Abstract
Background
Myocardial dysfunction is frequently described as an underlying cause of mortality in traumatic brain injury (TBI) known as brain-cardiac link. However the impact on prognosis of a disease remains uncertain.
Objectives
The current study aimed at investigating the correlation between TBI and cardiac troponin I (cTnI) rise and in-hospital mortality rate among patients with TBI.
Methods
In the current prospective study TBI patients with abbreviated injury scale score (AIS) > 3 and Glasgow coma scale (GCS) score ≤ 8 with cTnI measurement within the first 24 hours of admission were evaluated. Chi-square, Kruskal-Wallis, Mann-Whitney U and Logistic Regression tests were used for data analysis.
Results
A total of 166 eligible patients were studied .The mean age of the cases was 37.64 ± 17.21 years, largely under 65 (93.4%) and male (86.7%).The most common injuries were cerebral contusion (35.1%), while motor vehicle crash (MVC) was the most common cause of injuries (83.73%); 59 % of the patients showed detectable cTnI concentrations within 24 hours of admission; 65.7% of the patients expired; they showed higher levels of cTnI compared to survivors that showed lower levels, 0.148 ± 0.074 vs 0.057 ± 0.055, respectively (P < 0.001). Moreover, a significant association was observed between mortality rate and lower admission GCS 3.49 ± 1.08 vs 6.79 ± 1.66, respectively (P < 0.001).
Conclusions
Increased cTnI levels could be a predictor of mortality among patients with TBI. Its measurement and investigation for therapeutic strategies could lead to better management of these cases.
Keywords: Cardiac Troponin I, Traumatic Brain Injury, Mortality
1. Background
Traumatic brain injury (TBI) as a major health and socioeconomic concern, remains one of the main causes of mortality and morbidity among the cases with trauma worldwidethroughout the world (1-4). The incidence of TBI is rising especially in the developing countries with a significant financial annual burden due to more vehicle use. But in the developed countries, the traffic laws enforcement is associated with a lower rate of TBI incidence related to accidents. However the incidence of TBI following fall is increasing in these countries, due to the large old population (5). The prognosis of patients with TBI is dependent on two parts, first the damage at the time of initial trauma, and second one occurring during the following days (6, 7). Autonomic dysfunction is potentially involved in the secondary damage due to excessive adrenergic activity. This phenomenon leads to heart rate, blood pressure, and thermoregulation abnormalities that may result in myocardial dysfunction (8-10).
The association between central nervous system and cardiac damage is well described in the context of brain death, subarachnoid hemorrhage (SAH), Guillain Barre Syndrome, and seizure (11-19). Although the main etiology and pathophysiology are not well defined, several mechanisms are suggested including catecholamine release (20, 21), micro vascular dysfunction, and multi-vessel coronary artery spasm .However brain-cardiac interaction appears to be best described by catecholamine toxicity following sympathetic hyperactivity (22-24). In these cases, cardiac dysfunction may vary from quite asymptomatic conditions to severe myocardial dysfunction (25, 26). Publications frequently describe patients with TBI presenting sympatric discharge signs, including hypertension, tachycardia hyperthermia, and agitation (27). The association between the mentioned conditions and cardiac dysfunction created the concern that a similar relationship could be expected in patients with TBI. Although, established data supporting this correlation in some neurologic conditions are not scarce, available literature in the context of TBI and cardiac damage and its prognostic role is limited. Indeed, few studies show that troponin (cTnI) rise after TBI might be correlated with adverse prognosis. Furthermore, studies show that females with lower cardiac troponin concentrations are at a higher risk of cardiac damage compared to males (28).
To date, the vast majority of studies focus on evaluating the role of cTnI in coronary disease, thoracic trauma, and non TBI (29-31). Decavele et al. demonstrated the significance of cTnI elevation in prognostication of patients with thoracic trauma (32). Despite the advancement of knowledge regarding this association, sympathetic storm following TBI as the underlying cause of mortality and morbidity in such patients remains not well recognized and undertreated (27). Indeed, in setting the TBI, myocardial dysfunction results in additional complications for the patients, therefore, it should be considered and managed perfectly.
