Abstract
Purpose:
The natural history of non-clear cell renal cell carcinomas (non-ccRCC) following surgery with curative intent remains poorly defined, with post-operative surveillance informed by guidelines largely intended for clear cell RCC (ccRCC). We evaluated the patterns of relapse and potential implications for post-nephrectomy surveillance for patients with non-ccRCC enrolled in the largest randomized trial of adjuvant anti-angiogenic therapy for high-risk RCC (E2805).
Materials and Methods:
This was a retrospective analysis of patients with completely resected non-ccRCC. Participants received up to 54 weeks of post-operative therapy with sunitinib, sorafenib, or placebo, as well as surveillance imaging at standardized intervals for 10 years. For recurrence rates (RR) by site, the cumulative incidence was estimated accounting for competing risks. The adequacy of strict adherence to post-nephrectomy surveillance guidelines was evaluated.
Results:
403 non-ccRCC patients were enrolled, and 36% of non-ccRCC tumors recurred over a median follow-up period of 6.2 years. Five-year RRs were comparable between non-ccRCC and ccRCC (N = 1541) patients (34.6% vs 39.5%) [95% CIs (29.8 – 39.4) and (36.9 – 42.1), respectively]. However, non-ccRCC patients were significantly more likely to develop abdominal sites of relapse (5-year RR 26.4% vs 18.2%, p = 0.0008), and were significantly less likely to relapse in the chest (5-year RR 13.7% vs 20.9%, p = 0.0005). Current surveillance guidelines would capture approximately 90% of relapses at any site.
Conclusions:
Non-ccRCC may exhibit a distinct pattern of relapse when compared to conventional ccRCC. Our findings emphasize the importance of continued long-term imaging for patients with high-risk resected non-ccRCC.
Keywords: Non-clear cell, surveillance, nephrectomy, relapse, renal cell carcinoma
Introduction:
Non-clear cell renal cell carcinomas (non-ccRCC) represent a heterogeneous group of rare kidney cancers, accounting for approximately 25% of all RCCs. 1 Importantly, non-ccRCCs exhibit clinical behavior and disease biology that is distinct from conventional clear cell RCC (ccRCC), including a variety of genetic alterations and druggable pathways specific to non-ccRCC histologies. 2,3 However, despite these observed differences, the optimal management of non-ccRCCs remains unknown, largely owing to a paucity of clinical studies specific to this patient population. Across the non-ccRCC disease stage spectrum, current clinical management relies heavily on evidence extrapolated from well-established ccRCC treatment regimens, often despite recognition of suboptimal clinical outcomes.4,5
In particular, the natural history of non-ccRCC following surgery with curative-intent remains poorly defined, with post-operative surveillance strategies derived from consensus guidelines that are largely intended for ccRCC. 6,7 Prior reports describing clinical outcomes for patients with non-ccRCC primarily consist of small retrospective studies of heterogeneous populations (including patients with medullary carcinoma or collecting duct histologies), lack information regarding relapse patterns, or focus exclusively on patients with metastatic disease. 2,8,9 Furthermore, available post-surgical prognostic risk models focus primarily on ccRCC populations. 10 Therefore, an improved understanding of the patterns of relapse for resected non-ccRCC histologies is critical to inform patient counseling and optimal surveillance strategies for this understudied population.
We sought to evaluate the patterns of relapse and the implications for post-nephrectomy surveillance for patients with non-ccRCC enrolled in the first and largest randomized trial of adjuvant anti-angiogenic therapy for high-risk RCC.
Materials and Methods:
This was a retrospective analysis of all patients with non-ccRCC enrolled on ECOG-ACRIN E2805, which was a double-blind, placebo-controlled, randomized phase III trial of adjuvant sunitinib or sorafenib anti-angiogenic therapy in patients with resected local disease at high risk for recurrence (NCT 00326898). 11 Importantly, E2805 is the only reported phase III trial of adjuvant anti-angiogenic systemic therapy to include patients with non-ccRCC histologies. Study eligibility and treatment algorithms are as previously described. 11 Briefly, eligible patients with intermediate or high risk (≥ T1b Grade 3–4 N0) ccRCC or non-ccRCC within 12 weeks of complete primary tumor resection received up to 54 weeks of sunitinib, sorafenib, or placebo post-operative therapy. Protocol follow-up consisted of cross-sectional imaging of the chest, abdomen, and pelvis every 4.5 months during treatment, then every 6 months for 2 years, then at least annually for 10 years (regardless of pathologic tumor stage). 11 Central pathology review was conducted.
