Abstract
Background:
The normal small difference (3–5 mmHg) between arterial (partial pressure of carbon dioxide [PaCO2]) and end-tidal carbon dioxide pressure (ETPCO2) increases in children with congenital heart disease. The present study was conducted to evaluate the effect of corrective or palliative cardiac surgery on this difference (known as DPCO2).
Patients and Methods:
In a prospective study, 200 children (aged <12 years old) candidate for corrective or palliative cardiac surgery were studied. Using arterial blood gas measurement and simultaneous capnography, DPCO2 was calculated at various intra- and postoperative periods. DPCO2 values were compared within and between corrective or palliative procedures.
Results:
Corrective and palliative procedures were carried out on 154 and 46 patients, respectively. Initial DPCO2 was higher than normal values in corrective or palliative procedures (15.50 ± 13.1 and 10.75 ± 9.1 mmHg, respectively). DPCO2 was higher in patients who underwent palliative procedure, except early after procedure. The procedure did not have any effect on the final DPCO2 in palliative group. Although DPCO2 decrease was significant in the corrective group, it did not return to normal values. Operation time was longer, and the need to inotropic support was higher in corrective procedures; however, longer periods of ventilatory support were needed in the palliative group. Complication rate and Intensive Care Unit stay time were the same in two operation types.
Conclusions:
DPCO2 did not change after palliative cardiac procedures. DPCO2 decreased after corrective procedures; however, it did not return to normal values at early postoperative period. Thus, DPCO2 may not have any clinical value in monitoring the quality of corrective or palliative procedures.
Keywords: Arterial to end-tidal carbon dioxide pressure difference, congenital heart diseases, end-tidal carbon dioxide pressure, pediatric cardiac surgery
INTRODUCTION
In normal cardiopulmonary physiology, there is a small difference between arterial blood and end-tidal respiratory carbon dioxide tensions; this difference (DPCO2) is about 3–5 mmHg.[1,2] This small difference is due to the high solubility of the CO2 gas in alveolocapillary membrane. Similar to adults, there is such an acceptable correlation in neonates and children.[3,4,5,6] In some conditions and diseases, DPCO2 may increase significantly because of increase in thickness or decrease in effective surface of alveolocapillary membrane (increased dead space ventilation). McSwain measured DPco2 in 56 mechanically ventilated pediatric patients and calculated the dead space to tidal volume ratio (VD/VT). There was a strong correlation between end-tidal carbon dioxide pressure (ETPCO2) and partial pressure of arterial carbon dioxide (PaCO2) in various VD/VT ranges; DPCO2 increased predictably with increasing VD/VT ratio.[7] During anesthesia, increased dead space ventilation usually causes DPCO2 rise up to 10 mmHg. In children with congenital heart diseases (CHD), DPCO2 is abnormal; in fact, the rate of increase in DPCO2 is correlated with the severity of physiologic derangement. Choudhury et al. studied the DPCO2 value and effect size of hyperventilation on PaCO2 in children scheduled for correction of their underlying cardiac defect. They reported increased DPCO2 in both cyanotic and acyanotic patients and showed reduced effect of hyperventilation on PaCO2 setup. They also concluded that increased pulmonary artery pressure (PAP) or pulmonary blood flow (PBF) is important as right-to-left shunt in these findings.[8] CHDs are the most common inborn diseases with about 50% requirement for surgical intervention.[9] Incomplete surgical intervention (corrective or palliative) is the most common cause of early or late postoperative morbidity and mortality.[10,11] A review of the intraoperative transesophageal echocardiographic (IOTEE) reports of the patients who underwent ventricular septal defect (VSD) closure revealed that the rate of residual VSD is 37%.[9] Yang in a retrospective study reported the rate of residual VSD on IOTEE as 33%.[11] Most of residual VSDs are closing spontaneously;[11] however, early diagnosis can lead us to act in appropriate time. Although IOTEE is sensitive enough to detect most of the residual defects, its use, especially in small children, needs a high level of skill.[10,11,12,13] We hypothesized that after corrective or palliative surgeries, preoperative DPCO2 should have a normal value or be reduced, respectively. In a prospective clinical study on children with CHD who were candidate for cardiac surgery, simultaneous arterial blood PCO2 and end-tidal PCO2 were measured and DPCO2 was calculated and compared intra- and postoperatively between and within two corrective and palliative procedures.