2. Objectives
The present study aimed at investigating the association between serum cTnI levels as a biochemical marker of myocardial damage and mortality of the patients with TBI.
3. Methods
The current prospective study was approved by Guilan University of Medical Sciencesresearch ethics committee and conducted at Poorsina Hospital, a referral academic center affiliated to Guilan University of Medical Sciences. During 2017, patients above 18, diagnosed as TBI by computed tomography (CT) or magnetic resonance imaging (MRI), GCS ≤ 8, AIS > 3, and cTnI measurement performed during the first 24 hours of admission were enrolled in the study. All cases underwent standard monitoring including invasive and noninvasive blood pressure monitoring, SaO2, heart rate, and in case of intubation , the end tidal CO2. According to new guidelines, by the employment of proper hydration and vasopressor, systolic blood pressure (SBP) < 90mmHg was avoided. SBP ≥ 100mmHg was maintained for patients 50 to 69 years old and for patients 15 to 49 or above 70 years old SBP was kept ≥ 110mm Hg (3). Patients’ variables including gender, age, mechanism of trauma, GCS, cTnI levels, AIS, hospital and intensive unit care (ICU) length of stay and mortality rate were recorded. Troponin values were categorized as undetectable (< 0.06 ng/mL), and two levels of (0.06 - < 021 ng/mL) and severely elevated (> 0.21 ng/mL). The correlation between cTnI levels and mortality was investigated. To measure cTnI concentrations the enzyme-linked immune-sorbent sandwich assay (ELISA) (BioTek-ELX800) was employed. The severity of injury was evaluated by GCS and AIS. In order to analyze data, SPSS version 16 and chi-square, Kruskal-Wallis, Mann-Whitney U and logistic regression tests, were employed.
4. Results
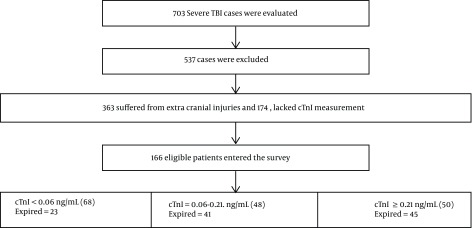
A total of 703 cases with severe TBI (AIS > 3 and GCS ≤ 8) were admitted during the study period; 363 of them that had extra cranial injuries and 174 that lacked cTnI measurement during the first 24 hours were excluded. Finally a total of 166 eligible patients were selected for the study. Admission characteristics of the patients are presented in Table 1. The mean age of the patients was 37.64 ± 17.21 years, largely under 65 (93.4%) and male (86.7%). The most common injuries were cerebral contusion (35.1%), followed by subdural hematoma (29.51%), epidural hematoma (27.1%), subarachnoid hemorrhage (25.3%) while motor vehicle crash (MVC) was the most common cause of injuries (83.73%). History of cardiac diseases was relatively uncommon at 9.3%; 33.7% of the study population had surgical intervention. Detectable cTnI was observed in 59% of the patients within 24 hours of admission. 65.7% of patients expired; they showed higher levels of cTnI compared to survivors 0.148 ± 0.074 vs 0.057 ± 0.055, respectively (P < 0.001). Moreover a significant association was observed between mortality rate and lower GCS at admission 3.49 ± 1.08 vs 6.79 ± 1.66, respectively (P < 0.001). On the whole mortality was significantly associated with age above 65 years, injury type, male gender, higher AIS, lower admission GCS, and reduced hospital stay (P < 0.05) (Table 2 ). The flow diagram of the patients is presented in Figure 1.