The Kaplan-Meier method was used to estimate disease-free survival (DFS), defined as the time from randomization to disease recurrence, development of a second primary cancer, or death from any cause. The log-rank test was used to evaluate survival differences between groups. Disease recurrence and sites of relapse were per investigator-assessment. Relapse sites in the chest included pulmonary parenchyma, thoracic lymphadenopathy, and pleural disease. Abdominal relapse sites included the nephrectomy bed, abdominopelvic lymphadenopathy, hepatic mass, abdominal wall, and peritoneal disease. For recurrence rates (RR) by site, the cumulative incidence was estimated accounting for competing risks, including recurrence at other sites, development of a second primary cancer, or death. Gray’s test was used to compare the incidence between groups. Multivariable Fine-Gray competing risks regression models were used to assess the effect of non-ccRCC histology on the observed clinical relapse pattern (chest vs abdominal relapse). Differences were considered significant at a p-value < 0.05. The current NCCN and AUA recommendations were used to evaluate the adequacy of strict adherence to post-nephrectomy consensus surveillance guidelines.6,7
Results:
Overall, 403 patients with non-ccRCC were enrolled in E2805 and included for analysis (N = 135 sunitinib, N = 130 sorafenib, N = 138 placebo). Patient characteristics at study entry are displayed in Table 1. Forty-seven percent (191/403) of patients were categorized as very-high risk by UCLA International Staging System (UISS) prognostic criteria. 12 The majority of patients (63%) underwent an open surgical approach, and 93% underwent a radical nephrectomy.
Table 1.
Characteristics of Patients with Non-Clear Cell RCC Enrolled on E2805 (by Adjuvant Treatment Arm)
| N (%) | Placebo n = 138 |
Sorafenib n = 130 |
Sunitinib n = 135 |
Total n = 403 |
|---|---|---|---|---|
| Male | 92 (67) | 85 (65) | 86 (64) | 263 (65) |
| Race | ||||
| White | 118 (86) | 114 (88) | 117 (87) | 349 (87) |
| Black | 14 (10) | 10 (8) | 11 (8) | 35 (9) |
| Asian | 2 (1) | 3 (2) | 3 (2) | 8 (2) |
| Other | 3 (2) | 0 (0) | 1 (1) | 4 (1) |
| Missing | 1 (1) | 3 (2) | 3 (2) | 7 (2) |
| Age - mean (SD) | 54 (± 12) | 55 (± 12) | 54 (± 13) | 54 (± 12) |
| ECOG PS | ||||
| 0 | 110 (80) | 104 (80) | 110 (81) | 324 (80) |
| 1 | 28 (20) | 26 (20) | 25 (19) | 79 (20) |
| Radical versus Partial Nephrectomy | ||||
| Radical | 131 (95) | 119 (92) | 126 (93) | 376 (93) |
| Partial | 7 (5) | 11 (8) | 9 (7) | 27 (7) |
| Surgical approach as stratified | ||||
| Open | 87 (63) | 82 (63) | 86 (64) | 255 (63) |
| Laparoscopic | 51 (37) | 48 (37) | 49 (36) | 148 (37) |
| Surgical approach as reported by surgeon | ||||
| Open | 84 (61) | 75 (58) | 83 (61) | 242 (60) |
| Laparoscopic | 54 (39) | 55 (42) | 52 (39) | 161 (40) |
| Histology | ||||
| Chromophobe | 29 (21) | 43 (33) | 40 (30) | 112 (28) |
| Mixed | 31 (22) | 22 (17) | 33 (24) | 86 (21) |
| Papillary | 59 (43) | 51 (39) | 39 (29) | 149 (37) |
| Unclassified | 19 (14) | 14 (11) | 23 (17) | 56 (14) |
| Sarcomatoid features | ||||
| Yes | 22 (16) | 21 (16) | 24 (18) | 67 (17) |
| No | 116 (84) | 109 (84) | 110 (81) | 335 (83) |
| Missing | 0 (0) | 0 (0) | 1 (1) | 1 (0) |
| UISS risk group | ||||
| Intermediate high | 72 (52) | 69 (53) | 71 (53) | 212 (53) |
| Very high | 66 (48) | 61 (47) | 64 (47) | 191 (47) |
Abbr. ECOG, Eastern Cooperative Oncology Group; PS, performance status; UISS, UCLA International Staging System
Thirty six percent (144/403) of non-ccRCC tumors recurred and were detected over a median follow-up period of 6.2 years. Five-year RRs (95% CI) were 22.0% (11.5, 28.1) and 48.6% (41.0, 55.7) for intermediate-high and very-high UISS risk groups, respectively. 12 In keeping with the overall E2805 trial findings, there were no significant differences in DFS or overall survival (OS) across treatment groups for patients with non-ccRCC histologies (Figure 1a/1b, log-rank p = 0.28 and 0.44, respectively).
