Abstract
The Polymerase Associated Factor 1 complex (Paf1C) is a multifunctional regulator of eukaryotic gene expression important for the coordination of transcription with chromatin modification and post-transcriptional processes. In this study, we investigated the extent to which the functions of Paf1C combine to regulate the Saccharomyces cerevisiae transcriptome. While previous studies focused on the roles of Paf1C in controlling mRNA levels, here, we took advantage of a genetic background that enriches for unstable transcripts, and demonstrate that deletion of PAF1 affects all classes of Pol II transcripts including multiple classes of noncoding RNAs (ncRNAs). By conducting a de novo differential expression analysis independent of gene annotations, we found that Paf1 positively and negatively regulates antisense transcription at multiple loci. Comparisons with nascent transcript data revealed that many, but not all, changes in RNA levels detected by our analysis are due to changes in transcription instead of post-transcriptional events. To investigate the mechanisms by which Paf1 regulates protein-coding genes, we focused on genes involved in iron and phosphate homeostasis, which were differentially affected by PAF1 deletion. Our results indicate that Paf1 stimulates phosphate gene expression through a mechanism that is independent of any individual Paf1C-dependent histone modification. In contrast, the inhibition of iron gene expression by Paf1 correlates with a defect in H3 K36 trimethylation. Finally, we showed that one iron regulon gene, FET4, is coordinately controlled by Paf1 and transcription of upstream noncoding DNA. Together, these data identify roles for Paf1C in controlling both coding and noncoding regions of the yeast genome.
Keywords: Paf1 complex, RNA polymerase II, histone modifications, chromatin, noncoding RNA
IN the context of chromatin, accurate and controlled transcription by RNA polymerase II (Pol II) requires the functions of many regulatory factors. One highly conserved regulatory factor is Paf1C, which in yeast is composed of Paf1, Ctr9, Leo1, Rtf1, and Cdc73 (Jaehning 2010; Crisucci and Arndt 2011; Tomson and Arndt 2013). Paf1C associates with Pol II during transcription elongation and regulates both transcriptional and post-transcriptional processes, including the cotranscriptional deposition of histone modifications and the nuclear export of RNAs (Tomson and Arndt 2013; Fischl et al. 2017; Van Oss et al. 2017). Histone modifications dependent on Paf1C include H3 lysine 4 di- and trimethylation (H3 K4me2/3), H3 K79me2/3, and H2B K123 mono-ubiquitylation (ub) in Saccharomyces cerevisiae (H2B K120 in humans). Paf1C facilitates the deposition of H2B K123ub via its Rtf1 subunit (Piro et al. 2012; Van Oss et al. 2016), which interacts directly with the ubiquitin-conjugating enzyme Rad6 through its histone modification domain (Van Oss et al. 2016). H2BK123ub is a prerequisite to H3 K4me2/3 and H3 K79me2/3, modifications catalyzed by the histone methyltransferases Set1 and Dot1, respectively (Dover et al. 2002; Sun and Allis 2002). Paf1C also promotes the deposition of H3 K36me3 by the Set2 histone methyltransferase (Krogan et al. 2003b; Chu et al. 2007). Consistent with the binding of Paf1C to the Pol II elongation machinery (Qiu et al. 2006, 2012; Amrich et al. 2012; Wier et al. 2013; Xu et al. 2017; Vos et al. 2018), the histone modifications dependent on Paf1C are found at regions of active transcription (Smolle and Workman 2013).
The absence of specific histone modifications in Paf1C mutants is associated with transcriptional defects. These defects include the transcriptional read-through of terminators found at the 3′ ends of Pol II-transcribed small nucleolar RNA (snoRNA) genes (Sheldon et al. 2005; Terzi et al. 2011; Tomson et al. 2011, 2013). In addition to promoting a histone modification state that facilitates transcription termination, Paf1C physically associates with proteins implicated in transcription termination and RNA 3′-end formation (Nordick et al. 2008; Rozenblatt-Rosen et al. 2009). The importance of Paf1C in regulating snoRNA termination supports a functional interaction with the Nrd1-Nab3-Sen1 (NNS) transcription termination pathway (Arndt and Reines 2015; Porrua and Libri 2015), which is responsible for the termination of snoRNAs and other noncoding transcripts including cryptic unstable transcripts (CUTs) in yeast (Schulz et al. 2013). Many of these same noncoding transcripts are rapidly degraded by the nuclear exosome through a process mediated by the Trf4/Trf5-Air1/Air2-Mtr4 polyadenylation complex (TRAMP) (Schmid and Jensen 2008). For example, loss of Trf4, the polyA polymerase subunit of the TRAMP complex that adds short polyA tails to transcripts destined for degradation or processing by the nuclear exosome, has been shown to stabilize CUTs and snoRNAs in S. cerevisiae (LaCava et al. 2005; Vaňáčová et al. 2005; Wyers et al. 2005; Thiebaut et al. 2006; Nishimura et al. 2009; Xu et al. 2009).
Despite growing understanding of the molecular functions of Paf1C, few studies have probed how these functions lead to a transcriptional outcome. Moreover, little is known about the roles of Paf1C in controlling noncoding transcription. To begin to address these questions, we sought to comprehensively investigate the importance of Paf1C in modulating the S. cerevisiae transcriptome, taking advantage of a genetic background that allows enhanced detection of unstable transcripts. To this end, we used strand-specific whole-genome tiling arrays to measure steady state RNA levels in PAF1 and paf1∆ strains that contain or lack the TRAMP subunit gene TRF4. We found that deletion of PAF1 affects all classes of Pol II transcripts including both stable and unstable noncoding RNAs (ncRNAs) and antisense transcripts. Comparisons with published NET-seq experiments, which detect Pol II-engaged, nascent transcripts (Harlen and Churchman 2017), indicate that most, but not all, changes in steady state transcript abundance in the paf1∆ background can be attributed to altered transcription. Analysis of subsets of protein-coding genes suggests that Paf1 represses the transcription of some genes through facilitating H3 K36me3 and stimulates the transcription of other genes independently of any single Paf1C-dependent histone modification. Finally, we report a regulatory mechanism governing the FET4 locus, which incorporates both CUT transcription and Paf1. Together, these data support a role for Paf1C in multiple regulatory mechanisms that collectively and broadly impact the Pol II transcriptome.
Materials and Methods
Yeast strains and culturing methods
All S. cerevisiae strains used in this study are listed in Table 1 and are isogenic to the FY2 strain, which is a GAL2+ derivative of S288C (Winston et al. 1995). Deletion of specific loci was achieved by one-step gene disruption (Lundblad et al. 2001), and confirmed by PCR. Genetic crosses were conducted as described (Rose et al. 1991). Cells were grown to log phase at 30° in rich media (YPD) supplemented with 400 μM tryptophan and harvested by centrifugation. Cell pellets were washed once with sterile water, flash frozen, and stored at −80° prior to RNA isolation for RT-qPCR and Northern blotting experiments.
Table 1. Yeast strains used in this study.
| Straina | Genotype |
|---|---|
| KY292 (FY118b) | MATa his4-912∂ lys2-128∂ leu2∆1 ura3-52 trp1∆63 |
| KY307 (FY838b) | MATα his3∆200 lys2∆202 leu2∆1 ura3-52 |
| KY457 | MATa rtf1∆::URA3 leu2∆1 ura3-52 trp1∆63 |
| KY508 (FY737b) | MATa his3∆200 lys2-128∂ leu2∆1 ura3-52 snf2∆::HIS3 |
| KY583 (GHY280c) | MATa his3∆200 lys2-128∂ leu2∆1 ura3-52 trp1∆63 chd1∆::HIS3 |
| KY632 | MATα his3∆200 lys2-128∂ leu2∆1 ura3-52 chd1∆::URA3 |
| KY802 | MATa his3∆200 lys2-173R2 ura3∆(0 or 52) paf1∆::URA3 |
| KY804 | MATα his3∆200 leu2∆2(0 or 1) ura3(∆0 or -52) paf1∆::URA3 |
| KY884 | MATa his3∆200 lys2-173R2 leu2∆1 ura3-52 trp1∆63 isw2∆::HIS3 |
| KY901 | MATα his3∆200 lys2-128∂ leu2∆1 ura3-52 trp1∆63 isw1∆::HIS3 |
| KY907 | MATa his3∆200 lys2-128∂ leu2∆1 ura3-52 set1∆::HIS3 |
| KY914 | MATα his3∆200 lys2-173R2 leu2∆1 ura3-52 set2∆::HIS3 |
| KY934 | MATα his3∆200 leu2∆1 trp1∆63 dot1∆::HIS3 |
| KY938 | MATα his3∆200 leu2∆1 trp1∆63 set1∆::HIS3 |
| KY972 | MATα his3∆200 lys2-128∂ leu2∆1 ura3-52 swr1∆::KANMX |
| KY1021 | MATa his4-912∂ lys2-128∂ leu2∆1 trp1∆63 |
| KY1235 | MATa his3∆200 lys2-173R2 ura3-52 rco1∆::HIS3MX6 |
| KY1250 | MATa his3∆200 leu2∆1 ura3-52 set2∆::HIS3 paf1∆::KANMX |
| KY1683 | MATα his3∆200 lys2-128∂ leu2∆1 trp1∆63 |
| KY1702 | MATa leu2∆0 ura3∆0 paf1∆::KANMX |
| KY1952 | MATα his4-912∂ lys2-128∂ trp1∆63 leu2∆1 bre1∆::KANMX |
| KY2012 | MATa leu2∆0 ura3∆0 trf4∆::CLONAT |
| KY2016 | MATa leu2∆0 ura3∆0 trf4∆::CLONAT paf1∆::KANMX |
| KY2027 | MATα ura3-52 (hta2-htb2)∆::KANMX |
| KY2045 | MATα his3∆200 leu2∆1 trp1∆63 rad6∆::KANMX |
| KY2167 | MATα ura3∆0 HTA1-htb1-K123R (hta2-htb2)∆::KANMX |
| KY2239 | MATα his4-912∂ lys2-128∂ trp1∆63 ctr9∆::KANMX |
| KY2241 | MATa his4-912∂ lys2-128∂ trp1∆63 cdc73∆::KANMX |
| KY2243 | MATα his4-912∂ lys2-128∂ leu2∆1 trp1∆63 rtf1∆::KANMX |
| KY2244 | MATa his4-912∂ lys2-128∂ ura3-52 trp1∆63 leo1∆::URA3 |
| KY2271 | MATα his4-912∂ lys2-128∂ leu2∆1 trp1∆63 paf1∆::KANMX |
| KY2276 | MATa leu2∆0 ura3∆0 |
| KY2845 | MATa leu2∆0 ura3∆0 trf4∆::CLONAT paf1∆::KANMX FET4::HIS3 TTS at -400 |
| KY2846 | MATa leu2∆0 ura3∆0 paf1∆::KANMX FET4::HIS3 TTS at -400 |
| KY2851 | MATa his3∆200 leu2∆0 ura3∆0 trf4∆::CLONAT FET4::HIS3 TTS at -400 |
| KY3460 | MATa his3∆200 lys2-128∂ leu2∆1 ura3-52 trp1∆63 arp8∆::HIS3 |
| KY3461 | MATa his3∆200 his4-912∂ leu2∆1 lys2-173R2 ura3-52 ino80::HIS3 |
| KY3462 | MATa his3∆200 leu2∆1 ura3-52 trp1∆63 paf1∆::URA3 |
| KY3463 | MATa his3∆200 lys2-128∂ leu2∆1 ura3-52 isw1∆::HIS3 |
| KY3464 | MATa his3∆200 leu2∆1 ura3-52 ade8 paf1∆::URA3 |
| KY3465 | MATa his3∆200 leu2∆1 ura3-52 ade8 arp8∆::HIS3 |
| KY3466 | MATa his3∆200 leu2∆0 ura3∆0 FET4::HIS3 TTS at -400 |
All strains derived from S288C.
FY strains were provided by Fred Winston.
GHY strains were provided by Grant Hartzog.
RNA isolation
RNA was extracted by the hot phenol extraction method (Collart and Oliviero 1993). Briefly, frozen cells were suspended in 400 μl of TES extraction buffer (10 mM Tris-Cl pH 7.5, 10 mM EDTA, 0.5% SDS) and 400 μl of acid phenol, followed by incubation at 65° for 1 hr. The aqueous phase was collected and re-extracted using acid phenol and then chloroform. Extracted RNA was combined with 40 μl of 3 M sodium acetate and 1 ml of 100% ethanol, mixed, and placed at −80° for at least 1 hr. Precipitated RNA was collected by centrifugation and suspended in RNase-free water before quantification and quality check by agarose gel electrophoresis.
Northern blot analysis
For Northern blot analyses, 10–20 µg of total RNA were separated on a gel containing 2% agarose, 6.5% formaldehyde, and 1× MOPS for 500 V hr and then transferred to a Nytran supercharge nylon transfer membrane (#10416296; Schleicher & Schuell BioScience, Dassel, Germany) prior to hybridization with radiolabeled DNA probes. DNA probes were generated by PCR corresponding to the following genomic regions relative to the +1 nucleotide of the annotated coding sequence for the FET4 gene: CUT 793/794 (−479 to −114) and FET4 (+261 to +651). Detection of SCR1 (−181 to +284) served as a loading control. Oligonucleotides used to generate these probes are listed in Supplemental Material, Table S1. Probes were made using [α32P]-dATP (single labeling) or [α32P]-dATP and [α32P]-dTTP (double labeling). Signals were quantified using a Typhoon FLA 7000 phosphorimager (GE, Boston, MA) and normalized to the SCR1 internal loading control using ImageJ software.
