Abstract
Portal vein thrombosis (PVT) is a common complication of cirrhosis sometimes implicated in hepatic decompensation. There are no consistent epidemiologic data to suggest an increased risk of thrombotic complications in nonalcoholic steatohepatitis (NASH); however, research suggests an increased risk of thrombosis. Our aim was to examine the independent association between NASH cirrhosis and PVT in patients who underwent liver transplantation (LT) in a cross-sectional study. Data on all LTs occurring in the United States between January 1, 2003 and December 31, 2012 were obtained from the United Network for Organ Sharing. Multivariable models were constructed to assess the statistical associations and risk factors for the development of PVT. A total of 33,368 patients underwent transplantation. Of these, 2096 (6.3%) had PVT. Of the patients with PVT, 12.0% had NASH. When we compared these patients to a composite of all other causes of cirrhosis, an increased prevalence of PVT was again found, with 10.1% having PVT at the time of transplantation versus 6.0% without NASH (P< 0.001). The strongest risk factor independently associated with a diagnosis of PVT in a multivariable analysis was NASH cirrhosis (odds ratio, 1.55; 95% confidence interval, 1.33–1.81; P< 0.001). NASH cirrhosis appears to predispose a patient to PVT independently of other risk factors. These epidemiological findings provide support for the idea that NASH is a prothrombotic state, and they should lead to more research in treatment and prevention in this population.
Nonalcoholic steatohepatitis (NASH) is the most severe form of nonalcoholic fatty liver disease (NAFLD) and is characterized by steatosis, inflammation, cell injury, and fibrosis.1 It is thought to also underlie many cases of cryptogenic cirrhosis.2 With increasing obesity in industrialized nations, prevalence rates for NAFLD are approximately 20%;3 this corresponds to 28 to 31 million adults worldwide.3 NASH is expected to become the leading indication for liver transplantation (LT) in the United States by 2025.4 Chronic ongoing inflammation from NASH results in lipid-based oxidative injury and necroapoptosis.5–8 This may lead to activation of the coagulation system and a resultant in vitro hypercoaguable state because procoagulant levels of plasminogen activator inhibitor 1 (PAI-1) and factor VIII have been shown to be elevated, whereas anticoagulant levels of protein C are decreased in NASH cirrhosis.9 NASH has also been independently associated with ischemic heart disease,10 but there are no consistent epidemiological or clinical data to support increased rates of venous thrombosis in NASH. In addition to macro-vascular disease, intrahepatic microthrombosis in NASH may play a role in the progression of fibrosis and parenchymal extinction,11 and the presence of thrombotic risk factors is associated with higher degrees of fibrosis and inflammatory stages on biopsy.12
The relationship between NASH and macrovascular venous thrombosis, particularly portal vein thrombosis (PVT), is less well documented, but it has been described in a limited number of patients.13 Although there is an ongoing debate regarding its clinical significance and the appropriate therapy, several studies indicate an adverse effect on clinical outcomes with or without transplantation.14,15 The prevalence of PVT in patients with cirrhosis has been reported to be as high as 26%16 and as high as 36% at explant examination in patients undergoing LT, as suggested by the presence of thickened walls on direct examination, which is consistent with previous thrombosis.17 The incidence of PVT is variable but can be as high as 16%.18 With the increasing incidence of NASH and the longer transplantation wait list and survival times of patients with cirrhosis, it is reasonable to presume that thrombotic complications, including PVT, will continue to increase in incidence. The importance of PVT is just beginning to be fully understood because its presence can lead to not only clinical deterioration, complications affecting quality of life, and hepatic decompensation but also increased mortality after LT 15,19
In this retrospective, nationwide US epidemiological cohort study, we aimed to examine whether or not there is an association between NASH and PVT in patients who undergo LT.
