Abstract
Autoimmunity is an identified factor for development of end-stage renal disease (ESRD). Regulatory T-cells (Tregs) play a fundamental role in preventing autoimmunity. This study aimed to determine Treg frequency and its effects on cytokine profile of ESRD patients with and without systemic lupus erythematosus (SLE). Moreover, this study also determines how Treg number is affected by blood transfusion and gender. Peripheral blood mononuclear cells were isolated from 26 ESRD and 10 healthy subjects and stained with anti-CD4, anti-CD25, and anti-FoxP3 antibodies. Treg frequencies in ESRD patients with and without blood transfusion were determined by flow cytometry. Antibodies against human leukocyte antigens (HLAs) were investigated by panel-reactive antibodies screening. Tumor growth factor (TGF)-β1, interleukin (IL)-4, IL-10, TNF-α, IL-17A, and interferon (IFN)-γ serum levels in participants were measured by enzyme-linked immunoasorbent assay (ELISA). ESRD patients with SLE, unlike the patients without SLE, showed a significant reduction in Treg percentage compared to healthy subjects (P < 0.01). All women had a reduced number of Tregs compared to men. Treg number was significantly decreased in ESRD patients with HLA antibodies (P < 0.05). Blood transfusion enhanced Treg development in ESRD patients without SLE, unlike the patients with SLE (P < 0.05). ESRD patients with low Treg showed a reduction in TGF-β1 and IL-4 and an increase in TNF-α and IL-17A levels compared to control groups (P < 0.05–0.0001). However, no change was observed in IL-10 and IFN-γ levels. Treg frequency was negatively associated with the age of patients (P < 0.01), while this association was not observed in healthy subjects. Based on these findings, it can be observed that reduction in Treg number may contribute to ESRD development in patients with SLE.
Keywords: blood transfusion, end-stage renal disease, regulatory T-cells, systemic lupus erythematosus
Introduction
End-stage renal disease (ESRD) is the last stage of chronic kidney disease that kidney functions reach below 10% of its normal ability.1 In most cases, ESRD is caused by autoimmune disorders and other health problems including diabetes, high blood pressure, genetic disorders, nephrotic syndrome, and urinary tract problems. Hemodialysis, peritoneal dialysis, and kidney transplantation are the most common treatments for ESRD.2,3
In autoimmune disorder, a condition in which tolerance to self-antigens is defect, autoantibodies against self-antigens bind to circulating antigens and produce immune complexes (antigen–antibody complexes) which may dispose in different tissues, especially blood vessel wall and glomerular basement membrane (GBM).4,5 This process can be subjected to a number of responses, including activation of complement and recruitment of inflammatory cells to the sites of immune complex deposition. Complement activation leads to further cellular infiltration, cytokine secretion, and vasoactive mediator production of endogenous and infiltrating cells, which include T-cells, neutrophils, macrophages, and platelets. In turn, inflammatory mediators affect the function of renal cells and thereby participate in the pathogenesis of renal diseases.4,6,7
Regulatory T-cells (Tregs), a subgroup of CD4+ T-cells, play an indispensable role in peripheral tolerance to self-antigens.8 These cells are characterized by the expression of several markers including cytotoxic T lymphocyte antigen-4 (CTLA-4), glucocorticoid-induced TNF receptor (GITR), CD25, and forkhead family transcription factor (FoxP3).8 Treg participates in downregulating immune responses to a verity of self-antigens and preventing the development of autoimmune diseases.8,9 Previous studies have evidenced that Tregs exert their immunosuppressive functions by the mechanism of cell–cell interaction and secretion of inhibitory cytokines such as interleukin (IL)-10, transforming growth factor-beta1 (TGF-β1), and IL-35.9 Extensive data of the literature have shown that the reduced number and impaired function of Tregs contribute to the pathogenesis of various autoimmune disorders such as rheumatoid arthritis (RA), multiple sclerosis (MS), and systemic lupus erythematosus (SLE).8,10
Given the role of Tregs in the maintenance of immunological self-tolerance, the aim of this study was to investigate whether Treg frequency in peripheral blood of ESRD patients with lupus nephritis differs from ESRD patients with health problems including hypertension, diabetes, Alport syndrome, recurrent respiratory infections, and nephritic syndrome. We also determined how the blood transfusion influences Treg number in ESRD patients. Furthermore, cytokine profile of ESRD patients with low Treg was compared with the patients with normal Treg and healthy subjects.
Materials and methods
Subjects
A total of 26 ESRD patients suffered from lupus nephritis and health problems were recruited among those referred to a transplantation center, Isfahan, Iran from May 2018 to December 2018. ESRD, lupus nephritis, and health problems were diagnosed by specialist according to clinical and laboratory diagnostic criteria (Table 1). All ESRD patients were on dialysis and in the remission phase of lupus nephritis. The blood sampling was carried out at least 48 h after the last dialysis. Patients with SLE reached in ESRD due to lupus nephritis (type VI) and had a score of 4 for SLE disorder. The first manifestations for lupus nephritis in ESRD patients were the presence of red blood cells (RBCs), white blood cells (WBCs), and renal tubular epithelial cell (RTE) casts in urine. ESRD patients with lupus nephritis did not receive any immunosuppressive agents after starting dialysis and at the time of the study. A total of 10 healthy volunteers without any history of health problems and autoimmune abnormalities were also participated as a control group. The study was approved by the Ethics Committee of Isfahan University of Medical Sciences that was in accordance with the Declaration of Helsinki for medical research involving human subjects. The informed consent was obtained from all participants before entering the study. Table 2 shows the demographic and laboratory characteristics of ESRD and healthy subjects.
Table 1.