Figure 1. Disease-Free Survival (A) and Overall Survival (B) Across Treatment Arms for Patients with Non-ccRCC.
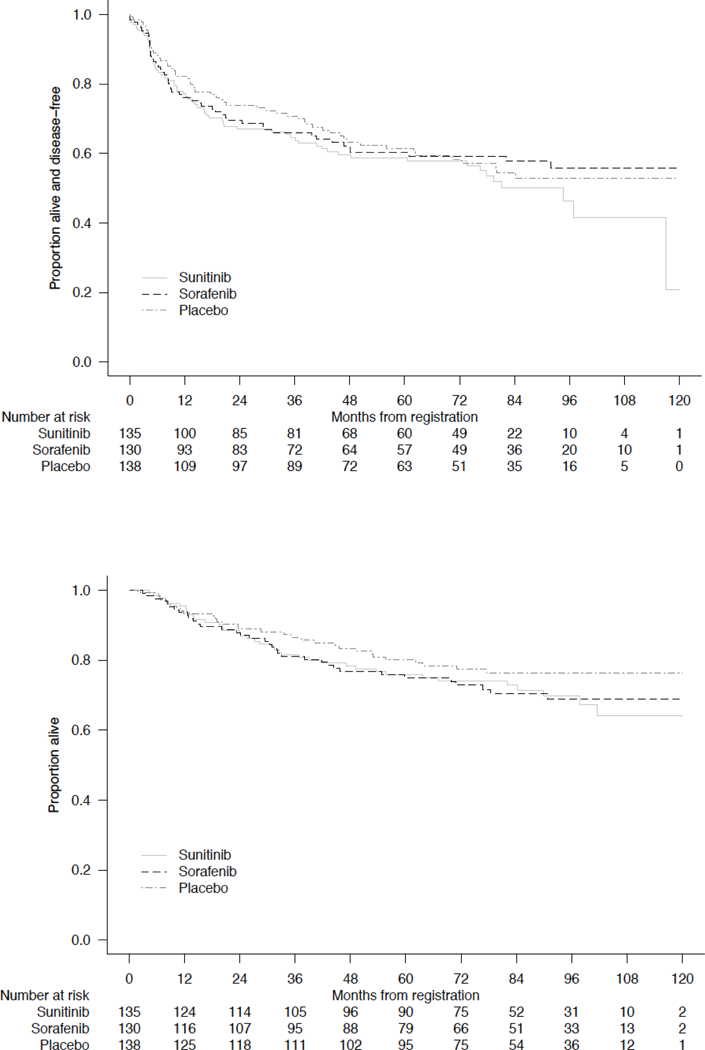
Log-rank p value = 0.23 for DFS and 0.44 or OS (stratified by all randomization stratification factors except tumor histology)
Baseline characteristics for non-ccRCC and ccRCC (N = 1541) patients were comparable and are displayed in Table 2. Five-year RRs were comparable between non-ccRCC and ccRCC patients (34.6% vs 39.5%) [95% CIs (29.8 – 39.4) and (36.9 – 42.1), respectively] (Figure 2a). However, significant differences were observed with regard to patterns of relapse. Among non-ccRCC patients, abdominal recurrences were most frequently identified, including lymph node (39%), nephrectomy bed (17%), and liver (13%). When compared to patients with ccRCC, non-ccRCC patients were significantly more likely to develop abdominal sites of relapse (5-year RR 26.4% vs 18.2%) [95% CIs (22.0 – 31.0) and (16.2 – 20.3), respectively, p = 0.0008], and were significantly less likely to relapse in the chest (5-year RR 13.7% vs 20.9%) [95% CIs (10.4 – 17.4) and (18.8 – 23.1), respectively, p = 0.0005] (Figure 2b/2c). Sites of recurrence by non-ccRCC histologic subtype are displayed in Table 3. On multivariate analysis accounting for known prognostic factors (including tumor stage, nodal stage, and sarcomatoid features), non-ccRCC histology was associated with reduced risk of chest relapse (HR 0.57, 95% CI 0.43 – 0.76, p < 0.001) and increased risk for abdominal relapse (HR 1.22, 95% CI 0.96 – 1.53, p = 0.099) (Table 4).