PATIENTS AND METHODS
This study was a single-center, cross-sectional study without any additional intervention (the invasive blood sampling and respiratory capnometry performed using previously placed instruments those are routine in cardiac surgery). The study first was approved by the Research Ethical Board of Tabriz University of Medical Sciences and then the investigators got a Registration number (IRCT201606011127N5) from Iranian Registry committee of Clinical Trials (www.irct.ir). Written informed consent approval was obtained from all parents preoperatively.
After approval from the local institutional ethics committee and obtaining written preoperative informed consent from all parents, infants and children (<12 years) with CHD, candidate for elective cardiac surgery, were studied prospectively in a about 12-month period from May 2014 to May 2015. The purposed sample size was 200 cases. Patients with preoperative diabetes mellitus, renal, hepatic, cerebral, or respiratory diseases were excluded from the study. Patients’ cyanosis and clubbing severity were graded by an anesthesiologist colleague using the following scales and graphs [Figures 1 and 2].
Figure 1.
The visual analog scale of cyanosis severity
Figure 2.
Grading of the clubbing severity
The patients were prepared for surgery as a routine practice. Considering body weight, premedication was done by midazolam or promethazine syrup or intramuscular morphine 30 min before induction of anesthesia. Anesthesia was induced intravenously using ketamine, 2 mg/kg; midazolam, 0.1 mg/kg; fentanyl, 3 μg/kg; and cisatracurium, 0.2 mg/kg. Trachea was intubated after 3–5 min (using an appropriately sized cuffed or uncuffed endotracheal tube). In any case without intravenous line in situ, the child was first sedated by intramuscular ketamine, and then intravenous access was done. Basic monitoring started simultaneously with anesthesia induction. Then, arterial and central venous catheterizations were done. Mechanical ventilation performed using a Dräger Fabius anesthesia machine in pressure- or volume-controlled modes. Anesthesia was maintained with continuous infusions of midazolam 50 μg/kg/h, fentanyl 5 μg/kg/h, and cisatracurium 2 μg/kg/min. Mainstream capnography (Novametrix CO2 SMO Model 7100 ETCO2 SpO2 Monitor) was performed intra- and postoperatively, continued to tracheal extubation.
Arterial blood gas (ABG) was analyzed in 10 min after induction (T1), about 10 min before (T2), 10 min after cardiopulmonary bypass (CPB) (T3), and at the end of surgery (T4), intraoperatively. ETPCO2 was recorded simultaneously. In off-pump surgeries, T2 and T3 were considered as 10 min before and 10 min after the procedure, respectively. Only ABG parameters and ETPCO2 of first three ICU stay days up to preextubation period were used postoperatively (T5). Data of the surgery, CPB, and hemodynamic status were collected in intra- and postoperative periods and compared regarding corrective and palliative procedures
Statistics
The data were analyzed using SPSS statistical software (version 16.0. Chicago, SPSS Inc). Normally distributed parametric variables were compared, using the independent groups’ Student's t-test, and Chi-square test was used for nonparametric data. ETPCO2 changes during the study were analyzed via repeated measures of ANOVA. The two-way ANOVA test was used to compare the differences over time between the two groups and paired groups’ t-test was used to compare basic and preextubation DPCO2 values in various palliative procedures. P < 0.05 was considered statistically significant.
RESULTS
Two hundred children (96 male and 104 female) aged 1–144 months were enrolled in this study. Corrective and palliative procedures were done on 154 (77%) and 46 (23%) patients, respectively. The operations covered a wide range of palliative and corrective procedures. The CHDs were as follows: tetralogy of Fallot (TOF), VSD, pulmonary stenosis (PS), atrial septal defect (ASD), VSD/PS, ASD/PS, ASD/tricuspid stenosis, transposition of great arteries (TGA), patent ductus arteriosus, atrioventricular septal defect, aortic stenosis, total anomalous pulmonary venous connection, and coarctation of aorta. Patients who underwent palliative procedures were younger than corrective group patients (P = 0.012). Clubbing severity was the same in two groups (P = 0.243); however, cyanosis was more severe in the palliative group (P = 0.001). CPB was used more in corrective than palliative procedures (96.8% vs. 30.4%). The study showed that the operation time and requirement to inotropic support were high in corrective procedures; nevertheless, longer period of ventilatory support was needed in the palliative group. Intensive Care Unit (ICU) stay time and morbidity and mortality rates were identical in both groups [Tables 1 and 2].
Table 1.
