Summary
Radiation therapy plays an integral role in the management of head and neck cancers (HNCs). While most HNC patients have historically been treated with photon-based radiation techniques such as intensity modulated radiation therapy (IMRT), there is a growing awareness of the potential clinical benefit of proton therapy over IMRT in the definitive, postoperative and reirradiation settings given the unique physical properties of protons. Intensity modulated proton therapy (IMPT), also known as “pencil beam proton therapy,” is a sophisticated mode of proton therapy that is analogous to IMRT and an active area of investigation in cancer care. Multifield optimization IMPT allows for high quality plans that can target superficially located HNCs as well as large neck volumes while significantly reducing integral doses. Several dosimetric studies have demonstrated the superiority of IMPT over IMRT to improve dose sparing of nearby organs such as the larynx, salivary glands, and esophagus. Evidence of the clinical translation of these dosimetric advantages has been demonstrated with documented toxicity reductions (such as decreased feeding tube dependency) after IMPT for patients with HNCs. While there are relative challenges to IMPT planning that exist today such as particle range uncertainties and high sensitivity to anatomical changes, ongoing investigations in image-guidance techniques and robust optimization methods are promising. A systematic approach towards utilizing IMPT and additional prospective studies are also necessary in order to more accurately estimate the clinical benefit of IMPT over IMRT and passive proton therapy on a case-by-case basis for patients with sub-site specific HNCs.
Keywords: Head and neck, Radiotherapy, IMPT, IMRT, proton therapy
Introduction
Radiation therapy (RT) has been well established as an essential tool to treat head and neck cancers (HNCs) in various clinical settings. While most HNC radiation treatments have been and continue to be photon-based, advancements in diagnostic imaging, RT planning and delivery have largely modernized the field. Intensity modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT) plans can yield highly conformal dose distributions resulting in toxicity reduction, faster treatment times, and use of less monitor units.1–3 However, the physical properties of photons present inherent challenges in our ability to dose escalate safely while respecting normal tissue tolerance constraints.
Particle therapy as a form of cancer treatment was originally proposed by Robert Wilson in 1946.4 Compared to photons which continually deposit dose throughout tissue and exhibit an exit dose, the dose distribution of protons forms a Bragg peak, denoting maximal dose deposition at a finite tissue depth followed by a sharp dose falloff with no exit dose. This dosimetric difference, in turn, can theoretically translate into a clinically relevant therapeutic advantage.5 IMPT is a sophisticated technique of proton delivery that allows for greater degrees of freedom to produce optimized dose distributions which are essential when treating large volumes such as a primary head and neck sites with simultaneous coverage of the (ipsilateral or bilateral) neck.