Reverse transcription quantitative polymerase chain reaction
A total of 10 μg of RNA from each sample to be used in reverse transcription quantitative polymerase chain reaction (RT-qPCR) was treated with TURBO DNase (AM1907; Ambion, Thermo Fisher, Waltham, MA) following the manufacturer’s instructions. To ensure that there was no DNA contamination after the DNase treatment, 1 μl of DNase-treated RNA was subjected to 40 cycles of PCR and analyzed by agarose gel electrophoresis. All samples used in RT-qPCR showed no PCR product after 40 cycles. Reverse transcription reactions were performed on 1 μg of DNase-treated RNA using the RETROScript Reverse Transcription Kit (AM1710; Ambion, Thermo Fisher) following the manufacturer’s instructions.
RT-qPCR experiments were performed in technical duplicate, and all strains were tested in at least biological triplicate. Reactions were prepared in a volume of 20 μl using Maxima SYBR Green/ROX qPCR Master Mix (2×) (#K0221; Thermo Fisher) following the manufacturer’s instructions. Each 20 μl reaction was then divided into two, 10-μl reactions, which were analyzed on a StepOne Real-Time PCR System (Thermo Fisher) beginning with a hold at 95° for 10 min followed by 40 cycles of 95° for 15 sec, and 58° for 1 min, and finally terminating with the generation of a melt curve. Efficiencies were determined for all primer sets by measuring Ct values across a series of six 10-fold dilutions starting with 250 ng/μl and ending with 2.5 pg/μl. RT-qPCR data were analyzed using the mathematical formula developed by Pfaffl (2001) and normalized to SCR1 levels. RT-qPCR primers and their efficiencies are listed in Table S1.
Affymetrix tiling array analysis
All RNA samples used in tiling array analysis were prepared using established methods (Juneau et al. 2007; Perocchi et al. 2007), and quality was assessed by agarose gel electrophoresis. RNA samples (100 μg total) were DNase treated (#EN0521; Fermentas, Waltham, MA) and purified using an RNeasy kit (#74104; Qiagen, Hilden, Germany). RNA was reverse transcribed into cDNA in the presence of 6 ng/μl actinomycin D to prevent antisense artifacts (Perocchi et al. 2007), fragmented, and labeled with a 3′ biotin tag. Affymetrix custom tiling arrays (A-AFFY-116, Affymetrix Custom Array, S. cerevisiae Tiling Steinmetz, GEO Platform ID: GPL4563) were used to quantify gene expression (David et al. 2006; Huber et al. 2006; van Bakel et al. 2013). Arrays were processed and scanned using published methods (van Bakel et al. 2013).
Generation of annotation files for the tilingArray R package
Following the guidelines provided with the davidTiling Bioconductor package (David et al. 2006), tiling array probes were mapped to the S. cerevisiae genome (S288C version = R64-2-1) (Cherry et al. 2012; Engel et al. 2014). Briefly, the probe FASTA file was extracted from the array design file and used as input for MUMmer3.23 (Kurtz et al. 2004), along with the chromosome FASTA files for the S288C genome. Both the output of MUMmer3.23 and the S288C genome annotations were read into R (R Development Core Team 2016), and a slightly modified version of the makeProbeAnno.R script was used to generate an up-to-date probe annotation file for use with the tilingArray package (Huber et al. 2006). All R packages used in this study can be found at www.bioconductor.org (Gentleman et al. 2004), and R scripts can be found at https://github.com/mae92/Paf1C-Transcriptome-Analysis-Code/tree/master/R_Code.
Variance stabilizing normalization
CEL files for 12 tiling arrays were read into R as a single expression set using the readCel2eSet() function of the tilingArray package. The probe intensities for all 12 arrays were log2 transformed and normalized (variance stabilized normalization R code available at: https://github.com/mae92/Paf1C-Transcriptome-Analysis-Code/tree/master/R_Code) to minimize batch effects using the vsn package (Huber et al. 2002). This normalization method, as opposed to spike-in methods, assumes that the expression of most genomic regions will be equivalent between experiments, and therefore, the extent of expression changes overall in the paf1∆ strains may be underestimated.
Mapping probe intensities to probe positions across the S. cerevisiae genome
The expression set containing the normalized log2 transformed probe intensities was used as input for the segChrom() function of the tilingArray package, and the locations of probes across the genome were extracted for use in downstream analysis using basic R commands (R code can be found at: https://github.com/mae92/Paf1C-Transcriptome-Analysis-Code/tree/master/R_Code). Probe locations were averaged for triplicate samples and these averaged values were used to generate BedGraph files, which were converted into bigWig files for visualization in the Integrative Genomics Viewer (IGV) from the Broad Institute (Thorvaldsdottir et al. 2013).
Annotation-guided differential expression analysis
Normalized log2 transformed probe intensity values were extracted from the tilingArray output. Using a custom file (available at: https://github.com/mae92/Paf1C-Transcriptome-Analysis-Code/blob/master/Transcript_Annotations/combined.fix.csv) containing transcript annotations (listed in Table S2) from the Saccharomyces Genome Database (SGD) and recent studies of novel ncRNA transcripts (Cherry et al. 1998; Xu et al. 2009; Yassour et al. 2010; van Dijk et al. 2011; Schulz et al. 2013; Venkatesh et al. 2016), we calculated the average log2 intensity values for probes spanning each annotation. This process was carried out using an in-house Python script (available at: https://github.com/mae92/Paf1C-Transcriptome-Analysis-Code/tree/master/Python_Code) that calculates the average intensity of all probes occupying a given annotation found in the annotation file. Average log2 intensity values for all transcripts in each replicate and strain background were loaded into the limma package, where a linear model was used to determine statistical significance. Log2 fold change, and P values for all transcripts tested, were extracted from the output for each strain comparison. These P values were adjusted using Benjamini and Hochberg’s false discovery rate (FDR) (Benjamini and Hochberg 1995) method using top.table command in limma (R code for limma analysis can be found at: https://github.com/mae92/Paf1C-Transcriptome-Analysis-Code/tree/master/R_Code) (Ritchie et al. 2015). Significantly differentially expressed genes (adjusted P value <0.05) that were present in both comparisons (paf1∆ vs. WT and paf1∆ trf4∆ vs. trf4∆) were loaded into SGD’s YeastMine database (Balakrishnan et al. 2012), where additional annotation and gene ontology information could be extracted. Gene ontology results are shown in Table 2. Plots of differential expression data were produced in R.
Table 2. Gene ontology results for genes that showed increased or decreased expression (1.5-fold) in both paf1Δ to WT and paf1Δ trf4Δ to trf4Δ comparisons.
| Increased | ||
|---|---|---|
| Biological process | P value | n |
| Siderophore transport [GO:0015891] | 3.26E−04 | 5 |
| Iron chelate transport [GO:0015688] | 5.91E−04 | 5 |
| Iron coordination entity transport [GO:1901678] | 5.33E−03 | 5 |
| Glycerol transport [GO:0015793] | 2.29E−02 | 4 |
| Iron ion homeostasis [GO:0055072] | 3.25E−02 | 7 |
| Cellular component | ||
| Integral component of plasma membrane [GO:0005887] | 2.77E−03 | 10 |
| Intrinsic component of plasma membrane [GO:0031226] | 7.38E−03 | 10 |
| Extracellular region [GO:0005576] | 2.00E−02 | 9 |
| Plasma membrane part [GO:0044459] | 2.22E−02 | 11 |
| Decreased | ||
|---|---|---|
| Biological process | P value | n |
| Small molecule metabolic process [GO:0044281] | 1.95E−09 | 50 |
| Small molecule biosynthetic process [GO:0044283] | 6.07E−08 | 30 |
| Single-organism biosynthetic process [GO:0044711] | 8.96E−07 | 42 |
| Oxoacid metabolic process [GO:0043436] | 1.32E−06 | 33 |
| Organic acid metabolic process [GO:0006082] | 1.39E−06 | 33 |
| Organic acid biosynthetic process [GO:0016053] | 9.25E−06 | 21 |
| Carboxylic acid biosynthetic process [GO:0046394] | 9.25E−06 | 21 |
| Single-organism metabolic process [GO:0044710] | 6.55E−05 | 61 |
| Branched-chain amino acid biosynthetic process [GO:0009082] | 9.27E−05 | 7 |
| Polyphosphate metabolic process [GO:0006797] | 7.04E−04 | 6 |
| Carboxylic acid metabolic process [GO:0019752] | 2.44E−03 | 27 |
| Cellular amino acid biosynthetic process [GO:0008652] | 4.44E−03 | 14 |
| Branched-chain amino acid metabolic process [GO:0009081] | 7.73E−03 | 7 |
| Alpha-amino acid biosynthetic process [GO:1901607] | 1.30E−02 | 13 |
| Organic hydroxy compound metabolic process [GO:1901615] | 4.57E−02 | 13 |
| Cellular component | ||
| Vacuolar transporter chaperone complex [GO:0033254] | 1.08E−03 | 4 |
De novo differential expression analysis
BedGraph files were created containing normalized log2 probe intensity values (averaged for the three biological replicate arrays) mapped to the yeast genome. Differentially expressed transcripts identified by the de novo differential expression analysis were defined by a six-step process (Figure S2). (1) Average log2 (probe intensity) was calculated for three biological replicates. (2) The data were smoothed by averaging across a sliding window of 20 tiling array probes (roughly 160 bp). (3) The log2 fold change (experimental vs. control) was calculated across the entire genome. (4) All regions where the absolute value of the observed fold change was >1 (log2 fold change of 0) were identified. (5) Regions of the genome with an absolute change of 1.5-fold (log2 fold change of 0.58) were identified, and any of these regions <80 bp long (a length comparable to the shortest snoRNA) were excluded. (6) The two lists of regions from steps 4 and 5 were intersected to yield a list of extended differentially expressed regions where some portion of the transcript had an absolute fold change of 1.5-fold or greater. The transcripts defined by this method were then treated as their own list of transcript annotations for use in the comparisons described herein. This was done using a combination of AWK (Aho et al. 1979) and the BedTools suite (Quinlan and Hall 2010; Quinlan 2014). The shell script used to define differentially expressed transcripts can be found at https://github.com/mae92/Paf1C-Transcriptome-Analysis-Code/tree/master/Shell_Code and R code used to generate the input BedGraph files can be found at https://github.com/mae92/Paf1C-Transcriptome-Analysis-Code/tree/master/R_Code.
Analysis of published datasets
Next generation sequencing datasets from previous studies (Churchman and Weissman 2011; Van Oss et al. 2016; Harlen and Churchman 2017) were obtained in BedGraph format directly from the authors or FASTQ format from NCBI SRA database and converted into BigWig format for use with DeepTools (Ramírez et al. 2014, 2016). Files received in BedGraph format were converted using the University of California Santa Cruz (UCSC) Genome Browser (Kent et al. 1976) utility BedGraphToBigWig. Files downloaded from the SRA database in FASTQ format were mapped to the S. cerevisiae genome (S288C version = R64-2-1) (Cherry et al. 2012; Engel et al. 2014) using HISAT2 (Kim et al. 2015), and converted to BAM format using Samtools (Li et al. 2009). BAM files were converted to Wig format using the bam2wig utility (found at https://github.com/MikeAxtell/bam2wig), and converted to BigWig format using the UCSC utility WigToBigWig. Heatmaps were plotted using computeMatrix and plotHeatmap tools in the deepTools package by summing the tag counts using 50-bp bins.
Statistical analysis
At least three biological replicates were performed for every assay shown in this manuscript including tiling arrays. Each biological replicate is a pure yeast culture derived from a single colony initiated from a single cell of a given strain. Tiling array data analyzed using the limma package were subjected to the standard limma workflow, which utilizes linear modeling and an empirical Bayes method to determine differentially expressed genes from as little as three biological replicates. The limma p values were adjusted for multiple comparisons using Benjamini and Hochberg’s FDR (Benjamini and Hochberg 1995). All RT-qPCR and northern blot P values were generated using an unpaired, two-sided, Student’s t-test, assuming equal variance carried out between the mutant strain and the wild-type strain.
Data availability
Strains are available upon request. Tiling array data (raw CEL files, BedGraph files, annotation-guided differential expression results, and files containing annotations for differentially expressed transcripts defined by our de novo analysis in BED6 file format) have been deposited in the Gene Expression Omnibus database under accession number GSE122704. Code used for analysis of tiling array data has been uploaded to the following GitHub repository (https://github.com/mae92/Paf1C-Transcriptome-Analysis-Code). Tables S1–S6 and Figures S1–S7 are available via FigShare. Supplemental material available at FigShare: https://doi.org/10.25386/genetics.7796837.
Results
Deletion of PAF1 affects coding and noncoding transcripts genome-wide
To investigate the impact of Paf1C on the S. cerevisiae transcriptome, we used high-resolution whole-genome tiling arrays to measure steady state RNA levels in S. cerevisiae strains deleted for the PAF1 gene, which encodes a core member of Paf1C important for complex integrity (Mueller et al. 2004; Deng et al. 2018). Additionally, to assess the Paf1-dependency of unstable ncRNAs in these experiments, we deleted TRF4 in both PAF1 and paf1∆ strains. We chose to delete TRF4 instead of the nuclear exosome subunit gene, RRP6, because in our previous survey of CUTs upstream of protein-coding genes, we observed enhanced CUT levels at four out of six loci in the trf4∆ background relative to the rrp6∆ background (Raupach et al. 2016).
