Abstract
Biliary tract cancers (BTC) represent an aggressive disease with a dismal prognosis. Gemcitabine in combination with cisplatin is the standard first-line palliative treatment for advanced BTC. There is no established treatment following progression on gemcitabine-cisplatin. In this article, we present two cases for individuals with advanced BTC who were treated with pembrolizumab and the tumors have completely resolved.
Keywords: cholangiocarcinoma, immunotherapy, gallbladder carcinoma, targeted therapy
Introduction
Biliary tract cancers (BTC) include intrahepatic bile duct cancers, extrahepatic bile duct cancers, and gallbladder cancers.1 These adenocarcinomas all arise from the biliary epithelium. Gallbladder cancer is the predominant type, accounting for 60–70% of cases with the reminder distributed between the intra- and extrahepatic biliary trees.1 There are more than 186,000 new cases of BTC diagnosed worldwide each year.2 Early lymph node involvement and early distant metastases are characteristics of BTC.3,4 In addition, the lack of a serosal layer in the gallbladder adjacent to the liver enables hepatic invasion and metastatic progression.3,4 These factors prevent up to 90% of patients from receiving curative intent surgery and contribute to its miserable prognosis.3,4
Gemcitabine in combination with cisplatin is the standard first-line palliative treatment for advanced BTC, with a median survival time of 11.2–11.7 months.5 There is no established treatment following progression on gemcitabine-cisplatin. In this article, we present two cases for individuals with advanced BTC who were treated with pembrolizumab, both patients showed complete disease resolution.
Cases presentation
Case 1
A 70-year-old female, known case of gout, presented to our center through emergency department with 2 weeks history of right upper quadrate (RUQ) pain. The pain was sudden with no radiation, relieved by analgesia, not related to food. There was no fever, no vomiting, no change in bowel habit or stool color, and no jaundice. No history of trauma. No remarkable surgical history prior to her complaint. She was not a smoker. The physical examination disclosed only an abdominal tenderness in the RUQ.
Laboratory investigation revealed mild leukocytosis with normal liver enzymes, normal bilirubin level, and normal liver function panel. Also, the tumor marker tests were performed after awhile and revealed elevation in cancer antigen 19–9 (CA19-9) (291.6 U/mL).
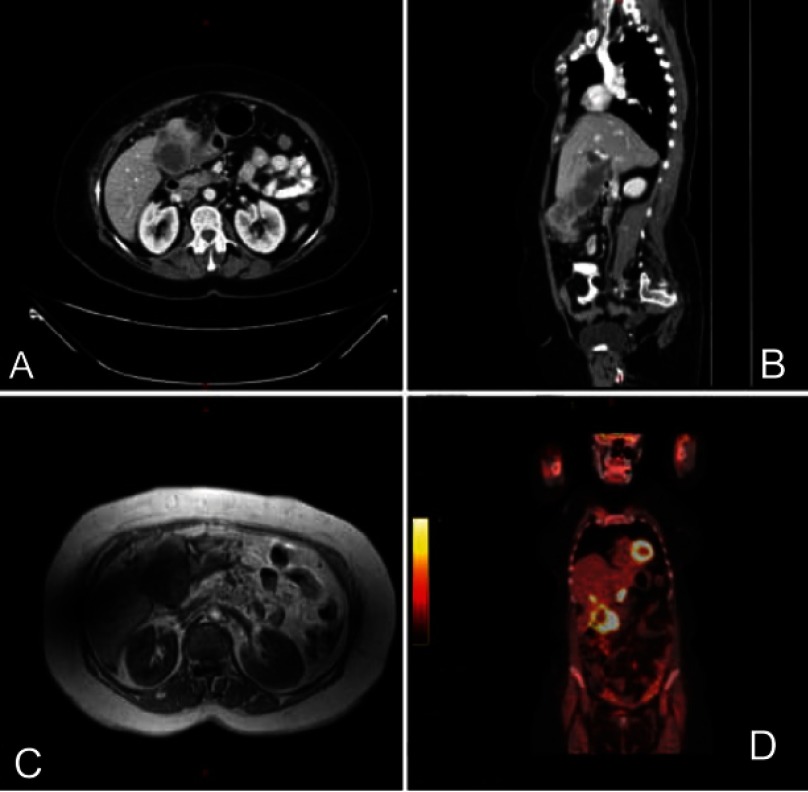
Radiological studies and interventions were conducted. Abdominal ultrasound revealed distended gallbladder with stone and suspicion of soft tissue mass. Then, an enhanced computerized tomography (CT) scan of the chest, abdomen, and pelvis was performed and demonstrated an irregular enhancing diffuse wall thickening of the gallbladder mainly at the fundus with an exophytic mass lesion measuring about 5×6 cm abutting the hepatic flexure with fatty infiltration of the liver and hypodense hepatic lesions noted in the gallbladder bed with largest measuring about 1.3 cm in diameter. Also, the proximal transverse colon showed wall thickening with regional lymphadenopathy, Figure 1(A and B). Then, the patient underwent positron emission tomography (PET) scan that also demonstrated intensely increased uptake involving the gallbladder wall as well as exophytic mass coming from the gallbladder strongly suspicious for primary malignancy. Also, there are multiple foci of increased uptake in the liver at the gallbladder bed representing direct invasion of the primary gallbladder tumor into liver. The multiple enlarged lymph nodes seen on CT images were hypermetabolic on PET scan which was consistent with metastatic lymphadenopathy, Figure 1(D).