Comparison of the data in palliative and corrective procedures
| Procedural group | Total | P | ||
|---|---|---|---|---|
| Palliative (n=46) | Corrective (n=154) | |||
| Gender (male/female) | 21 (25) | 75 (79) | 96 (104) | 0.716 |
| Age (month), mean±SD | 11.31±18.5 | 22.58±28.2 | 19.99±26.6 | 0.012* |
| Weight (kg), mean±SD | 8.26±6.6 | 11.99±9.3 | 11.13±8.9 | 0.013* |
| Height (cm), mean±SD | 68.33±13.8 | 77.29±21.1 | 75.23±20.0 | 0.007* |
| Hemoglobin (g/dl), mean±SD | 13.55±3.2 | 13.17±2.9 | 13.25±3.0 | 0.449 |
| WBC (×1000), mean±SD | 10.43±3.5 | 9.83±3.1 | 9.971±3.2 | 0.268 |
| Platelet (×1000), mean±SD | 279.13±105.3 | 296.92±96.9 | 292.83±98.9 | 0.286 |
| Severity of cyanosis, n (%) | ||||
| Acyanotic | 16 (34.8) | 102 (66.3) | 118 (59) | 0.001* |
| Grade 1 | 8 (17.4) | 23 (14.9) | 31 (15.5) | |
| Grade 2 | 10 (21.8) | 14 (9.1) | 24 (12) | |
| Grade 3 | 7 (15.2) | 8 (5.2) | 15 (7.5) | |
| Grade 4 | 5 (10.8) | 7 (4.5) | 12 (6) | |
| Using CPB (yes/no) | 14/32 | 149/5 | 163/37 | 0.001* |
| Operation time (min), mean±SD | 202.56±67.9 | 254.90±75.2 | 243.18±76.6 | 0.001* |
| Mechanical ventilation time (h), mean±SD | 34.77±40.0 | 22.74±25.4 | 25.43±29.6 | 0.019* |
| ICU stay (day), mean±SD | 6.28±6.3 | 5.36±3.6 | 5.56±4.4 | 0.225 |
| Need to inotropic support, n (%) | 27 (58.7) | 122 (79.2) | 149 (74.5) | 0.005* |
| Morbidity, n (%) | 22 (47.8) | 53 (34.4) | 75 (37.5) | 0.099 |
| Mortality, n (%) | 4 (8.7) | 8 (5.2) | 12 (6) | 0.320 |
*P<0.05. SD: Standard deviation, WBC: White blood cell, CPB: Cardiopulmonary bypass, ICU: Intensive Care Unit
Table 2.
Arterial to end-tidal carbon dioxide pressure differences (DPCO2) in palliative or corrective procedures at various periods
| Procedures | DPCO2 (mmHg) | |||||||
|---|---|---|---|---|---|---|---|---|
| After induction | PreCPB* | PostCPB† | End surgery | ICU day 1 | ICU day 2 | ICU day 3 | Before extubation | |
| Palliative (n=46) | 15.50±13.1 (n=46) | 14.61±12.9 (n=46) | 12.30±11.2 (n=46) | 12.22±12.1 (n=46) | 13.21±9.4 (n=46) | 13.66±10.6 (n=27) | 13.61±7.0 (n=12) | 13.74±6.7 (n=46) |
| Corrective (n=154) | 10.75±9.1 (n=154) | 10.02±9.1 (n=154) | 10.86±7.5 (n=154) | 10.89±8.5 (n=154) | 10.12±8.1 (n=154) | 9.52±7.9‡ (n=72) | 9.1±5.3‡ (n=23) | 8.70±4.6‡ (n=154) |
| Total | 11.84±10.3 (n=200) | 11.08±10.3 (n=200) | 11.24±8.4 (n=200) | 11.20±8.9 (n=200) | 10.83±8.4 (n=200) | 10.47±8.5 (n=99) | 10.14±5.8 (n=35) | 9.81±5.5 (n=200) |
| P | 0.006§ | 0.007§ | 0.313 | 0.403 | 0.030§ | 0.038§ | 0.040§ | 0.001§ |
*In off-pump operations, the value was measured 10 min before the procedure, †In off-pump operations, the value was measured 10 min after the procedure, ‡Significant difference with basic (after induction) value (P<0.01), §Significant differences between palliative and corrective groups. CPB: cardiopulmonary bypass, ICU: Intensive Care Unit, DPCO2: difference PCO2
Table 2 and Figure 3 represent the DPCO2 at various periods of the study. Except immediately after CPB and at the end of surgery, there were significant differences between palliative and corrective groups. DPCO2 was higher, almost at anytime, in patients who underwent palliative procedure. Changes in DPCO2 were not significant in the palliative group at any time; however, comparing with the primary value, DPCO2 decreased significantly in the corrective group patients prior to the extubation period. Table 3 shows the basic and preextubation DPCO2 values in various palliative procedures; the type of palliative procedure did not have any effect on final DPCO2.