Diagnosis criteria for ESRD patient selection.
| Clinical criteria | Laboratory criteria |
|---|---|
| Anorexia | Cr > 1.4 |
| Weight loss | BUN > 26 |
| Morning nausea | GFR < 15 |
| Refractory acidosis | Hyper kalmia |
| Neurologic symptoms such as asterixis | Decrease in kidney size in MRI imaging |
| Pruritus | FBS |
| Uremic pericarditis |
ESRD: end-stage renal disease; Cr: creatinine; BUN: blood urea nitrogen; GFR: glomerular filtration rate; MRI: magnetic resonance imaging; FBS: fasting blood sugar.
Table 2.
The demographic and laboratory characteristics of ESRD and healthy subjects.
| Demographic and laboratory parameters | ESRD patients with health problems (n = 14) | ESRD patients with SLE (n = 12) | Healthy subjects (n = 10) |
|---|---|---|---|
| Age, year (range of age) | 41.7 ± 13.5 (18–67) | 48.8 ± 14.4 (29–74) | 44.1 ± 4.6 (30–54) |
| Gender (male/female) | 7/7 | 6/6 | 5/5 |
| Glomerular filtration rate (GFR) | 7.58 ± 0.74 | 7.66 ± 0.79 | 98 ± 4.08 |
| Urea | 120 ± 2.3 | 119 ± 3.7 | 27.25 ± 1.97 |
| Creatinine | 8.01 ± 1.02 | 7.78 ± 1.13 | 0.92 ± 0.059 |
| Blood urea nitrogen (BUN) | 49.49 ± 3.05 | 49.48 ± 2.86 | 12.75 ± 0.92 |
| Fasting blood sugar (FBS) | 106 ± 37 | 93 ± 11 | 88 ± 4 |
ESRD: end-stage renal disease; SLE: systemic lupus erythematosus.
Sample collection and peripheral blood mononuclear cells isolation
Heparinized venous blood samples (5 mL) were collected from ESRD and healthy subjects. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by Ficoll density centrifugation according to the manufacturer’s guideline (Lymphodex, Germany). The isolated cells were washed twice with 0.15 M phosphate-buffered saline (PBS), and the cells were counted using a Bürker Neubauer chamber (haemocytometer, 40443001; Hecht Assistent, Germany). Cell viability was determined by trypan blue dye exclusion.
Flow cytometry
To assess the percentage of circulating Tregs in ESRD patients and healthy subjects, the isolated cells were stained with fluorescein isothiocyanate (FITC) anti-human CD4 and phycoerythrin (PE) anti-human CD25 antibodies (eBiosciences, USA) for 25 min at 4°C. Isotype-matched control antibodies were used as negative controls. Fixation and permeabilization of the cells were performed according to the manufacturer’s guideline (eBiosciences, USA). The cells were then subjected to intracellular staining with PE/cyanine 5 (PE/Cy5) anti-human FoxP3 or isotype-matched control antibodies (eBiosciences, USA) for 30 min at 4°C. The stained cells were washed with PBS and centrifuged at 200×g for 5 min at room temperture. The percentage of the stained cells was measured by a FACSCalibur system (Becton Dickinson, USA). In this regard, lymphocyte population was gated using forward and side scatter in order to exclude debris or non-lymphocyte populations from the analysis of CD4+ cells. Afterwards, the CD4+ cells were gated to determine the percentage of CD25+ FoxP3+ cells. The gating strategy was carried out using FlowJo software (v10.1; FlowJo, USA). In this study, CD4+, CD25+, and FoxP3+ cells were considered as Tregs.
Investigation of circulating reactive antibodies against human leukocyte antigens
To detect human leukocyte antigen (HLA) antibodies in ESRD patients, panel-reactive antibody screening was performed using a complement-dependent cytotoxicity assay. PBMCs (n = 24) obtained from heparinized whole blood of healthy subjects were seeded in 72-well plates at a density of 3 × 103 cells/well. To set the panel-reactive antibody screening up, the serum samples were isolated from whole blood of ESRD patients and incubated at 63°C for 3 min in order to inactivate the complement components. Afterwards, the serum samples (1 µL) were added to the wells containing PBMCs and then incubated at room temperature. After 30-min incubation, 5 µL of rabbit complement (Inno-Train, Germany) was added to each well and incubated for 60 min at room temperature. To fix antibody–antigen complexes, 4 µL of formalin was added to the wells containing the serum samples, PBMCs, and complement components. The cells were then stained with 2 µL of eosin Y (Merck, Germany). Subsequently, cell death and cell viability were measured using an invert microscope (Wilovert, Leitz, USA).
Cytokine assay
To study the possible effect of Tregs on the cytokine profile of ESRD patients, circulating Treg percentage was determined in healthy subjects and ESRD patients with low and normal Treg. The values of Treg in the patients were used as criteria to define the normal and low Treg groups. The patients with the values of Treg less than the median of the healthy subjects were considered as ESRD patients with low Treg, while the patients with the values of Tregs higher than the median of the healthy subjects served as ESRD patients with normal Treg. Afterwards, the serum samples were obtained from whole blood of participants, and the levels of IL-17A, tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), IL-4, TGF-β1, and IL-10 were measured using an enzyme-linked immunoasorbent assay (ELISA) kit (Mabtech, Sweden) according to the manufacturer’s instructions.
Statistical analysis
Data analysis was performed by GraphPad Prism 6 (GraphPad Software, USA). The results are expressed as mean ± standard error of mean (SEM). The groups with normal distribution were compared using one-way analysis of variance (ANOVA) and unpaired t-tests, while Mann–Whitney and Kruskal–Wallis tests were used to compare the groups with non-normal distribution. Pearson’s test was used to determine the correlation coefficients of the data with normal distribution and Spearman’s test in the case of non-normal distribution. P value <0.05 was considered statistically significant.