Figure 1.
(A) CT scan – axial view at diagnosis. Exophytic mass arising from the gallbladder and invade the liver. (B) CT scan: sagittal view at diagnosis. Exophytic mass arising from the gallbladder and invade the transverse colon. (C) MRCP at diagnosis indicating a mass from the gallbladder mostly represented a primary tumor. (D) PET scan showing hypermetabolic lesion from the gallbladder. Abbreviations: CT, computed tomography; MRCP, Magnetic resonance cholangiopancreatography; PET, positron emission tomography.
While the lower gastrointestinal endoscopy was obtained, and it was unremarkable, the possibility of colon cancer was excluded. Also, she was offered magnetic cholangiopancreatography which yielded a diffuse wall thickening of dilated gallbladder mainly at the fundus with a heterogeneously enhancing soft tissue mass lesion arising from its wall measuring 4.7×6.7 cm. The mass extended downward and appears inseparable from the proximal transverse colon which appeared thickened and edematous with few enlarged regional lymph nodes in the porta hepatis and precaval nodes. The mass manifested invasion to segment VIb of the liver as well. Also, there were two large stones and multiple enhancing polyps inside the gallbladder. Moreover, few well-defined high T2 signal intensity lesions were seen in the liver at the gallbladder bed the largest of which measured about 1.3 cm, Figure 1(C). After that, percutaneous biopsy was taken from the mass which demonstrated invasive moderately differentiated adenocarcinoma consistent with gallbladder origin.
Multidisciplinary team was consulted, and the decision was to attempt radical surgery by an expert surgeon. Open cholecystectomy, partial hepatectomy with transverse colectomy, and lymph node dissection were performed. Microscopic examination revealed solid sheets of tumor cells invaded the gallbladder and liver tissue. The tumor cells were bizarre shape, hyperchromatic, high nuclear/cytoplasm ratio, prominent nucleoli, and high mitotic index. There is geographic necrosis detected. The tumor cells were immunoreactive for cytokeratin (CK)19, CK7 and CK5/6 and immunonegative for CK20, and hepatocyte-paraffin stain. The tumor was poorly differentiated (grade 3). The surgical margins were negative. The tumor exhibited lymphovascular and perineural invasion. The TNM stage for the cancer was 4B (T=3, N=2, M=0). The histopathological results confirmed the aforementioned diagnosis. Unfortunately, analysis of microsatellite instability was not available.
