]. A recent model derived from a random population-based cohort of subjects from Olmsted County undergoing screening for BE, reported an AUROC of 0.71 [71]. None of the available models are currently recommended for clinical use given the need for further refinement and definition of a threshold for screening. Further refinement and modifications may be needed to increase AUROC values to perhaps more than 0.90, which may be a more optimal threshold for widespread adoption into clinical practice. Recent Gastroenterology society recommendations outlining criteria for screening are listed in Table 1. These recommendations need to be carefully weighed on a case by case basis taking into account patient’s co-morbidities, performance status, and after careful discussion of the pros and cons of screening.
Table 1:
| Society (year) |
Demographic profile to be considered for screening |
|---|---|
| ACG (2016) | Male with either >5 years of GERD or >weekly GERD symptoms AND ≥2 other risk factors: Age >50 years, central obesity (waist circumference >102 cm or WHR >0.9), Caucasian race, active/history of smoking, first-degree relative with BE or EAC. Screening in females not recommended unless multiple risk factors are present. |
| BSG (2014) | GERD and at least 3 risk factors (age >50 years, Caucasian race, male sex, and obesity); threshold lowered in those with family history. |
| ASGE (2012) | Male sex, white race, age >50 years, chronic GERD, family history of BE, smoking, and obesity. |
| AGA (2011) | Male sex, white race, age >50 years, chronic GERD, hiatal hernia, obesity. |
ACG, American College of Gastroenterology; BSG, British Society of Gastroenterology; ASGE, American Society of Gastrointestinal Endoscopy; AGA, American Gastroenterological Association; GERD, Gastroesophageal reflux symptoms; WHR, Waist to hip ratio; BE, Barrett’s Esophagus; EAC, Esophageal adenocarcinoma
Summary and Future directions:
Despite recent advances, several challenges to BE screening remain. With regards to tools for screening, the capsule sponge and TNE are the most studied in both the community and secondary care settings. Larger community based trials are currently ongoing using these techniques. Comparative data on participation rates and patient preferences are needed to determine best approaches for BE screening. Data on novel portable and disposable TNE techniques are also promising, but further data on its feasibility for use in the community as well as its cost-effectiveness is needed. Furthermore, its acceptance by physicians remains low [75]. Novel non-invasive biomarkers identifiable in blood, saliva, and breath would be ideal for widespread application, but they are currently in the early stages of research.
In order to reduce the number of “missed” patients in a screening program, BE risk prediction models with high sensitivity are needed to be used as an inexpensive pre-selection tool for screening. The accuracy of current models is unlikely to be sufficient for widespread clinical use (AUROC 0.6–0.85). Furthermore, these scoring tools require external validation in unselected populations, testing in females, and likely addition of other circulating biomarkers. Iterative refinements of such models combined with a simple screening test may potentially make screening for BE more cost-effective. As a result, it is likely that the use of prediction models based on multiple risk factors, will emerge as the means of identifying at risk populations for BE and EAC.
Any screening program is likely to result in an increased workload and downstream costs to the healthcare systems which are challenging to justify. The best approach for management of screening-diagnosed patients with NDBE remains unclear, particularly as the majority does not progress to cancer. Performing routine endoscopic surveillance in those subjects will likely lead to escalating costs potentially without substantial benefit. Therefore, markers and models to predict individuals at increased risk of progression to EAC are needed. Ideally, subjects with a low risk of progression could be discharged from surveillance while those at a higher risk could be placed in intensive surveillance with advanced imaging or offered ablation to decrease the risk of developing EAC. A conceptual flow diagram outlining an approach to decrease the incidence of and mortality from EAC is presented in Figure 2. This represents a future vision of how this process could potentially be effective in reducing EAC incidence and mortality.