Results
Description of subjects
A total of 26 ESRD subjects (mean age of 45 ± 14.15, mean ± standard deviation, aged 18–74 years) participated in the study. The most common clinical manifestations among ESRD patients were high blood pressure (hypertension) and lupus nephritis (Table 3). Of the 26 ESRD patients, 12 had SLE, while 14 did not (Table 3). All ESRD patients with SLE had lupus nephritis (type VI; Table 3). Of the 12 ESRD patients with health problems, 10 had hypertension, 1 had diabetes, nephritic syndrome, Alport syndrome, and recurrent respiratory infections (Table 3). Table 3 depicts the clinical characteristics of ESRD subjects.
Table 3.
The clinical characteristics of ESRD patients with SLE or other health problems.
| Clinical characteristics | ESRD patients with SLE | ESRD patients with health problems |
|---|---|---|
| SLE | 12 | 0 |
| High blood pressure (hypertension) | 0 | 10 |
| Diabetes | 0 | 1 |
| Alport syndrome | 0 | 1 |
| Recurrent respiratory infection | 0 | 1 |
| Nephritic syndrome | 0 | 1 |
ESRD: end-stage renal disease; SLE: systemic lupus erythematosus.
The frequency of circulating Treg in ESRD and healthy subjects
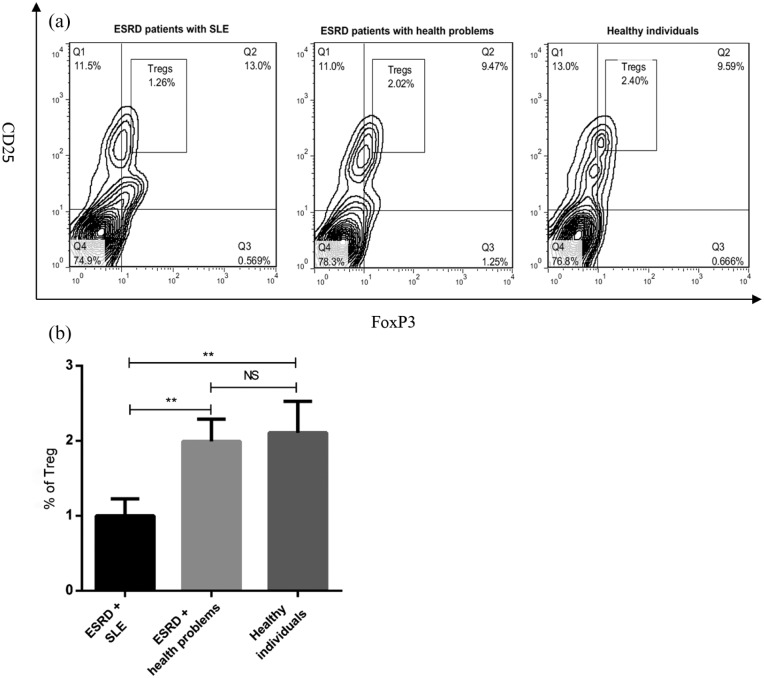
To determine the frequency of Tregs in the peripheral blood of ESRD and healthy individuals, the percentage of circulating Tregs was measured using flow cytometry. Our data revealed that Treg number was significantly lower in ESRD patients with SLE than healthy subjects (P < 0.01, Figure 1(b)). There was a significant difference in Treg frequency between ESRD patients with SLE and the patients suffered from health problems (P < 0.01, Figure 1(b)). As shown in Figure 1(a) and (b), no significant difference was observed in the number of Tregs between ESRD patients with health problems and healthy individuals.
Figure 1.
The percentage of Tregs in peripheral blood of ESRD and healthy subjects. PBMCs were isolated from whole blood of healthy subjects (n = 10) and ESRD patients with SLE (n = 12) or health problems (n = 14). The cells were stained with anti-CD4, anti-CD25, and anti-foxP3 antibodies. The frequency of Tregs in PBMCs was determined by flow cytometry (a) and then analyzed (b). All data are represented as mean ± SEM. (**P < 0.01).