Table 1. Selected Characteristics of the Study Populationa.
| Characteristics | Total | Survived | Expired | P Value |
|---|---|---|---|---|
| Age, y | 37.64 ± 17.21 | 57 (32.5) | 109 (67.5) | < 0.001 |
| ≤ 65 | 155 (93.4 ) | 45 (29) | 110 (71) | |
| > 65 | 11 (6.6) | 2 (18.2) | 9 (81.8) | < 0.001 |
| Gender | 166 | 54 (35.4) | 112 (67.5) | |
| Male | 144 (86.7) | 51 (35.4) | 93 (64.6) | |
| Female | 22 (13.3 ) | 6 (27.3) | 16 (72.7) | < 0.001 |
| Causes of trauma | 0.055 | |||
| Motor vehicle crash (MVC) | 139 (83.73) | 46 (33.09) | 98 (70.50) | |
| Fall | 20 (12.04) | 9 (45) | 11 (55) | |
| Other | 7 (4.21) | 4 (57.14) | 3 (42.85) | |
| Admission GCS score | 5.75 ± 1.44 | 6.79 ± 1.66 | 3.49 ± 1.08 | < 0.001 |
| 3 - 5 | 72 (43. 4) | 10 (13.91) | 62 (86.18) | |
| 6 - 8 | 94 (56.6) | 46 (48.93) | 48 (51.06) | |
| AIS | 3.71 ± 1.41 | 3.22 ± 0.56 | 4.34 ± 1.12 | < 0.001 |
| Types of injuries | ||||
| Subdural hematoma | 49 (29.51) | 11 (22.4) | 38 (77.6) | 0.037 |
| Subarachnoid hemorrhage | 30 (25.3) | 10 (33.3) | 20 (66.7) | 0.89 |
| Epidural hematoma | 45 (27.10) | 19 (42.2) | 26 (57.8) | 0.192 |
| Cerebral contusion | 97 (58.43) | 41 (42.3) | 56 (57.7) | 0.011 |
| Pneumocephalus | 13 (7.83) | 6 (46.2) | 7 (53.8) | 0.35 |
| Skull fracture | 42 (25.30) | 12 (24.95) | 30 (75.05) | 0.710 |
| Length of ICU stay, d | 4.8 ± 7.7 | 7.61 ± 12.44 | 3.33 ± 2.13 | 0.883 |
| Length of hospital stay, d | 7.03 ± 8.85 | 12.85 ± 12.8 (3.2, 61) | 3.99 ± 2.91 (0.5, 4.1) | < 0.001 |
| Cardiac troponin I | 0.057 ± 0.055 | 0.148 ± 0.074 | < 0.001 | |
| Normal (< 0.06 ng/mL) | 79 (47.59) | 39 (73.41) | 40 (13) | |
| Elevated (≥ 0.06 ng/mL) | 87 (52.40) | 16 (18.39) | 71 (81.60) | |
| Surgical intervention | 56 (33.7) | 21 (37.5) | 35 (62.5) | 0.54 |
| Preexisting cardiac diseases | 15 (9.3) | 7 (46.66) | 8 (53.33) | 0.912 |
aValues are expressed as mean ± SD or No. (%).
Table 2. Characteristics of the Study Population by cTnI Levels Cardiac Troponin I (cTnI)a.
| Characteristics | < 0.06 ng/mL (68) | 0.06 - 0.21 ng/mL (48) | ≥ 0.21 ng/mL (50) | P Value |
|---|---|---|---|---|
| GCS | < 0.001 | |||
| 3 - 5 | 3 (4.2) | 24 (33.3) | 45 (62.5) | |
| 6 - 8 | 65 (69.1) | 24 (25.5) | 5 (5.3) | |
| AIS | 3.22 ± 0.54 | 4.06 ± 0.91 | 4.88 ± 1.13 | < 0.001 |
| Length of ICU stay, d | 5.14 ± 9.17 | 6.6 ± 8.5 | 2.6 ± 2.84 | < 0.001 |
| Length of hospital stay, d | 8.24 ± 9.41 | 10.57 ± 1.152 | 3.35 ± 3.89 | 0.742 |
| Preexisting cardiac diseases | 9 (11.39) | 4 (10.25) | 6 (12.5) | 0.767 |
| Mortality | 23 (33.8) | 41 (85.4) | 45 (90) | < 0.001 |
aValues are expressed as mean ± SD or No. (%).