Table 2.
Characteristics of Patients with Non-Clear Cell RCC versus Clear Cell RCC
| Non-CC n = 403 |
Clear Cell n = 1,540 |
Total n = 1,943 |
|
|---|---|---|---|
| Male | 263 (65) | 1,046 (68) | 1,309 (67) |
| Race | |||
| White | 349 (87) | 1,423 (92) | 1,772 (91) |
| Black | 35 (9) | 50 (3) | 85 (4) |
| Asian | 8 (2) | 35 (2) | 43 (2) |
| Other/Unknown | 11 (3) | 32 (2) | 43 (2) |
| Age - mean (SD) | 54 (± 12) | 56 (± 10) | 56 (± 11) |
| ECOG PS | |||
| 0 | 324 (80) | 1,205 (78) | 1,529 (79) |
| 1 | 79 (20) | 335 (22) | 414 (21) |
| Surgical approach as stratified | |||
| Open | 255 (63) | 919 (60) | 1,174 (60) |
| Laparoscopic | 148 (37) | 621 (40) | 769 (40) |
| Surgical approach as reported by surgeon | |||
| Open | 242 (60) | 869 (56) | 1,111 (57) |
| Laparoscopic | 161 (40) | 671 (44) | 832 (43) |
| Nephrectomy type | |||
| Radical | 376 (93) | 1,461 (95) | 1,837 (95) |
| Partial | 27 (7) | 79 (5) | 106 (5) |
| Histology | |||
| Chromophobe | 112 (28) | 0 (0) | 112 (6) |
| Clear cell | 0 (0) | 1,540 (100) | 1,540 (79) |
| Mixed | 86 (21) | 0 (0) | 86 (4) |
| Papillary | 149 (37) | 0 (0) | 149 (8) |
| Unclassified | 56 (14) | 0 (0) | 56 (3) |
| Sarcomatoid features | |||
| Yes | 67 (17) | 104 (7) | 171 (9) |
| No | 335 (83) | 1,433 (93) | 1,768 (91) |
| Missing | 1 (0) | 3 (0) | 4 (0) |
| UISS risk group | |||
| Intermediate high | 212 (53) | 761 (49) | 973 (50) |
| Very high | 191 (47) | 779 (51) | 970 (50) |
| Arm | |||
| Sunitinib | 135 (33) | 512 (33) | 647 (33) |
| Sorafenib | 130 (32) | 519 (34) | 649 (33) |
| Placebo | 138 (34) | 509 (33) | 647 (33) |
Abbr. ECOG, Eastern Cooperative Oncology Group; PS, performance status; UISS, UCLA International Staging System; Non-CC, non-clear cell
Figure 2.