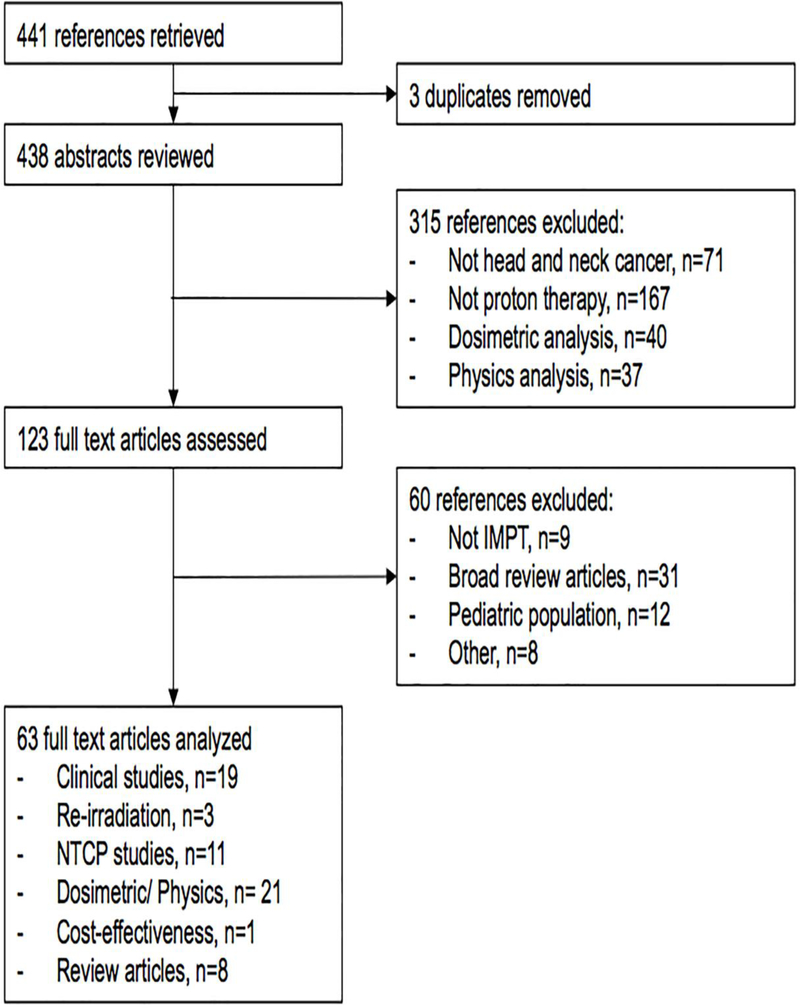
Herein, we present a critical review of existing literature regarding the evolution and application of proton therapy, specifically IMPT, for the management of HNCs. An assessment of technological limitations and proposal of the future directions for IMPT are also reported. Key proton therapy references cited in this review article were derived from a thorough PubMed query (Fig. 1). Four hundred and forty one references were retrieved using the following formula: “intensity modulated proton therapy” or “impt” or “pencil beam scanning” or “active scanning” or “scanning beam proton therapy” or “scanned protons” and “head and neck cancer”. Out of these, 63 full text articles focusing on dosimetric or clinical studies between IMRT and IMPT, normal tissue complication probability (NTCP) models, re-irradiation studies, and cost-effectiveness analyses were analyzed for content for inclusion in this review.
Figure 1. Critical IMPT reference search algorithm.
Abbreviations: IMPT, intensity modulated proton therapy; NTCP, normal tissue complication probability.
Dosimetric Advantages of Proton Therapy
The difference in depth dose curves between photons and protons is depicted in Figure 2. As mentioned earlier, protons exhibit a Bragg peak (or spot) characterized by a sharp increase in dose at the end of the particle range with subsequent absence of dose beyond this range. In comparison, the dose fall off with photons is typically around a few percent per centimeter depending on the medium.6 Therefore, the rapid fall-off dose of proton beams make them ideal for treating tumors with intracranial extension or those located in critical areas such as the periorbital region, skull base, and/or cavernous sinus. Scattering foils, energy modulation techniques, and brass apertures can be utilized to create spread-out Bragg peak modulated (SOBP) fields conformed to cover a delineated three-dimensional (3D) target volume on CT.7 This form of RT modality, known as passively scattered proton therapy (PSPT), has been widely studied and used for primary and recurrent HNCs.8–14 To further corroborate its usage, a recent systematic review and meta-analysis performed by Patel el al. evaluating 43 paranasal sinus and nasal cavity patient cohorts suggested an improvement in 5-year disease free survival (DFS) and locoregional control (LRC) with proton therapy compared to IMRT.15
Figure 2.
Dose depth curves for photons and protons. The spread out Bragg peak is the therapeutic radiation distribution created by the sum of multiple individual Bragg peaks (such as the orange single proton Bragg peak) ranging at different depths. Note the rapid falloff of dose after the Bragg peak compared to the photon beam which exhibits an exit dose.