PATIENTS AND METHODS
Study Design and Recipient Characteristics
Data on all LTs occurring in the United States between January 1, 2003 and December 31, 2012 were obtained from the Organ and Transplantation Network (OPTN) with permission from the United Network for Organ Sharing (UNOS). Only transplant candidates who were listed for transplantation at or above the age of 18 years were included in the analysis. All transplants for acute liver failure, status 1 candidates (urgent retransplantation), malignancy (hepatocellular carcinoma, cholangiocarcinoma, and hepatoblastoma), and recipients with transjugular intrahepatic portosystemic shunts were excluded from the analysis. Because the definition of PVT in this data set relied on explant examinations and direct surgical observations, only transplant candidates who successfully underwent transplantation were included in this analysis. In the transplantation data set, the etiology of cirrhosis was characterized into 1 of 79 different diagnoses on the basis of clinical information available at the time of the initial evaluation and listing. In this article, transplant recipients were categorized into 1 of 2 disease etiology categories: (1) recipients with diagnosis code 4214 (“Cirrhosis: Fatty Liver [NASH]”) were categorized as NASH cirrhosis, and (2) recipients with all other cirrhosis codes were categorized as non-NASH. Recipients with diagnosis code 4208 (“Cirrhosis: Cryptogenic–Idiopathic”) and diagnosis code 4213 (“Cirrhosis: Cryptogenic [Idiopathic]”) were categorized as cryptogenic cirrhosis and were excluded from the analysis because of the potential miscoding of NASH recipients as cryptogenic. Baseline covariate characteristics were reviewed, and they included recipient characteristics (age at listing and at LT, sex, and ethnicity), etiology of liver disease, severity of liver disease based on the Model for End-State Liver Disease (MELD) score, laboratory values (bilirubin, international normalized ratio [INR], creatinine, and albumin), portal hypertension manifestations (ascites and hepatic encephalopathy), and NASH risk factors (diabetes and body mass index [BMI]).
Outcomes Definition
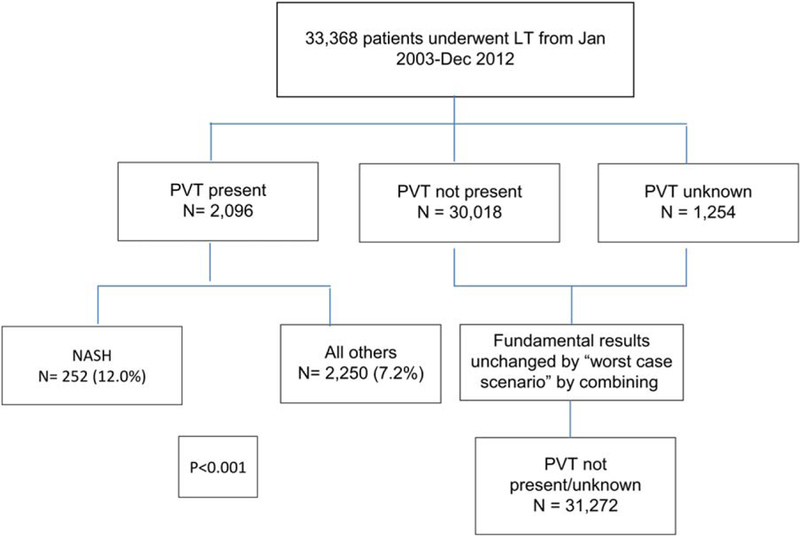
Analyses were performed that compared recipients with NASH cirrhosis to the non-NASH group. PVT was categorized as “present,” “not present,” or “unknown,” and this was based on explant examinations and direct surgical observations. The character and extent (partial or complete) of PVT were not indicated in the data set. An initial analysis showed that 30,018 recipients (90.0%) were categorized as having PVT not present, and 2096 (6.3%) were categorized as definitively having PVT at transplantation; 1254 (3.8%) were categorized in the data set as having an “unknown” PVT status. A worst-case/best-case scenario analysis was performed with all of the “unknown” recipients characterized as definite PVT, and conversely, all were characterized as no PVT. The results of these separate analyses did not change the fundamental conclusions of this article; therefore, for the purpose of this analysis, all recipients categorized as “unknown” were considered to not have PVT. Data were incomplete to sufficiently review regarding previous episodes, the presence of concurrent inherited thrombophilic disorder, and/or the treatment of PVT.