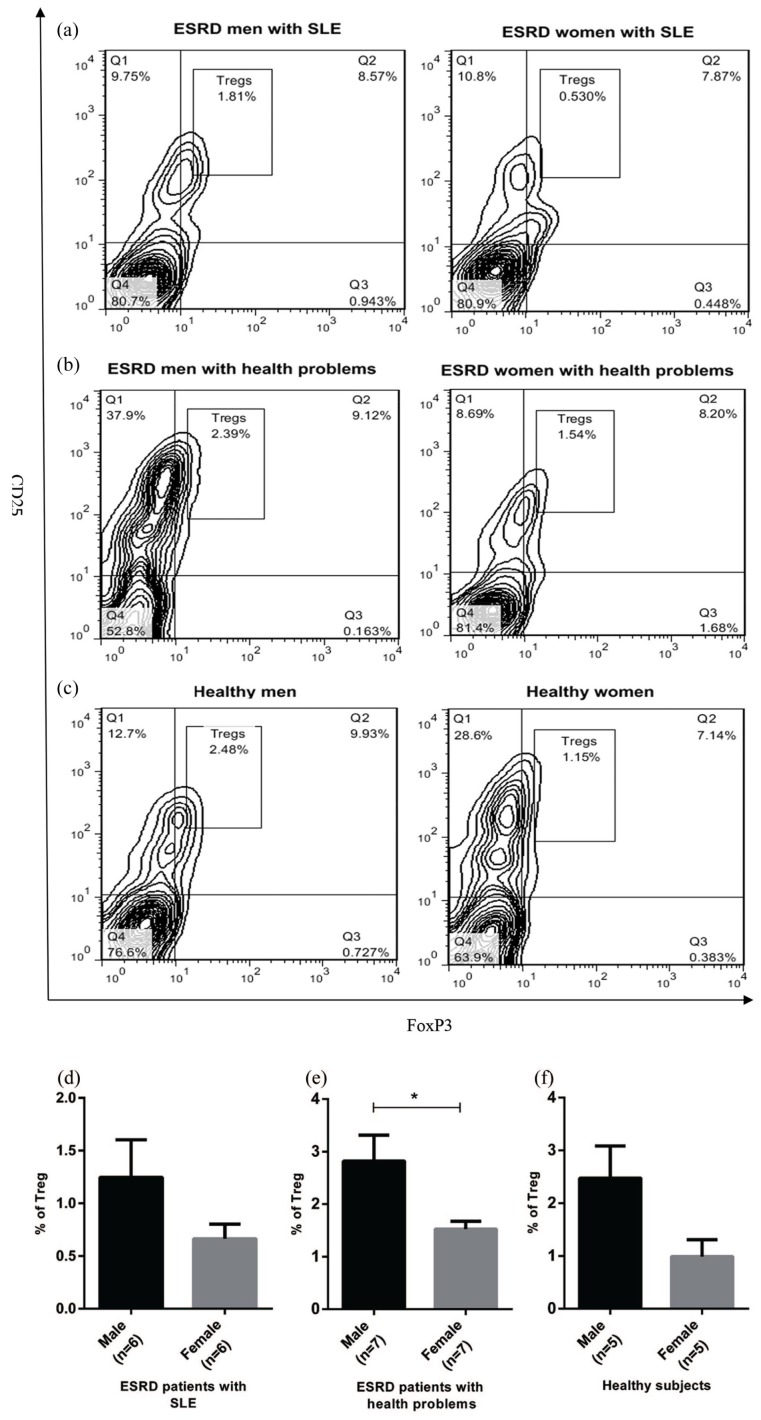
The effect of gender on Treg frequency in peripheral blood of ESRD and healthy subjects
Given that previous reports have demonstrated that autoimmune diseases are more common in women than men,11,12 the effect of gender on the number of circulating Treg in ESRD and healthy individuals was evaluated. Flow cytometry results indicated that all women participated in the study had a reduced number of Tregs in peripheral blood compared to men (Figure 2(a)–(f)). Interestingly, ESRD women with health problems showed a significant reduction in circulating Tregs compared to ESRD men with health problems (P < 0.05, Figure 2(b) and (e)).
Figure 2.
Sex effect on circulating Treg number in ESRD and healthy subjects. PBMCs from healthy subjects (n = 10) and ESRD patients with SLE (n = 12) and health problems (n = 14) were stained with anti-CD4, anti-CD25, and anti-foxP3 antibodies. The percentage of Tregs in participants was determined by flow cytometry (a-c) and later analyzed (d-f). The results of Mann-Whitney test revealed that the frequency of Tregs was lower in women than men. Each bar in (d-f) shows mean ± SEM. (*P < 0.05).
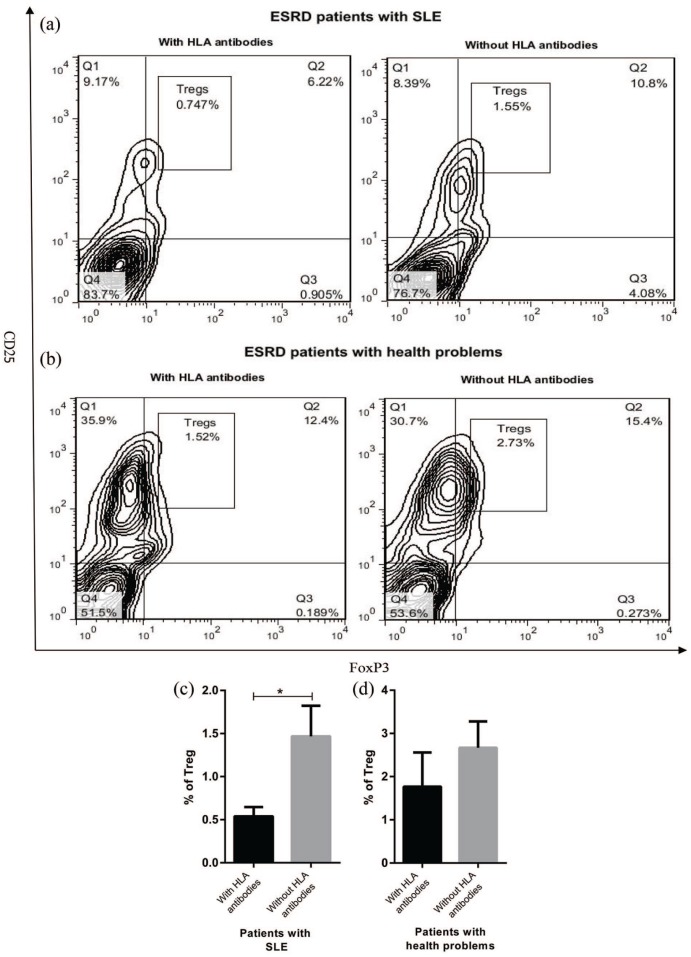
Circulating Treg percentage in ESRD patients with and without panel-reactive antibodies
Since Tregs play a fundamental role in regulating the immune system and inhibiting production of antibodies against different antigens, Treg frequency in ESRD patients who had panel-reactive antibodies was compared to those who did not have. ESRD patients with SLE who were positive for HLA antibodies indicated a significant reduction in the number of Tregs compared to those did not have HLA antibodies (P < 0.05, Figure 3(a) and (c)). Similar to ESRD patients with SLE, the patients suffered from health problems and HLA antibodies showed a decreased frequency of circulating Tregs; however, this reduction was not statistically significant (Figure 3(b) and (d)).
Figure 3.