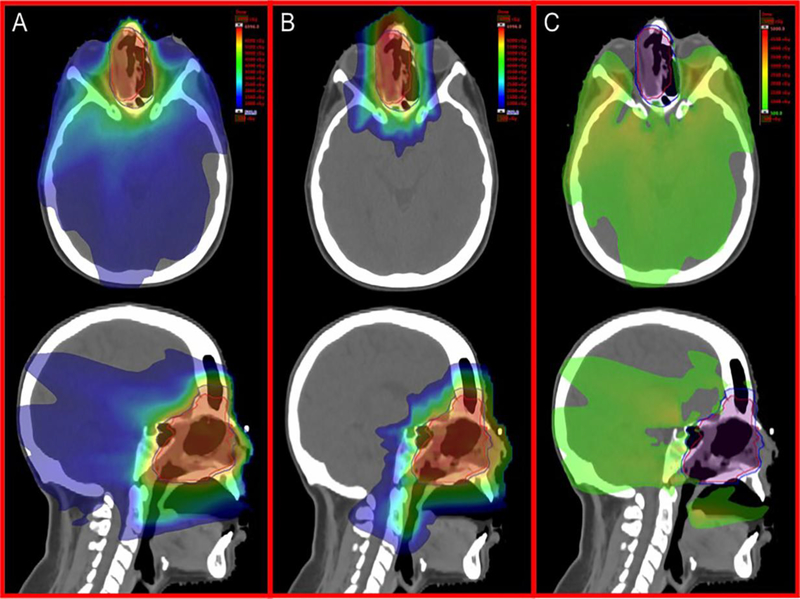
IMPT, also commonly referred to as “pencil beam” or “active scanning”, is a more sophisticated and complex technical mode of delivering RT. Proton “pencil” beams originating from the accelerator are manipulated to treat tumors in layers of spots at varying depths by altering the following: number of protons (local dose deposition), energy (local penetration), and magnetic deflection (off-axis coverage).16 Unlike IMRT that achieves a conformal dose distribution through the use of multiple beam arrangements (or arcs) and mechanical multileaf collimators, IMPT relies on electromagnetic control of the pencil beam to achieve comparable target coverage while reducing the integral dose bath. Therefore, IMPT is promising over IMRT for HNC treatments that desire dose escalation while sparing organs-at-risk (OARs). IMPT is also advantageous over PSPT as it eliminates the need for patient-specific devices (i.e. compensators) that can be costly and labor intensive to produce and use during daily treatments. Figure 3 illustrates comparative VMAT and IMPT treatment plans for a patient with olfactory neuroblastoma.
Figure 3.
Comparative VMAT and IMPT treatment plans for a patient with olfactory neuroblastoma. Representative axial and sagittal images for A) IMRT, B) IMPT, and C) subtraction (IMRT-IMPT) plans are shown.
IMPT Planning Optimization and Limitations
Currently, most commercially available proton beam systems employ therapeutic beam energy ranges from 70 to 250 MeV which translates to water-equivalent thickness depths of over 4 cm.17 As HNC tumors tend to be more superficially located, range shifters or patient-specific bolus must be utilized with IMPT, or alternatively, high quality plans without range shifters can be achieved through the use of multifield optimization (MFO-IMPT).18–21 MFO-IMPT plans are now typically recommended for treating HNCs and can be generated using three to four fields (typically a left and right anterior oblique beam and a single posterior beam ± a vertex beam) subjected to multi-criteria, inverse optimization algorithms that optimize all spots from all fields simultaneously.19,22,23 Patient-specific apertures can also be implemented with IMPT for a sharper lateral penumbra and further dose reductions to OARs.24
While IMPT plans have been shown to result in both dosimetric advantages and clinically acceptable treatment-related toxicities compared to IMRT or PSPT,18,19 there are certain limitations that should be acknowledged with this technology. Firstly, IMPT is highly sensitive to uncertainties in particle range and anatomical changes, the latter of which can be related to interfractional variations in patient positioning, intrafractional organ motion, or target volume changes throughout treatment (i.e. tumor regression or patient weight loss)25. With IMRT planning, simple margin expansions to account for anatomical changes and setup errors are quite effective in ensuring adequate target coverage by the prescribed dose. PSPT can account for clinical uncertainties by widening apertures and by compensator smearing.26 For IMPT, a greater focus is required on improving robust optimization methods and using frequent imaging to verify treatment plans and/or trigger adaptive planning during treatment.27–32 Using a dataset of 31 locally advanced HNC cases, Stutzer et al. demonstrated the importance of verification CTs with IMPT. In this study where recalculated IMRT or IMPT plans were created on CT scans taken at week 4 of therapy, recalculated IMPT plans showed deterioration of clinical target volume (CTV) coverage and an increase in hotspots within and outside target volumes.33 Based on reported data, rates of adaptive planning with IMPT can be expected to range from 16% to 38%.34,35
Limitations in proton beam energy switching time, scanning-spot optimization algorithms, and RBE uncertainties with proton therapy also exist and may affect the accuracy of IMPT planning.16,36–38 However, these are all areas that are actively being investigated including the development of linear energy transfer-guided optimization and novel methods to seamlessly integrate scanning-spot optimization with beam orientation optimization.39–42 Establishing and standardizing efficient patient-specific quality assurance protocols are also crucial for successful utilization of IMPT in the clinic.43