Statistics
Recipients with PVT were compared to those without PVT statistically for multiple factors, including demographics, waiting list characteristics, medical comorbidities, transplantation characteristics, and outcomes. Multivariable models were constructed to assess statistical associations and risk factors for the development of PVT. Individual factors were included in the multi-variable model if they were statistically significant at P < 0.20 in the univariate analysis, were clinically important, or had been shown in the literature to be important.21,22 Univariate comparisons were performed with the Student t test, Wilcoxon sign rank test, chisquare test, or Fisher’s exact test as appropriate. Multivariable models were constructed with logistic regressions and analyses of the maximum likelihood estimates. All statistical tests for significance were 2-sided, and a significance level of P ≤ 0.05 was considered statistically significant. All data set manipulation and statistical analyses were performed with SAS (version 9.3; Cary, NC). No transplants involving prisoners were included in this analysis. Because the OPTN data set is deidentified, institutional review board approval was not required for this study.
RESULTS
A total of 33,368 patients met the criteria for inclusion in our study analysis (Fig. 1). Of these, 2096 patients (6.3%) had PVT at the time of LT, 30,018 did not have PVT, and 1254 recipients had an unknown status but were included in the non-PVT group. Background demographics, the severity of liver disease (including manifestations of portal hypertension), and laboratory values were, in general, statistically similar or with marginal clinically important differences for patients with and PVT, with several exceptions (Table 1). Patients with PVT had a higher MELD at time of allocation (23 versus 22; P< 0.001) and had more advanced ascites (35.1% versus 31.6%; P = 0.001). A lower percentage of African Americans was found in the PVT group (7.5% for the PVT group versus 10.3% for the non-PVT group; P < 0.001). Patients with PVT were more likely to have diabetes, a risk factor for NASH (28.1% versus 21.7%; P< 0.0001), and had a slightly higher BMI (28.2 versus 27.9 kg/m2; P = 0.0354).
Figure 1.
Study enrollment.
TABLE 1.
Baseline Characteristics of 33,368 Patients With and Without PVT at the Time of Transplantation
| PVT (n = 2096) | No PVT (n = 31,272) | P Value | |
|---|---|---|---|
| Recipient characteristics | |||
| Age at listing, years | 53.14 (52.72–53.55) | 52.14 (52.03–52.26) | <0.001 |
| Age at transplant, years | 53.80 (53.38–54.21) | 52.60 (52.49–52.71) | <0.001 |
| Sex, male | 1424 (66.70) | 20,832 (66.62) | 0.21 |
| African American race | 158 (7.54) | 3217 (10.29) | <0.001 |
| Etiology of liver disease | |||
| Alcohol alone | 331 (15.79) | 4881 (15.61) | 0.82 |
| Autoimmune disease | 76 (3.63) | 1045 (3.34) | 0.48 |
| Cholestatic disease | 183 (8.73) | 3237 (10.35) | 0.018 |
| Hepatitis B | 50 (2.39) | 866 (2.77) | 0.30 |
| HCV | 820 (39.12) | 13,282 (42.47) | <0.001 |
| NASH | 252 (12.02) | 2250 (7.19) | <0.001 |
| Other | 384 (18.32) | 5711 (18.26) | 0.95 |
| Severity of liver disease | |||
| MELD score at listing | 19.45 (19.10–19.81) | 19.71 (19.61–19.81) | 0.20 |
| MELD score at transplantation | 23.29 (22.89–23.69) | 22.64 (22.53–22.75) | <0.001 |
| Laboratory values | |||
| Serum bilirubin, mg/dL | 9.15 (8.66–9.65) | 9.38 (9.25–9.51) | 0.39 |
| INR | 1.92 (1.88–1.96) | 1.88 (1.87–1.89) | 0.14 |
| Serum albumin, g/dL | 2.96 (2.93–2.99) | 2.96 (2.95–2.96) | 0.75 |
| Creatinine, g/dL | 1.66 (1.60–1.71) | 1.67 (1.65–1.69) | 0.67 |
| On dialysis at transplantation | 274 (13.09) | 4873 (10.39) | 0.12 |
| Portal hypertension manifestations | |||
| Ascites grade > 2 at transplant | 735 (35.07) | 9886 (31.61) | 0.001 |
| Hepatic encephalopathy > 2 at transplant | 253 (12.07) | 3487 (11.15) | 0.20 |
| NASH risk factors | |||
| Diabetes | 588 (28.05) | 6772 (21.66) | <0.001 |
| BMI at transplant, kg/m2 | 28.16 (27.91–28.42) | 27.89 (27.83–27.95) | 0.04 |
NOTE: Data are given as n (%) or mean (95% CI).