Treg percentage in ESRD patients with and without HLA antibodies. ESRD patients with SLE (n = 12) or health problems (n = 14) were divided into two groups according to the presence or absence of panel-reactive antibodies. PBMCs were isolated from ESRD patients (with and without HLA antibodies) and stained with anti-CD4, anti-CD25, and anti-foxP3 antibodies. The percentage of the stained cell was measured by (a and b) flow cytometry and (c and d) later analyzed. Each bar in (c) and (d) shows mean ± SEM (*P < 0.05).
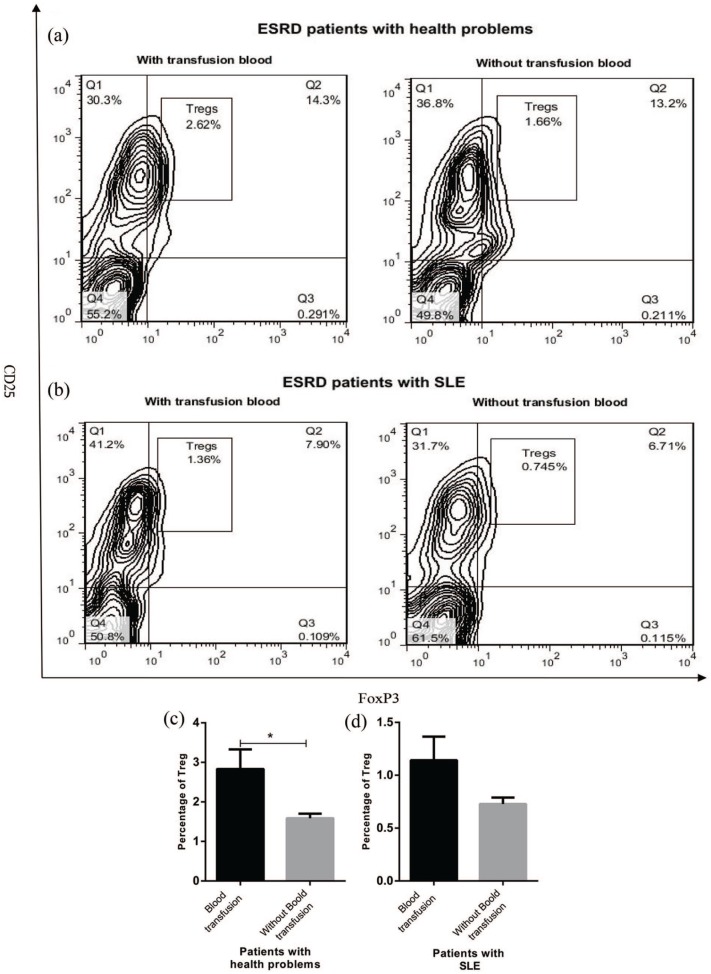
Blood transfusion effect on the percentage of circulating Tregs in ESRD patients
Regarding the fact that some ESRD patients experienced blood transfusion during hemodialysis, the effect of blood transfusion on Treg frequency in peripheral blood of ESRD patients was evaluated. The results demonstrated that ESRD patients with health problems that received blood samples had an increased number of Tregs in peripheral blood compared to those who did not receive (P < 0.05, Figure 4(a) and (c)). However, blood transfusion failed to enhance Treg number in ESRD patients with SLE (Figure 4(b) and (d)).
Figure 4.
The blood transfusion effect on number of circulating Tregs in ESRD patients. Patients with SLE (n = 12) and health problems (n = 14) were divided into two groups based on blood sample. The frequency of Tregs in PBMCs of ESRD patients was assessed by flow cytometry after staining the cells with anti-CD4, anti-CD25, and anti-foxP3 antibodies (a and b) and then analyzed (c and d). The results are shown as mean ± SEM (*P < 0.05).
The assessment of the cytokine profile of ESRD and healthy subjects
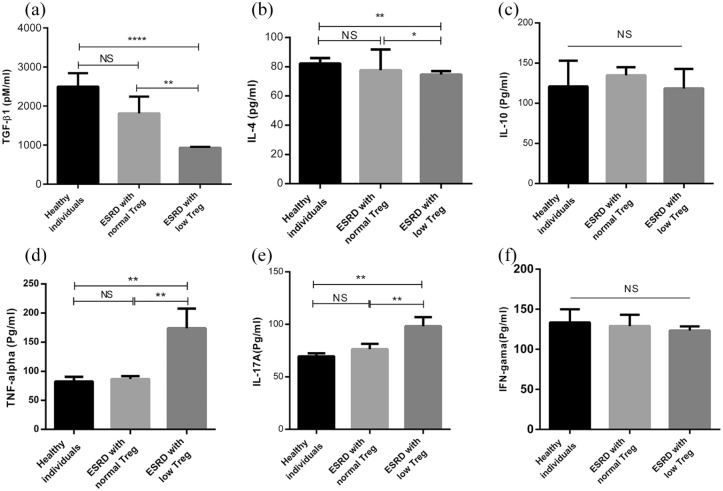
The serum levels of TGF-β1, IL-10, IL-4, TNF-α, IFN-γ, and IL-17A were measured in ESRD and healthy individuals. As shown in Figure 5(a) and (b), ESRD patients with low Treg had significant reductions in the levels of TGF-β1 and IL-4 compared to healthy individuals (P < 0.0001–0.05), while these reductions were not observed in ESRD patients with normal Treg (Figure 5(a) and (b)). In spite of the changes in the levels of TGF-β1 and IL-4, no statistically significant change was observed in the IL-10 level in ESRD patients (Figure 5(c)). TNF-α and IL-17A levels were significantly higher in ESRD patients with low Treg than the patients with normal Treg and healthy subjects (P < 0.01, Figure 5(d) and (e)). However, there was no significant difference in the levels of TNF-α and IL-17A between ESRD patients with normal Treg and healthy subjects (Figure 5(d) and (e)). Unexpectedly, no significant change was observed in the IFN-γ level in the peripheral blood of ESRD and healthy individuals (Figure 5(f)).