Figure 2:
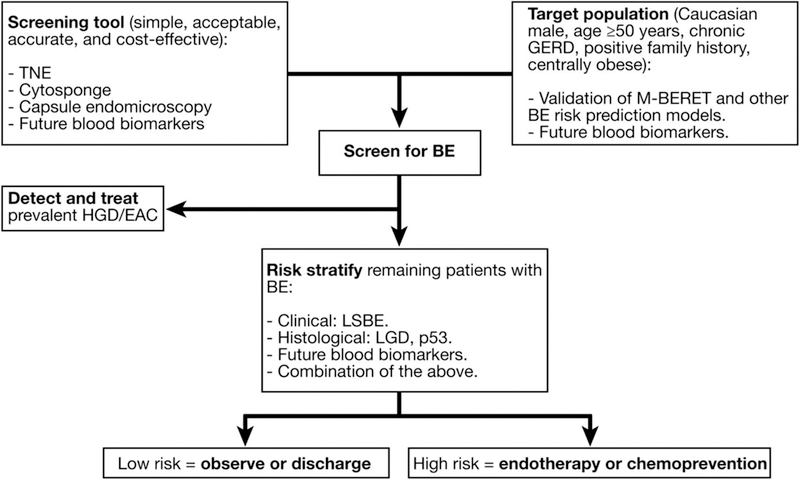
Proposed approach to reducing incidence and mortality from EAC. (Reproduced with permission from Sami SS, et al [76]. Copyright Elsevier). BE, Barrett’s Esophagus; EAC, esophageal adenocarcinoma; GERD, gastroesophageal reflux disease; HGD, high grade dysplasia; LGD, low grade dysplasia; LSBE, long segment Barrett’s Esophagus; TNE, transnasal endoscopy.
In summary, in addition to the several exciting advances, challenges to the widespread application of BE screening remain. Nevertheless, this area remains ripe for research, particularly focusing on the comprehensive evaluation of emerging minimally-invasive and novel screening tools as well as deriving and validating BE and EAC risk prediction models.
Acknowledgments
Grant support:
Grant NCI U54 CA163004 received by Prasad Iyer
Human and Animal Rights:
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Abbreviations:
- AUROC
receiver operating characteristic curve
- BE
Barrett’s Esophagus
- BMI
body mass index
- EAC
esophageal adenocarcinoma
- GERD
gastroesophageal reflux disease
- GWAS
genome-wide association study
- HGD
high grade dysplasia
- LGD
low grade dysplasia
- LOH
loss of heterozygosity
- LSBE
long segment Barrett’s Esophagus
- M-BERET
Michigan Barrett’s Esophagus pREdiction Tool
- NDBE
nondysplastic Barrett’s Esophagus
- OSA
obstructive sleep apnea
- OR
odds ratio
- SIM
small intestinal metaplasia
- TFF3
Trefoil Factor 3
- TNE
transnasal endoscopy
- ECE
esophageal capsule endoscopy
References:
- 1.Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiology Biomarkers and Prevention. 2010;19(6):1468–70. [DOI] [PubMed] [Google Scholar]
- 2.Auvinen MI, Sihvo EI, Ruohtula T, Salminen JT, Koivistoinen A, Siivola P et al. Incipient angiogenesis in Barrett’s epithelium and lymphangiogenesis in Barrett’s adenocarcinoma. J Clin Oncol. 2002;20(13):2971–9. [DOI] [PubMed] [Google Scholar]
- 3.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97(2): 142–6. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 4.Prasad GA, Wu TT, Wigle DA, Buttar NS, Wongkeesong LM, Dunagan KT et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology. 2009;137(3):815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P, Katzka DA, Gupta N, Ajani J, Buttar N, Chak A et al. Quality indicators for the management of Barrett’s esophagus, dysplasia, and esophageal adenocarcinoma: international consensus recommendations from the American Gastroenterological Association Symposium. Gastroenterology. 2015;149(6):1599–606. doi: 10.1053/j.gastro.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dulai GS, Guha S, Kahn KL, Gornbein J, Weinstein WM. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: A systematic review. Gastroenterology. 2002;122(1):26–33. [DOI] [PubMed] [Google Scholar]
- 7.Chak A, Faulx A, Eng C, Grady W, Kinnard M, Ochs-Balcom H et al. Gastroesophageal reflux symptoms in patients with adenocarcinoma of the esophagus or cardia. Cancer. 2006;107(9):2160–6. [DOI] [PubMed] [Google Scholar]
- 8.Hvid-Jensen F, Pedersen L, Drewes AM, Sorensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365(15):1375–83. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 9.Jung KW, Talley NJ, Romero Y, Katzka DA, Schleck CD, Zinsmeister AR et al. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett’s esophagus: a population-based study. American Journal of Gastroenterology. 