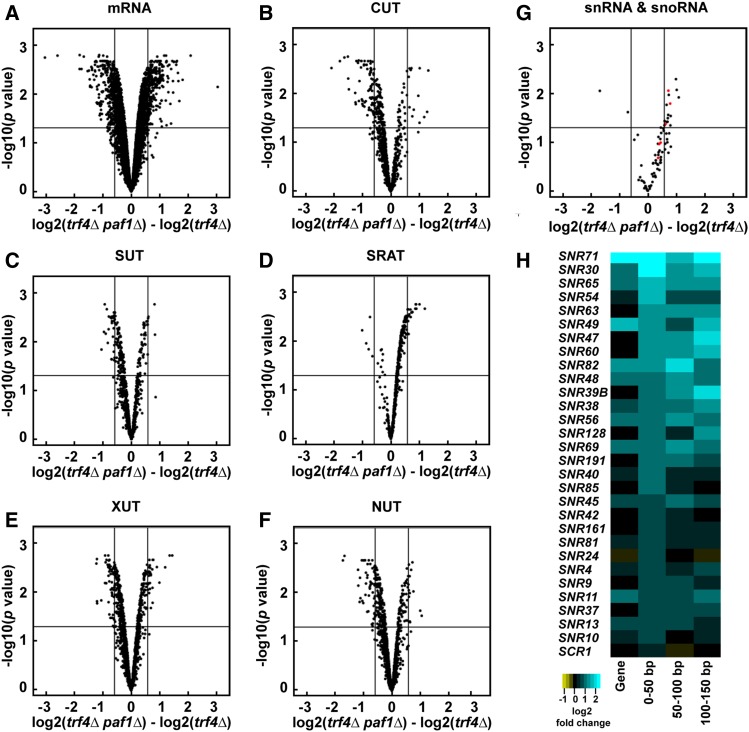
When compared to a trf4Δ strain, the paf1Δ trf4Δ double mutant revealed wide-ranging effects on all Pol II transcript classes examined: mRNAs, small nuclear RNAs (snRNAs), snoRNAs, CUTs, stable unannotated transcripts (SUTs; Xu et al. 2009), Xrn1-dependent unstable transcripts (XUTs; van Dijk et al. 2011), Nrd1-unterminated transcripts (NUTs; Schulz et al. 2013), and Set2-repressed antisense transcripts (SRATs; Venkatesh et al. 2016) (Figure 1, A–G and Table S3). In general, levels of snRNAs, snoRNAs, and SRATs increased in the paf1Δ trf4Δ double mutant relative to the trf4Δ single mutant (Figure 1, D and G and Table S3) indicating that, in wild-type cells, Paf1 suppresses their transcription or destabilizes the transcripts. In the case of SRATs, increased transcript levels are consistent with a requirement for Paf1 in facilitating H3 K36me3—a modification that negatively regulates transcription (Churchman and Weissman 2011; Kim et al. 2016; Venkatesh et al. 2016) by activating the Rpd3S histone deacetylase complex and by inhibiting histone exchange (Carrozza et al. 2005; Joshi and Struhl 2005; Keogh et al. 2005; Govind et al. 2010; Venkatesh et al. 2012). Levels of many CUTs, SUTs, XUTs, and NUTs decreased upon deletion of PAF1 (Figure 1, B, C, E, and F and Table S3). For NUTs and CUTs, these changes in transcript abundance suggest that Paf1 impacts NNS-dependent termination beyond the snoRNA genes. At protein-coding genes, Paf1 positively and negatively affects mRNA levels in a locus-specific manner (Figure 1A and Table S3), in agreement with previous studies (Shi et al. 1996; Porter et al. 2005; Cao et al. 2015; Chen et al. 2015; Yu et al. 2015; Yang et al. 2016; Fischl et al. 2017).
Figure 1.
Deletion of PAF1 affects all Pol II transcript classes. (A–G) Volcano plots graphing statistical significance (y-axis) against expression change (x-axis) between paf1Δ trf4Δ and trf4Δ strains (KY2016 and KY2012, respectively) for the indicated Pol II transcript classes. In (G), snRNAs and snoRNAs are shown in red and black, respectively. Each point represents an individual transcript. Tiling array probe intensities were averaged over annotated regions using a custom Python script and an average log2 fold change and P value were calculated using the limma R package. The horizontal line indicates an FDR adjusted P value of 0.05 and the vertical lines indicate a 1.5-fold change in expression (log2 fold change of 0.58). Counts and percentages of differentially expressed transcripts shown here are listed in Table S3. (H) Heatmap of log2 fold change in expression between paf1Δ and WT strains (KY1702 and KY2276, respectively) for the 29 most affected snoRNA genes. The snoRNA gene bodies and regions 0–50, 50–100, and 100–150 bp downstream of their annotated 3′ ends are plotted and sorted by the 0–50 bp region.
For many snoRNA genes, we detected an increase in RNA levels downstream of the annotated gene in the paf1Δ and paf1∆ trf4∆ strains relative to wild type and trf4∆ strains, respectively (Figure 1H and Figure S1). This observation is consistent with previous studies showing Paf1 is required for efficient termination at many snoRNA genes (Sheldon et al. 2005; Tomson et al. 2013). Note that the log2 fold change values calculated for any particular snoRNA gene and its downstream region do not always agree. In many cases, RNA levels mapping to the gene body do not change even when downstream changes are observed, suggesting that read-through transcription is occurring at these loci (Figure 1H). Interestingly, through k-means clustering analysis, we also identified snoRNA genes that are relatively unaffected by deletion of PAF1 (Figure S1). Further studies will be needed to elucidate the mechanistic distinctions between the Paf1-dependent and Paf1-independent snoRNA genes.
De novo differential expression analysis reveals effects of Paf1 on antisense transcripts
As an independent analysis and to facilitate detection of unannotated transcripts, we performed a de novo differential expression analysis of our tiling array data. Here, we relied on the data to reveal the boundaries of differentially expressed transcripts instead of using predetermined annotations. Strains lacking PAF1 were compared to control strains on a probe-by-probe basis. Genomic regions with a 1.5-fold or greater difference in expression between paf1∆ and PAF1 strains were selected as differentially expressed and extended until the expression difference was no longer observed (see Figure S2 and Materials and Methods for a detailed description of this analysis).
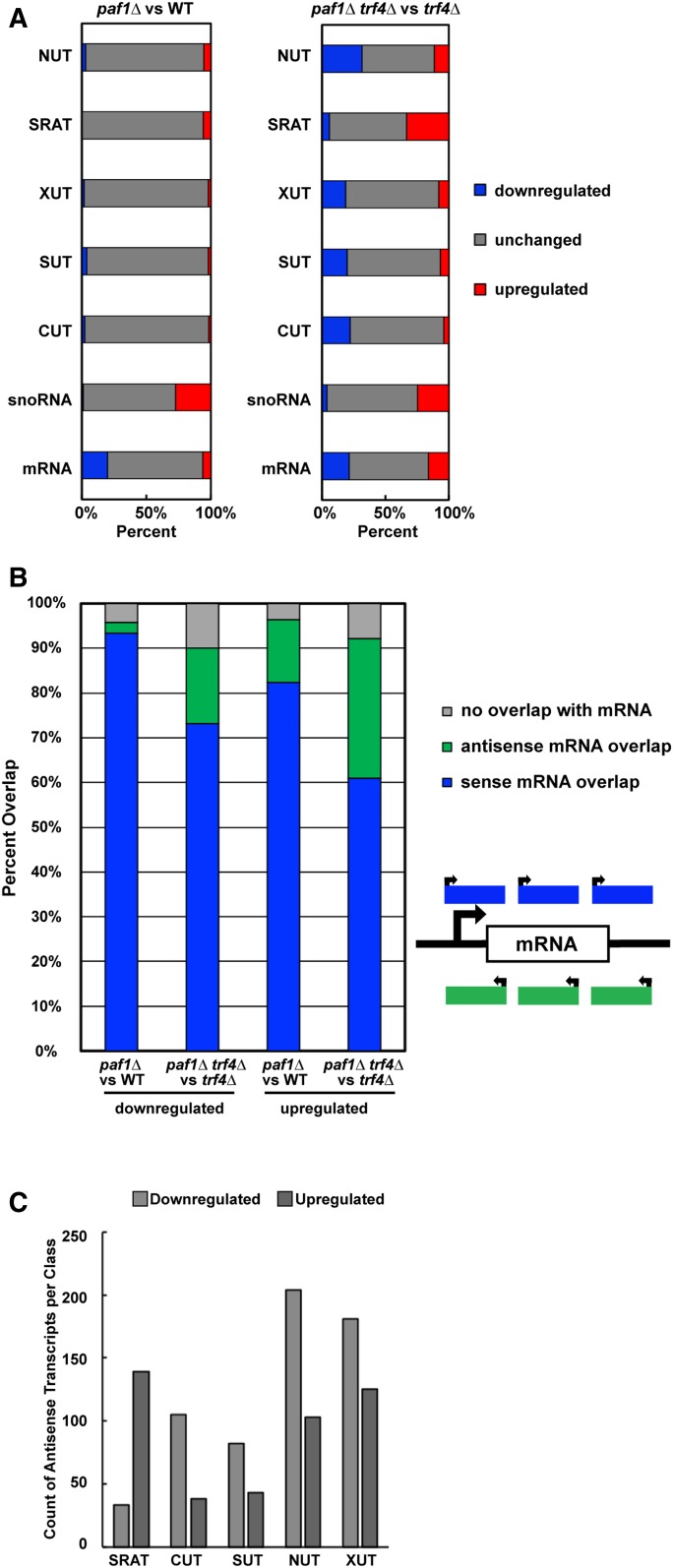
Confirming the accuracy of the de novo analysis, we found that nearly all the mRNAs identified as differentially expressed in the paf1∆ trf4∆ strain by our annotation-guided analysis (585 mRNAs;1.5-fold or greater expression change relative to trf4∆ strain) were also detected by the de novo analysis (Figure S3A). We note that, compared to the annotation-guided analysis, a larger number of differentially expressed transcripts that overlap mRNAs on the sense strand were detected in the de novo analysis. This observation is not due to technical differences, but rather a consequence of multiple de novo transcripts overlapping with a single annotated mRNA and the increased sensitivity of the analysis. Additionally, we found that the length distribution of all transcripts identified in the de novo analysis was similar to that reported in SGD for mRNAs (Figure S3B), confirming that we were not calling exceedingly long or short transcripts. Further, separation of the de novo analysis data by transcript class revealed effects of paf1∆ on ncRNAs and coding RNAs similar to those observed in the annotation-guided analysis (compare Figure 2A to Figure 1, A–G and Table S4). In the paf1∆ trf4∆ strain, for example, SRATs and snoRNAs were predominantly upregulated, and other ncRNAs were predominantly downregulated. When viewed as a whole, the de novo analysis detected far more differentially expressed transcripts in the paf1∆ trf4∆ strain (relative to the trf4∆ control strain) than in the paf1∆ strain (relative to the PAF1 control strain) (Figure S3C). Therefore, a functional TRAMP complex, which promotes processing and degradation of unstable transcripts, obscures many of the transcriptional effects of deleting PAF1.
Figure 2.
Paf1 positively and negatively regulates antisense transcription. (A) Horizontally stacked bar graphs showing the percentage of each transcript class (listed in Table S2) found to overlap with a differentially expressed transcript identified in paf1∆ or paf1∆ trf4∆ strains by de novo analysis (counts and percentages listed in Table S4). (B) Vertically stacked bar graph plotting percentage of transcripts, identified in the de novo analysis, that overlap with mRNA coding regions on the sense or antisense strand. These data are also presented in Table S5. (C) Bar graph summarizing the overlap between differentially expressed antisense transcripts detected by the de novo analysis and previously annotated noncoding RNAs (see sums in Table S6 for counts).
Unstable ncRNAs are often found near mRNA loci in tandem or antisense orientations (Neil et al. 2009; Xu et al. 2009; van Dijk et al. 2011; Schulz et al. 2013; Castelnuovo et al. 2014). Murray et al. (2015) demonstrated that regions of high antisense transcription are deficient in H2BK123ub, H3K4me3, H3K79me3, and H3K36me3, while regions of low antisense transcription are enriched for H3K79me2. Levels of all these histone modifications are affected by PAF1 deletion. Therefore, deletion of PAF1 and loss of Paf1C-dependent histone modifications may generate a chromatin landscape that promotes antisense transcription at some loci and represses it at others. Interestingly, in the de novo analysis, we observed enrichment of many transcripts oriented antisense to mRNA loci in the paf1∆ trf4∆ strain, relative to the trf4∆ strain, and found that Paf1 both positively and negatively regulates antisense transcript levels in S. cerevisiae (Figure 2B and Table S5). Deeper analysis revealed that many of the antisense transcripts detected by the de novo analysis overlapped with previously annotated noncoding transcripts (Figure 2C), consistent with earlier studies showing that many noncoding transcripts are oriented antisense to genes (Xu et al. 2009; van Dijk et al. 2011; Schulz et al. 2013; Venkatesh et al. 2016). This suggests that a large portion of the ncRNA differential expression profile observed in the paf1∆ trf4∆ strain results from antisense transcription.
To investigate the antisense transcriptional landscape further, we plotted sense and antisense transcript levels relative to the transcription start site (TSS) and transcription end site (TES) of protein-coding genes at which we detected an absolute change of ≥1.5-fold in antisense transcription overlapping the gene (Figure S4, A and B). For both the antisense-downregulated class and the antisense-upregulated class, k-means clustering analysis revealed five clusters differing in the patterns and levels of antisense transcription relative to sense transcription. (Note that when these clusters are broken down by overlap with various ncRNA classes, no one cluster is dominated by an individual ncRNA class (Figure S4, C and D and Table S6). A small number of protein-coding genes show an apparent anti-correlation between sense and antisense transcription in the paf1∆ trf4∆ mutant (cluster 1 in Figure S4A and cluster 5 in Figure S4B). However, for most genes experiencing a 1.5-fold or greater increase in antisense transcription, a clear relationship between antisense and sense transcript levels was not detected. This result agrees with previous work on antisense transcription (Murray et al. 2015). Further, a plot of sense and antisense transcript levels for all protein-coding genes suggests that antisense transcription is not governing the changes we detect in sense transcription for most genes (Figure S4E).