Figure 5.
Cytokine profile of healthy individuals and ESRD patients with low and normal Treg. The patients were divided into two groups including (1) ESRD patients with low Treg which had Treg values less than the median of the control group and (2) ESRD patients with normal Treg which had Treg values higher than the median of the control group. (a) TGF-β1, (b) IL-4, (c) IL-10, (d) TNF-α, (e) IL-17A, and (f) IFN-γ levels in ESRD (n = 26) and healthy subjects (n = 10) were measured by ELISA. All data are represented as mean ± SEM (*P < 0.05, **P < 0.01, ****P < 0.0001).
Correlation of Treg number with the demographic and laboratory characteristics of ESRD patients
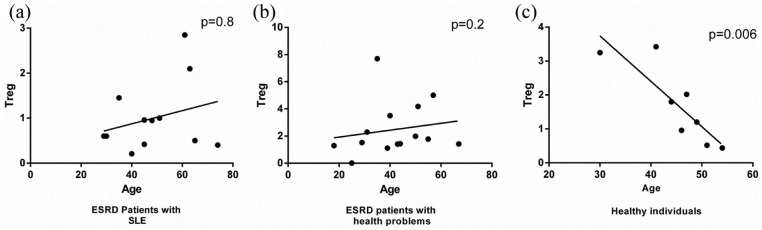
To explore the association of Treg frequency with the demographic and laboratory parameters of ESRD patients, statistical correlation analyses were performed. The results of Spearman’s test showed a weak positive correlation between the number of Tregs and the age of ESRD patients (with or without SLE), which was not statistically significant (Figure 6(a) and (b)). In contrast, we observed that Treg frequency was negatively associated with the age of healthy subjects (Figure 6(c), P < 0.01, odds ratio (OR) = −0.8565640, 95% confidence interval (CI) = −0.9735954 to −0.3829888). Other results of Pearson’s and Spearman’s tests revealed that there was no significant association between circulating Treg frequency and laboratory parameters including the cytokine profile (IL-17A, IL-10, IFN-γ, TNF-α, TGF-β1, and IL-4) of the patients, serum levels of blood urea nitrogen (BUN), fasting blood sugar (FBS), urea, creatinine, and glomerular filtration rate (GFR) in ESRD patients.
Figure 6.
Correlation of Treg frequency with the age of ESRD and healthy subjects. (a) and (b) The results of Spearman’s test showed a weak positive association between Treg percentage and age of ESRD patients. (c) There was a significant negative correlation between Treg number and age of healthy individuals (P < 0.01).
Discussion
ESRD occurs in people with chronic kidney disease that patient gradually loses the functions of kidney over time.1 Autoimmune disorder is one of the identified causes of the disease.13,14 Tregs have a crucial role in the maintenance of natural self-tolerance and prevention of autoimmune disorders.8,9 We therefore focused on determining Treg percentage and its effects on the cytokine profile of ESRD patients with and without SLE. In addition, how Treg frequency is influenced by the blood transfusion and sex.
Previous studies have shown that ESRD patients display impaired immune responses and have overactivated but functionally compromised immune system.15 Several lines of evidence indicated that patients with ESRD had a defect in the function or reduction in the number of Tregs.15,16 It is reported that large proportion of ESRD patients experience a reduced number of circulating Tregs due to increase in apoptosis resulting from their continuous activation by uraemic toxins and oxidized low-density lipoproteins.15,16,17 However, in our knowledge, the difference in the frequency of Tregs between ESRD individuals with SLE or other health problems has not yet been investigated. Thus, the critical question was whether there is a significant difference in Treg number of two groups of the patients. The results of this study indicated that ESRD patients with SLE experience a reduced number of Tregs compared to the patients suffered from other health problems and healthy subjects. This finding suggests that the reduction in the frequency of circulating Tregs may contribute to the pathogenesis of ESRD through the breakdown of peripheral tolerance to self-antigens and activation of different cells of the immune system such as autoreactive B cells, which result in the damage of GBM and subsequently loss of functions of kidney at all.
Having considered that one of the functions of Tregs is the regulation of antibody production against different antigens,18 circulating Treg frequency in ESRD patients with antibody production against HLAs was compared with those who did not have. Our data demonstrated that the patients with panel-reactive antibodies had a significant reduction in circulating Tregs compared to those who were negative for these antibodies, although this reduction in ESRD patients without SLE was not statistically significant. These results were additional confirmation regarding the reduced number of Tregs in patients with ESRD who can participate in the pathogenesis of the disease through the breakdown of immunological self-tolerance and production of antibodies against various soluble antigens.
In the next step, the effect of blood transfusion on the frequency of Tregs in patients with ESRD was evaluated. We found that blood transfusion significantly induced the development of Tregs in ESRD patients without SLE. This observation is consistent with other reports showing that donor-specific blood transfusion can be considered as a useful approach to promote hyporesponsiveness and graft acceptance.19,20 Many experimental transplant models have been demonstrated that preoperative blood transfusion significantly affects graft outcome through affecting Tregs. It is reported that pre-exposure to alloantigen can result in the generation of regulatory cells and enhance alloantigen-specific immunoregulatory activity of these cells, which lead to control the effector arms of the immune system.20,21 In contrast to the effect of blood transfusion on Treg generation in ESRD patients without SLE, our results showed that blood transfusion failed to induce Treg production in ESRD patients suffered from SLE. The difference observed in the blood transfusion effect on Treg frequency in patients with and without SLE provides evidence to indicate that increased number of Treg in the patients was not derived from the blood donor. Therefore, it is likely that ESRD patients with SLE had a defect in the generation of Tregs. However, further studies and more information are required to confirm this finding and clarify the molecular mechanisms involved in this possible defect.