Figure 3.
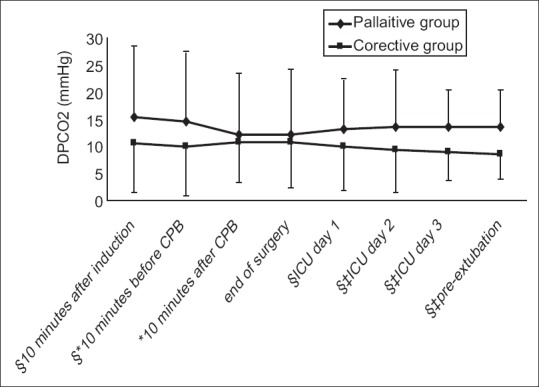
Arterial-end-tidal carbon dioxide pressure differences in palliative or corrective procedures at various times. *In off-pump operations, the value was measured 10 min before the procedure. † In off-pump operations, the value was measured 10 min after the procedure. ‡ Significant difference with basic (after induction) value (P < 0.01). § Significant differences between palliative and corrective groups
Table 3.
Basic and final arterial to end-tidal carbon dioxide pressure differences (DPCO2) in various palliative procedures
| Palliative procedure | n (%) | After induction | Before extubation | P |
|---|---|---|---|---|
| Annular patch | 4 (8.7) | 16.00±12.7 | 13.50±3.4 | 0.717 |
| Blalock-Taussig’s shunt | 8 (17.4) | 14.25±16.6 | 15.13±6.2 | 0.793 |
| Annular patch/Blalock-Taussig’s shunt | 10 (21.7) | 14.30±13.4 | 12.90±6.4 | 0.769 |
| Total cavopulmonary connection | 2 (4.3) | 9.00±1.4 | 12.50±0.7 | 0.087 |
| Pulmonary artery banding | 6 (13.0) | 19.50±13.6 | 13.5±7.7 | 0.364 |
| Pulmonary artery banding/atrial septectomy | 4 (8.7) | 17.50±10.72 | 9.75±1.5 | 0.202 |
| Glenn’s shunt | 12 (26.1) | 15.50±12.9 | 13.63±6.5 | 0.676 |
| Total | 46 | 15.50±13.1 | 13.74±6.7 | 0.375 |
DPCO2: difference PCO2
DISCUSSION
The current study covered a wide range of children aged 1–144 months with a wide spectrum of cardiac anomalies. In the majority of patients (77%), corrective procedures were performed. There was a trend in performing palliative procedures in younger and more cyanotic children. This was predictable because traditionally palliative procedures are performed in patients with more complex anomalies or low weight patients.
In patients with normal cardiopulmonary function, there is a good correlation between ETPCO2 and arterial carbon dioxide pressure (PaCO2); thus, end-tidal capnometry is widely used in clinical practice to estimate PaCO2. Today, the end-tidal capnometry has become a basic and standard procedure for respiratory monitoring during anesthesia and recovery,[14,15] ICU,[16,17,18] and at emergency departments (ED).[19,20] Takano et al. studied the utility of the portable capnometer in general wards or in-home care practice in spontaneously breathing patients and reported that expiratory capnometry gives a reliable estimate of PaCO2 and can be useful to evaluate the respiratory condition of spontaneously breathing patients.[21] Yosefy et al. aimed to verify whether ETPCO2 can accurately predict PaCO2 and variables that may affect this correlation in patients who referred to ED for respiratory distress. They reported a good correlation between ETCO2 and PaCO2, and showed that DPCO2 had an inverse correlation with age.[19] In some anesthetized patients or other patients with increased pulmonary dead space, DPCO2 may increase significantly.[7] In most CHD patients, because of abnormal cardiopulmonary physiology, DPCO2 increases; thus, it is often recommended to monitor PaCO2 directly for respiratory setup.[16,22]
CHDs are the most common congenital diseases. In order to improve cardiopulmonary physiology and/or quality of life, many corrective or palliative surgical procedures are used. Conventionally, it is predicted that after corrective or palliative surgeries, DPCO2 should reach a normal value or be reduced, respectively. Bhat and Abhishek reported a strong correlation between mainstream ETCO2 and PaCO2 in mechanically ventilated newborns. In neonates with lung disease, this correlation was weak; surfactant therapy improved the correlation.[4] Mehta et al. showed a correlation between increase in DPCO2 and the severity of lung disease in neonates and children receiving mechanical ventilation. They recommended that blood gases should be measured in these patients until the lung disease is healed.[17]