Paf1 regulates transcript abundance at the transcriptional level
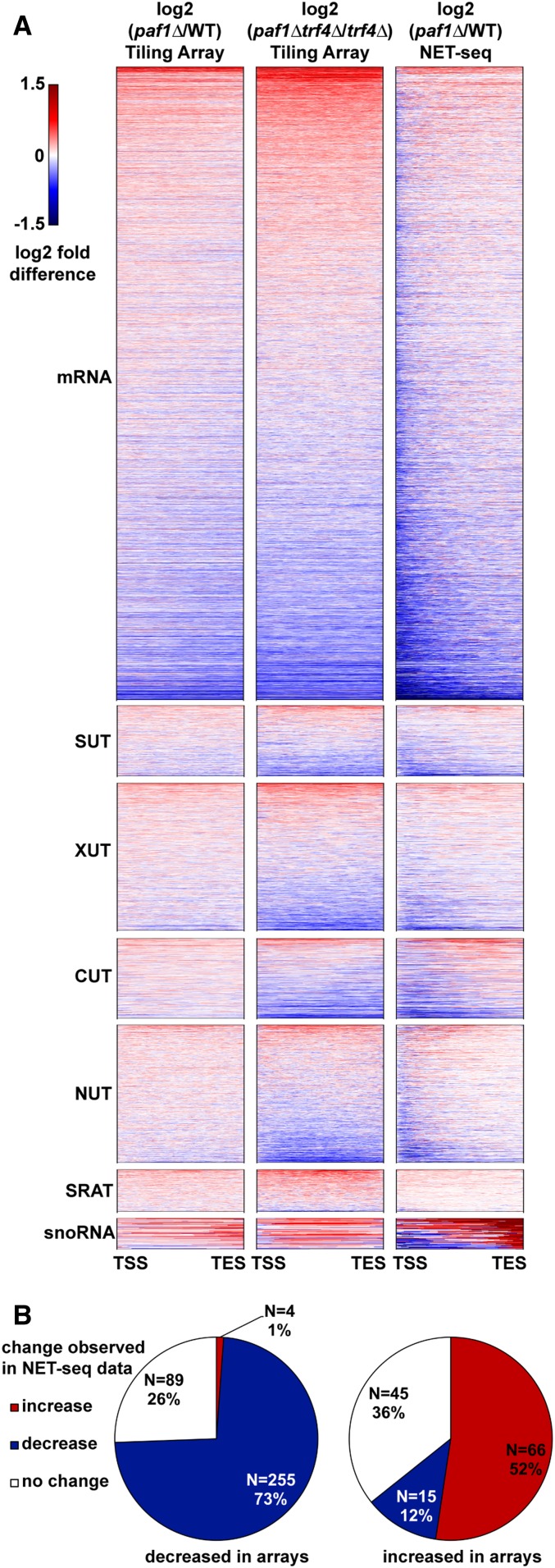
Paf1C has been shown to regulate both transcriptional and post-transcriptional processes at protein-coding genes (Porter et al. 2005; Van Oss et al. 2016; Fischl et al. 2017). To determine if changes in RNA levels detected by our tiling array analysis occurred at the transcriptional level, we compared our results to published NET-seq data (Harlen and Churchman 2017). Tiling array data comparing paf1∆ and PAF1 strains or paf1∆ trf4∆ and trf4∆ strains were used to generate heatmaps for comparison to paf1∆ NET-seq data (Figure 3A). Overall, we observed similarity between the paf1∆ trf4∆ tiling array data and the paf1∆ NET-seq data, indicating that Paf1 is regulating the abundance of many transcripts, including unstable ncRNAs and mRNAs, through an effect on transcription (Figure 3, A and B). However, our analysis also indicates, that at some genes, Paf1 is likely playing a post-transcriptional role. For example, for the majority of Paf1-stimulated protein-coding genes (73%), a decrease in steady state RNA levels in paf1∆ cells was reflected in reduced nascent transcript levels (Figure 3B). In contrast, a smaller fraction of Paf1-repressed genes (52%) showed a corresponding increase in NET-seq signal in the paf1∆ background (Figure 3B). Therefore, both positive and negative effects of Paf1 occur at the transcriptional level at many loci, but for protein-coding genes repressed by Paf1, a larger fraction appear to be post-transcriptionally regulated.
Figure 3.
Paf1 regulates many of its target loci at the transcriptional level. (A) Heatmaps plotting log2 fold-change in transcript levels detected by tiling array for paf1∆ vs. WT (KY1702 vs. KY2276) and paf1∆ trf4∆ vs. trf4∆ (KY2016 vs. KY2012) as well as a paf1∆ vs. WT NET-seq comparative analysis (Harlen and Churchman 2017). Previously annotated coding and noncoding transcripts were scaled so that each row in the heatmap represents a single transcript from transcription start site (TSS) to transcription end site (TES). (B) Pie charts showing the direction of change in NET-seq data (Harlen and Churchman 2017) for mRNAs that increased or decreased expression by at least 1.5-fold in the paf1∆ vs. WT comparison as measured by tiling array. Direction of change in NET-seq was determined by summing the reads in the first 500 bp of protein-coding genes in both WT and paf1∆ NET-seq datasets and calculating a fold-change (1.5-fold cutoff).
One possible difference between Paf1-stimulated and Paf1-repressed genes is related to their level of expression in wild-type cells. To investigate this possibility, we used ChIP-exo data from Van Oss et al. (2016) to analyze Pol II occupancy (Rpb3 subunit) at protein-coding genes with absolute expression changes of 1.5-fold or greater in a paf1∆ background as measured by our tiling array analysis. The Rpb3 ChIP-exo data indicate that, in general, Paf1-stimulated genes are more highly transcribed than Paf1-repressed genes (Figure S5, A and B). Similarly, Paf1 occupancy is higher at Paf1-stimulated genes compared to Paf1-repressed genes, consistent with the known association of Paf1C with Pol II. In agreement with the Rpb3 ChIP-exo data, analysis of transcript abundance in wild-type strains further demonstrated that Paf1 generally plays a positive role at highly active genes (Figure S5, A–C). Since defects in Paf1C cause a disruption in telomeric silencing (Krogan et al. 2003a; Ng et al. 2003a), we also analyzed the chromosomal locations of Paf1-regulated genes. Our analysis revealed broad chromosomal distribution of Paf1-stimulated and Paf1-repressed genes, in both TRF4 and trf4∆ backgrounds, with no obvious bias toward telomeres (Figure S6, A–C).
Paf1 stimulates the expression of phosphate homeostasis genes through a mechanism independent of its effects on individual histone modifications
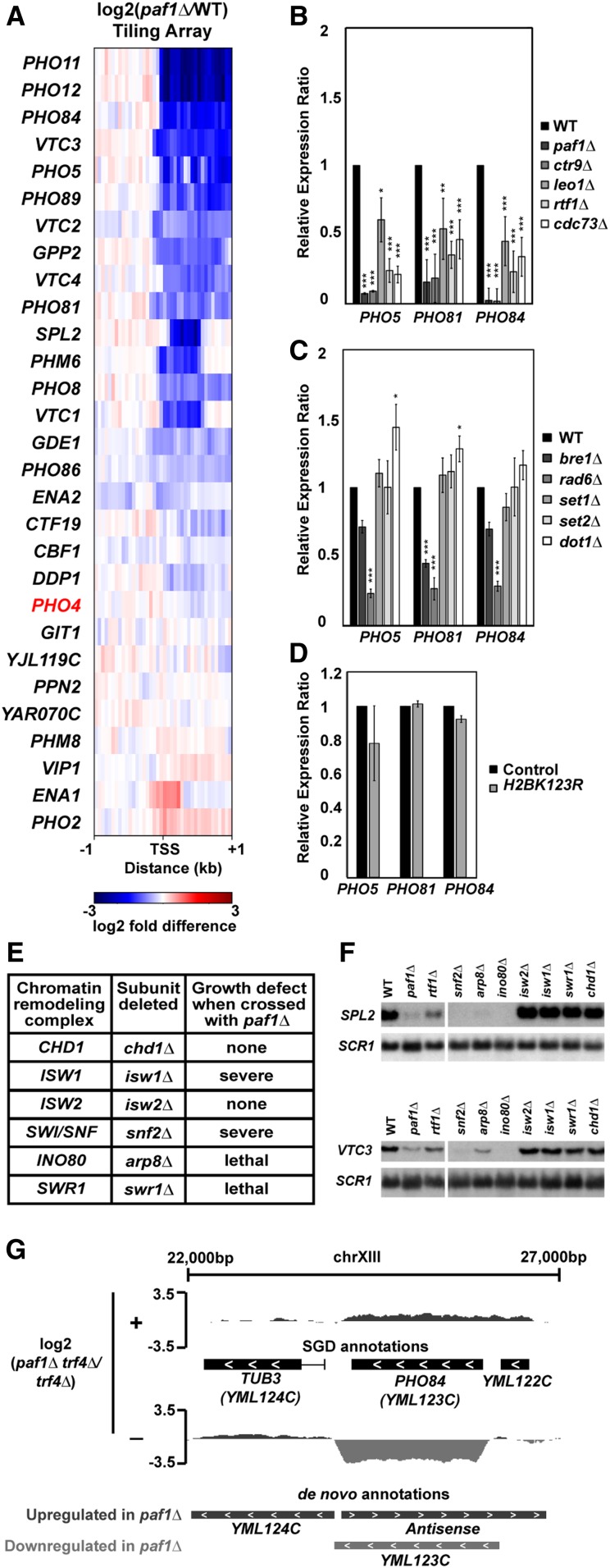
Gene ontology analysis (Ashburner et al. 2000) revealed an enrichment in phosphate homeostasis genes among the genes where expression decreased upon deletion of PAF1 in both the TRF4 and trf4∆ backgrounds (Table 2). Given the wealth of information on the importance of chromatin structure in regulating genes in the phosphate regulon (Korber and Barbaric 2014), we explored the mechanism of Paf1 involvement at these genes. Our tiling array data show that many but not all genes activated by the Pho4 transcription factor (Zhou and O’Shea 2011) are downregulated in the absence of Paf1 (Figure 4A), indicating that the effects of Paf1 are unlikely to be due to a loss of Pho4 function. Consistent with this, PHO4 transcript levels are not strongly affected in the paf1∆ strain (Figure 4A).
Figure 4.
Paf1 positively regulates many phosphate homeostasis genes. (A) Heatmap of expression differences observed in a paf1Δ strain (KY1702) relative to a WT strain (KY2276) at Pho4-responsive genes (Zhou and O’Shea 2011). (B–D) RT-qPCR analysis of phosphate gene expression in strains lacking (B) individual Paf1C subunits (KY1021, KY2271, KY2239, KY2243, KY2241, and KY2244), (C) histone modification enzymes (KY1683, KY2045, KY1952, KY938, KY914, KY934) or (D) H2B K123. In (D), RNA levels in the H2B K123R mutant (KY2167) were compared to the appropriate WT control strain (KY2027). Relative expression ratio is calculated using primer efficiency, normalization to the RNA polymerase III transcript SCR1 and a comparison to a WT strain (Pfaffl 2001). Error bars represent SEM and all statistically significant results are reported as asterisks (0.01 < P < 0.05 = *, 0.001 < P < 0.01 = **, 0 < P < 0.001 = ***). All P values were derived from a Student’s t-test between the mutant strain and WT. (E) Cumulative data from crosses between a paf1∆ strain and strains deleted for chromatin remodeling factors. Following tetrad analysis of the following crosses, growth defects of double mutants were determined: chd1∆ paf1∆ = KY583 × KY804; isw1∆ paf1∆ = KY3464 × KY901; isw2∆ paf1∆ = KY884 × KY804; snf2∆ paf1∆ = KY508 × KY804; arp8∆ paf1∆ = KY3460 × KY804; swr1∆ paf1∆ = KY3462 × KY972. (F) Northern blot analysis of SPL2 and VTC3 RNA levels. Strains used in this analysis were KY292, KY802, KY457, KY508, KY3465, KY3461, KY884, KY3463, KY972, and KY632. SCR1 serves as a loading control. (G) Genome browser view showing antisense transcription at the PHO84 locus. The browser view shows smoothed differential expression tracks (log2(paf1∆ trf4∆/trf4∆), 160 bp sliding window) with both SGD and de novo transcript annotations. Plus (+) and minus (−) symbols refer to DNA strand. The PHO84 gene is oriented right to left.
To assess the contribution of individual Paf1C members to the expression of phosphate homeostasis genes, we performed RT-qPCR analysis of RNA isolated from paf1∆, ctr9∆, cdc73∆, rtf1∆, and leo1∆ strains. The RT-qPCR results generally agreed with our tiling array results. RNA levels for PHO5, PHO81, and PHO84 were significantly decreased in the absence of any single Paf1C subunit with deletions of PAF1 and CTR9 causing the greatest effects (Figure 4B). Occupancy of Paf1 on PHO5, PHO81, and PHO84, as demonstrated by ChIP-exo analysis (VanOss et al. 2016) argues for a direct role of Paf1 in regulating these genes (Figure S5D).