Clinical Experience with IMPT by Head and Neck Sub-sites
While definitive concurrent chemoradiation is standard of care for the majority of nonsurgical, locally advanced head and neck cases,44 RT techniques are constantly evolving and now include proton therapy as an alternative to IMRT. Multiple studies published in the past few years have demonstrated the ability of proton therapy, mainly PSPT, to significantly reduce toxicities such as acute dysgeusia and mucositis compared to photon-based therapies, regardless of primary sub-site or whether only unilateral neck radiation was delivered.45 The cumulative clinical experience with IMPT for HNCs, however, is still relatively new and therefore the primary focus of this review article. Table 1 summarizes several retrospective and prospective studies investigating clinical and toxicity outcomes after IMPT for the treatment of head and neck cancers of various sub-sites.46–48 Of note, several units to describe proton dose have been used throughout the literature but for the purposes of this review, all will be reported as the relative biological effectiveness (RBE)-weighted absorbed dose, GyRBE, which is the latest International Commission on Radiation Units and Measurements (ICRU) recommendation.49 GyRBE, defined as the product of absorbed proton dose and the constant relative biological effectiveness (RBE) value of 1.1, is a concept that estimates the photon dose that would produce the same therapeutic effect as the proton dose under identical conditions.
Table 1.
Studies evaluating IMPT for Head and Neck Cancers
| Study | HN Site | Study Type | Accrual Period | No. of Patients | Median follow up | Outcomes(years) | Toxicities (n, %) and interventions |
|---|---|---|---|---|---|---|---|
| Gunn35 (2016) | OPC | PS | 2011–2014 | 50 IMPT | 29 mo | LRC2.4: 92% OS2: 94.5% PFS2: 88.6% |
Acute: G3 mucositis (29, 58%), G3 dermatitis (23, 46%). Acute vs. late G3 dysphagea (12, 24% vs. 6,12%). G-tube placed in 11 (22%) patients. |
| Blanchard54 (2016) | OPC | RS | 2010–2014 | A) 50 IMPT B) 100 IMRT |
32 mo | A) OS3: 94.3% B) OS3: 89.3% |
G-tube or weight loss >20% (A vs. B) after 3-mo: (9, 18%) vs. 34 (34%) and after 12-mo: 4 (8%) vs. 22 (25%). |
| Lewis63 (2016) | NPC | PS | 2011–2013 | 10 IMPT | 24.5 mo | LRC2: 100% OS2: 88.9% |
Acute: G3 dermatitis (4, 44%), G3 mucositis (1, 11%). Chronic: G2 xerostomia (2, 22%). No chronic G3-G5 toxicities. |
| Holliday64 (2015) | NPC | RS | 2011–2013 | A) 10 IMPT B) 20 IMRT |
21.6 mo | A) LC2: 100% B) LC2: 95% |
G-tube insertion (A vs. B): 2 (20%) vs. 13 (65%). Swallowing dysfunction (A vs. B): 0 vs 3 (15%). |
| Holliday68 (2014) | Sinonasal | PS | 2011–2013 | 16 IMPT | 10.5 mo | LC: 87.5% | Acute: G2 dermatitis (13, 81%), G3 dermatitis (1, 6%), G2 mucositis (5, 31%), G2 dysgeusia (2, 13%). |
| Holliday68 (2014) | Parotid | PS | 2011–2013 | 13 IMPT | 13.2 mo | LC: 100% OS: 100% |
Acute: G2 dermatitis (9, 69%), G3 dermatitis (4, 31%). |
| Holliday68 (2014) | Periorbital | PS | 2011–2013 | 21 proton (9 IMPT) | 27.5 mo | LC: 100% | Acute: G3 dermatitis (7, 33%). Chronic: Visual changes (5, 24%), G3 keratopathy (3, 14%). |
| Holliday (2016) | Periorbital | PS | 2008–2014 | 6 IMPT, 14 PSPT | 27.1 mo | LC: 100% | Chronic: G3 epiphora (3, 15%), G3 exposure keratopathy (3, 15%). Decreased visual acuity (4, 20%) |
| Ares (2009) | BOS | RS | 1998–2005 | 20 IMPT, 44 scanningψ | 38 mo | LC5: 81%a and 94%b OS 100%a and 91%b |
Chronic: G3 optic neuropathy (1, 2%), G4 optic neuropathy (1, 2%), G3 brain necrosis (2, 3%). |
| Phan11 (2016) | Recurrence | RS | 2011–2015 | 15 PSPT, 45 (75%) IMPT | 13.6 mo | OS1: 83.8% PFS1: 60.1% |
Acute G3 toxicities in 18 (30%). G-tube insertion: 13 (22%). |
Abbreviations: BOS, base of skull tumors; G-tube, gastrostomy tube; Mo, months; NPC, nasopharyngeal carcinoma; OPC, oropharyngeal cancer; OS, overall survival; PFS, progression free survival; PS, prospective study; PSPT, passive scatter proton therapy; RS, retrospective study; SCC, squamous cell carcinoma.