2011;106(8):1447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadri SR, Lao-Sirieix P, O’Donovan M, Debiram I, Das M, Blazeby JM et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. BMJ. 2010;341:c4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross-Innes CS, Debiram-Beecham I, O’Donovan M, Walker E, Varghese S, Lao-Sirieix P et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett’s esophagus: a multi-center case-control study. PLoS Med. 2015;12(1):e1001780. doi: 10.1371/journal.pmed.1001780. **Large case control study showing the Cytosponge-TFF3 test to be safe, acceptable, and has accuracy comparable to other screening tests. This test may be a simple and inexpensive approach to screen patients for Barrett’s esophagus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peery AF, Hoppo T, Garman KS, Dellon ES, Daugherty N, Bream S et al. Feasibility, safety, acceptability, and yield of office-based, screening transnasal esophagoscopy (with video). Gastrointestinal Endoscopy. 2012;75(5):945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shariff MK, Bird-Lieberman EL, O’Donovan M, Abdullahi Z, Liu X, Blazeby J et al. Randomized crossover study comparing efficacy of transnasal endoscopy with that of standard endoscopy to detect Barrett’s esophagus. Gastrointestinal Endoscopy. 2012;75(5):954–61. [DOI] [PubMed] [Google Scholar]
- 14.Sami SS, Dunagan K, Johnson ML, Schleck C, Shah N, Zinsmeister A et al. A randomized comparative effectiveness trial of novel endoscopic techniques and approaches for Barrett’s esophagus screening in the community. Am J Gastroenterol. 2015;110(1):148–58. **Study showed that community mobile unit screening with disposable transnasal endoscopy has equivalent participation rates and effectiveness to hospital screening. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63(1):7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 16.Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140(3):1084–91. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 17.Evans JA, Early DS, Fukami N, Ben-Menachem T, Chandrasekhara V, Chathadi KV et al. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc. 2012;76(6):1087–94. doi: 10.1016/j.gie.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Shaheen NJ, Falk GW, Iyer PG, Gerson LB. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol. 2016;111(1):30–50. doi: 10.1038/ajg.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Visrodia K, Singh S, Krishnamoorthi R, Ahlquist DA, Wang KK, Iyer PG et al. Systematic review with meta-analysis: prevalent vs. incident oesophageal adenocarcinoma and high-grade dysplasia in Barrett’s oesophagus. Aliment Pharmacol Ther. 2016;44(8):775–84. doi: 10.1111/apt.13783. [DOI] [PubMed] [Google Scholar]
- 20.Corley DA, Mehtani K, Quesenberry C, Zhao W, de Boer J, Weiss NS. Impact of Endoscopic Surveillance on Mortality From Barrett’s Esophagus-Associated Esophageal Adenocarcinomas. Gastroenterology. 2013;145(2):312–9.e1. doi: 10.1053/j.gastro.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sikkema M, de Jonge PJ, Steyerberg EW, Kuipers EJ. Risk of esophageal adenocarcinoma and mortality in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2010;8(3):235–44. doi: 10.1016/j.cgh.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Visrodia K, Singh S, Krishnamoorthi R, Ahlquist DA, Wang KK, Iyer PG et al. Magnitude of Missed Esophageal Adenocarcinoma After Barrett’s Esophagus Diagnosis: A Systematic Review and Meta-analysis. Gastroenterology. 2016;150(3):599–607.e7; quiz e14–5. doi: 10.1053/j.gastro.2015.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper GS, Yuan Z, Chak A. Association of prediagnosis endoscopy with stage and survival in adenocarcinoma of the esophagus and gastric cardia. . Cancer. 2002;95(1):32–8. [DOI] [PubMed] [Google Scholar]