Given the prominent role of Paf1C in promoting transcription-coupled histone modifications, we asked if loss of these modifications could explain the gene expression changes we observed in the Paf1C mutant strains. To this end, we performed RT-qPCR assays on RNA prepared from strains lacking the H2Bub enzymes Rad6 and Bre1, the H3 K4 methyltransferase Set1, the H3 K36 methyltransferase Set2, or the H3 K79 methyltransferase Dot1 (Figure 4C). RNA levels for PHO5, PHO81, and PHO84 decreased in the rad6Δ and bre1Δ strains, which, like an rtf1∆ strain (Van Oss et al. 2016), are severely deficient in H2Bub. However, deletion of PAF1 and CTR9 had a greater impact on the transcription of these genes than deletion of either BRE1, which encodes the ubiquitin ligase for H2B K123, or RTF1, which encodes the primary Paf1C determinant of H2Bub (Figure 4, B and C). The larger effect of rad6∆ compared to bre1∆ suggests that, as a ubiquitin conjugating enzyme, Rad6 may play roles in PHO gene regulation beyond catalyzing H2Bub. In agreement with this, we observed only a slight decrease in PHO5, PHO81, and PHO84 RNA levels in a H2B K123R mutant compared to the control strain (Figure 4D). Other than a slight, but statistically significant, upregulation of PHO5 and PHO81 in the dot1Δ strain, loss of individual H3 methyltransferases did not alter transcription of the PHO genes (Figure 4C). Therefore, the loss of individual Paf1C-mediated histone modifications does not explain the strong reduction in phosphate homeostasis gene expression observed in paf1∆ and ctr9∆ mutants.
The absence of a clear effect of Paf1C-dependent histone modifications on PHO gene regulation prompted us to investigate other connections between Paf1 and chromatin. Previous work by Batta et al. (2011) showed reduced nucleosome occupancy within coding regions in a paf1∆ strain, and the importance of nucleosome occupancy changes for PHO gene expression have been well documented (Barbaric et al. 2007; Korber and Barbaric 2014). Therefore, we investigated genetic interactions between Paf1 and chromatin remodeling factors. Genetic crosses were performed between strains lacking Paf1 and strains mutated for the following chromatin remodeling factors: Chd1, Isw1, Isw2, Swi/Snf, Ino80, and Swr1 (Figure 4E). We observed synthetic lethality or severe synthetic growth defects in double mutants containing paf1∆, and a deletion of SWR1, ISW1, SNF2, or ARP8, which encodes a subunit of the Ino80 complex. While the molecular basis for these genetic interactions is unclear, it is likely that Paf1C and chromatin remodeling factors regulate the expression of a shared group of genes. To test this idea for genes in the Pho4 regulon, we focused on two genes, SPL2 and VTC3, known to be stimulated by Ino80 (Ohdate et al. 2003). Northern analysis revealed greatly reduced VTC3 and SPL2 expression in cells lacking PAF1, SNF2, ARP8, or INO80 (Figure 4F). Although deletion of SWR1 or ISW1 did not affect SPL2 or VTC3 mRNA levels, it is possible that other Paf1-dependent genes are regulated by these remodeling factors. Taken together, these data suggest that Paf1C and chromatin remodeling factors work in parallel to maintain gene expression levels required for cell viability and phosphate homeostasis.
A well-studied example of locus-specific antisense control of transcription occurs at the PHO84 gene in yeast (Castelnuovo et al. 2013). At this locus, accumulation of an antisense transcript in an rrp6∆ strain leads to repression of the sense transcript through a mechanism dependent on particular histone modifications (Castelnuovo et al. 2014). In light of the changes in antisense RNAs detected in the paf1∆ background (Figure 2B and Figure S4), we examined our de novo differential expression data for evidence of Paf1-regulated antisense transcription at PHO84 (Figure 4G). When comparing the paf1∆ trf4∆ mutant to the trf4∆ control strain, we observed increased antisense and decreased sense transcript levels across the PHO84 gene. Interestingly, PHO84 fell into one of the two clusters of genes for which an anticorrelation between sense and antisense transcription was observed in the paf1∆ trf4∆ mutant (Figure S4B, cluster 5). These data suggest that, at the PHO84 gene, deletion of PAF1 elevates antisense transcription and coordinately decreases sense transcription.
Paf1 represses iron homeostasis gene expression in part through its role as a facilitator of H3 K36me3
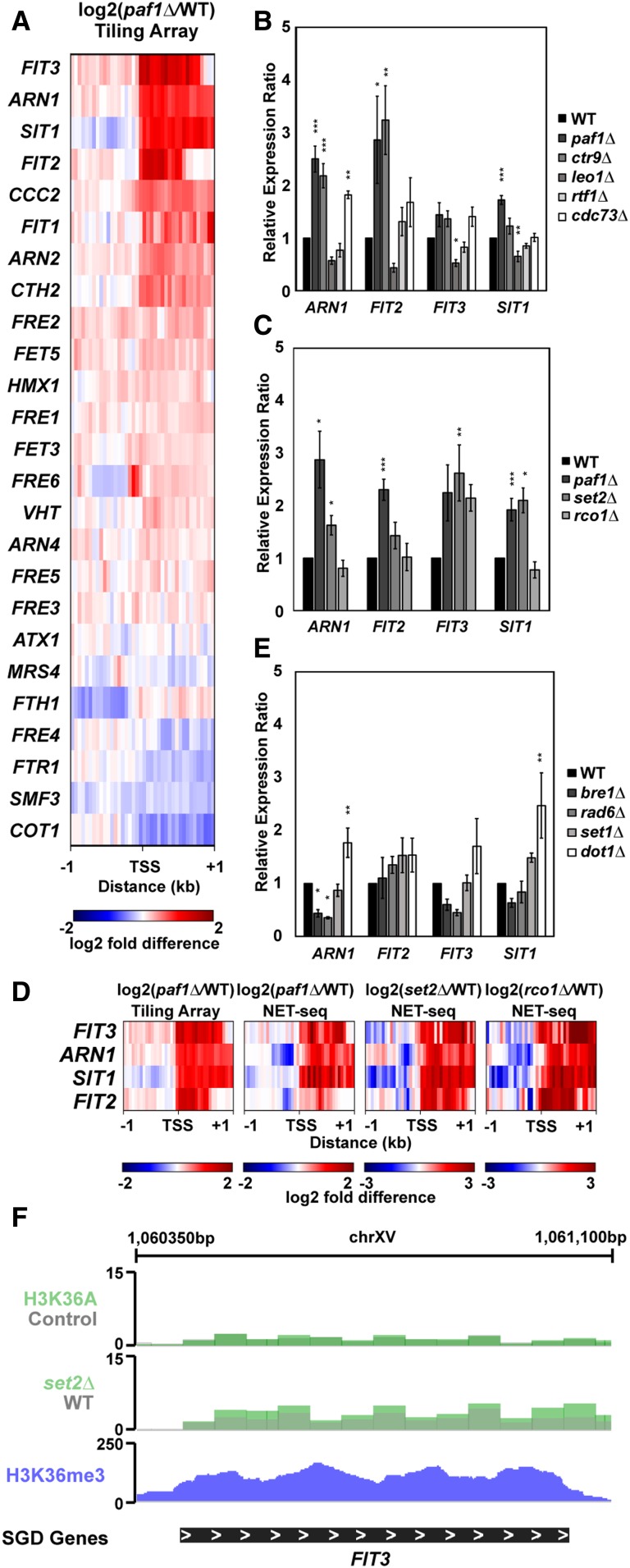
Gene ontology analysis of genes that increased expression in paf1Δ strains revealed enrichment for genes in various iron-related processes (Table 2). As with the phosphate genes, an additional motivation for choosing iron homeostasis genes for follow-up experiments was the extent to which they have been characterized in the literature (Yamaguchi-Iwai et al. 2002; Rutherford and Bird 2004; Courel et al. 2005; Kaplan and Kaplan 2009; Cyert and Philpott 2013). Our tiling array analysis of genes that are normally activated by the Aft1 and Aft2 transcription factors in iron-limiting conditions (Cyert and Philpott 2013) revealed that many but not all of these genes are repressed by Paf1 in iron-replete media (Figure 5A).
Figure 5.
Paf1 represses iron homeostasis genes. (A) Heatmap of expression differences observed in a paf1Δ strain (KY1702) relative to a WT strain (KY2276) at Aft1 and Aft2 responsive genes involved in maintenance of iron homeostasis (Cyert and Philpott 2013). (B and C) RT-qPCR analysis of the indicated genes in strains lacking (B) individual Paf1C subunits (KY1021, KY2271, KY2239, KY2243, KY2241, and KY2244) or (C) genes in the Set2/Rpd3S pathway (KY307, KY914, KY1702, and KY1235). Calculation of the relative expression ratio and statistical testing were performed as in Figure 4. (D) Heatmaps of expression differences between mutant yeast strains and their respective WT strains in tiling array (this study) and NET-seq (Churchman and Weissman 2011; Harlen and Churchman 2017) datasets. (E) RT-qPCR results for iron homeostasis genes in strains lacking enzymes that catalyze Paf1C-associated histone modifications (KY1683, KY2045, KY1952, KY938, and KY934). (F) Genome browser view of the FIT3 locus showing H3 K36A and set2∆ RNA-seq data from Venkatesh et al. (2016) and H3 K36me3 occupancy data from Weiner et al. (2015).
To further investigate this subset of genes, we performed RT-qPCR analysis on RNA isolated from strains lacking individual Paf1C members (Figure 5B). A reproducible increase in expression was observed for ARN1, FIT2, FIT3, and SIT1 in paf1∆ and ctr9∆ strains. Occupancy of Paf1 on these genes suggests that Paf1 is playing a direct role in mediating their repression (Figure S5D). With the exception of SIT1, deletion of CDC73 also led to derepression of these genes. In contrast, whereas Paf1, Ctr9, and Cdc73 repress the transcription of ARN1, FIT2, and FIT3, Leo1 appears to play a stimulatory role at these genes, while Rtf1 has little effect. Together, these data demonstrate that individual Paf1C subunits differentially regulate iron-responsive genes.
The Set2 histone methyltransferase catalyzes H3 K36me3—a modification that is dependent on Paf1 and Ctr9 (Krogan et al. 2003b; Chu et al. 2007). This epigenetic mark leads to the activation of the Rpd3S histone deacetylase complex and inhibition of histone exchange, generating a repressed chromatin state (Carrozza et al. 2005; Joshi and Struhl 2005; Keogh et al. 2005; Govind et al. 2010; Churchman and Weissman 2011; Venkatesh et al. 2012, 2016; Kim et al. 2016). In the set2Δ strain, RNA levels for FIT3 and SIT1 increased to those observed in the paf1Δ strain, suggesting that Paf1 represses these genes through stimulating H3 K36me3 (Figure 5C). This conclusion is supported by our observation that a set2∆ paf1∆ double mutant recapitulates the increased expression of FIT3 and SIT1 observed in the set2∆ and paf1∆ single mutants (Figure S7). Further, NET-seq data (Churchman and Weissman 2011; Harlen and Churchman 2017) indicate that the increase in steady state mRNA levels for FIT3 and SIT1 in paf1∆, set2∆, and rco1∆ strains is associated with an increase in transcription (Figure 5D).
For two other genes, ARN1 and FIT2, the level of derepression observed in the paf1∆ strain was greater than that observed in the set2∆ strain, despite evidence from NET-seq data (Figure 5D; Churchman and Weissman 2011; Harlen and Churchman 2017) that loss of Set2 strongly increases transcription of these genes. Similarly, with the exception of FIT3, steady state mRNA levels in a strain lacking the Rpd3S subunit Rco1 did not reflect the increase in transcription detected by NET-seq (Figure 5, C and D). One likely explanation for the difference between the steady-state mRNA measurements (Figure 5C) and the nascent transcript data (Figure 5D) is that mRNA levels for the iron-responsive genes are post-transcriptionally regulated, possibly through a degradation pathway that involves Paf1. This conclusion is in line with observations made through our de novo analysis (Figure 3B), which indicated a post-transcriptional role for Paf1 at genes where it negatively regulates mRNA levels, and with previous descriptions of RNA degradation pathways that target mRNAs in the iron regulon (Lee et al. 2005; Puig et al. 2005).
In addition to the Set2/Rpd3S pathway, we tested other histone modifiers for a role in iron gene repression by examining ARN1, FIT2, FIT3, and SIT1 expression in bre1Δ, rad6Δ, set1Δ, and dot1Δ strains by RT-qPCR (Figure 5E). With the exception of the dot1Δ mutation, which elevated ARN1 and SIT1 transcript levels, none of these mutations led to a significant derepression of the iron genes. The prominent role of Set2 in mediating FIT3 repression is further supported by the enrichment of H3 K36me3 on this gene (Weiner et al. 2015) and the derepression of FIT3 RNA levels in set2∆ and H3 K36A mutants (Venkatesh et al. 2016) (Figure 5F). Taken together, these results suggest that Paf1 represses expression of genes in the iron regulon by inhibiting transcription, most likely by facilitating histone marks such as H3 K36me3, and by influencing RNA stability.