In this study, 44 patients received proton therapy using a spot-scanning technique.
Five-year LC and OS rates for chordomas.
Five-year LC and OS rates for chondrosarcomas.
Oropharyngeal Cancers (OPC)
With over 51,500 new cases diagnosed in the United States in 2018 alone, cancers of the oropharynx collectively exhibit an average 5-year OS of 65% despite aggressive medical interventions with traditional RT techniques.50,51 Early efforts to improve locoregional control of OPCs by using proton therapy as a boost with photons were first reported by Slater et al. in 2005.52 In this prospective study from 1991 to 2002, a total of 29 patients with localized Stage II-IV OPC received accelerated photon and proton therapy to a total dose of 75.9 GyRBE in 45 fractions over 5.5 weeks (50.4 GyRBE in 28 fractions of photon-based RT followed by a PSPT concomitant boost of 25.5 GyRBE in 17 fractions delivered to the primary tumor and involved neck nodes). The 2- and 5-year locoregional control (LRC) rates were 93% and 84% respectively, while the corresponding disease-free survival rates were 81% and 65%, respectively. Of equal importance with improved outcomes, this treatment was well tolerated with late grade 3 toxicities reported in 3 (11%) patients.
Over time, the focus has shifted from treating large volume OPCs with a combined RT modality approach to IMPT only. In 2016, Gunn et al. reported favorable disease control and toxicity profiles when using IMPT to treat 50 patients with OPC, 98% of whom had clinical stage III/IV disease and 64% of whom were treated with concurrent systemic therapy. After a median follow up of 29 months, LRC was 92%, and 2-year OS and progression-free survival (PFS) rates were high at 95% and 89%, respectively.35 The same institution published on patient reported outcomes after concurrent chemoradiation using IMPT (n=35) or IMRT (n=46) techniques. No differences in acute and chronic phase-symptom burden were detected between both groups. However, during the subacute recovery phase (3 months after treatment completion), patient reported symptom burden was significantly lower with IMPT.53 Moreover, after a 2:1 case matched analysis of 50 IMPT and 100 IMRT patients, IMPT was associated with significantly reduced rates of feeding tube dependency and severe weight loss at 3 months (hazard ratio, HR 0.44 [95% confidence intervals, CI: 0.19–1]; p=0.05) and at 12 months (HR 0.23 [0.07–0.73]; p=0.01) post RT.54 These observed toxicity reductions with IMPT are likely reflective of a clinical translation of dose reduction to several OPC substructures including the oral cavity, larynx, salivary glands, and esophagus.55–58 Such favorable findings with IMPT have triggered an ongoing clinical trial (NCT01893307) evaluating IMPT versus IMRT for the management of OPC. Lastly, additional studies investigating IMPT use in the postoperative setting for OPC patients is warranted, although there is evidence to suggest potential maintenance of the dosimetric superiority of IMPT compared to IMRT or VMAT.59
Nasopharynx
Nasopharyngeal carcinoma (NPC) is thought to be chemoradiosensitive; therefore, RT plays a crucial role in both the definitive and postoperative settings. This particular region of the head and neck presents unique challenges for treatment as surrounding neurological structures can be affected by high doses of radiation and result in hearing impairment, optic neuropathy, or temporal lobe necrosis.60 Given these dose-limiting structures, several studies have demonstrated the feasibility of improving tumor coverage and reducing integral dose to OARs with MFO-IMPT relative to IMRT and helical tomotherapy.61,62
The University of Texas MD Anderson Cancer Center (Houston, TX) has published most of the existing clinical data on IMPT use for NPC. LRC and OS at 2 years was excellent at 100% and 89%, respectively, for a cohort of 10 NPC patients treated with platinum-based concurrent chemoradiation using IMPT (prescribed dose of 70 GyRBE in 33 fractions).63 The most common acute grade 3 toxicity was dermatitis (n=4) and only one patient suffered from acute grade 3 mucositis. No chronic grade 3 or higher toxicities were seen. Furthermore, a dosimetric comparison between treatment IMPT plans and theoretical IMRT plans in this study suggest potential dosimetric benefits with IMPT as significant differences in OAR doses favored IMPT in 13 out of 15 cases. Furthermore, a 2:1 case-matched analysis comparing this prospective data with 20 IMRT NPC cases found significantly lower rates of gastrostomy tube insertion with IMPT (20% vs. 65%; p=0.02),64 a noteworthy finding that supports the preferential usage of IMPT for management of these anatomically challenging cancers.