Incomplete corrections or nonadequate palliative operations are common reasons for postoperative mortality and morbidity.[10,11,23] Hanna et al. in a retrospective review of IOTEE on 690 patients reported a residual VSD with significant left-to-right shunt in 260 patients (37%). Twenty-four patients (9.2%) returned to CPB again in the same surgery.[10] Furthermore, most of these defects are trivial and resolve spontaneously, but early diagnosis could lead us to a correct way. Guzeltas et al. reviewed the perioperative TEE records of 265 pediatric patients with CHD. In 5 (1.8%) patients, the surgical plan was changed following preoperative TEE; in 12 patients (4.5%), CPB reinitiated because of residual defects identified by IOTEE. They concluded that perioperative TEE causes a significant reduction in mortality and morbidity.[13] IOTEE is a sensitive modality to detect residual problems; however, it requires high level of skill, especially in younger children. In Hanna's study, 125 of residual VSDs were not detected by IOTEE, and from 13 defects requiring reoperation during the same hospitalization, only five were detected by IOTEE.[10]
In infants and children with normal cardiopulmonary physiology, DPCO2 is low and ETPCO2 is used as a valuable predictor of PaCO2. Tingay et al. in mechanically ventilated neonates without lung disease showed an acceptable agreement between ETPCO2 and PaCO2 postsurgically.[24] Trevisanuto et al. reported a good correlation between mainstream ETPCO2 and PaCO2 in 143 very low birth weight newborns (VLBWN); however, the agreement was poor and negatively influenced by the severity of pulmonary disease. They concluded that capnography should not replace PaCO2 in ventilated VLBWN, but it may exert a role to detect trends of PaCO2.[25] Naidu, in review of seven studies about using ETPCO2 in mechanically ventilated neonates, showed a good correlation between ETPCO2 and PaCO2. He concluded that ETPCO2 is a valuable trending tool, and especially in patients with underlying lung disease, it cannot be completely replaced with the gold standard serial ABG analyses.[26] Amuchou Singh and Singhal in a retrospective study showed a good correlation and agreement between EtCO2 and PaCO2 in mechanically ventilated extremely low birth weight newborns.[27] In such as lung disease in most children with CHD, DPCO2 may increase.[14,19]
CHDs increase lung dead space, intra- or extrapulmonary shunts, and ventilation/perfusion mismatching (V/Q mismatch).[28] Although the main effects of these problems reflect on O2 exchange (cyanosis), in lower degrees, CO2 exchange may be affected. In anesthetized patients, because of mechanical ventilation, muscle paralysis, and positioning, DPCO2 increases. It is expected that the DPCO2 rises more significantly in CHDs; the present study indicated this (11.84 mmHg vs. normal 1–5 mmHg value). Right-to-left intracardiac shunt is the most common explanation for this finding in CHDs.[28] Lungs are bypassed and venous blood with high CO2 content flows directly to arterial side, increasing DPCO2. In increased PBF, blood runs very speedy through capillaries without enough time for gas exchanges in alveolocapillary system; this may increase the DPCO2. The main purpose of surgery is to correct the cardiopulmonary physiology. Thus, the present study hypothesized that DPCO2 should reach normal values or decrease after successful corrective or palliative procedures. The findings showed that palliative procedures do not have any corrective effect on DPCO2, and corrective procedures have only an imperfect effect on DPCO2, which may be due to the continuation of extrapulmonary shunts or increased PBF. In most patients with preoperative pulmonary artery hypertension (PAH) (generally PBF increases too), a few days or weeks for remission of PAH are required. Thus, in such cases, DPCO2 may be higher than normal in early postoperative period. The results support the findings of Choudhury and Short. Choudhury studied the effect of hyperventilation on DPCO2 in children with CHD who were scheduled for correction of their CHD. They concluded that DPCO2 can be increased in both cyanotic and acyanotic diseases. They showed that, as in right-to-left shunting, increased PAP and PBF cause disturbance in carbon dioxide homeostasis, and hyperventilation has little effect on reducing PaCO2.[8] Short studied the ability of arterial oxygen saturation in prediction of DPCO2 in children with congenital cyanotic heart disease during cardiac surgery.[28] They reported that observed values were much greater than predicted DPCO2, concluding that in such pulmonary hypo-perfusion with right-to-left intracardiac shunting, pulmonary hyper-perfusion caused by large left-to-right shunts increases the DPCO2.