FET4 is differentially regulated by Paf1 and upstream CUT transcription
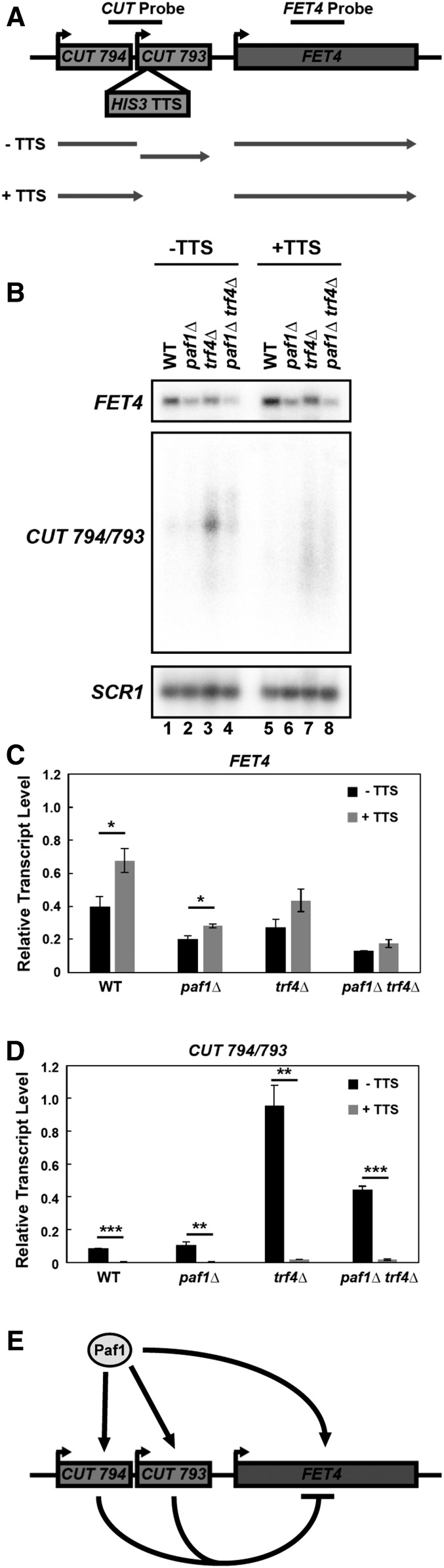
To investigate the interplay between Paf1 and noncoding DNA transcription on a protein-coding gene in the iron regulon, we focused on the FET4 gene, which encodes a low affinity iron transporter in S. cerevisiae (Dix et al. 1994). Two CUTs have been annotated upstream of the FET4 coding region (Xu et al. 2009; Raupach et al. 2016) (Figure 6A). We hypothesized that CUT 794/793 transcription regulates FET4 transcription possibly in a PAF1-dependent manner. To test this, we generated strains containing a transcription termination sequence (TTS) upstream of the FET4 gene positioned to stop transcription of both upstream CUTs in wild type, paf1∆, trf4∆, and paf1∆ trf4∆ backgrounds.
Figure 6.
The FET4 locus is regulated by Paf1 and transcription of ncDNA upstream of the coding region. (A) Diagram of the FET4 locus and the position of a transcription termination sequence (HIS3 TTS) inserted 400 bp upstream of the FET4 start codon to block CUT 794/793 transcription. (B) Northern analysis of FET4 mRNA, CUT 794/793 and SCR1 RNA (loading control) from WT, paf1Δ, trf4Δ, and paf1Δ trf4Δ strains without the inserted TTS (KY2276, KY1702, KY2012, KY2016) or with the TTS (KY3466, KY2846, KY2851, KY2845). (C and D) Quantification of northern blot results for FET4 and CUT 794/793 normalized to SCR1. Error bars represent SEM and all statistically significant results are reported as asterisks that represent P values from Students t-test as in Figure 4. (E) Diagram of the observed effects of PAF1 and CUT794/793 at the FET4 locus.
Northern analysis showed that deletion of PAF1 reduced FET4 transcript levels (Figure 6B top blot, cf. lanes 1 and 2 and lanes 3 and 4; Figure 6C) and CUT794/793 levels (Figure 6B middle blot, compare lanes 3 and 4; Figure 6D). When the TTS was inserted upstream of FET4 (+TTS), CUT levels decreased and FET4 transcript levels increased in all conditions tested, suggesting that transcription of the upstream CUT inhibits expression of the coding region (Figure 6, B–D). This is reminiscent of the inhibitory effect of noncoding transcription upstream of the well-studied SER3 gene (Martens et al. 2004). Interestingly, even when CUT transcription was blocked, FET4 transcript levels were reduced in the paf1∆ background. These data suggest that CUT transcription upstream of the FET4 promoter negatively regulates transcription of the FET4 gene and that Paf1 has a dual role in regulating FET4 by stimulating expression of both the ORF and the inhibitory CUTs 794/793 (Figure 6E).
Discussion
The many roles of Paf1C and the pleiotropic phenotypes conferred by deletion of individual Paf1C subunits (Betz et al. 2002) suggest that this conserved transcription elongation complex regulates the expression of many genetic loci. While previous studies focused on the regulation of mRNAs (Shi et al. 1996; Penheiter et al. 2005; Porter et al. 2005; Batta et al. 2011; van Bakel et al. 2013; Cao et al. 2015; Chen et al. 2015; Yang et al. 2016; Fischl et al. 2017; Harlen and Churchman 2017), here we sought to identify the full cohort of Paf1-regulated transcripts, both coding and noncoding, by exploiting a genetic background deficient in the TRAMP/exosome-dependent RNA degradation pathway and by performing de novo transcript identification analyses. Our high-resolution tiling array experiments revealed differential expression of transcripts in all Pol II transcribed RNA classes in strains deleted for PAF1. A comparison of our paf1∆ trf4∆ tiling array data with published paf1∆ NET-seq (Harlen and Churchman 2017) data demonstrated that Paf1 regulates many coding and ncRNAs at the transcriptional level, and that the presence of a functional TRAMP complex obscures many of these transcriptional effects.
Our study revealed both positive and negative roles of Paf1 in regulating ncRNA levels. For many transcripts in the CUT, NUT, XUT, and SUT classes, Paf1 stimulates their expression. For two other important classes of ncRNAs, snoRNAs and SRATs, Paf1 functions primarily as a negative regulator. The elevation in SRAT expression was not unexpected given the importance of Paf1 for H3 K36me3 (Krogan et al. 2003b; Chu et al. 2007)—a mark important for the maintenance of a repressive chromatin environment. In our previous work, we showed that Paf1 is important for snoRNA termination (Sheldon et al. 2005; Tomson et al. 2011, 2013). We recapitulate those results here, and identify additional snoRNA loci that exhibit transcription termination defects in the absence of Paf1 (Figure 1H). These results, together with our finding that Paf1 impacts the transcription of many CUTs and NUTs, extends the functional connections between Paf1 and the machinery that terminates and processes these ncRNAs, including the NNS machinery and the nuclear exosome. Similar to a paf1∆ strain, snoRNA 3′ ends are extended in rrp6 and nrd1 mutants, which lack subunits of the nuclear exosome and NNS, respectively (Schulz et al. 2013; Fox et al. 2015). In contrast, while NUTs and CUTs are elevated in nrd1 and rrp6 mutants, levels of many of these unstable ncRNAs are decreased in strains deleted for PAF1. The reduced levels of these RNAs in paf1∆ strains are likely due, at least in part, to the stimulatory effect Paf1 has on their transcription (Figure 3).
By performing a de novo differential expression analysis of our tiling array data, we uncovered effects of Paf1 on antisense transcription (Figure 2B). Interestingly, many of the histone modifications promoted by Paf1C are reduced in regions experiencing higher levels of antisense transcription, but still others are present at high levels in these same regions (Murray et al. 2015). The loss of Paf1C-promoted histone modifications may therefore contribute to changes in antisense transcription (Castelnuovo et al. 2014; Murray et al. 2015). Indeed, we found instances of both increased and decreased antisense transcription in our paf1∆ strains. Although some global anticorrelation exists between sense and divergent antisense transcription initiating from nucleosome depleted regions (Xu et al. 2009; Churchman and Weissman 2011), antisense transcription does not universally correlate or anticorrelate with sense transcription (Murray et al. 2015). Our results agree with this observation (Figure S4E), but also point to a small subset of genes where sense and antisense transcription appear to be anticorrelated when PAF1 is deleted (Figure S4A cluster 1 and S4B cluster 5).
One gene that fits into this category is PHO84 (Figure S4B cluster 5; Figure 4G). Our data suggest a role for Paf1 in preventing antisense transcription at PHO84 independently of its functional connections with the TRAMP/exosome pathway, as we detect higher antisense and lower sense transcript levels in the paf1∆ trf4∆ strain relative to the trf4∆ strain (Figure 4G). The ability to detect changes in antisense transcription was enhanced by the absence of Trf4. This observation agrees with studies on PHO84 and other genes, which showed elevated antisense transcription in the absence of Rrp6 (Castelnuovo et al. 2013). With respect to PHO84, the mechanism by which Paf1 facilitates sense and represses antisense transcription remains undefined. Although previous studies showed that set1∆ strongly upregulates PHO84 sense transcription in the presence of RRP6 (Castelnuovo et al. 2014) and that Paf1C is required for Set1-dependent H3 K4 methylation (Krogan et al. 2003a; Ng et al. 2003b), our strand-specific tiling array data showed that paf1∆ strongly downregulates PHO84 sense transcription (Figure 4A). Similarly, our results do not ascribe the stimulatory effect of Paf1 on PHO5 and PHO81 to any single Paf1C-dependent histone modification or an obvious change in antisense transcription; however, it remains possible that the individual modifications function redundantly in promoting the expression of these genes.
At many genes that are normally induced in iron-limiting conditions, Paf1 plays a repressive role under iron-replete conditions. For the four strongly upregulated genes examined, deletion of PAF1 increased steady state RNA levels as well as nascent transcript levels, arguing that Paf1 is controlling the transcription of these genes. The increase in transcription in the paf1∆ strain correlates with a decrease in Set2 function, as shown by NET-seq data (Churchman and Weissman 2011; Harlen and Churchman 2017). Indeed, in our tiling array experiments, we detected a global increase in SRAT transcription in a paf1Δ strain (Figure 1D and Figure 2A). Interestingly, when comparing steady state RNA levels and nascent transcript levels, we noted an apparent post-transcriptional effect of Paf1 (Figure 3B and Figure 5, C and D). With respect to the iron metabolism genes, we saw a strong overlap between Paf1-repressed mRNAs and Rnt1-repressed mRNAs (Lee et al. 2005). Rnt1 is a double-stranded RNA endonuclease that cleaves RNAs with a particular stem-loop structure (Chanfreau et al. 2000), and, in iron replete conditions, executes an RNA degradation pathway for mRNAs that encode iron uptake proteins (Lee et al. 2005). While other explanations are possible, the overlap between Paf1-and Rnt1-repressed mRNAs suggests that the post-transcriptional role of Paf1 at iron regulon genes may involve a functional interaction with Rnt1. A recent study showed that the rate of transcription elongation can influence the folding and processing of histone pre-mRNAs (Saldi et al. 2018), raising the possibility that deletion of PAF1 might alter the rate of elongation in a way that affects the folding of substrates for Rnt1 or another RNA processing factor. Together, our results suggest that through stimulating Set2-mediated H3 K36 methylation, Paf1 represses genes in the iron regulon, but has an additional role in reducing the stability of these mRNAs.
Numerous examples of protein-coding gene regulation by ncDNA transcription or ncRNAs have been observed (Castelnuovo and Stutz 2015). Adding to this body of evidence, we investigated the regulatory mechanisms governing expression of FET4, which encodes a low affinity iron transporter. Our analysis indicates that FET4 is regulated by the expression of upstream CUTs and by Paf1. Insertion of a transcription termination sequence upstream of FET4 decreased CUT794/793 levels and increased FET4 transcription. Deletion of PAF1 reduced both CUT794/793 and FET4 transcript levels. Together with these targeted experiments, our tiling array results on the paf1∆ strain also revealed a stimulatory effect of Paf1 on FET4 mRNA levels. However, we note that our tiling array analysis of the paf1∆ trf4∆ strain indicated that, in some circumstances, Paf1 can repress FET4 expression. Previous studies have shown that genetic background and growth conditions can influence the levels of FET4 mRNA and the noncoding RNAs adjacent to or overlapping FET4 (CUT794/793 and the SUT322 antisense ncRNA) (Xu et al. 2009). Since our tiling array and northern blotting experiments used RNA from yeast grown on separate days, it is possible that slight differences in media may be responsible for differences in expression dynamics at the FET4 locus, highlighting the intricacies of the regulatory system operating at this gene. Collectively, our results add to the interesting list of telomere-proximal metal-responsive genes under the control of noncoding transcription (Toesca et al. 2011).
The complexity of the transcription process and its regulation by chromatin provides numerous opportunities for multifunctional transcription factors, like Paf1C, to regulate gene expression. Our study reveals genome-wide effects of Paf1 on both coding and noncoding RNAs and provides mechanistic explanations for its diverse effects on specific classes of protein-coding genes. An understanding of the locus-specific effects of Paf1C will be an important step in elucidating the numerous connections of this complex to gene expression changes that cause human disease (Tomson and Arndt 2013; Karmakar et al. 2018).
Acknowledgments
We thank Margaret Shirra, Elizabeth Hildreth, Christine Cucinotta, Matthew Hurton, and Paul Cantalupo for helpful discussions. We are grateful to Frank Pugh and Stirling Churchman, and members of their laboratories, for assistance with analysis of published genomic data, and to Chenchen Zhu and Lars Steinmetz for advice on building a probe annotation file for the tiling array analysis. This research was supported by the National Institutes of Health (NIH) Grant R01 GM52593 to K.M.A., Canada Research Chairs Program (CRC) funds to C.N., and funds from the University of Pittsburgh to M.T.L. M.A.E. is supported by a predoctoral fellowship from the NIH (F31GM129917).
Footnotes
Supplemental material available at FigShare: https://doi.org/10.25386/genetics.7796837.