- 24.Cooper GS, Kou TD, Chak A. Receipt of previous diagnoses and endoscopy and outcome from esophageal adenocarcinoma: a population-based study with temporal trends. . Am J Gastroenterol. 2009; 104(6):1356–62. [DOI] [PubMed] [Google Scholar]
- 25.Das A, Singh V, Fleischer DE, Sharma VK. A Comparison of Endoscopic Treatment and Surgery in Early Esophageal Cancer: An Analysis of Surveillance Epidemiology and End Results Data. Am J Gastroenterol. 2008;103(6):1340–5. [DOI] [PubMed] [Google Scholar]
- 26.Wani S, Drahos J, Cook MB, Rastogi A, Bansal A, Yen R et al. Comparison of endoscopic therapies and surgical resection in patients with early esophageal cancer: a population-based study. Gastrointest Endosc. 2014;79(2):224–32 e1. doi: 10.1016/j.gie.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett C, Vakil N, Bergman J, Harrison R, Odze R, Vieth M et al. Consensus Statements for Management of Barrett’s Dysplasia and Early-Stage Esophageal Adenocarcinoma, Based on a Delphi Process. Gastroenterology. 2012;143(2):336–46. doi:S0016–5085(12)00614–2 [pii] 10.1053/j.gastro.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inadomi JM, Somsouk M, Madanick RD, Thomas JP, Shaheen NJ. A cost-utility analysis of ablative therapy for Barrett’s esophagus. Gastroenterology. 2009;136(7):2101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heberle CR, Omidvari AH, Ali A, Kroep S, Kong CY, Inadomi JM et al. Cost Effectiveness of Screening Patients With Gastroesophageal Reflux Disease for Barrett’s Esophagus With a Minimally Invasive Cell Sampling Device. Clin Gastroenterol Hepatol. 2017; 15(9): 1397–404. doi: 10.1016/j.cgh.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.UK National Screening Commitee, Criteria for appraising the viability, effectiveness and appropriateness of a screening programme. http://wwwscreeningnhsuk/criteria. 2009.
- 31.Falk GW, Chittajallu R, Goldblum JR, Biscotti CV, Geisinger KR, Petras RE et al. Surveillance of patients with Barrett’s esophagus for dysplasia and cancer with balloon cytology. Gastroenterology. 1997;112(6):1787–97. [DOI] [PubMed] [Google Scholar]
- 32.Rader AE, Faigel DO, Ditomasso J, Magaret N, Burm M, Fennerty MB. Cytological screening for Barrett’s esophagus using a prototype flexible mesh catheter. Digestive Diseases and Sciences. 2001;46(12):2681–6. [DOI] [PubMed] [Google Scholar]
- 33.Benaglia T, Sharples LD, Fitzgerald RC, Lyratzopoulos G. Health benefits and cost effectiveness of endoscopic and nonendoscopic cytosponge screening for Barrett’s esophagus. Gastroenterology. 2013;144(1):62–73 e6. doi: 10.1053/j.gastro.2012.09.060. [DOI] [PubMed] [Google Scholar]
- 34.Iyer P, Johnson ML, Lansing R, Yab TC, Taylor WR, Pophali PA et al. Discovery, Validation and Feasibility Testing of Highly Discriminant DNA Methylation Markers for Detection of Barrett’s Esophagus Using a Capsule Sponge Device. Gastroenterology. 2017;150(4):S66–S7. doi: 10.1016/s0016-5085(16)30344-4. [DOI] [Google Scholar]
- 35.Mallick R, Patnaik SK, Wani S, Bansal A. A Systematic Review of Esophageal MicroRNA Markers for Diagnosis and Monitoring of Barrett’s Esophagus. Dig Dis Sci. 2016;61(4):1039–50. doi: 10.1007/s10620-015-3959-3. [DOI] [PubMed] [Google Scholar]