Several differences were noted with respect to the etiology of chronic liver disease. Of those patients with PVT, 12.0% had underlying NASH cirrhosis, whereas 7.2% did in the non-PVT group. Cholestatic liver disease was less common in patients with PVT (8.7% versus 10.4%; P = 0.02). Recipients with hepatitis C virus (HCV; 39.1% in HCV group versus 42.5% in all others; P < 0.001) were found at a lower frequency in the PVT group. When we compared patients with NASH cirrhosis to a composite of all other causes of cirrhosis, an increased prevalence of PVT was again found, with 10.1% having PVT at the time of LT versus 6.0% without NASH cirrhosis (P< 0.001).
In a multivariable analysis of risk factors for PVT, the strongest risk factor independently associated with a diagnosis of PVT was NASH cirrhosis (odds ratio [OR], 1.55; 95% confidence interval [CI], 1.33–1.81; P < 0.001; Table 2). This was highly significant. Interestingly, African American race was independently associated with an inverse relationship with PVT (OR, 0.76; 95% CI, 0.64–0.90; P< 0.001). Although statistically significant in the univariate analysis, the association only approached significance in the multivariable analysis (OR, 0.91; 95% CI, 0.82–1.00; P = 0.06).
TABLE 2.
Multivariate Analysis for Predictors of PVT at Transplantation
| OR | 95% CI | P Value | |
|---|---|---|---|
| NASH cirrhosis | 1.55 | 1.33–1.81 | <0.001 |
| HCV | 0.91 | 0.82–1.00 | 0.058 |
| Cholestatic disease | 0.87 | 0.73–1.03 | 0.10 |
| Sex, male | 1.08 | 0.98–1.19 | 0.11 |
| African American | 0.76 | 0.64–0.90 | <0.001 |
| race | |||
| Age, years | 1.01 | 1.00–1.02 | <0.001 |
| Final MELD score | 1.01 | 1.00–1.01 | 0.04 |
| BMI, kg/m2 | 1.00 | 0.99–1.01 | 0.10 |
| Ascites > 2 | 1.11 | 1.00–1.23 | 0.05 |
| Encephalopathy > 2 | 0.98 | 0.85–1.14 | 0.82 |
| Final INR | 1.00 | 0.95–1.05 | 0.96 |
NOTE: An OR >1.0 indicates a relative risk for development of PVT, whereas an OR < 1.0 indicates protection from development of PVT.
DISCUSSION
Although cardiovascular disease is the leading cause of death worldwide and also for NASH, an epidemiological link outside metabolic syndrome risk factors has not been definitively proven. Hypercoagulability and thrombosis were initially identified by studies on the natural history of NASH.7 Although we know that synergistic risk factors such as diabetes, obesity, and dyslipidemia lead to NASH, it is thought that chronic repetitive inflammation produces lipid-based oxidative injury and necroapoptosis, which may lead to activation of the coagulation system and a hypercoagulable state in laboratory and pathophysiology studies.5,6,23,24 To date, marginal evidence exists from epidemiological studies suggesting a clinically applicable thrombophilic state, and the majority of evidence supports arterial rather than venous thromboembolic disease. A clinically significant association or causal relationship has yet to be firmly established.