Communicating editor: O. Rando
Literature Cited
- Aho A. V., Kernighan B. W., Weinberger P. J., 1979. Awk a pattern scanning and processing language. Softw. Pract. Exper. 9: 267–279. 10.1002/spe.4380090403 [DOI] [Google Scholar]
- Amrich C. G., Davis C. P., Rogal W. P., Shirra M. K., Heroux A., et al. , 2012. Cdc73 subunit of Paf1 complex contains C-terminal Ras-like domain that promotes association of Paf1 complex with chromatin. J. Biol. Chem. 287: 10863–10875. 10.1074/jbc.M111.325647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt K. M., Reines D., 2015. Termination of transcription of short noncoding RNAs by RNA polymerase II. Annu. Rev. Biochem. 84:381–404. 10.1146/annurev-biochem-060614-034457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M., Ball C. A., Blake J. A., Botstein D., Butler H., et al. , 2000. Gene ontology: tool for the unification of biology. Nat. Genet. 25: 25–29. 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan R., Park J., Karra K., Hitz B. C., Binkley G., et al. , 2012. YeastMine: an integrated data warehouse for Saccharomyces cerevisiae data as a multipurpose tool-kit. Database (Oxford) 2012: 1–8. 10.1093/database/bar062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaric S., Luckenbach T., Schmid A., Blaschke D., Hörz W., et al. , 2007. Redundancy of chromatin remodeling pathways for the induction of the yeast PHO5 promoter in vivo. J. Biol. Chem. 282: 27610–27621. 10.1074/jbc.M700623200 [DOI] [PubMed] [Google Scholar]
- Batta K., Zhang Z., Yen K., Goffman D. B., Franklin Pugh B., 2011. Genome-wide function of H2B ubiquitylation in promoter and genic regions. Genes Dev. 25: 2254–2265. 10.1101/gad.177238.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y., 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 57: 289–300. [Google Scholar]
- Betz J. L., Chang M., Washburn T. M., Porter S. E., Mueller C. L., et al. , 2002. Phenotypic analysis of Paf1/RNA polymerase II complex mutations reveals connections to cell cycle regulation, protein synthesis, and lipid and nucleic acid metabolism. Mol. Genet. Genomics 268: 272–285. 10.1007/s00438-002-0752-8 [DOI] [PubMed] [Google Scholar]
- Cao Q.-F., Yamamoto J., Isobe T., Tateno S., Murase Y., et al. , 2015. Characterization of the human transcription elongation factor Rtf1: evidence for nonoverlapping functions of Rtf1 and the Paf1 complex. Mol. Cell. Biol. 35: 3459–3470. 10.1128/MCB.00601-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrozza M. J., Li B., Florens L., Suganuma T., Swanson S. K., et al. , 2005. Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell 123: 581–592. 10.1016/j.cell.2005.10.023 [DOI] [PubMed] [Google Scholar]
- Castelnuovo M., Stutz F., 2015. Role of chromatin, environmental changes and single cell heterogeneity in non-coding transcription and gene regulation. Curr. Opin. Cell Biol. 34: 16–22. 10.1016/j.ceb.2015.04.011 [DOI] [PubMed] [Google Scholar]
- Castelnuovo M., Rahman S., Guffanti E., Infantino V., Stutz F., et al. , 2013. Bimodal expression of PHO84 is modulated by early termination of antisense transcription. Nat. Struct. Mol. Biol. 20: 851–858. 10.1038/nsmb.2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelnuovo M., Zaugg J. B., Guffanti E., Maffioletti A., Camblong J., et al. , 2014. Role of histone modifications and early termination in pervasive transcription and antisense-mediated gene silencing in yeast. Nucleic Acids Res. 42: 4348–4362. 10.1093/nar/gku100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanfreau G., Buckle M., Jacquier A., 2000. Recognition of a conserved class of RNA tetraloops by Saccharomyces cerevisiae RNase III. Proc. Natl. Acad. Sci. USA 97: 3142–3147. 10.1073/pnas.97.7.3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F. X., Woodfin A. R., Gardini A., Rickels R. A., Marshall S. A., et al. , 2015. PAF1, a molecular regulator of promoter-proximal pausing by RNA polymerase II. Cell 162: 1003–1015. 10.1016/j.cell.2015.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry J. M., Adler C., Ball C., Chervitz S. A., Dwight S. S., et al. , 1998. SGD: saccharomyces genome database. Nucleic Acids Res. 26: 73–79. 10.1093/nar/26.1.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry J. M., Hong E. L., Amundsen C., Balakrishnan R., Binkley G., et al. , 2012. Saccharomyces genome database: the genomics resource of budding yeast. Nucleic Acids Res. 40: D700–D705. 10.1093/nar/gkr1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y., Simic R., Warner M. H., Arndt K. M., Prelich G., 2007. Regulation of histone modification and cryptic transcription by the Bur1 and Paf1 complexes. EMBO J. 26: 4646–4656. 10.1038/sj.emboj.7601887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchman L. S., Weissman J. S., 2011. Nascent transcript sequencing visualizes transcription at nucleotide resolution. Nature 469: 368–373. 10.1038/nature09652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart, M. A., and S. Oliviero, 1993 Preparation of yeast RNA. Curr. Protoc. Mol. Biol. 23: 13.12.1–13.12.5. 10.1002/0471142727.mb1312s23 10.1002/0471142727.mb1312s23 [DOI] [PubMed] [Google Scholar]
- Courel M., Lallet S., Camadro J.-M., Blaiseau P.-L., 2005. Direct activation of genes involved in intracellular iron use by the yeast iron-responsive transcription factor Aft2 without its paralog Aft1. Mol. Cell. Biol. 25: 6760–6771. 10.1128/MCB.25.15.6760-6771.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisucci E. M., Arndt K. M., 2011. The roles of the Paf1 complex and associated histone modifications in regulating gene expression. Genet. Res. Int. 2011: 1–15. 10.4061/2011/707641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert M. S., Philpott C. C., 2013. Regulation of cation balance in Saccharomyces cerevisiae. Genetics 193: 677–713. 10.1534/genetics.112.147207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David L., Huber W., Granovskaia M., Toedling J., Palm C. J., et al. , 2006. A high-resolution map of transcription in the yeast genome. Proc. Natl. Acad. Sci. USA 103: 5320–5325. 10.1073/pnas.0601091103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P., Zhou Y., Jiang J., Li H., Tian W., et al. , 2018. Transcriptional elongation factor Paf1 core complex adopts a spirally wrapped solenoidal topology. Proc. Natl. Acad. Sci. USA 115: 9998–10003. 10.1073/pnas.1812256115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix D. R., Bridgham J. T., Broderius M. A., Byersdorfer C. A., Eide D. J., 1994. The FET4 gene encodes the low affinity Fe(II) transport protein of Saccharomyces cerevisiae. J. Biol. Chem. 269: 26092–26099. [PubMed] [Google Scholar]
- Dover J., Schneider J., Tawiah-Boateng M. A., Wood A., Dean K., et al. , 2002. Methylation of histone H3 by COMPASS requires ubiquitination of histone H2B by Rad6. J. Biol. Chem. 277: 28368–28371. 10.1074/jbc.C200348200 [DOI] [PubMed] [Google Scholar]
- Engel S. R., Dietrich F. S., Fisk D. G., Binkley G., Balakrishnan R., et al. , 2014. The reference genome sequence of Saccharomyces cerevisiae: then and now. G3 (Bethesda) 4: 389–398. 10.1534/g3.113.008995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl H., Howe F. S., Furger A., Mellor J., 2017. Paf1 has distinct roles in transcription elongation and differential transcript fate. Mol. Cell 65: 685–698.e8. 10.1016/j.molcel.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. J., Gao H., Smith-Kinnaman W. R., Liu Y., Mosley A. L., 2015. The exosome component Rrp6 is required for RNA polymerase II termination at specific targets of the Nrd1-Nab3 pathway. PLoS Genet. 11: e1004999 10.1371/journal.pgen.1004999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman R., Carey V., Bates D., Bolstad B., Dettling M., et al. , 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5: R80 10.1186/gb-2004-5-10-r80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind C. K., Qiu H., Ginsburg D. S., Ruan C., Hofmeyer K., et al. , 2010. Phosphorylated Pol II CTD recruits multiple HDACs, including Rpd3C(S), for methylation-dependent deacetylation of ORF nucleosomes. Mol. Cell 39: 234–246. 10.1016/j.molcel.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlen K. M., Churchman L. S., 2017. Subgenic Pol II interactomes identify region‐specific transcription elongation regulators. Mol. Syst. Biol. 13: 900 10.15252/msb.20167279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber W., von Heydebreck A., Sültmann H., Poustka A., Vingron M., 2002. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics 18: S96–S104. 10.1093/bioinformatics/18.suppl_1.S96 [DOI] [PubMed] [Google Scholar]
- Huber W., Toedling J., Steinmetz L. M., 2006. Transcript mapping with high-density oligonucleotide tiling arrays. Bioinformatics 22: 1963–1970. 10.1093/bioinformatics/btl289 [DOI] [PubMed] [Google Scholar]
- Jaehning J. A., 2010. The Paf1 complex: platform or player in RNA polymerase II transcription? Biochim. Biophys. Acta. Gene Regul. Mech. 1799: 379–388. 10.1016/j.bbagrm.2010.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A. A., Struhl K., 2005. Eaf3 chromodomain interaction with methylated H3–K36 links histone deacetylation to pol II elongation. Mol. Cell 20: 971–978. 10.1016/j.molcel.2005.11.021 [DOI] [PubMed] [Google Scholar]
- Juneau K., Palm C., Miranda M., Davis R. W., 2007. High-density yeast-tiling array reveals previously undiscovered introns and extensive regulation of meiotic splicing. Proc. Natl. Acad. Sci. USA 104: 1522–1527. 10.1073/pnas.0610354104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan C. D., Kaplan J., 2009. Iron acquisition and transcriptional regulation. Chem. Rev. 109: 4536–4552. 10.1021/cr9001676 [DOI] [PubMed] [Google Scholar]
- Karmakar S., Dey P., Vaz A. P., Bhaumik S. R., Ponnusamy M. P., et al. , 2018. PD2/PAF1 at the crossroads of the cancer network. Cancer Res. 78: 313–319. 10.1158/0008-5472.CAN-17-2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent W., Sugnet C., Furey T., Roskin K., 2002. The human genome browser at UCSC. Genome Res. 12: 996–1006. 10.1101/gr.229102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh M. C., Kurdistani S. K., Morris S. A., Ahn S. H., Podolny V., et al. , 2005. Cotranscriptional Set2 methylation of histone H3 lysine 36 recruits a repressive Rpd3 complex. Cell 123: 593–605. 10.1016/j.cell.2005.10.025 [DOI] [PubMed] [Google Scholar]
- Kim D., Langmead B., Salzberg S. L., 2015. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12: 357–360. 10.1038/nmeth.3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Lee B. B., Oh Y. M., Zhu C., Steinmetz L. M., et al. , 2016. Modulation of mRNA and lncRNA expression dynamics by the Set2–Rpd3S pathway. Nat. Commun. 7: 13534 10.1038/ncomms13534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korber P., Barbaric S., 2014. The yeast PHO5 promoter: from single locus to systems biology of a paradigm for gene regulation through chromatin. Nucleic Acids Res. 42: 10888–10902. 10.1093/nar/gku784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan N. J., Dover J., Wood A., Schneider J., Heidt J., et al. , 2003a The Paf1 complex is required for histone H3 methylation by COMPASS and Dot1p: linking transcriptional elongation to histone methylation. Mol. Cell 11: 721–729. 10.1016/S1097-2765(03)00091-1 [DOI] [PubMed] [Google Scholar]
- Krogan N. J., Kim M., Tong A., Golshani A., Cagney G., et al. , 2003b Methylation of histone H3 by Set2 in Saccharomyces cerevisiae is linked to transcriptional elongation by RNA polymerase II. Mol. Cell. Biol. 23: 4207–4218. 10.1128/MCB.23.12.4207-4218.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz S., Phillippy A., Delcher A. L., Smoot M., Shumway M., et al. , 2004. Versatile and open software for comparing large genomes. Genome Biol. 5: R12 10.1186/gb-2004-5-2-r12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCava J., Houseley J., Saveanu C., Petfalski E., Thompson E., et al. , 2005. RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell 121: 713–724. 10.1016/j.cell.2005.04.029 [DOI] [PubMed] [Google Scholar]
- Lee A., Henras A. K., Chanfreau G., 2005. Multiple RNA surveillance pathways limit aberrant expression of iron uptake mRNAs and prevent iron toxicity in S. cerevisiae. Mol. Cell 19: 39–51. 10.1016/j.molcel.2005.05.021 [DOI] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., et al. , 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25: 2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad V., Hartzog G., Moqtaderi Z., 2001. Manipulation of cloned yeast DNA. Curr. Protoc. Mol. Biol. 13: Unit 13.10 10.1002/0471142727.mb1310s39 [DOI] [PubMed] [Google Scholar]
- Martens J. A., Laprade L., Winston F., 2004. Intergenic transcription is required to repress the Saccharomyces cerevisiae SER3 gene. Nature 429: 571–574. 10.1038/nature02538 [DOI] [PubMed] [Google Scholar]
- Mueller C. L., Porter S. E., Hoffman M. G., Jaehning J. A., 2004. The Paf1 complex has functions independent of actively transcribing RNA polymerase II. Mol. Cell 14: 447–456. 10.1016/S1097-2765(04)00257-6 [DOI] [PubMed] [Google Scholar]
- Murray S. C., Haenni S., Howe F. S., Fischl H., Chocian K., et al. , 2015. Sense and antisense transcription are associated with distinct chromatin architectures across genes. Nucleic Acids Res. 43: 7823–7837. 10.1093/nar/gkv666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil H., Malabat C., D’Aubenton-Carafa Y., Xu Z., Steinmetz L. M., et al. , 2009. Widespread bidirectional promoters are the major source of cryptic transcripts in yeast. Nature 457: 1038–1042. 10.1038/nature07747 [DOI] [PubMed] [Google Scholar]
- Ng H. H., Dole S., Struhl K., 2003a The Rtf1 component of the Paf1 transcriptional elongation complex is required for ubiquitination of histone H2B. J. Biol. Chem. 278: 33625–33628. 10.1074/jbc.C300270200 [DOI] [PubMed] [Google Scholar]
- Ng H. H., Robert F., Young R. A., Struhl K., 2003b Targeted recruitment of Set1 histone methylase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Mol. Cell 11: 709–719. 10.1016/S1097-2765(03)00092-3 [DOI] [PubMed] [Google Scholar]