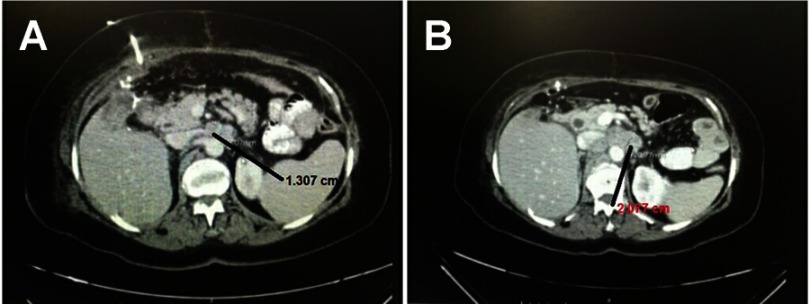
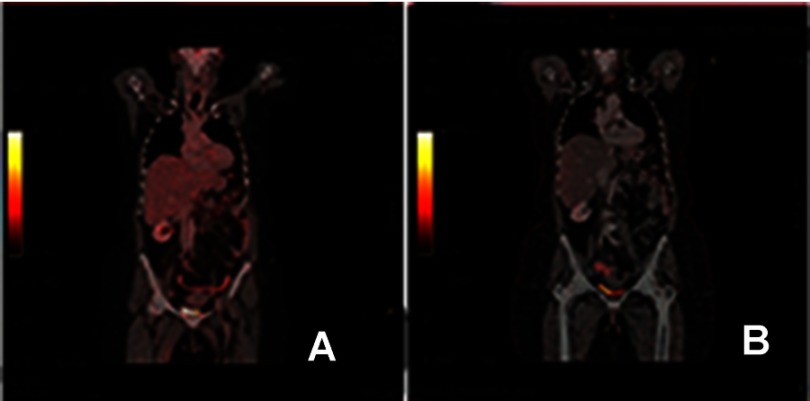
However, CT scan was performed 1 month postoperatively and disclosed a recurrent local cancer with relapse of the liver, colon metastasis, and lymph nodes. Other radiological examinations were not available and the clinical status of the patient did not allow us to take biopsy. Another CT scan two months postoperatively confirms the progression of the relapsed disease, Figure 2(A and B). Consequently, and after obtaining the consent from the patient, she commenced a pembrolizumab regimen for the advanced tumor. The patient’s clinical performance was poor. In addition, she refused chemotherapy. For these two reasons we consulted the patients and offered the pembrolizumab. The pembrolizumab was administered in eight doses. The doses were 3 weeks apart. The total duration of treatment was 21 weeks. Each dose was 100 mg (half the complete dose due to financial issues as this drug is expensive for the patients). PET scan after the eighth cycle revealed a complete resolution of the tumor, Figure 3(A). The last PET scan was performed 2 years after the last dose of pembrolizumab and indicated no disease. The patient was clinically and radiologically free after 2 years follow-up, Figure 3(B).
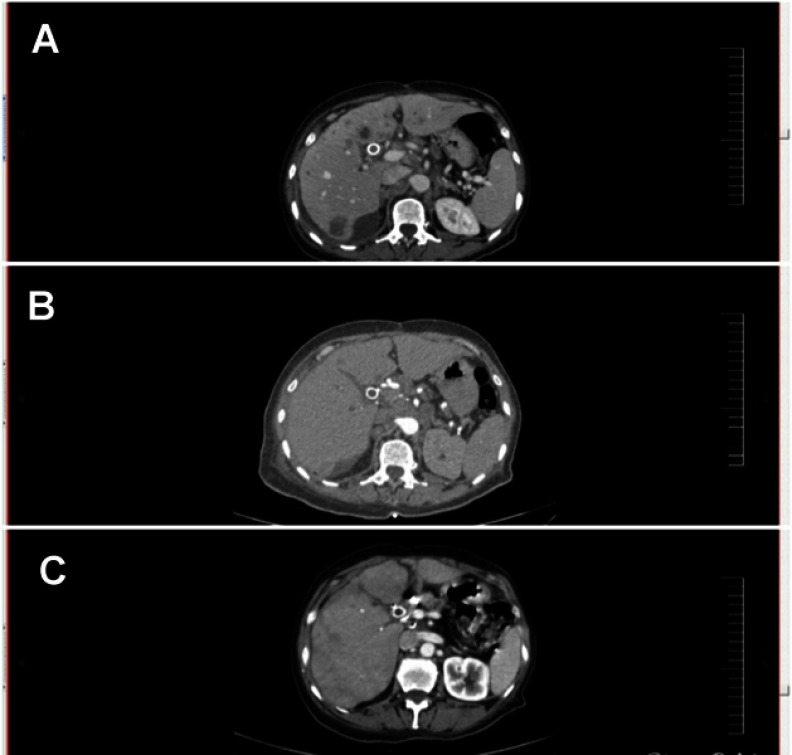
Figure 2.