In our study based on data from a large national database, we document a cross-sectional association showing an increased risk of PVT in patients with NASH cirrhosis who undergo LT. This difference was seen despite adjustments for known risk factors for NASH, including diabetes and overweight/obesity, which also was independently predictive of risk for PVT despite the etiology of cirrhosis. This further supports the in vivo data for a hypercoagulable state existing in NASH patients,5–8 which may predispose them to clot formation. We further suggest that the impact of systemic inflammation predisposing patients to venous thrombus generation and endothelial dysfunction is underestimated. Recent data examining plasma for levels of procoagulants and anticoagulants in patients across the spectrum of NAFLD, including those with cirrhosis, support this.9 Although the exact clinical implications of this finding remain unclear, the authors postulate that the imbalance may be due to increased factor VIII and reduced protein C levels, which may lead to the downstream hallmark effects of adverse cardiovascular events and liver fibrosis in NASH patients,9 although the link to fibrosis has yet to be established because previous studies have failed to show a correlation between fibrosis on liver biopsy and an increase in factor VIII.25 However, levels of PAI-1 have been shown to significantly correlate with increasing severity of steatosis, lobular inflammation, ballooning, and fibrosis.25 We suggest that the imbalance of procoagulants and anticoagulants leads to PVT and that future study to better define this exact mechanism is warranted. A prevalence of 6.3% for PVT in all patients undergoing LT is expected on the basis of what has been previously published across the literature, albeit this is on the low end.
Our findings that African American race was associated with a lower prevalence of PVT was somewhat surprising because, in general, it is felt that African Americans are predisposed to venous thromboembolic disease, perhaps through higher levels of factor VIII and von Willebrand factor and lower protein C levels.26 What seems more likely is a dropout bias because these data are based on explant examinations. This is in alignment with previously demonstrated racial disparities in LT that affect African Americans and the findings of higher wait list mortality for African Americans.27
Our study has several weaknesses. Although it is based on a large data set compiled across greater than a decade of transplantation, it is nonetheless retrospective. Additionally, missing data are an issue with large data sets. We presumed that patients with an “unknown PVT status” did not have PVT, and this may have led to an intrinsic bias and an underreporting of the true prevalence of PVT in our population. However, we performed a best-case/worse-case scenario analysis, and our fundamental conclusions were unchanged. Furthermore, PVT may prevent LT at several centers, and our analysis was unable to account for a potential dropout and selection bias because of this. Large data sets are also dependent on the accuracy of diagnostic coding. Additionally, our population considered only those patients who underwent LT. There is a large volume of patients undergoing LT who may have PVT and were not included in our analysis, and the generalizability of these findings to all patients with NASH and PVT, including non-LT candidates, remains unclear. Furthermore, the UNOS database does not differentiate between partial and complete PVT, and this may serve as a confounder in outcomes analysis. The UNOS database also does not contain information on anticoagulant use for other comorbid venous thromboembolic or cardiac disease, which may offer some protection against PVT. Nor does the data set contain information on inherited thrombophilic states, which could also lead to the development of PVT. Additionally, we examined data obtained at the time of LT, which may or may not correlate with the presence of PVT at the time the patient was initially listed.
In light of our findings that suggest an increased risk of PVT in NASH cirrhosis, more study is needed in establishing pathophysiology and mechanisms for this finding and for means to prevent or treat PVT. A recent unblinded, single-center randomized controlled trial demonstrated that daily prophylactic dosing of low-molecular-weight heparin for 12 months prevented the development of PVT in patients with compensated cirrhosis at 48 weeks; this persisted through follow-up at 5 years in comparison with the standard of care.28 Furthermore, the authors demonstrated significantly less hepatic decompensation in the low-molecular-weight heparin arm (P< 0.001) and a survival benefit.31 Although this study has several flaws, it nevertheless remains promising for providing a therapeutic target to prevent PVT and leading to increased transplant-free survival. Our findings of increased PVT prevalence in NASH cirrhosis may offer a high-risk group for further clinical studies on anticoagulation and PVT.