Normal small systemic to pulmonary collateral blood flow may get significantly high values in patients with underdeveloped native pulmonary circulation. This abnormal pulmonary flow comes from aorta and other systemic arteries can affect patient's DPCO2 that may continue for a long time after cardiac surgery.[29,30] ETPCO2 reflects the amount of blood flow from lungs circulation; thus, in low cardiac output states, DPCO2 may be affected in low cardiac output patients. Our study did not consider this important aspect.
The current results showed a higher initial DPCO2 in palliative group patients, which may be due to more severe cardiopulmonary derangement in these patients, as they were younger and had more severe cyanosis. Another important finding was similar DPCO2 in palliative and corrective groups in early post-CPB period. Conventionally, immediately after weaning from CPB, there are a lot of problems; most of them usually recover in a few minutes or hours. Thus, in early post-CPB period, the definitive results of intervention may not be apparent, and DPCO2 should not be used as an indicator of incomplete surgery or replace IOTEE.
Furthermore, in many pathologic conditions, DPCO2 increases, but there is a stable reliable relationship between ETPCO2 and PaCO2. In the current study, DPCO2 was very unstable in palliative group, supporting Lazzel and Tugrul studies.[22,31] Lazzel and Burrows studied the stability of DPCO2 during various times of surgery in children with various types of CHD and reported that it is generally stable intraoperatively although some patients may demonstrate large individual variations.[22] In children with cyanotic CHD, DPCO2 was not stable. They concluded that ETPCO2 cannot be used during surgery to reliably estimate PaCO2 in children with cyanotic CHD. The unstable DPCO2 in cyanotic CHD is also reflected in Tugrul et al.'s study;[31] in a prospective clinical study, they investigated the relationship between ETPCO2 and PBF augmentation achieved by insertion of a Blalock-Taussig shunt in cyanotic children with TOF. They reported a significant negative correlation between DPCO2 and arterial oxygen saturation and recommended that ETPCO2 alterations offer an alternative intraoperative tool to monitor PBF during systemic to pulmonary shunt procedures. Russell and Graybeal reported that ETPCO2 does not provide a stable reflection of PaCO2 during craniotomy;[14] however, some others do not agree with him.[16,32] Heines et al. studied it in mechanically ventilated postcardiac surgery patients and concluded that there is a good correlation between ETPCO2 and PaCO2, but it is not valid to estimate PaCO2.[16] Therefore, expiratory capnography cannot be used to replace serial blood gas analyses completely and may be solely a good cardiopulmonary trend monitor. In other words, an abnormal DPCO2 is a reflection of abnormal cardiopulmonary physiology.
CONCLUSIONS
DPCO2 is higher than normal values in all CHDs. It does not change after palliative cardiac procedures. DPCO2 decreases significantly after corrective procedures; however, it does not return to normal values at early postoperative period. Thus, DPCO2 does not have any clinical value in monitoring the quality of corrective or palliative procedures. ETPCO2 cannot completely be replaced with the gold standard technique (serial blood gas analyses) in pediatric cardiac surgeries.
Contribution details
EB, SF, and AY were contributed to the concept of study. They planned the study frame. EB and SF were responsible for preoperative visit and premedication. MM, SA, and YE provided the intraoperative anesthetic management. EB, SF, and AY managed the patient as long they were in postcardiac Pediatric Intensive Care Unit. EB, SF, and YE participated in the data collection and recording in perioperative period. EB and SF had the responsibility of to supervise the study and controlled data validity periodically. EB and SF by close working with a social medicine specialist were responsible for data handling and analyzing. EB, SF, SA, and YE prepared the manuscript for publish. All authors reviewed and approved the final version of the manuscript.