Nasal Cavity and Paranasal Sinuses
Tumors of the nasal cavity and paranasal sinuses are primarily managed surgically followed by postoperative RT (PORT) with or without systemic therapy based on high-risk pathologic features such as nodal involvement or close/positive margins. Local control and OS are highly correlated with the extent of surgical resection; however as demonstrated by Resto et al.,65 PORT delivered with proton therapy can allow for safer dose escalation and improved survival rates compared to historical photon-based outcomes. Using a retrospective cohort of 102 patients with locally advanced sinonasal malignancies that were treated with a combination of proton and photon beam RT, Resto et al. reported 5-year LC rates of 95%, 82%, and 87% as well as 5-year OS rates of 90%, 53%, and 49% in patients who underwent a complete resection, partial resection, or biopsy only, respectively. Similarly for nonsurgical patients, definitive PSPT has been shown to provide adequate 2- to 3-year OS rates over 60% and LC rates of 70–95% for various histological subtypes and extent of disease.66,67 In order to compare charged particle therapy (protons or carbon ions) to photon therapy for treatment of sinonasal tumors, Patel and colleagues performed a meta-analysis using data collected from 43 cohorts from 41 non-comparative observational studies.15 The authors found a significantly improved OS at 5 years with charged particle therapy (relative risk, RR 1.51, 95% CI 1.14–1.99; p=0.0038) as well as improved LRC at longest follow up. Moreover, a subanalysis of proton therapy to IMRT showed significantly higher 5-year DFS (RR, 1.44; 95% CI 1.01–2.05; p=0.045) and LRC at longest follow up (RR 1.26, 95% CI 1.05–1.51; p=0.011) with proton therapy. While these findings are supportive of proton therapy use for tumors of the nasal cavity and paranasal sinuses, additional prospective investigations are necessary as most of the studies included in this analysis are retrospective and potentially biased. Besides, caution must be taken during the RT planning phase since proton therapy as with IMRT can also result in significant toxicities such as osteonecrosis, brain necrosis, cataracts and retinopathy.67
Long term outcomes associated with IMPT use for treatment of sinonasal tumors is still in its infancy with limited data available in the current literature. One notable prospective study by Holliday et al. included 16 patients with sinonasal tumors out of which 13 (81%) of patients received IMPT in the postoperative setting.68 The median radiation dose delivered was 62 GyRBE and treatment was well tolerated with no reported grade 4 or 5 toxicities. In the acute setting, the most common treatment-related toxicity was grade 2 dermatitis (13 patients, 81%) followed by grade 2 mucositis (5 patients, 31%), and grade 2 dysgeusia (2 patients, 13%).
Salivary Gland Tumors
Major and minor salivary gland tumors are a relatively rare diagnosis with only 2,000–2,500 cases diagnosed in the United States per year and representing approximately 5–8% of all head and neck cancers.69 While the parotid is the most common site to harbor disease, the main therapeutic approach remains the same for all salivary gland tumors, which is surgical intervention followed by PORT for adverse features. With regards to the efficacy of proton therapy in general, the Patel meta-analysis which showed improved OS and DFS with particle therapy over photon therapy was based on multiple studies that included patients with minor salivary gland tumors.15 Due to the rarity of this diagnosis, however, there is a low volume of published evidence demonstrating the potential benefit of IMPT over PSPT or IMRT to treat these cancers. A small study that investigated 13 patients with parotid cancers demonstrated excellent 1-year LC and OS rates with IMPT.68 In this study, acute grade 2 and grade 3 dermatitis was reported in 9 and 4 patients, respectively. Furthermore, no significant chronic toxicities were reported which is promising when considering RT options for treating the unilateral head and neck.