In conclusion, the association between NASH and a hypercoaguable state is an ever-expanding field of research. Existing epidemiological evidence demonstrates an independent effect of NASH on arterial clot formation in the macrovasculature; laboratory data confirm inflammation and coagulation changes in the liver. We have provided the first large-scale observational data showing that NASH predisposes patients to PVT through an undetermined mechanism. The identification of this mechanism is impaired by a lack of effective laboratory measures of the coagulation cascade and platelet function and a lack of progress in further defining parenchymal extinction and the effect of anticoagulation on fibrosis. Further exploration of the therapeutic and prophylactic treatment of PVT in NASH is greatly needed and will hopefully improve clinical outcomes and survival.
Acknowledgments
Jonathan G. Stine and Patrick G. Northup planned and conducted the study, collected and/or interpreted the data, drafted the article, and gave final approval; Neeral L. Shah, Curtis K. Argo, Shawn J. Pelletier, and Stephen H. Caldwell drafted the article and gave final approval.
Patrick G. Northup is the guarantor of the article.
This work was supported in part by grant funding from the National Institutes of Health. This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Abbreviations:
- BMI
body mass index
- CI
confidence interval
- HCV
hepatitis C virus
- INR
international normalized ratio
- LT
liver transplantation
- MELD
Model for End-State Liver Disease
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- OPTN
Organ and Transplantation Network
- OR
odds ratio
- PAI-1
plasminogen activator inhibitor 1
- PVT
portal vein thrombosis
- UNOS
United Network for Organ Sharing
Footnotes
Potential conflict of interest: Nothing to report.
REFERENCES
- 1.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865–873. [DOI] [PubMed] [Google Scholar]
- 2.Caldwell SH, Lee VD, Kleiner DE, Al-Osaimi AM, Argo CK, Northup PG, Berg CL . NASH and cryptogenic cirrhosis: a histological analysis. Ann Hepatol 2009;8:346–352. [PMC free article] [PubMed] [Google Scholar]
- 3.Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 2013;178:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011;141:1249–1253. [DOI] [PubMed] [Google Scholar]
- 5.Targher G, Bertolini L, Rodella S, Lippi G, Franchini M, Zoppini G, et al. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity (Silver Spring) 2008;16:1394–1399. [DOI] [PubMed] [Google Scholar]
- 6.Targher G, Zoppini G, Moghetti P, Day CP. Disorders of coagulation and hemostasis in abdominal obesity: emerging role of fatty liver. Semin Thromb Hemost 2010;36:41–48. [DOI] [PubMed] [Google Scholar]
- 7.Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis 2009;13: 511–531. [DOI] [PubMed] [Google Scholar]
- 8.Cigolini M, Targher G, Agostino G, Tonoli M, Muggeo M, De Sandre G. Liver steatosis and its relation to plasma haemostatic factors in apparently healthy men—role of the metabolic syndrome. Thromb Haemost 1996;76:69–73. [PubMed] [Google Scholar]
- 9.Tripodi A, Fracanzani AL, Primignani M, Chantarangkul V, Clerici M, Mannucci PM, et al. Procoagulant imbalance in patients with non-alcoholic fatty liver disease. J Hepatol 2014;61:148–154. [DOI] [PubMed] [Google Scholar]
- 10.Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes 2005;54:3541–3546. [DOI] [PubMed] [Google Scholar]
- 11.Wanless IR, Shiota K. The pathogenesis of nonalcoholic steatohepatitis and other fatty liver diseases: a four-step model including the role of lipid release and hepatic venular obstruction in the progression to cirrhosis. Semin Liver Dis 2004;24:99–106. [DOI] [PubMed] [Google Scholar]