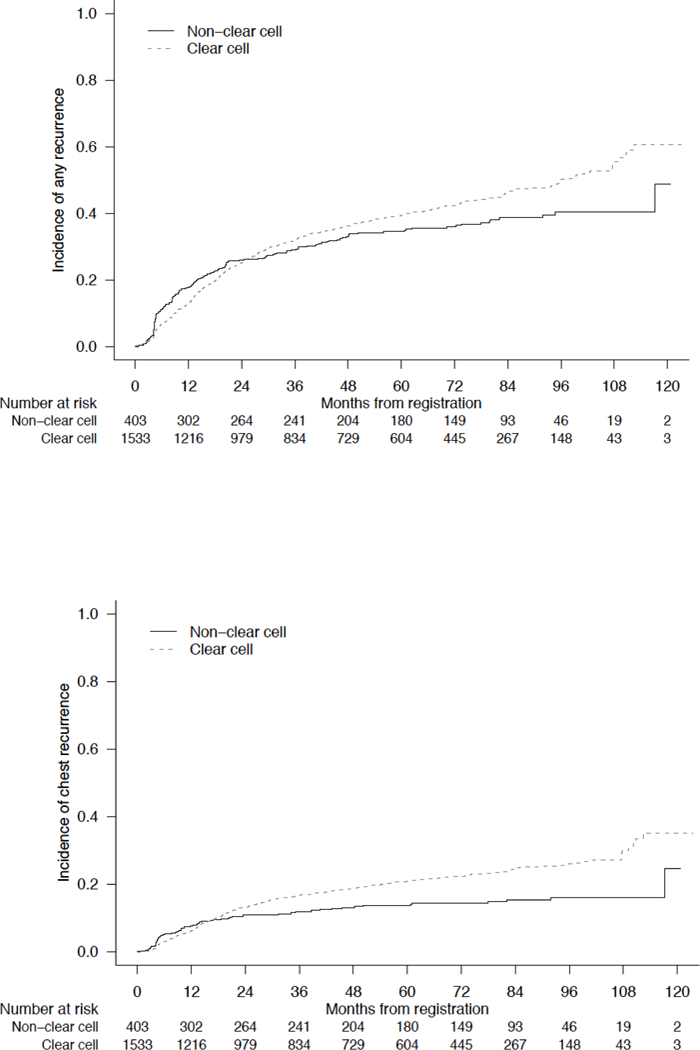
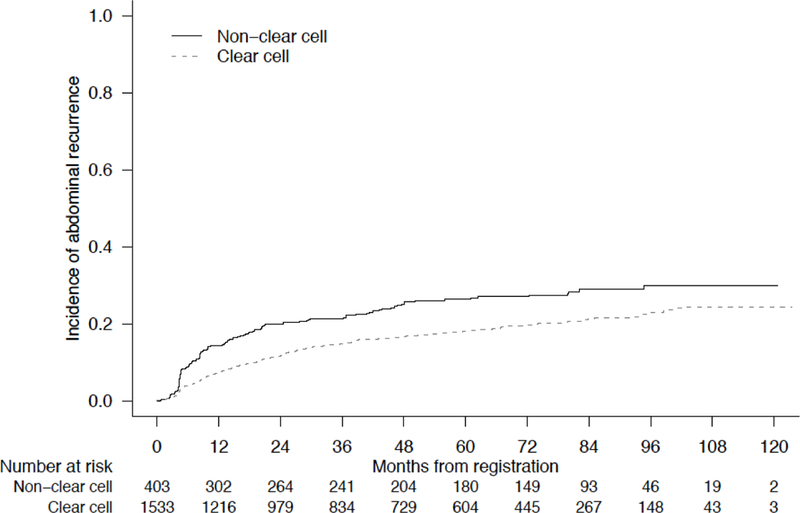
Cumulative Incidence of Disease Relapse at (A) Any Site, (B) Chest, or (C) Abdomen, Stratified by Clear Cell versus Non-Clear Cell Histology
Table 3.
Recurrence Site by Non-ccRCC Histologic Subtypea
| N(%) | Chromophobe n = 17 |
Mixed n = 48 |
Papillary n = 54 |
Unclassified n = 25 |
Total n = 144 |
|---|---|---|---|---|---|
| Lung | 2 (12) | 23 (48) | 18 (33) | 7 (28) | 50 (35) |
| Liver | 3 (18) | 9 (19) | 4 (7) | 3 (12) | 19 (13) |
| Brain | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 1 (1) |
| Abdominopelvic Lymph Node | 7 (41) | 15 (31) | 21 (39) | 13 (52) | 56 (39) |
| Bone | 2 (12) | 5 (10) | 0 (0) | 4 (16) | 11 (8) |
| Nephrectomy Bed | 2 (12) | 8 (17) | 9 (17) | 5 (20) | 24 (17) |
| Otherb | 4 (24) | 12 (25) | 15 (28) | 5 (20) | 36 (25) |
– Relapse site at first report of recurrence. Some patients recurred at more than one site.