(A) CT scan – axial view 1 month after the operation demonstrating local recurrence. Lymph node was detected and measured 1.3 cm. (B) CT scan – axial view 2 months after the operation demonstrating local recurrence and the same node became 2 cm.
Figure 3.
(A) PET scan at the end of the eighth cycle indicating complete resolution of the tumor. (B) PET scan after 2 years the immunotherapy showing no disease. Abbreviation: PET, positron emission tomography.
Case 2
A 51-year-old female, not known to have any medical illness, presented to our center with painless progressive yellowish discoloration of her face. Associated with prurities, dark urine, and pale stool. No previous history of biliary colic or similar episodes. The patient stated significant weight loss, anorexia, and asthenia. No history of contact with hepatitis patient. Neither blood transfusion nor drug abuse was reported. No travel history. No previous surgical history. No family history of malignancies. She was not a smoker. On examination, yellowish discoloration of whole body was noted. No abdominal tenderness or masses felt. Laboratory investigation was conducted and disclosed a direct hyperbilirubinemia with a total bilirubin of 200 umol/L and direct bilirubin of 196 umol/L. In addition, alkaline phosphatase and gamma-glutamyl transferase were significantly elevated (972 and 973 U/L, respectively). The aspartate aminotransferase and alanine aminotransferase were mildly elevated. The complete blood count revealed microcytic anemia that was found to be due to iron deficiency. Normal coagulation profile was yielded. Lipase and amylase levels were normal. The CA 19.9 was significantly elevated.
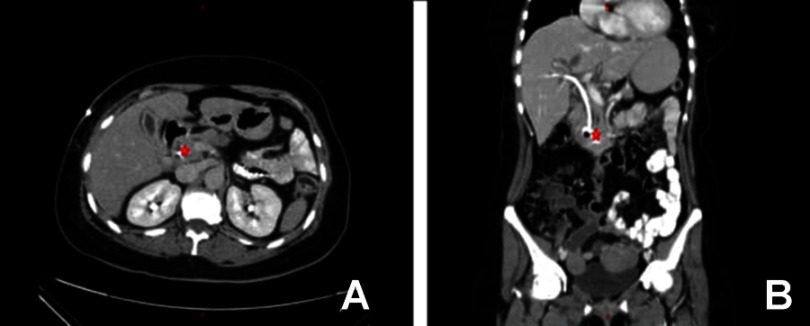
She underwent a series of radiological studies and interventions. Abdominal ultrasound revealed a severe dilation of the common bile duct with a diameter of 15 mm with intrahepatic and pancreatic duct dilatation. After that, endoscopic retrograde cholangiopancreatography (ERCP) was done and showed a completely invaded ampulla of vater by a vegetating mass with no visible orifice or bile flow. Subsequently, the biliary tree could not be visualized. Multiple biopsies were taken from the ampullary mass which demonstrated the presence of invasive moderately differentiated adenocarcinoma consistent with biliary origin. As the ERCP failed to relieve the obstructions, she underwent percutaneous transhepatic cholangiography that also demonstrated an intra- and extrahepatic biliary dilation. Internal and external drainage were achieved. Chest–abdomen–pelvis CT scan revealed the presence of periampullary mass lesion that invades the second part of duodenum with regional lymphadenopathy, Figure 4(A and B). Also, PET scan was conducted, and a hypermetabolic mass measured 3.5×2.5 cm was located around ampulla of vater with perihepatic, celiac, retropancreatic, aortic, and precaval lymphadenopathy. These findings were consistent with distal cholangiocarcinoma with regional invasion.
Figure 4.
(A) CT scan – axial view at the time of diagnosis and after PTC indicating mass lesion near the ampulla of Vatter. (B) CT scan coronal view at the time of diagnosis and after PTC indicating mass lesion near the ampulla of Vatter. The stent of the PTC is obvious.
Abbreviations: CT, computed tomography; PTC, Percutaneous transhepatic cholangiography.