Periorbital Tumors
Due to the complexity of the surrounding anatomy, tumors of the orbit and periorbital regions have been traditionally managed with orbit exenteration in order to ensure widely negative surgical margins. However, a multidisciplinary orbit-sparing treatment approach is now generally recommended when feasible in order to preserve some visual function while maintaining high rates of local control.70–72 El-Sawy and colleagues have published their institution’s experience with globe-sparing treatment for patients with primary lacrimal sac or nasolacrimal duct carcinoma. In this retrospective study of 14 surgical patients (9 limited surgery, 1 biopsy only, and 4 orbital exenteration due to extensive orbital tissue involvement), 13 patients underwent PORT with protons or IMRT (median dose, 60 Gy) and 8 patients received chemotherapy. The globe was spared in all 10 patients after a median follow up of 27 months, and 9 (90%) patients either maintained or saw an improvement in their baseline visual acuity.70
In a slightly larger cohort of 20 patients with epithelial tumors of the orbit/ocular adnexa, Holliday et al demonstrated the feasibility of achieving disease control after orbit-sparing surgery followed by proton therapy.71 The median radiation dose was 60 GyRBE, and 6 (30%) patients received IMPT using an eye-deviation technique for patients with lateralized tumors. After a median follow-up of 27.1 months, no patient experienced a local recurrence (1 regional, 1 distant), and the treatment was overall well tolerated with the exception of some cases of grade 3 epiphora (3 patients, 15%) and grade 3 exposure keratopathy (3 patients, 15%). All patients maintained vision in the ipsilateral eye with only four (20%) patients experiencing a decrease in visual acuity. One interesting finding the authors noted was an association of grade 3 or higher chronic ocular toxicities with a higher max dose to the ipsilateral cornea (median 46.3 GyRBE vs. median 37.4 GyRBE, P=0.017). Additional investigations are warranted to identify the most optimal patient setup, IMPT planning specifications, and sub-ocular structure radiation dose tolerance limits in order to provide the most favorable outcomes with IMPT for this patient population.
Base of Skull Tumors
Tumors located at the base of skull (BOS) are challenging to manage as surgical interventions are often not possible for patients with locally advanced disease, and efforts to deliver ablative doses to the tumor can be hindered by the need to respect the tolerance limits of nearby structures such as the brain and brainstem. From a dosimetric standpoint, IMPT may again offer a benefit over IMRT. Leeman et al. recently reported dose-volume characteristics for IMRT and IMPT plans in the preoperative and postoperative settings for two patients with BOS disease. In one particular case, an 80year old male with adenocarcinoma of the ethmoid sinus with extensive involvement of the anterior BOS and paranasal sinuses, IMPT resulted in either comparable or significantly improved dose sparing of several OARs in the preoperative setting compared to IMRT (brainstem max dose, 35.8 GyRBE vs. 60 Gy; right/left cochlea: 9.6/7.1 GyRBE vs. 38.4/35.8 Gy).73 The dosimetric advantage conferred by IMPT persisted in the postoperative setting for this case as well as for the second case of locally advanced chondrosarcoma of the sphenoid sinus.
The first long-term report on the use of proton therapy, particularly IMPT, for chordomas and chondrosarcomas was reported by Ares et al. in 2009.74 A total of 64 patients with BOS disease were treated with either a spot-scanning proton therapy technique (n=44) or IMPT (n=20). The median total dose delivered was 73.5 GyRBE and 68.4 GyRBE for chordomas and chondrosarcomas, respectively. With this treatment, 5-year LC rates were high at 81% for chordomas and 94% with chondrosarcomas, and 5-year OS were even higher at 100% and 91%, respectively. Treatment-related toxicities were limited and no brainstem toxicity was reported, making IMPT an ideal RT technique for treatment of BOS tumors.