- 36.Bus P, Kestens C, Ten Kate FJ, Peters W, Drenth JP, Roodhart JM et al. Profiling of circulating microRNAs in patients with Barrett’s esophagus and esophageal adenocarcinoma. J Gastroenterol. 2016;51(6):560–70. doi: 10.1007/s00535-015-1133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie Z, Chen G, Zhang X, Li D, Huang J, Yang C et al. Salivary microRNAs as promising biomarkers for detection of esophageal cancer. PLoS One. 2013;8(4):e57502. doi: 10.1371/journal.pone.0057502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar S, Huang J, Abbassi-Ghadi N, Mackenzie HA, Veselkov KA, Hoare JM et al. Mass Spectrometric Analysis of Exhaled Breath for the Identification of Volatile Organic Compound Biomarkers in Esophageal and Gastric Adenocarcinoma. Ann Surg. 2015;262(6):981–90. doi: 10.1097/sla.0000000000001101. [DOI] [PubMed] [Google Scholar]
- 39.Chan DK, Zakko L, Visrodia KH, Leggett CL, Lutzke LS, Clemens MA et al. Breath Testing for Barrett’s Esophagus Using Exhaled Volatile Organic Compound Profiling With an Electronic Nose Device. Gastroenterology. 2017;152(1):24–6. doi: 10.1053/j.gastro.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Eliakim R, Sharma VK, Yassin K, Adler SN, Jacob H, Cave DR et al. A prospective study of the diagnostic accuracy of PillCam ESO esophageal capsule endoscopy versus conventional upper endoscopy in patients with chronic gastroesophageal reflux diseases. Journal of Clinical Gastroenterology. 2005;39(7):572–8. [DOI] [PubMed] [Google Scholar]
- 41.Sharma P, Wani S, Rastogi A, Bansal A, Higbee A, Mathur S et al. The diagnostic accuracy of esophageal capsule endoscopy in patients with gastroesophageal reflux disease and Barrett’s esophagus: a blinded, prospective study. American Journal of Gastroenterology. 2008;103(3):525–32. [DOI] [PubMed] [Google Scholar]
- 42.Bhardwaj A, Hollenbeak CS, Pooran N, Mathew A. A meta-analysis of the diagnostic accuracy of esophageal capsule endoscopy for Barrett’s esophagus in patients with gastroesophageal reflux disease. American Journal of Gastroenterology. 2009;104(6):1533–9. [DOI] [PubMed] [Google Scholar]
- 43.Chang JY, Talley NJ, Locke GR 3rd, Katzka DA, Schleck CD, Zinsmeister AR et al. Population screening for barrett esophagus: a prospective randomized pilot study. Mayo Clinic Proceedings. 2011. ;86(12):1174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubenstein JH, Inadomi JM, Brill JV, Eisen GM. Cost utility of screening for Barrett’s esophagus with esophageal capsule endoscopy versus conventional upper endoscopy. Clinical Gastroenterology & Hepatology. 2007;5(3):312–8. [DOI] [PubMed] [Google Scholar]
- 45.Ramirez FC, Akins R, Shaukat M. Screening of Barrett’s esophagus with string-capsule endoscopy: a prospective blinded study of 100 consecutive patients using histology as the criterion standard. Gastrointestinal Endoscopy. 2008;68(1):25–31. [DOI] [PubMed] [Google Scholar]
- 46.Sami SS, Subramanian V, Ortiz-Fernández-Sordo’ J, Saeed A, Ragunath K. The utility of ultrathin endoscopy as a diagnostic tool for barrett’s oesophagus (BO). Systematic review and meta-analysis United European Gastroenterology Week; Berlin 2013. [Google Scholar]
- 47.Shariff MK, Bird-Lieberman EL, O’Donovan M, Abdullahi Z, Liu X, Blazeby J et al. Randomized crossover study comparing efficacy of transnasal endoscopy with that of standard endoscopy to detect Barrett’s esophagus. Gastrointest Endosc. 2012;75(5):954–61. [DOI] [PubMed] [Google Scholar]
- 48.Jobe BA, Hunter JG, Chang EY, Kim CY, Eisen GM, Robinson JD et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett’s esophagus: a randomized and blinded comparison. Am J Gastroenterol. 2006;101(12):2693–703. [DOI] [PubMed] [Google Scholar]