Financial support and sponsorship
The study was financially supported by grants from the Medical Research center of Tabriz University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Nakata Y, Goto T, Uezono S, Sasaki F, Morita S. Relationship between end-tidal and arterial carbon dioxide partial pressure using a cuffed oropharyngeal airway and a tracheal tube. Br J Anaesth. 1998;80:253–4. doi: 10.1093/bja/80.2.253. [DOI] [PubMed] [Google Scholar]
- 2.Casati A, Fanelli G, Cappelleri G, Albertin A, Anelati D, Magistris L, et al. Arterial to end-tidal carbon dioxide tension difference in anaesthetized adults mechanically ventilated via a laryngeal mask or a cuffed oropharyngeal airway. Eur J Anaesthesiol. 1999;16:534–8. doi: 10.1046/j.1365-2346.1999.0534a.x. [DOI] [PubMed] [Google Scholar]
- 3.Rozycki HJ, Sysyn GD, Marshall MK, Malloy R, Wiswell TE. Mainstream end-tidal carbon dioxide monitoring in the neonatal Intensive Care Unit. Pediatrics. 1998;101:648–53. doi: 10.1542/peds.101.4.648. [DOI] [PubMed] [Google Scholar]
- 4.Bhat YR, Abhishek N. Mainstream end-tidal carbon dioxide monitoring in ventilated neonates. Singapore Med J. 2008;49:199–203. [PubMed] [Google Scholar]
- 5.Wu CH, Chou HC, Hsieh WS, Chen WK, Huang PY, Tsao PN, et al. Good estimation of arterial carbon dioxide by end-tidal carbon dioxide monitoring in the neonatal Intensive Care Unit. Pediatr Pulmonol. 2003;35:292–5. doi: 10.1002/ppul.10260. [DOI] [PubMed] [Google Scholar]
- 6.Nangia S, Saili A, Dutta AK. End tidal carbon dioxide monitoring – Its reliability in neonates. Indian J Pediatr. 1997;64:389–94. doi: 10.1007/BF02845211. [DOI] [PubMed] [Google Scholar]
- 7.McSwain SD, Hamel DS, Smith PB, Gentile MA, Srinivasan S, Meliones JN, et al. End-tidal and arterial carbon dioxide measurements correlate across all levels of physiologic dead space. Respir Care. 2010;55:288–93. [PMC free article] [PubMed] [Google Scholar]
- 8.Choudhury M, Kiran U, Choudhary SK, Airan B. Arterial-to-end-tidal carbon dioxide tension difference in children with congenital heart disease. J Cardiothorac Vasc Anesth. 2006;20:196–201. doi: 10.1053/j.jvca.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 9.van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, et al. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241–7. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 10.Hanna BM, El-Hewala AA, Gruber PJ, Gaynor JW, Spray TL, Seliem MA, et al. Predictive value of intraoperative diagnosis of residual ventricular septal defects by transesophageal echocardiography. Ann Thorac Surg. 2010;89:1233–7. doi: 10.1016/j.athoracsur.2009.10.058. [DOI] [PubMed] [Google Scholar]
- 11.Yang SG, Novello R, Nicolson S, Steven J, Gaynor JW, Spray TL, et al. Evaluation of ventricular septal defect repair using intraoperative transesophageal echocardiography: Frequency and significance of residual defects in infants and children. Echocardiography. 2000;17:681–4. doi: 10.1046/j.1540-8175.2000.00681.x. [DOI] [PubMed] [Google Scholar]
- 12.Allen HD, Driscoll DJ, Shaddy RD, Feltes TF. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2001. Moss and Adams' Heart Disease in Infants, Children and Adolescents: Including the Fetus and Young Adult; pp. 524–42. [Google Scholar]
- 13.Guzeltas A, Ozyilmaz I, Tanidir C, Odemis E, Tola HT, Ergul Y, et al. The significance of transesophageal echocardiography in assessing congenital heart disease: Our experience. Congenit Heart Dis. 2014;9:300–6. doi: 10.1111/chd.12139. [DOI] [PubMed] [Google Scholar]
- 14.Russell GB, Graybeal JM. The arterial to end-tidal carbon dioxide difference in neurosurgical patients during craniotomy. Anesth Analg. 1995;81:806–10. doi: 10.1097/00000539-199510000-00025. [DOI] [PubMed] [Google Scholar]
- 15.Campbell FA, McLeod ME, Bissonnette B, Swartz JS. End-tidal carbon dioxide measurement in infants and children during and after general anaesthesia. Can J Anaesth. 1994;41:107–10. doi: 10.1007/BF03009801. [DOI] [PubMed] [Google Scholar]