Multidisciplinary team was informed, and the decision was to go for radical surgery by an experienced surgeon. Whipple procedure was performed with pancreaticoduodenectomy, distal gastrectomy, and a dissection of 20 regional lymph nodes were done. Microscopic examination demonstrated multiple fragments of duodenal and ampullary mucosa infiltrated by a tumor composed of moderately differentiated glands and tubules, lined by pleomorphic cells with hyperchromatic nuclei and prominent nucleoli. Areas of central necrosis are also detected. The tumor cells are immunoreactive for CK19, CK20, and CKX2 immunostains and immunonegative for CK7 immunostain. The tumor was moderately differentiated (grade 2). The surgical margins were negative. The tumor exhibited lymphovascular and perineural invasion. The TNM stage was 3B (T=3, N=1, M=0). The resultant histopathological examination confirmed the aforementioned diagnosis of distal cholangiocarcinoma with regional lymph nodes metastasis. Unfortunately, analysis of microsatellite instability was not available.
While the surgery was radical and achieved R0 resection, the decision was to commence the patient on gemcitabine-cisplatin chemotherapy. This decision was based on the results that the lymph nodes were invaded by the tumor. Shortly after the surgery, the patient started chemotherapy. After the second cycle, the gemcitabine-cisplatin regimen was stopped due to two main reasons. The follow-up CT scan revealed a progression of the disease as a hepatic metastasis, Figure 5(A). Also, the patient developed acute heart failure as a side effect of the chemotherapy. As a result, and after obtaining a consent from the patient, she started a pembrolizumab regimen. The pembrolizumab was administered in eight doses. The doses were 3 weeks apart. The total duration of treatment was 21 weeks. Each dose was 100 mg (half the complete dose due to financial issues as this drug is expensive for the patients). Whole body CT scan during and after the eighth cycle revealed decrease and complete resolution of the tumor, Figure 5(B and C). The last CT scan was performed 2 years after the last dose of pembrolizumab and demonstrated no relapse.
Figure 5.
(A) CT scan axial view after the second cycle of the chemotherapy indicating the progression of the disease by liver metastasis. (B) CT scan axial view during the treatment with pembrolizumab indicating clearance of most of the liver lesions. (C) CT scan axial view at the end of the immunotherapy demonstrating resolution of the disease.
Discussion
To our knowledge, this is a study to report a complete resolution of advanced BTC after treatment by a monotherapy of pembrolizumab. Bang et al reported in their clinical trial of four patients who had partial response, 4 patients had stable disease, and 12 patients had progressive disease as their best response.6 Czink et al reported a partial response of extrahepatic cholangiocarcinoma after treatment with pembrolizumab in a patient with tumor displayed DNA mismatch repair deficiency and microsatellite instability but lacked programmed death ligand 1 (PD-L1) expression.7 Arkenau et al studied the combination of ramucirumab and pembrolizumab which did not demonstrate an improvement in survival.8
Pembrolizumab is a humanized monoclonal antibody against programmed death 1 (PD-1) that has antitumor activity, with increased activity in tumors that express PD-L1. The programmed cell death protein 1 (PD1) is one of the checkpoints that regulates the immune response.9,10 Ligation of PD1 with its ligands PDL results in transduction of negative signals to T-cells. PD1 expression is an important mechanism contributing to the exhausted effector T-cell phenotype.9,10 The expression of PD1 on effector T-cells and PDL on neoplastic cells enables tumor cells to evade anti-tumor immunity.9,10 Blockade of PD1 is an important immunotherapeutic strategy for cancers.11,12
Pembrolizumab was approved by the FDA for the treatment of advanced melanoma and non-small cell lung cancer.13–15 In refractory melanoma, pembrolizumab induced overall response rates of 21–34%.13 It was superior to another immune checkpoint inhibitor ipilimumab in stage III/IV unresectable melanoma.13 In refractory non-small cell lung cancer, pembrolizumab induced overall response rates of 19–25%.14,15 Also, pembrolizumab was found to be effective in the treatment of the following malignancies: recurrent or metastatic cervical cancer, recurrent locally advanced gastric or gastroesophageal junction adenocarcinoma, recurrent or metastatic head and neck squamous cell carcinoma, primary mediastinal large B-cell lymphoma in adult or pediatric patients with refractory disease, adult and pediatric patients with refractory classical Hodgkin lymphoma, locally advanced or metastatic urothelial carcinoma.16–21
BTC represents an aggressive disease with a dismal prognosis, hence the development of novel treatment strategies is fundamental.22 The classical chemotherapeutic regimens include gemcitabine and oxaliplatin, gemcitabine and cisplatin, 5-fluorouracil and oxaliplatin, or single agent options as gemcitabine and capecitabine.22 A number of clinical trials with targeted therapies have been completed in recent years.23 A main problem that has emerged is the large number of driver mutations with small patient subsets for each target.23 Also, differences across intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, and gallbladder adenocarcinoma are the main limitations.23 Bang et al reported in their clinical trial four patients had partial response as they were treated by a monotherapy of pembrolizumab.6 In the presenting cases, we used pembrolizumab as a palliative agent after the progression of the BTC. The surprising results were the complete resolution of the disease on 2 years of follow-up. The main limitation point in our study is the lack of the genetic and molecular analysis.