– Peritoneum, intramuscular, or subcutaneous relapses
Table 4.
Multivariable Modela of Non-ccRCC and Risk of Abdominal (A) or Chest Relapse (B)
| A) Abdomen Relapse | |||
|---|---|---|---|
| HR | 95% CI | p | |
| Non-CC | 1.22 | (0.96, 1.53) | 0.099 |
| Sarcomatoid features | 2.06 | (1.53, 2.76) | < 0.001 |
| T-stage 3 or 4 | 1.30 | (1.05, 1.61) | 0.015 |
| N-stage 1 or 2 | 2.90 | (2.21, 3.81) | < 0.001 |
| B) Chest Relapse | |||
| HR | 95% CI | p | |
| Non-CC | 0.57 | (0.43, 0.76) | <0.001 |
| Sarcomatoid features | 1.87 | (1.36, 2.57) | < 0.001 |
| T-stage 3 or 4 | 1.36 | (1.10, 1.68) | 0.005 |
| N-stage 1 or 2 | 1.36 | (0.96, 1.91) | 0.080 |
– Multivariable Fine-Gray competing risks regression models to assess the effect of non-ccRCC histology on the observed clinical relapse pattern.
No significant differences were observed in the sites of relapse for either non-ccRCC or ccRCC patients based on assigned adjuvant treatment group (sunitinib, sorafenib, or placebo) (Table 5). To further assess the potential impact of assigned adjuvant treatment on observed relapse patterns, additional exploratory analysis was restricted to the population of non-ccRCC and ccRCC patients randomized to placebo treatment. This additional analysis also indicated differences in relapse pattern by RCC histology. Among patients randomized to placebo treatment, 5-year RRs were comparable between non-ccRCC and ccRCC histologies (34.6% vs 37.1%) [95% CIs (26.5 – 42.9) and (32.7 – 41.5), respectively]. However, when compared to ccRCC, patients with non-ccRCC had numerically higher rates of abdominal relapse (5-year RR 27.8% vs 16.0%) and lower rates of chest relapse (5-year RR 13.6% vs 19.3%).
Table 5.
Recurrence Site by Assigned Adjuvant Treatmenta
| Sunitinib | Sorafenib | Placebo | |
|---|---|---|---|
| Lung | 132 (20) | 127 (20) | 129 (20) |
| Liver | 25 (4) | 22 (3) | 28 (4) |
| Brain | 15 (2) | 7 (1) | 7 (1) |
| Abdominopelvic Lymph Node |
56 (9) | 53 (8) | 46 (7) |
| Bone | 30 (5) | 28 (4) | 36 (6) |
| Nephrectomy Bed | 41 (6) | 44 (7) | 35 (5) |
| Otherb | 60 (9) | 62 (10) | 57 (9) |
– Relapse site at first report of recurrence. Some patients recurred at more than one site.
– Peritoneum, intramuscular, or subcutaneous relapses
Based on strict adherence to consensus guidelines, surveillance imaging for 5.3 years would be required to capture 95% of non-ccRCC abdominal recurrences. Strict adherence to current NCCN or AUA guidelines would successfully capture approximately 91% of relapses at any site, regardless of UISS risk stratification (Table 6). Nine percent of non-ccRCC recurrences (13/144) occurred beyond 5 years from surgery, including papillary (N=6), chromophobe (N=3), and mixed (N=4) histologic subtypes.
Table 6.