In an attempt to discover the effect of Tregs on the cytokine profile of patients with ESRD, the levels of some cytokines which play fundamental role in the induction and/or regulation of the immune system were investigated. We observed that ESRD patients with low Treg showed significant reductions in the levels of TGF-β1 and IL-4 compared to the patients with normal Treg and healthy individuals. Although the comparison of cytokine profile of ESRD patients with low and normal Treg has not been preformed so far, there are some studies showing the levels of different cytokines in ESRD patients. In line with notion, Stefoni et al.22 showed that ESRD patients had lower serum values of TGF-β1 than healthy control group. On the contrary, some reports have indicated that hyperexpression of TGF-β1 provides a mechanism for the increased prevalence of renal failure in African Americans (Blacks) compared to Whites.23 Other data of this study revealed that TNF-α and IL-17A levels were significantly increased in patients with low Treg than those with normal Treg and healthy group. In agreement with these findings, it has been demonstrated that many patients with ESRD have the increased serum levels of inflammatory cytokines including TNF-α, IL-1, and IL-6.24–26 Considering the fact that inflammation is a highly common condition among ESRD patients and anti-inflammatory mediators may influence the risk for cardiovascular events in ESRD patients,27,28 the levels of IL-10 and IFN-γ as pro- and anti-inflammatory cytokines, respectively, in peripheral blood of patients with ESRD were also assessed. We found that ESRD patients with low Treg had IL-10 and IFN-γ levels similar to ESRD patients with normal Treg and healthy control. These findings suggest that inflammation as a prevalent condition in ESRD patients is mainly mediated by the stimulation of pro-inflammatory cytokines (TNF-α and IL-17A) production and inhibition of anti-inflammatory cytokines (TGF-β1 and IL-4) secretion of different cells of the immune system. However, these data provide another evidence to indicate that the reduction in Treg number may contribute to the development of ESRD through the enhancement of inflammation. However, it should be noted that additional studies are required to confirm changes observed in the production of anti- and pro-inflammatory cytokines and determine other possible mediators and/or non-immunologic mechanisms involved in inflammation among ESRD patients with low Treg.
Given that previous studies have demonstrated that the frequency of circulating Tregs can be affected by age,8 we examined whether the demographic and laboratory parameters influence the number of Tregs in peripheral blood of participants. Our results revealed that circulating Treg percentage was lower in female participants than men. In line with this result, we observed that this reduction was statistically significant in ESRD women with health problems. Numerous studies have provided convincing evidence that autoimmune diseases such as SLE, MS, RA, and Hashimoto’s thyroiditis are more prevalent in women than men.12,29,30 The result of this study may explain one of the causes why the prevalence of autoimmunity in female is more than male.11,12,29,31 The results of Pearson’s and Spearman’s tests showed that there was an inverse association between Treg number and the age of healthy subjects, while this correlation was not observed in patients with ESRD. This finding is in contrast with our previous studies and other reports that showed the frequency of Tregs in peripheral blood of patients with MS increases with age.8,32 This discrepancy could be attributed to the type of subjects used in these studies. Other data of this study indicated that Treg percentage was not associated with the cytokine profile and the demographic and laboratory characteristics of patients.
Overall, this study provides evidence to show that Treg frequency in ESRD patients with SLE significantly differs from the patients without SLE. Furthermore, the results revealed that the blood transfusion had a potent effect on Treg generation in ESRD patients with health problems, unlike ESRD patients with SLE. In addition, ESRD patients with low Treg showed a shift in the cytokine profile from anti-inflammatory toward pro-inflammatory cytokines and had the increased HLA antibodies in peripheral blood. Although these observations suggest that the reduced number of Treg may contribute to the development of ESRD in patients suffering from SLE, a limitation of the study was a lack of a control group with SLE but no ESRD. Therefore, it should be noted that this limitation will be considered to confirm these findings in future studies.
Acknowledgments
The authors would like to thank all individuals who participated in this study. We also thank Isfahan Kidney Diseases Research Center for its help and support of the study.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was financially supported by Isfahan University of Medical Sciences (IUMS) under Grant No. 394365.
ORCID iD: Hossein Motedayyen  https://orcid.org/0000-0002-4863-1771
https://orcid.org/0000-0002-4863-1771
References
- 1. Peralta CA, Shlipak MG, Judd S, et al. (2011) Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA 305(15): 1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bauer A, Limperger V, Nowak-Gottl U. (2016) End-stage renal disease and thrombophilia. Hamostaseologie 36(2): 103–107. [DOI] [PubMed] [Google Scholar]
- 3. Wu C, Chen S, Ho C, et al. (2015) End-stage renal disease after hypertensive disorders in pregnancy. Obstetric Anesthesia Digest 35: 74. [DOI] [PubMed] [Google Scholar]
- 4. Weening JJ, D’Agati VD, Schwartz MM, et al. (2004) The classification of glomerulonephritis in systemic lupus erythematosus revisited. Journal of the American Society of Nephrology 15(2): 241–250. [DOI] [PubMed] [Google Scholar]