Conclusion
BTC represents an aggressive disease with a dismal prognosis. Pembrolizumab could have a large effect on the survival of patients with these aggressive tumors or even curative effect. Clinical trials focusing on this type of therapy are recommended.
Ethics and patient consent
The patient has provided written informed consent for the case details and images to be published. Institutional approval was not required.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21177 [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. doi: 10.2147/CLEP.S37357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu AX, Hezel AF. Development of molecularly targeted therapies in biliary tract cancers: reassessing the challenges and opportunities. Hepatology. 2011;53:695–704. doi: 10.1002/hep.24145 [DOI] [PubMed] [Google Scholar]
- 5.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721 [DOI] [PubMed] [Google Scholar]
- 6.Bang YJ, Doi T, Braud FD, et al. 525 Safety and efficacy of pembrolizumab (MK 3475) in patients (pts) with advanced biliary tract cancer: interim results of KEYNOTE-028. Eur J Cancer. 2015;51:S112. [Google Scholar]
- 7.Czink E, Kloor M, Goeppert B, et al. Successful immune checkpoint blockade in a patient with advanced stage microsatellite-unstable biliary tract cancer. Cold Spring HarbMol Case Stud. 2017;3: pii:a001974. doi: 10.1101/mcs.a001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arkenau HT, Martin-Liberal J, Calvo E, et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced or metastatic biliary tract cancer: nonrandomized, Open-Label, Phase I Trial (JVDF). Oncologist. 2018;23:1407–e136. theoncologist.2018–0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517 [DOI] [PubMed] [Google Scholar]
- 11.Kwok G, Yau TC, Chiu JW, Tse E, Kwong YL. Pembrolizumab (Keytruda). Hum Vaccin Immunother. 2016;12:2777–2789. doi: 10.1080/21645515.2016.1199310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1 positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 13.Robert C, Ribas A, Wolchok JD, et al. Anti-programmeddeath- receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2 [DOI] [PubMed] [Google Scholar]
- 14.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl JMed. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 15.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 16.Frenel JS, Le Tourneau C, O’Neil B, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1 positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J Clin Oncol. 2017;35:4035–4041. doi: 10.1200/JCO.2017.74.5471 [DOI] [PubMed] [Google Scholar]
- 17.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–965. doi: 10.1016/S1470-2045(16)30066-3 [DOI] [PubMed] [Google Scholar]
- 18.Joshi SS, Maron SB, Catenacci DV. Pembrolizumab for treatment of advanced gastric and gastroesophageal junction adenocarcinoma. Future Oncol. 2018;14:417–430. doi: 10.2217/fon-2017-0436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zinzani PL, Ribrag V, Moskowitz CH, et al. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinallarge B-cell lymphoma. Blood. 2017;130:267–270. doi: 10.1182/blood-2016-12-758383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen R, Zinzani PL, Fanale MA, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic hodgkin lymphoma. J Clin Oncol. 2017;35:2125–2132. doi: 10.1200/JCO.2016.72.1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan E, Abou-Alfa GK, Lowery MA. Systemic therapy for biliary cancers. Chin Clin Oncol. 2016;5(5):65. doi: 10.21037/cco [DOI] [PubMed] [Google Scholar]
- 23.Hezel AF, Deshpande V, Zhu AX. Genetics of biliary tract cancers and emerging targeted therapies. J Clin Oncol. 2010;28:3531–3540. doi: 10.1200/JCO.2009.27.4787 [DOI] [PMC free article] [PubMed] [Google Scholar]