Recurrence Successfully Captured by Consensus Guidelines (NCCN or AUA)a
| N (%) | |
|---|---|
| UISS risk group | |
| Intermediate high | 43 (89.6) |
| Very high | 88 (91.7) |
| Site of recurrence | |
| Any site | 131 (91.0) |
| Chest | 49 (89.1) |
| Abdomen | 91 (92.9) |
– Strict adherence to consensus surveillance guideline through 5 years post-nephrectomy
Discussion:
There remains limited knowledge regarding the natural history, patterns of relapse, and optimal surveillance and management for patients with non-ccRCC. 1 Prior reports on the patterns of relapse for resected RCC are largely limited to long-term follow-up of ccRCC. 13 To our knowledge, this is the largest evaluation of the natural history of non-ccRCC following curative-intent nephrectomy with standardized patient follow-up and annotation of sites of relapse. Our findings indicate that while overall 5-year RRs were comparable between non-ccRCC and ccRCC patients, those with non-ccRCC appear to demonstrate a distinct pattern of relapse characterized by more abdominal site recurrence and less frequent chest relapse. In addition, no differences in site of relapse were identified based on adjuvant treatment group, which is in keeping with reported findings in similar adjuvant studies restricted to only ccRCC patients.14
Characterizing the patterns of relapse for patients at high risk of recurrence may have important implications for operative technique and for post-operative surveillance. The apparent increased rate of abdominal site relapse with non-ccRCC raises the hypothesis of potential benefit from intensified abdominal surveillance and/or consideration of additional local therapy. For example, given the current lack of highly effective systemic therapies for non-ccRCC histologies, there may be a greater role for more extensive local surgery, such as empiric lymphadenectomy. Indeed, 39% of non-ccRCC patients in this cohort developed first relapse within abdominal lymph nodes. In addition, intensified abdominal surveillance may allow for the identification of disease relapse at earlier stages, therefore potentially enabling metastatectomy or other local salvage therapies. 15,16 For example, although abdominal imaging with ultrasound, CT, or MRI are each allowable as surveillance modalities per AUA guidelines, our findings suggest a potential advantage for cross-sectional imaging over ultrasound imaging for more sensitive abdominal surveillance in this population with apparent higher risk of abdominal relapse.
Furthermore, controversy exists regarding the optimal surveillance duration following treatment for localized RCC, and current guidelines counsel a risk-stratified approach with imaging for approximately 5 years in higher-risk subgroups. 6,7,17 However, there is limited information regarding the adequacy of these recommendations for non-ccRCC subgroups. The AUA and NCCN guidelines do not distinguish surveillance based on histology and leave aspects of follow-up protocols to physician discretion. Although the standardized surveillance imaging in the E2805 protocol generally mirrors standard consensus guidelines, a significant distinction is the prolonged routine cross-sectional imaging surveillance conducted through 10 years for patients on E2805. As 90% of clinical recurrences would potentially be detected by strict adherence to AUA or NCCN guidelines, our data indicates that current guidelines are largely adequate, but emphasize the importance of long-term follow-up beyond 5 years in order to capture approximately 10% of observed late non-ccRCC recurrences. For example, such prolonged surveillance, particularly of the abdomen, may be warranted in younger and healthier individuals. Importantly, the observed relapse patterns and potential implications for population-based surveillance and management cannot be definitively evaluated from this study and will require careful validation and evaluation of impact on patient outcomes and societal costs.
A limitation of this analysis is the heterogeneous nature of non-ccRCC and the potential for variable clinical courses based on individual non-ccRCC subtypes (i.e. papillary type 1 vs type 2). It is possible that the observed increased risk for abdominal relapse may be largely driven by specific histologic subtypes within the non-ccRCC category. However, given the rarity of these distinct subtypes, future follow-up of individual histology-dependent outcomes will require ongoing pooled and cooperative efforts. In addition, higher pathologic tumor stage has been recognized as a prognostic feature for abdominal site relapse in prior RCC cohorts. 2,13 Therefore, differences in the distribution of tumor stage across histologies may influence observed patterns of relapse. However, the proportions of patients within each UISS risk group, which accounts for primary tumor staging, was similar across ccRCC and non-ccRCC histologies, therefore indicating a lack of confounding by tumor stage. 11 The E2805 study included a relatively high risk cohort of completely resected RCC patients, and therefore these findings may not be generalizable to resected non-ccRCC patients with estimated lower recurrence rates. Finally, although no differences were found according to the assigned adjuvant therapy or placebo, it is possible that this analysis had limited power to detect differences in relapse pattern by treatment group. However, consistent findings within analyses restricted to placebo-treated patients further strengthen the observed differences in relapse pattern. Further, recently reported findings from similar adjuvant anti-angiogenic therapy studies also indicate lack of effect of adjuvant therapy on observed relapse patterns.14
Conclusions:
This is the largest, standardized evaluation of the natural history of non-ccRCC following curative-intent nephrectomy. Our findings suggest that non-ccRCC exhibits a distinct pattern of relapse when compared to conventional ccRCC, and emphasize the utility of continued long-term imaging for patients with high-risk resected non-ccRCC.