Figure 1. Consort diagram of the patients.
5. Discussion
The current study aimed to investigate the correlation between TBI and cTnI rise and also its significant prognostic role. It was hypothesized that a positive correlation existed between cardiac damage and mortality rate in patients with TBI.
Cardiac troponin I, which is a regulatory protein and a biomarker for myocardial injury also rises in several non-cardiac conditions including sepsis, pulmonary embolism, chronic renal failure, and non-traumatic brain injury (29, 32, 33). It is supposed that the rise of circulating catechol amines, after brain damage, results in the elevation of cTnI levels. Studies revealed that in patients with TBI, hypothalamic- adrenal is activated resulting in increased systemic catechol amines with clinical manifestation of tachycardia hypertension, diaphoresis, mydriasis, and tachypnea. The occurrence of excessive sympathetic release after TBI is completely established and in animal and human studies both plasma and urine concentrations of catecholamine are increased after TBI (27, 34). Therefore, it seems that similar to previous studies indicating the cardiac- brain interaction due to excessive sympathetic discharge following brain damage, the same association can be described among patients with TBI. Heffernan et al. reported that patients with TBI benefit from beta adrenergic blocker therapy, which emphasizes the significant negative role of sympathetic hyper activity among these cases (27).
Indeed, after a severe TBI, a massive sympathetic release causes peripheral vascular resistance, and potentially left ventricle de-compensation occurs (18, 19). Clinically, this phenomena is known as neurogenic stunned myocardium, which is presented with reversible left ventricular systolic dysfunction, cardiogenic shock, and pulmonary edema (8, 35). Lee et al. performed serial measurements of epinephrine and norepinephrine (NE) plasma levels in cases with TBI and reported a threefold increase in NE concentrations in them. This condition lasted for ten days but took a period of six months to reach normal levels (20). Surprisingly, among the survived patients, the normalization of GCS was parallel to their NE serum levels dropping to normal statue. Furthermore, they reported that in patients with no improvement, plasma NE levels raised up to seven times higher than as high as normal. Plasma NE concentrations within the first 48 hours after injury predicted GCS at the first week, survival rate, and ventilator days (21, 27). This study supported the prognostic value and the merit of cTnI data in patients with TBI. Salim et al. investigated the association between elevated cTnI and adverse outcomes in patients with TBI. They studied 420 cases with severe TBI and reported that cTnI serum levels were correlated with the severity of injury, and could be an independent predictor of poor prognosis among such cases. In this regard, they found that TBI with elevated cTnI benefited from beta blocker administration (29). In line with the current study results, Stephen S et al. in a retrospective study, reviewed the patients above 18 with isolated TBI. They found that patients with higher cTnI levels had significantly higher risk of mortality compared to the ones with undetectable cTnI. They introduced cTnI as a biomedical marker for hospital mortality in patients with TBI (36). In contrast to this work, Serri et al. in a prospective observational study, reported no significant association between TBI and cardiac dysfunction. But the differences between the two studies should be considered. In their study, patients with underlying cardiac disease were excluded. The lower mean age of their cases and higher median GCS scores should be considered as well. In summary, it seems that their studied patients were younger and healthier. Furthermore, the current study criterion to evaluate cardiac statue was different, since the current study measured cTnI, whereas they did left ventricle ejection fraction. Of course they declared that their results might be due to young age, and no cardiovascular risk factors of patients (1). In spite of available confirming studies demonstrating the neuro-cardiac axis due to sympathetic release, underlying cardiac disease should be considered. Furthermore, it should be noted that in TBI victims with fever, tachycardia, tachypnea, and hypertension, in addition to sympathetic storm, other diagnosis including sepsis, not controlled pain and pulmonary emboli should be ruled out. Other conditions such as malignant hyperthermia, thyroid storm and pheochromocytoma crisis can also mimic this clinical feature (26, 27). According to the results of the current study, routine measurement of cTnI levels in patients with TBI at admission time and during the first 24 hours should be part of the hospital policy. Then TBI cases with raised cTnI levels would be managed with special cardiac care.