- 49.Sami SS, Subramanian V, Ortiz-Fernandez-Sordo J, Saeed A, Singh S, Guha IN et al. Performance characteristics of unsedated ultrathin video endoscopy in the assessment of the upper GI tract: systematic review and meta-analysis. Gastrointest Endosc. 2015;82(5):782–92. doi: 10.1016/j.gie.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 50.Moriarty JP, Shah ND, Rubenstein JH, Blevins CH, Johnson M, Katzka DA et al. Costs associated with Barrett’s esophagus screening in the community: an economic analysis of a prospective randomized controlled trial of sedated versus hospital unsedated versus mobile community unsedated endoscopy. Gastrointest Endosc [Epub ahead of print] 2017. April 25. doi: 10.1016/j.gie.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sami S, Pophali P, Halland M, DiPietro M, Enders F, Fernandez-Sordo JO et al. Feasibility and Performance Characteristics of a Novel Disposable Transnasal Capsule Device for Barrett’s Oesophagus Screening: A Prospective International Multicentre Trial. Gut. 2017;66(suppl 2):OC-003. doi: 10.1016/j.gie.2017.03.101. [DOI] [Google Scholar]
- 52.Alashkar B, Faulx AL, Hepner A, Pulice R, Vemana S, Greer KB et al. Development of a Program to train Physician Extenders to Perform Transnasal Esophagoscopy and Screen for Barrett’s Esophagus. Clin Gastroenterol Hepatol. 2013;October 23 [Epub ahead of print]. doi: 10.1016/j.cgh.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gora MJ, Sauk JS, Carruth RW, Gallagher KA, Suter MJ, Nishioka NS et al. Tethered capsule endomicroscopy enables less invasive imaging of gastrointestinal tract microstructure. Nat Med. 2013;19(2):238–40. doi: 10.1038/nm.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor JB, Rubenstein JH. Meta-analyses of the effect of symptoms of gastroesophageal reflux on the risk of Barrett’s esophagus. The American journal of gastroenterology. 2010;105(8):1729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. The New England journal of medicine. 1999;340(11):825–31. [DOI] [PubMed] [Google Scholar]
- 56.Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett’s esophagus in asymptomatic individuals. Gastroenterology. 2002;123(2):461–7. [DOI] [PubMed] [Google Scholar]
- 57.Ward EM, Wolfsen HC, Achem SR, Loeb DS, Krishna M, Hemminger LL et al. Barrett’s esophagus is common in older men and women undergoing screening colonoscopy regardless of reflux symptoms. American Journal of Gastroenterology. 2006;101(1):12–7. [DOI] [PubMed] [Google Scholar]
- 58.Zagari RM, Fuccio L, Wallander MA, Johansson S, Fiocca R, Casanova S et al. Gastro-oesophageal reflux symptoms, oesophagitis and barrett’s oesophagus in the general population: The Loiano-Monghidoro study. Gut. 2008;57(10):1354–9. [DOI] [PubMed] [Google Scholar]
- 59.Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54(5):710–7. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balasubramanian G, Singh M, Gupta N, Gaddam S, Giacchino M, Wani SB et al. Prevalence and predictors of columnar lined esophagus in gastroesophageal reflux disease (GERD) patients undergoing upper endoscopy. Am J Gastroenterol. 2012;107(11):1655–61. doi: 10.1038/ajg.2012.299. [DOI] [PubMed] [Google Scholar]
- 61.Eloubeidi MA, Provenzale D. Clinical and demographic predictors of Barrett’s esophagus among patients with gastroesophageal reflux disease: a multivariable analysis in veterans. Journal of Clinical Gastroenterology. 2001;33(4):306–9. [DOI] [PubMed] [Google Scholar]
- 62.Rubenstein JH, Scheiman JM, Sadeghi S, Whiteman D, Inadomi JM. Esophageal adenocarcinoma incidence in individuals with gastroesophageal reflux: synthesis and estimates from population studies. Am J Gastroenterol. 2011;106(2):254–60. doi: 10.1038/ajg.2010.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong A, Fitzgerald RC. Epidemiologic risk factors for Barrett’s esophagus and associated adenocarcinoma. Clin Gastroenterol Hepatol. 2005;3(1):1–10. [DOI] [PubMed] [Google Scholar]