- Nishimura K., Fukagawa T., Takisawa H., Kakimoto T., Kanemaki M., 2009. An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat. Methods 53: 1689–1699. 10.1017/CBO9781107415324.004 [DOI] [PubMed] [Google Scholar]
- Nordick K., Hoffman M. G., Betz J. L., Jaehning J. A., 2008. Direct interactions between the Paf1 complex and a cleavage and polyadenylation factor are revealed by dissociation of Paf1 from RNA polymerase II. Eukaryot. Cell 7: 1158–1167. 10.1128/EC.00434-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohdate H., Lim C. R., Kokubo T., Matsubara K., Kimata Y., et al. , 2003. Impairment of the DNA binding activity of the TATA-binding protein renders the transcriptional function of Rvb2p/Tih2p, the yeast RuvB-like protein. Essential for Cell Growth. 278: 14647–14656. 10.1074/jbc.M213220200 [DOI] [PubMed] [Google Scholar]
- Penheiter K. L., Washburn T. M., Porter S. E., Hoffman M. G., Jaehning J. A., 2005. A posttranscriptional role for the yeast Paf1-RNA polymerase II complex is revealed by identification of primary targets. Mol. Cell 20: 213–223. 10.1016/j.molcel.2005.08.023 [DOI] [PubMed] [Google Scholar]
- Perocchi F., Xu Z., Clauder-Münster S., Steinmetz L. M., 2007. Antisense artifacts in transcriptome microarray experiments are resolved by actinomycin D. Nucleic Acids Res. 35: e128 10.1093/nar/gkm683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M. W., 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29: e45 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piro A. S., Mayekar M. K., Warner M. H., Davis C. P., Arndt K. M., 2012. Small region of Rtf1 protein can substitute for complete Paf1 complex in facilitating global histone H2B ubiquitylation in yeast. Proc. Natl. Acad. Sci. USA 109: 10837–10842. 10.1073/pnas.1116994109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrua O., Libri D., 2015. Transcription termination and the control of the transcriptome: why, where and how to stop. Nat. Rev. Mol. Cell Biol. 16: 190–202. 10.1038/nrm3943 [DOI] [PubMed] [Google Scholar]
- Porter S. E., Penheiter K. L., Jaehning J. A., 2005. Separation of the Saccharomyces cerevisiae Paf1 complex from RNA polymerase II results in changes in its subnuclear localization. Eukaryot. Cell 4: 209–220. 10.1128/EC.4.1.209-220.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig S., Askeland E., Thiele D. J., 2005. Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell 120: 99–110. 10.1016/j.cell.2004.11.032 [DOI] [PubMed] [Google Scholar]
- Qiu H., Hu C., Wong C.-M., Hinnebusch A. G., 2006. The Spt4p subunit of yeast DSIF stimulates association of the Paf1 complex with elongating RNA polymerase II. Mol. Cell. Biol. 26: 3135–3148. 10.1128/MCB.26.8.3135-3148.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H., Hu C., Gaur N. A., Hinnebusch A. G., 2012. Pol II CTD kinases Bur1 and Kin28 promote Spt5 CTR-independent recruitment of Paf1 complex. EMBO J. 31: 3494–3505. 10.1038/emboj.2012.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan A. R., 2014. BEDTools: the swiss-army tool for genome feature analysis. Curr. Protoc. Bioinformatics 47: 11.12.1–11.12.34. 10.1002/0471250953.bi1112s47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan A. R., Hall I. M., 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26: 841–842. 10.1093/bioinformatics/btq033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez F., Dündar F., Diehl S., Grüning B. A., Manke T., 2014. DeepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 42: W187–W191. 10.1093/nar/gku365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez F., Ryan D. P., Grüning B., Bhardwaj V., Kilpert F., et al. , 2016. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44: W160–W165. 10.1093/nar/gkw257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raupach E. A., Martens J. A., Arndt K. M., 2016. Evidence for regulation of ECM3 expression by methylation of histone H3 lysine 4 and intergenic transcription in Saccharomyces cerevisiae. G3 (Bethesda) 6: 2971–2981. 10.1534/g3.116.033118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team , 2016. R: A Language and Environment for Statistical Computing. R Found. Stat. Comput, Vienna. [Google Scholar]
- Ritchie M. E., Phipson B., Wu D., Hu Y., Law C. W., et al. , 2015. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43: e47 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Winston F., Heiter P., 1991. Methods in yeast genetics: a laboratory course manual. Biochem. Educ. 19: 101–102. 10.1016/0307-4412(91)90039-B [DOI] [Google Scholar]
- Rozenblatt-Rosen O., Nagaike T., Francis J. M., Kaneko S., Glatt K. A., et al. , 2009. The tumor suppressor Cdc73 functionally associates with CPSF and CstF 3′ mRNA processing factors. Proc. Natl. Acad. Sci. USA 106: 755–760. 10.1073/pnas.0812023106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford J. C., Bird A. J., 2004. Metal-responsive transcription factors that regulate iron, zinc, and copper homeostasis in eukaryotic cells. Eukaryot. Cell 3: 1–13. 10.1128/EC.3.1.1-13.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldi, T., N. Fong, and D. L. Bentley, 2018 Transcription elongation rate affects nascent histone pre-mRNA folding and 3′ end processing. Genes Dev. 32 297–308 (erratum Genes Dev. 32 592). https//.org/10.1101/gad.310896.117.min 10.1101/gad.310896.117.min [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M., Jensen T. H., 2008. The exosome: a multipurpose RNA-decay machine. Trends Biochem. Sci. 33: 501–510. 10.1016/j.tibs.2008.07.003 [DOI] [PubMed] [Google Scholar]
- Schulz D., Schwalb B., Kiesel A., Baejen C., Torkler P., et al. , 2013. Transcriptome surveillance by selective termination of noncoding RNA synthesis. Cell 155: 1075–1087. 10.1016/j.cell.2013.10.024 [DOI] [PubMed] [Google Scholar]
- Sheldon K. E., Mauger D. M., Arndt K. M., 2005. A requirement for the Saccharomyces cerevisiae Paf1 complex in snoRNA 3′ end formation. Mol. Cell 20: 225–236. 10.1016/j.molcel.2005.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Finkelstein A., Wolf A. J., Wade P. A., Burton Z. F., et al. , 1996. Paf1p, an RNA polymerase II-associated factor in Saccharomyces cerevisiae, may have both positive and negative roles in transcription. Mol. Cell. Biol. 16: 669–676. 10.1128/MCB.16.2.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolle M., Workman J. L., 2013. Transcription-associated histone modifications and cryptic transcription. Biochim. Biophys. Acta. Gene Regul. Mech. 1829: 84–97. 10.1016/j.bbagrm.2012.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z.-W., Allis C. D., 2002. Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature 418: 104–108. 10.1038/nature00883 [DOI] [PubMed] [Google Scholar]
- Terzi N., Churchman L. S., Vasiljeva L., Weissman J., Buratowski S., 2011. H3K4 trimethylation by Set1 promotes efficient termination by the Nrd1-Nab3-Sen1 pathway. Mol. Cell. Biol. 31: 3569–3583. 10.1128/MCB.05590-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut M., Kisseleva-Romanova E., Rougemaille M., Boulay J., Libri D., 2006. Transcription termination and nuclear degradation of cryptic unstable transcripts: a role for the Nrd1-Nab3 pathway in genome surveillance. Mol. Cell 23: 853–864. 10.1016/j.molcel.2006.07.029 [DOI] [PubMed] [Google Scholar]
- Thorvaldsdottir H., Robinson J. T., Mesirov J. P., 2013. Integrative genomics viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14: 178–192. 10.1093/bib/bbs017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toesca I., Nery C. R., Fernandez C. F., Sayani S., Chanfreau G. F., 2011. Cryptic transcription mediates repression of subtelomeric metal homeostasis genes. PLoS Genet. 7: e1002163 10.1371/journal.pgen.1002163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomson B. N., Arndt K. M., 2013. The many roles of the conserved eukaryotic Paf1 complex in regulating transcription, histone modifications, and disease states. Biochim. Biophys. Acta 1829: 116–126. 10.1016/j.bbagrm.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomson B. N., Davis C. P., Warner M. H., Arndt K. M., 2011. Identification of a role for histone H2B ubiquitylation in noncoding RNA 3′-end formation through mutational analysis of Rtf1 in Saccharomyces cerevisiae. Genetics 188: 273–289. 10.1534/genetics.111.128645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomson B. N., Crisucci E. M., Heisler L. E., Gebbia M., Nislow C., et al. , 2013. Effects of the Paf1 complex and histone modifications on snoRNA 3′-end formation reveal broad and locus-specific regulation. Mol. Cell. Biol. 33: 170–182. 10.1128/MCB.01233-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanácová Š., Wolf J., Martin G., Blank D., Dettwiler S., et al. , 2005. A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS Biol. 3: e189 10.1371/journal.pbio.0030189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bakel H., Tsui K., Gebbia M., Mnaimneh S., Hughes T. R., et al. , 2013. A compendium of nucleosome and transcript profiles reveals determinants of chromatin architecture and transcription. PLoS Genet. 9: e1003479 10.1371/journal.pgen.1003479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk E. L., Chen C. L., D’Aubenton-Carafa Y., Gourvennec S., Kwapisz M., et al. , 2011. XUTs are a class of Xrn1-sensitive antisense regulatory non-coding RNA in yeast. Nature 475: 114–117. 10.1038/nature10118 [DOI] [PubMed] [Google Scholar]
- Van Oss S. B., Shirra M. K., Bataille A. R., Wier A. D., Yen K., et al. , 2016. The histone modification domain of Paf1 complex subunit Rtf1 directly stimulates H2B ubiquitylation through an interaction with Rad6. Mol. Cell 64: 815–825. 10.1016/j.molcel.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oss S. B., Cucinotta C. E., Arndt K. M., 2017. Emerging insights into the roles of the Paf1 complex in gene regulation. Trends Biochem. Sci. 42: 788–798. 10.1016/j.tibs.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh S., Smolle M., Li H., Gogol M. M., Saint M., et al. , 2012. Set2 methylation of histone H3 lysine 36 suppresses histone exchange on transcribed genes. Nature 489: 452–455. 10.1038/nature11326 [DOI] [PubMed] [Google Scholar]
- Venkatesh S., Li H., Gogol M. M., Workman J. L., 2016. Selective suppression of antisense transcription by Set2-mediated H3K36 methylation. Nat. Commun. 7: 13610 10.1038/ncomms13610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos S. M., Farnung L., Boehning M., Wigge C., Linden A., et al. , 2018. Structure of activated transcription complex Pol II–DSIF–PAF–SPT6. Nature 560: 607–612. 10.1038/s41586-018-0440-4 [DOI] [PubMed] [Google Scholar]
- Weiner A., Hsieh T. H. S., Appleboim A., Chen H. V., Rahat A., et al. , 2015. High-resolution chromatin dynamics during a yeast stress response. Mol. Cell 58: 371–386. 10.1016/j.molcel.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier A. D., Mayekar M. K., Heroux A., Arndt K. M., VanDemark A. P., 2013. Structural basis for Spt5-mediated recruitment of the Paf1 complex to chromatin. Proc. Natl. Acad. Sci. USA 110: 17290–17295. 10.1073/pnas.1314754110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston F., Dollard C., Ricupero-Hovasse S. L., 1995. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11: 53–55. 10.1002/yea.320110107 [DOI] [PubMed] [Google Scholar]
- Wyers F., Rougemaille M., Badis G., Rousselle J. C., Dufour M. E., et al. , 2005. Cryptic Pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 121: 725–737. 10.1016/j.cell.2005.04.030 [DOI] [PubMed] [Google Scholar]
- Xu Y., Bernecky C., Lee C., Maier K. C., Schwalb B., et al. , 2017. Architecture of the RNA polymerase II-Paf1C-TFIIS transcription elongation complex. Nat. Commun. 8: 15741 10.1038/ncomms15741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Wei W., Gagneur J., Perocchi F., Clauder-Münster S., et al. , 2009. Bidirectional promoters generate pervasive transcription in yeast. Nature 457: 1033–1037. 10.1038/nature07728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Iwai Y., Ueta R., Fukunaka A., Sasaki R., 2002. Subcellular localization of Aft1 transcription factor responds to iron status in Saccharomyces cerevisiae. J. Biol. Chem. 277: 18914–18918. 10.1074/jbc.M200949200 [DOI] [PubMed] [Google Scholar]
- Yang Y., Li W., Hoque M., Hou L., Shen S., et al. , 2016. PAF complex plays ovel subunit-specific roles in alternative cleavage and polyadenylation. PLoS Genet. 12: 1–28. 10.1371/journal.pgen.1005794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassour M., Pfiffner J., Levin J. Z., Adiconis X., Gnirke A., et al. , 2010. Strand-specific RNA sequencing reveals extensive regulated long antisense transcripts that are conserved across yeast species. Genome Biol. 11: R87 10.1186/gb-2010-11-8-r87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Yang W., Ni T., Tang Z., Nakadai T., et al. , 2015. RNA polymerase II-associated factor 1 regulates the release and phosphorylation of paused RNA polymerase II. Science 350: 1383–1386. 10.1126/science.aad2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., O’Shea E. K., 2011. Integrated approaches reveal determinants of genome-wide binding and function of the transcription factor Pho4. Mol. Cell 42: 826–836. 10.1016/j.molcel.2011.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains are available upon request. Tiling array data (raw CEL files, BedGraph files, annotation-guided differential expression results, and files containing annotations for differentially expressed transcripts defined by our de novo analysis in BED6 file format) have been deposited in the Gene Expression Omnibus database under accession number GSE122704. Code used for analysis of tiling array data has been uploaded to the following GitHub repository (https://github.com/mae92/Paf1C-Transcriptome-Analysis-Code). Tables S1–S6 and Figures S1–S7 are available via FigShare. Supplemental material available at FigShare: https://doi.org/10.25386/genetics.7796837.