5.1. Suggestion
Future well planned multicenter surveys with larger sample sizes are warranted to confirm these findings.
5.2. Limitations
Plasma troponin levels were measured within the first 24 hours, but duration of cTnI elevation was not well known. In addition, the current study judgment was just based on cTnI levels, while electrocardiography, and echocardiography or other diagnostic methods might provide more information.
5.3. Conclusions
In patients with TBI, cTnI levels were elevated and associated with the severity of the damage and higher mortality rate. Well planned multicenter prospective studies are welcomed to answer several questions arising from this issue and optimization management of such patients.
Footnotes
Authors' Contribution: All authors were involved in preparing this manuscript. They read and approved it.
Conflict of Interests: The authors declared no conflict of interests.
Ethical Approval: The current prospective study was approved by Guilan University of Medical Sciences University Research Ethics Committee.
Funding/Support: Anesthesiology Research Center provided funding for this research.
Patient Consent: It is not declared by the authors.
Contributor Information
Siamak Rimaz, Email: smkrimaz@yahoo.com.
Ali Ashraf, Email: draliashraf@yahoo.com.
Shideh Marzban, Email: shideh_42@yahoo.com.
Mohammad Haghighi, Email: manesthesist@gmail.com.
Seyyed Mahdi Zia Ziabari, Email: smzz102186@gmail.com.
Gelareh Biazar, Email: gelarehbiazar1386@gmail.com.
Sheyda Rimaz, Email: rimaz_sh@ymail.com.
Samad Omidi, Email: samad.omidi84@yahoo.com.
References
- 1.Serri K, El Rayes M, Giraldeau G, Williamson D, Bernard F. Traumatic brain injury is not associated with significant myocardial dysfunction: An observational pilot study. Scand J Trauma Resusc Emerg Med. 2016;24(1) doi: 10.1186/s13049-016-0217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marik PE, Varon J, Trask T. Management of head trauma. Chest. 2002;122(2):699–711. doi: 10.1378/chest.122.2.699. [DOI] [PubMed] [Google Scholar]
- 3.Picetti E, Iaccarino C, Servadei F. Letter: Guidelines for the management of severe traumatic brain injury fourth edition. Neurosurgery. 2017;81(1):E2. doi: 10.1093/neuros/nyx086. [DOI] [PubMed] [Google Scholar]
- 4.Hosseini SH, Ayyasi M, Akbari H, Heidari Gorji MA. Comparison of glasgow coma scale, full outline of unresponsiveness and acute physiology and chronic health evaluation in prediction of mortality rate among patients with traumatic brain injury admitted to intensive care unit. Anesth Pain Med. 2017;7(5):e33653. doi: 10.5812/aapm.33653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM, Faul M, et al. Surveillance for traumatic brain injury-related deaths; United States, 1997-2007. Centers for Disease Control and Prevention; 2011. [PubMed] [Google Scholar]
- 6.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7(8):728–41. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 7.Skolnick BE, Maas AI, Narayan RK, van der Hoop RG, MacAllister T, Ward JD, et al. A clinical trial of progesterone for severe traumatic brain injury. N Engl J Med. 2014;371(26):2467–76. doi: 10.1056/NEJMoa1411090. [DOI] [PubMed] [Google Scholar]
- 8.Hinson HE, Sheth KN. Manifestations of the hyperadrenergic state after acute brain injury. Curr Opin Crit Care. 2012;18(2):139–45. doi: 10.1097/MCC.0b013e3283513290. [DOI] [PubMed] [Google Scholar]