Acknowledgements:
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Peter J. O’Dwyer, MD and Mitchell D. Schnall, MD PhD, Group Co-Chairs) and supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: CA180820, CA180794. The content is solely the responsibilities of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Presentation: This study was presented at the 2018 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL June 2018.
Footnotes
Disclosures: None.
Contributor Information
Vivek Narayan, University of Pennsylvania, Abramson Cancer Center, Philadelphia, PA.
Maneka Puligandla, Dana-Farber Cancer Institute, Boston, MA.
Naomi B. Haas, University of Pennsylvania, Abramson Cancer Center, Philadelphia, PA.
Pearl Subramanian, University of Pennsylvania, Philadelphia, PA.
Robert S. DiPaola, University of Kentucky, Lexington, KY.
Robert Uzzo, Fox Chase Cancer Center, Philadelphia, PA.
References:
- 1.Giles RH, Choueiri TK, Heng DY, et al. Recommendations for the management of rare kidney cancers. Eur Urol. 2017;72(6):974–983. [DOI] [PubMed] [Google Scholar]
- 2.Leibovich BC, Lohse CM, Crispen PL, et al. Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. J Urol. 2010;183(4):1309–1316. [DOI] [PubMed] [Google Scholar]
- 3.Vaishampayan U Evolving treatment paradigms in non-clear cell kidney cancer. Curr Treat Options Oncol. 2018;19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tannir NM, Jonasch E, Albiges L, et al. Everolimus versus sunitinib prospective evaluation in metastatic non-clear cell renal cell carcinoma (ESPN): A randomized multicenter phase 2 trial. Eur Urol. 2016;69(5):866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong AJ, Halabi S, Eisen T, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): A multicentre, open-label, randomised phase 2 trial. Lancet Oncol. 2016;17(3):378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 2.2017: Clinical practice guidelines in oncology. JNCCN J Nat Compr Cancer Netw. 2017;15(6):804–834. [DOI] [PubMed] [Google Scholar]
- 7.Donat SM, Diaz M, Bishoff JT, et al. Follow-up for clinically localized renal neoplasms: AUA guideline. J Urol. 2013;190(2):407–416. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Bacik J, Mariani T, Russo P, Mazumdar M, Reuter V. Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol. 2002;20(9):2376–2381. [DOI] [PubMed] [Google Scholar]
- 9.Aizer AA, Urun Y, McKay RR, Kibel AS, Nguyen PL, Choueiri TK. Cytoreductive nephrectomy in patients with metastatic non-clear-cell renal cell carcinoma (RCC). BJU Int. 2014;113(5 B):E67–E74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storkel S, Eble JN, Adlakha K, et al. Classification of renal cell carcinoma: Workgroup no. 1. Cancer. 1997;80(5):987–989. [DOI] [PubMed] [Google Scholar]
- 11.Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): A double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016;387(10032):2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam JS, Shvarts O, Leppert JT, Pantuck AJ, Figlin RA, Belldegrun AS. Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. J Urol. 2005;174(2):466–472. [DOI] [PubMed] [Google Scholar]
- 13.Stephenson AJ, Chetner MP, Rourke K, et al. Guidelines for the surveillance of localized renal cell carcinoma based on the patterns of relapse after nephrectomy. J Urol. 2004;172(1):58–62. [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Ravaud A, Patard J-, et al. Adjuvant sunitinib for high-risk renal cell carcinoma after nephrectomy: Subgroup analyses and updated overall survival results. Eur Urol. 2018;73(1):62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang CJ, Christie A, Lin M-, et al. Safety and efficacy of stereotactic ablative radiation therapy for renal cell carcinoma extracranial metastases. Int J Radiat Oncol Biol Phys. 2017;98(1):91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Littrup PJ, Bang HJ, Currier BP, et al. Soft-tissue cryoablation in diffuse locations: Feasibility and intermediate term outcomes. J Vasc Intervent Radiol. 2013;24(12):1817–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williamson TJ, Pearson JR, Ischia J, Bolton DM, Lawrentschuk N. Guideline of guidelines: Follow-up after nephrectomy for renal cell carcinoma. BJU Int. 2016;117(4):555–562. [DOI] [PubMed] [Google Scholar]