- 12.Papatheodoridis GV, Chrysanthos N, Cholongitas E, Pavlou E, Apergis G, Tiniakos DG, et al. Thrombotic risk factors and liver histologic lesions in non-alcoholic fatty liver disease. J Hepatol 2009;51:931–938. [DOI] [PubMed] [Google Scholar]
- 13.Davidson BR, Gibson M, Dick R, Burroughs A, Rolles K. Incidence, risk factors, management, and outcome of portal vein abnormalities at orthotopic liver transplantation. Transplantation 1994;57:1174–1177. [DOI] [PubMed] [Google Scholar]
- 14.Ponziani FR, Zocco MA, Senzolo M, Pompili M, Gasbarrini A, Avolio AW. Portal vein thrombosis and liver transplantation: Implications for waiting list period, surgical approach, early and late follow-up. Transplantation Rev 2014;28:92–101. [DOI] [PubMed] [Google Scholar]
- 15.Englesbe MJ, Kubus J, Muhammad W, Sonnenday CJ, Welling T, Punch JD, et al. Portal vein thrombosis and survival in patients with cirrhosis. Liver Transpl 2010; 16:83–90. [DOI] [PubMed] [Google Scholar]
- 16.Gayowski TJ, Marino IR, Doyle HR, Echeverri L, Mieles L, Todo S, et al. A high incidence of native portal vein thrombosis in veterans undergoing liver transplantation. J Surg Res 1996;60:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wanless IR, Wong F, Blendis LM, Greig P, Heathcote EJ, Levy G. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology 1995;21:1238–1247. [PubMed] [Google Scholar]
- 18.Zocco MA, Di Stasio E, De Cristofaro R, Novi M, Ainora ME, Ponziani F, et al. Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. J Hepatol 2009;51:682–689. [DOI] [PubMed] [Google Scholar]
- 19.Rodríguez-Castro KI, Porte RJ, Nadal E, Germani G, Burra P, Senzolo M. Management of nonneoplastic portal vein thrombosis in the setting of liver transplantation: a systematic review. Transplantation 2012;94: 1145–1153. [DOI] [PubMed] [Google Scholar]
- 20.Hibi T, Nishida S, Levi DM, Selvaggi G, Tekin A, Fan J, et al. When and why portal vein thrombosis matters in liver transplantation: a critical audit of 174 cases. Ann Surg 2013;259:760–766. [DOI] [PubMed] [Google Scholar]
- 21.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med 2008;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun GW, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariate analysis. J Clin Epidemiol 1996;49:907–916. [DOI] [PubMed] [Google Scholar]
- 23.Targher G, Chonchol M, Miele L, Zoppini G, Pichiri I, Muggeo M. Nonalcoholic fatty liver disease as a contributor to hypercoagulation and thrombophilia in the metabolic syndrome. Semin Thromb Hemost 2009;35:277–287. [DOI] [PubMed] [Google Scholar]
- 24.Caldwell S, Ikura Y, Dias D, Isomoto K, Yabu A, Moskaluk C, et al. Hepatocellular ballooning in NASH. J Hepatol 2010;53:719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verrijken A, Francque S, Mertens I, Prawitt J, Caron S, Hubens G, et al. Prothrombotic factors in histologically proven nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2014;59:121–129. [DOI] [PubMed] [Google Scholar]
- 26.Zakai NA, McClure LA. Racial differences in venous thromboembolism. J Thromb Haemost 2011;9:1877–1882. [DOI] [PubMed] [Google Scholar]
- 27.Reid AE, Resnick M, Chang Y, Buerstatte N, Weissman JS. Disparity in use of orthotopic liver transplantation among blacks and whites. Liver Transpl 2004;10:834–841. [DOI] [PubMed] [Google Scholar]
- 28.Villa E, Cammá C, Marietta M, Luongo M, Critelli R, Colopi S, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology 2012;143:1253–1260. [DOI] [PubMed] [Google Scholar]