- 9.Kothavale A, Banki NM, Kopelnik A, Yarlagadda S, Lawton MT, Ko N, et al. Predictors of left ventricular regional wall motion abnormalities after subarachnoid hemorrhage. Neurocrit Care. 2006;4(3):199–205. doi: 10.1385/NCC:4:3:199. [DOI] [PubMed] [Google Scholar]
- 10.Urbaniak K, Merchant AI, Amin-Hanjani S, Roitberg B. Cardiac complications after aneurysmal subarachnoid hemorrhage. Surg Neurol. 2007;67(1):21–8. doi: 10.1016/j.surneu.2006.08.065. discussion 28-9. [DOI] [PubMed] [Google Scholar]
- 11.Naidech AM, Kreiter KT, Janjua N, Ostapkovich ND, Parra A, Commichau C, et al. Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation. 2005;112(18):2851–6. doi: 10.1161/CIRCULATIONAHA.105.533620. [DOI] [PubMed] [Google Scholar]
- 12.Provencio JJ. Subarachnoid hemorrhage: A model for heart-brain interactions. Clev Clin J Med. 2007;74(Suppl_1):S86. doi: 10.3949/ccjm.74.Suppl_1.S86. [DOI] [PubMed] [Google Scholar]
- 13.Martorano PP, Bini G, Tanara L, Sinkovets L, Pelaia P, Pietropaoli P. [Subarachnoid hemorrhage and the heart.]. Minerva Anestesiol. 1998;64(5):231–3. (Ita). [PubMed] [Google Scholar]
- 14.Jeon IC, Chang CH, Choi BY, Kim MS, Kim SW, Kim SH. Cardiac troponin I elevation in patients with aneurysmal subarachnoid hemorrhage. J Korean Neurosurg Soc. 2009;46(2):99–102. doi: 10.3340/jkns.2009.46.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hravnak M, Frangiskakis JM, Crago EA, Chang Y, Tanabe M, Gorcsan J3, et al. Elevated cardiac troponin I and relationship to persistence of electrocardiographic and echocardiographic abnormalities after aneurysmal subarachnoid hemorrhage. Stroke. 2009;40(11):3478–84. doi: 10.1161/STROKEAHA.109.556753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inamasu J, Ito K, Sugimoto K, Watanabe E, Kato Y, Hirose Y. Cardiac wall motion abnormality associated with spontaneous intracerebral hemorrhage. Int J Cardiol. 2013;168(2):1667–9. doi: 10.1016/j.ijcard.2013.03.096. [DOI] [PubMed] [Google Scholar]
- 17.Sieweke N, Allendorfer J, Franzen W, Feustel A, Reichenberger F, Pabst W, et al. Cardiac troponin I elevation after epileptic seizure. BMC Neurol. 2012;12:58. doi: 10.1186/1471-2377-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernstein R, Mayer SA, Magnano A. Neurogenic stunned myocardium in Guillain-Barre syndrome. Neurology. 2000;54(3):759–62. doi: 10.1212/WNL.54.3.759. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen H, Zaroff JG. Neurogenic stunned myocardium. Curr Neurol Neurosci Rep. 2009;9(6):486–91. doi: 10.1007/s11910-009-0071-0. [DOI] [PubMed] [Google Scholar]
- 20.Lee VH, Oh JK, Mulvagh SL, Wijdicks EF. Mechanisms in neurogenic stress cardiomyopathy after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2006;5(3):243–9. doi: 10.1385/NCC:5:3:243. [DOI] [PubMed] [Google Scholar]
- 21.Naredi S, Lambert G, Eden E, Zall S, Runnerstam M, Rydenhag B, et al. Increased sympathetic nervous activity in patients with nontraumatic subarachnoid hemorrhage. Stroke. 2000;31(4):901–6. doi: 10.1161/01.STR.31.4.901. [DOI] [PubMed] [Google Scholar]
- 22.De'Ath HD, Rourke C, Davenport R, Manson J, Renfrew I, Uppal R, et al. Clinical and biomarker profile of trauma-induced secondary cardiac injury. Br J Surg. 2012;99(6):789–97. doi: 10.1002/bjs.8728. [DOI] [PubMed] [Google Scholar]