- 16.Heines SJ, Strauch U, Roekaerts PM, Winkens B, Bergmans DC. Accuracy of end-tidal CO2 capnometers in post-cardiac surgery patients during controlled mechanical ventilation. J Emerg Med. 2013;45:130–5. doi: 10.1016/j.jemermed.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Mehta H, Kashyap R, Trivedi S. Correlation of end tidal and arterial carbon dioxide levels in critically ill neonates and children. Indian J Crit Care Med. 2014;18:348–53. doi: 10.4103/0972-5229.133874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Razi E, Moosavi GA, Omidi K, Khakpour Saebi A, Razi A. Correlation of end-tidal carbon dioxide with arterial carbon dioxide in mechanically ventilated patients. Arch Trauma Res. 2012;1:58–62. doi: 10.5812/atr.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yosefy C, Hay E, Nasri Y, Magen E, Reisin L. End tidal carbon dioxide as a predictor of the arterial PCO2 in the emergency department setting. Emerg Med J. 2004;21:557–9. doi: 10.1136/emj.2003.005819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cinar O, Acar YA, Arziman I, Kilic E, Eyi YE, Ocal R, et al. Can mainstream end-tidal carbon dioxide measurement accurately predict the arterial carbon dioxide level of patients with acute dyspnea in ED. Am J Emerg Med. 2012;30:358–61. doi: 10.1016/j.ajem.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Takano Y, Sakamoto O, Kiyofuji C, Ito K. A comparison of the end-tidal CO2 measured by portable capnometer and the arterial PCO2 in spontaneously breathing patients. Respir Med. 2003;97:476–81. doi: 10.1053/rmed.2002.1468. [DOI] [PubMed] [Google Scholar]
- 22.Lazzell VA, Burrows FA. Stability of the intraoperative arterial to end-tidal carbon dioxide partial pressure difference in children with congenital heart disease. Can J Anaesth. 1991;38:859–65. doi: 10.1007/BF03036960. [DOI] [PubMed] [Google Scholar]
- 23.Sayadpour Zanjani K, Aarabi Moghadam MY. Residual defects after surgical repair of ventricular septal defects in children: Incidence, risk factors and follow-up. Acta Med Iran. 2008;46:495–500. [Google Scholar]
- 24.Tingay DG, Mun KS, Perkins EJ. End tidal carbon dioxide is as reliable as transcutaneous monitoring in ventilated postsurgical neonates. Arch Dis Child Fetal Neonatal Ed. 2013;98:F161–4. doi: 10.1136/fetalneonatal-2011-301606. [DOI] [PubMed] [Google Scholar]
- 25.Trevisanuto D, Giuliotto S, Cavallin F, Doglioni N, Toniazzo S, Zanardo V, et al. End-tidal carbon dioxide monitoring in very low birth weight infants: Correlation and agreement with arterial carbon dioxide. Pediatr Pulmonol. 2012;47:367–72. doi: 10.1002/ppul.21558. [DOI] [PubMed] [Google Scholar]
- 26.Naidu S. Is ETCO2 a predictor of PaCO2 in ventilated neonates on the neonatal Intensive Care Unit? Work Pap Health Sci. 2012;1:1. [Google Scholar]
- 27.Amuchou Singh S, Singhal N. Dose end-tidal carbon dioxide measurement correlate with arterial carbon dioxide in extremely low birth weight infants in the first week of life? Indian Pediatr. 2006;43:20–5. [PubMed] [Google Scholar]
- 28.Short JA, Paris ST, Booker PD, Fletcher R. Arterial to end-tidal carbon dioxide tension difference in children with congenital heart disease. Br J Anaesth. 2001;86:349–53. doi: 10.1093/bja/86.3.349. [DOI] [PubMed] [Google Scholar]
- 29.Liao PK, Edwards WD, Julsrud PR, Puga FJ, Danielson GK, Feldt RH, et al. Pulmonary blood supply in patients with pulmonary atresia and ventricular septal defect. J Am Coll Cardiol. 1985;6:1343–50. doi: 10.1016/s0735-1097(85)80223-0. [DOI] [PubMed] [Google Scholar]
- 30.Boshoff D, Gewillig M. A review of the options for treatment of major aortopulmonary collateral arteries in the setting of tetralogy of Fallot with pulmonary atresia. Cardiol Young. 2006;16:212–20. doi: 10.1017/S1047951106000606. [DOI] [PubMed] [Google Scholar]
- 31.Tugrul M, Camci E, Sungur Z, Pembeci K. The value of end-tidal carbon dioxide monitoring during systemic-to-pulmonary artery shunt insertion in cyanotic children. J Cardiothorac Vasc Anesth. 2004;18:152–5. doi: 10.1053/j.jvca.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Lobo D. The arterial to end-tidal carbon dioxide difference in neurosurgical patients during craniotomy. Anesth Analg. 1996;82:1113. doi: 10.1097/00000539-199605000-00063. [DOI] [PubMed] [Google Scholar]