- 64.Singh S, Sharma AN, Murad MH, Buttar NS, El-Serag HB, Katzka DA et al. Central Adiposity Is Associated With Increased Risk of Esophageal Inflammation, Metaplasia, and Adenocarcinoma: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2013;11(11):1399–412 e7. doi: 10.1016/j.cgh.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Juhasz A, Mittal SK, Lee TH, Deng C, Chak A, Lynch HT. Prevalence of Barrett esophagus in first-degree relatives of patients with esophageal adenocarcinoma. J Clin Gastroenterol. 2011;45(10):867–71. doi: 10.1097/MCG.0b013e31821f44a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Su Z, Gay LJ, Strange A, Palles C, Band G, Whiteman DC et al. Common variants at the MHC locus and at chromosome 16q24.1 predispose to Barrett’s esophagus. Nat Genet. 2012;44(10):1131–6. doi: 10.1038/ng.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Orloff M, Peterson C, He X, et al. Germline mutations in msr1, ascc1, and cthrc1 in patients with barrett esophagus and esophageal adenocarcinoma. JAMA. 2011;306(4):410–9. doi: 10.1001/jama.2011.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thrift AP, Kendall BJ, Pandeya N, Vaughan TL, Whiteman DC. A clinical risk prediction model for Barrett esophagus. Cancer Prevention Research. 2012;5(9):1115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thrift AP, Garcia JM, El-Serag HB. A multibiomarker risk score helps predict risk for Barrett’s esophagus. Clin Gastroenterol Hepatol. 2014;12(8):1267–71. doi: 10.1016/j.cgh.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rubenstein JH, Morgenstern H, Appelman H, Scheiman J, Schoenfeld P, McMahon LF Jr,. et al. Prediction of Barrett’s esophagus among men. Am J Gastroenterol. 2013;108(3):353–62. doi: 10.1038/ajg.2012.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crews NR, Johnson ML, Schleck CD, Enders FT, Wongkeesong LM, Wang KK et al. Prevalence and Predictors of Gastroesophageal Reflux Complications in Community Subjects. Dig Dis Sci. 2016;61(11):3221–8. doi: 10.1007/s10620-016-4266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Locke GR, Zinsmeister AR, Talley NJ. Can symptoms predict endoscopic findings in GERD? Gastrointest Endosc. 2003;58(5):661–70. [DOI] [PubMed] [Google Scholar]
- 73.Gerson LB, Edson R, Lavori PW, Triadafilopoulos G. Use of a simple symptom questionnaire to predict Barrett’s esophagus in patients with symptoms of gastroesophageal reflux. American Journal of Gastroenterology. 2001;96(7):2005–12. [DOI] [PubMed] [Google Scholar]
- 74.Thrift AP, Vaughan TL, Anderson LA, Whiteman DC, El-Serag HB. External Validation of the Michigan Barrett’s Esophagus Prediction Tool. Clinical Gastroenterology and Hepatology. 2017;15(7):1124–6. doi: 10.1016/j.cgh.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Atkinson M, Das A, Faulx A, Kinnard M, Falck-Ytter Y, Chak A. Ultrathin esophagoscopy in screening for Barrett’s esophagus at a Veterans Administration Hospital: easy access does not lead to referrals. American Journal of Gastroenterology. 2008;103(1):92–7. [DOI] [PubMed] [Google Scholar]
- 76.Sami SS, Ragunath K, Iyer PG. Screening for Barrett’s esophagus and esophageal adenocarcinoma: rationale, recent progress, challenges, and future directions. Clin Gastroenterol Hepatol. 2015;13(4):623–34. doi: 10.1016/j.cgh.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]