- 23.El-Menyar A, Goyal A, Latifi R, Al-Thani H, Frishman W. Brain-heart interactions in traumatic brain injury. Cardiol Rev. 2017;25(6):279–88. doi: 10.1097/CRD.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 24.Huttemann E, Schelenz C, Chatzinikolaou K, Reinhart K. Left ventricular dysfunction in lethal severe brain injury: Impact of transesophageal echocardiography on patient management. Intensive Care Med. 2002;28(8):1084–8. doi: 10.1007/s00134-002-1355-x. [DOI] [PubMed] [Google Scholar]
- 25.Borbely XI, Krishnamoorthy V, Modi S, Rowhani-Rahbar A, Gibbons E, Souter MJ, et al. Temporal changes in left ventricular systolic function and use of echocardiography in adult heart donors. Neurocrit Care. 2015;23(1):66–71. doi: 10.1007/s12028-014-0101-x. [DOI] [PubMed] [Google Scholar]
- 26.Prathep S, Sharma D, Hallman M, Joffe A, Krishnamoorthy V, Mackensen GB, et al. Preliminary report on cardiac dysfunction after isolated traumatic brain injury. Crit Care Med. 2014;42(1):142–7. doi: 10.1097/CCM.0b013e318298a890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heffernan DS, Inaba K, Arbabi S, Cotton BA. Sympathetic hyperactivity after traumatic brain injury and the role of beta-blocker therapy. J Trauma. 2010;69(6):1602–9. doi: 10.1097/TA.0b013e3181f2d3e8. [DOI] [PubMed] [Google Scholar]
- 28.Shah ASV, Ferry AV, Mills NL. Cardiac biomarkers and the diagnosis of myocardial infarction in women. Curr Cardiol Rep. 2017;19(5):40. doi: 10.1007/s11886-017-0839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salim A, Hadjizacharia P, Brown C, Inaba K, Teixeira PG, Chan L, et al. Significance of troponin elevation after severe traumatic brain injury. J Trauma. 2008;64(1):46–52. doi: 10.1097/TA.0b013e31815eb15a. [DOI] [PubMed] [Google Scholar]
- 30.Mortazavi MMT, Ganjpour Sales J, Nouri-Vaskeh M, Parish M, Abdolhosseynzadeh S. Perioperative cardiac troponin i levels in patients undergoing total hip and total knee arthroplasty: A single center study. Anesth Pain Med. 2018;8(6):e84228. doi: 10.5812/aapm.84228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Javaherforoosh Zadeh F, Moadeli M, Soltanzadeh M, Janatmakan F. Effect of remote ischemic preconditioning on troponin I in CABG. Anesth Pain Med. 2017;7(4):e12549. doi: 10.5812/aapm.12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Decavele M, Gault N, Gauss T, Pease S, Moyer JD, Paugam-Burtz C, et al. Cardiac troponin I as an early prognosis biomarker after trauma: A retrospective cohort study. Br J Anaesth. 2018;120(6):1158–64. doi: 10.1016/j.bja.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Jeremias A, Gibson CM. Narrative review: Alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med. 2005;142(9):786–91. doi: 10.7326/0003-4819-142-9-200505030-00015. [DOI] [PubMed] [Google Scholar]
- 34.Wilson NM, Wall J, Naganathar V, Brohi K, De'Ath HD. Mechanisms involved in secondary cardiac dysfunction in animal models of trauma and hemorrhagic shock. Shock. 2017;48(4):401–10. doi: 10.1097/SHK.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 35.Salem R, Vallee F, Depret F, Callebert J, Maurice JP, Marty P, et al. Subarachnoid hemorrhage induces an early and reversible cardiac injury associated with catecholamine release: One-week follow-up study. Crit Care. 2014;18(5):558. doi: 10.1186/s13054-014-0558-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai SS, Bonds BW, Hu PF, Stein DM. The role of cardiac troponin I in prognostication of patients with isolated severe traumatic brain injury. J Trauma Acute Care Surg. 2016;80(3):477–83. doi: 10.1097/TA.0000000000000916. [DOI] [PMC free article] [PubMed] [Google Scholar]