- 5. Pascual V, Farkas L, Banchereau J. (2006) Systemic lupus erythematosus: All roads lead to type I interferons. Current Opinion in Immunology 18(6): 676–682. [DOI] [PubMed] [Google Scholar]
- 6. Botto M, Dell’Agnola C, Bygrave AE, et al. (1998) Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nature Genetics 19(1): 56. [DOI] [PubMed] [Google Scholar]
- 7. Lerner R, Glassock R, Dixon FJ. (1967) The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. The Journal of Experimental Medicine 126(6): 989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sedaghat NMH, Etemadifar M, Zarkesh H, et al. (2018) Effect of fingolimod on the frequency of regulatory T cells in patients with relapsing-remitting multiple sclerosis. Journal of Immune Research 5(1): 1032. [Google Scholar]
- 9. Sadati ZA, Motedayyen H, Sherkat R, et al. (2019) Comparison of the percentage of regulatory T cells and their p-STAT5 expression in allergic and non-allergic common variable immunodeficiency patients. Immunological Investigations 48: 52–63. [DOI] [PubMed] [Google Scholar]
- 10. Brusko TM, Hulme MA, Myhr CB, et al. (2007) Assessing the in vitro suppressive capacity of regulatory T cells. Immunological Investigations 36(5–6): 607–628. [DOI] [PubMed] [Google Scholar]
- 11. Oertelt-Prigione S. (2012) The influence of sex and gender on the immune response. Autoimmunity Reviews 11: A479–A485. [DOI] [PubMed] [Google Scholar]
- 12. Lockshin MD. (2005) Sex differences in autoimmune disease. In: Lockshin M, Ware Branch D, Asherson RA. (eds) Handbook of systemic autoimmune diseases, vol. 4 Amsterdam: Elsevier, pp. 3–10. [Google Scholar]
- 13. Nossent HC, Swaak TJ, Berden JH. (1990) Systemic lupus erythematosus: Analysis of disease activity in 55 patients with end-stage renal failure treated with hemodialysis or continuous ambulatory peritoneal dialysis. The American Journal of Medicine 89: 169–174. [DOI] [PubMed] [Google Scholar]
- 14. Coplon NS, Diskin CJ, Petersen J, et al. (1983) The long-term clinical course of systemic lupus erythematosus in end-stage renal disease. The New England Journal of Medicine 308(4): 186–190. [DOI] [PubMed] [Google Scholar]
- 15. Hendrikx TK, van Gurp EA, Mol WM, et al. (2009) End-stage renal failure and regulatory activities of CD4+ CD25bright+ FoxP3+ T-cells. Nephrology, Dialysis, Transplantation 24(6): 1969–1978. [DOI] [PubMed] [Google Scholar]
- 16. Meier P, Golshayan D, Blanc E, et al. (2009) Oxidized LDL modulates apoptosis of regulatory T cells in patients with ESRD. Journal of the American Society of Nephrology 20(6): 1368–1384. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17. Meier P, Spertini F, Blanc E, et al. (2007) Oxidized low-density lipoproteins activate CD4+ T cell apoptosis in patients with end-stage renal disease through Fas engagement. Journal of the American Society of Nephrology 18(1): 331–342. [DOI] [PubMed] [Google Scholar]
- 18. Fujio K, Okamura T, Sumitomo S, et al. (2012) Regulatory T cell-mediated control of autoantibody-induced inflammation. Frontiers in Immunology 3: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pirenne J, Kitade H, Kawai M, et al. (2005) Regulatory cells, TH1/TH2 unbalance, and antibody-induced chronic rejection in operational tolerance induced by donor-specific blood transfusion. Transplantation 79: S25–S27. [DOI] [PubMed] [Google Scholar]
- 20. Bushell A, Karim M, Kingsley CI, et al. (2003) Pretransplant blood transfusion without additional immunotherapy generates CD25+ CD4+ regulatory T cells: A potential explanation for the blood-transfusion effect. Transplantation 76(3): 449–455. [DOI] [PubMed] [Google Scholar]
- 21. Wood KJ, Sakaguchi S. (2003) Regulatory lymphocytes: Regulatory T cells in transplantation tolerance. Nat Rev Immunol 3: 199. [DOI] [PubMed] [Google Scholar]
- 22. Stefoni S, Cianciolo G, Donati G, et al. (2002) Low TGF-β1 serum levels are a risk factor for atherosclerosis disease in ESRD patients. Kidney International 61: 324–335. [DOI] [PubMed] [Google Scholar]
- 23. August P, Sharma V, Ding R, et al. (2009) Transforming growth factor beta and excess burden of renal disease. Transactions of the American Clinical and Climatological Association 120: 61. [PMC free article] [PubMed] [Google Scholar]
- 24. Stenvinkel P, Ketteler M, Johnson RJ, et al. (2005) IL-10, IL-6, and TNF-α: Central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney International 67: 1216–1233. [DOI] [PubMed] [Google Scholar]
- 25. Turkmen K, Guney I, Yerlikaya FH, et al. (2012) The relationship between neutrophil-to-lymphocyte ratio and inflammation in end-stage renal disease patients. Renal Failure 34(2): 155–159. [DOI] [PubMed] [Google Scholar]
- 26. Samy E, Elrahman A, Shafei M, et al. (2016) The relation between the level of serum tumor necrosis factor–alpha and hemodialysis adequacy in diabetic and non diabetic patients on maintenance hemodialysis. Urology & Nephrology Open Access Journal 3: 00074. [Google Scholar]
- 27. Girndt M, Kaul H, Sester U, et al. (2002) Anti-inflammatory interleukin-10 genotype protects dialysis patients from cardiovascular events. Kidney International 62(3): 949–955. [DOI] [PubMed] [Google Scholar]
- 28. Kamimura MA, Draibe SA, Dalboni MA, et al. (2007) Serum and cellular interleukin-6 in haemodialysis patients: Relationship with energy expenditure. Nephrology, Dialysis, Transplantation 22(3): 839–844. [DOI] [PubMed] [Google Scholar]
- 29. Whitacre CC, Reingold SC, O’Looney PA, et al. (1999) A gender gap in autoimmunity: Task force on gender, multiple sclerosis and autoimmunity. Science 283: 1277–1278. [DOI] [PubMed] [Google Scholar]
- 30. Voskuhl R. (2011) Sex differences in autoimmune diseases. Biology of Sex Differences 2(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klein SL. (2004) Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunology 26: 247–264. [DOI] [PubMed] [Google Scholar]
- 32. Gregg R, Smith C, Clark F, et al. (2005) The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clinical and Experimental Immunology 140(3): 540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]