Reirradiation for Recurrent or New Primary HNCs
Substantial progress in RT techniques and its application for reirradiation of recurrent or new primary cancers of the head and neck have occurred over the last three decades. Earlier studies using conventional fractionation or brachytherapy in previously irradiated tissues often showed poor to modest local control but at a costly trade-off of highly morbid late complications such as soft tissue necrosis, osteoradionecrosis and carotid blowout.75–77 IMRT and stereotactic body radiotherapy (SBRT), the latter being a conformal photon-based modality capable of delivering ablative radiation doses over 1–5 fractions with greater dose fall-off than IMRT, have largely replaced traditional 2D/3D RT techniques. Reported 1- to 2-year LRC rates in the literature are approximately 20–60% with 3D RT, 50–60% with IMRT, and 40–80% with SBRT.77
The ability to deliver conformal definitive doses safely is highly correlative with improved local control and overall survival. Therefore, proton therapy is ideal from a dosimetric standpoint for such cases where minimizing dose exposure to reirradiated normal tissue is critical to reducing the risks of treatment-related toxicities. We have previously summarized the findings of four clinical reirradiation series using proton therapy for squamous cell carcinomas of the head and neck.78 The majority of these patients were treated with PSPT, and one study (n=92) reported a 1-year LC and OS of 75% and 65%, respectively, after a PSPT median dose of 60.6 GyRBE. However, noteworthy grade 3 or higher late skin and dysphagia toxicities were seen in 6 (8.7%) patients and 4 (7.1%) patients, respectively, as well as 2 cases of grade 5 bleeding.10
Compared to IMRT and PSPT, there is a lack of clinical and toxicity outcomes data after reirradiation using IMPT given the novelty of the technology. Most of the existing information regarding IMPT in the reirradiation setting for HNCs are dosimetric studies such as the one recently published by Eekers et al.79 They compared VMAT, IMPT, and ion therapy (IMIT) reirradiation plans prescribed to a second dose of 70 Gy/GyRBE for 25 patients. Compared to IMRT, IMPT plans were superior as they resulted in significantly reduced mean doses to 15 out of 22 (65%) OARs studied (brainstem D2, 2.7 GyRBE vs. 8.2 Gy; spinal cord D2, 6.7 GyRBE vs. 16.6 Gy; larynx mean dose, 27.2 GyRBE vs. 34.1 Gy). Phan et al have reported on clinical outcomes associated with reirradiation of HNCs with IMPT.11 From April 2011 to June 2015, 60 patients were treated with proton therapy (IMPT: n=45, 75%) out of which 35 (58%) patients received upfront surgery, and 44 (73%) received concurrent chemotherapy. The most common retreatment sites for the collective cohort were the oropharynx (40%), nasopharynx (8%), or the neck, orbit, or sinonasal regions (13% each). With a median follow-up of 13.6 months, the 1-year rates of locoregional failure-free survival, OS, PFS, and acute grade 3 toxicities for all patients were 68.4%, 83.8%, 60.1%, and 30%, respectively. While these results are promising, they still highlight the main barrier to reirradiation which is the high rates of toxicity, regardless of RT modality. Therefore, extensive prospective evaluation of IMPT for head and neck reirradiation is required.
Future directions
These are exciting times as proton therapy continues to be validated as a valuable treatment modality for patients with HNCs. The National Comprehensive Cancer Network (NCCN) guidelines already recognizes the dosimetric and clinical advantages protons provide for treating tumors near critical structures and recommends its usage when normal tissue constraints cannot be met with photon-based therapies.44 With the increase in proton therapy centers worldwide, IMPT will become more readily available, tentatively allowing for increased feasibility in performing larger prospective studies on clinical outcomes and cost-effectiveness analyses.80,81 Currently, it is still unclear which HNC patients will exhibit a significantly enhanced therapeutic ratio with IMPT over PSPT or IMRT. However in the absence of randomized clinical data, retrospective comparison plans using normal tissue complication probability (NTCP) modeling is providing insight on potential subgrouping categories to properly preselect patients for IMPT. Modern NTCP models take into account the incidence of various toxicities such as acute oral mucositis, aspiration, xerostomia, and dysphagia to compute corresponding NTCP values that can be compared by RT technique. One study has suggested risk reductions of dysphagia with IMPT over IMRT in patients with tumors located in the upper head and neck area as well as risk reductions of acute mucositis in patients with tumors in the larynx region.82 Additional NTCP models are being investigated to estimate the efficacy of IMPT for specific HNCs sub-sites or when IMPT is delivered in a mixed modality treatment.83–86
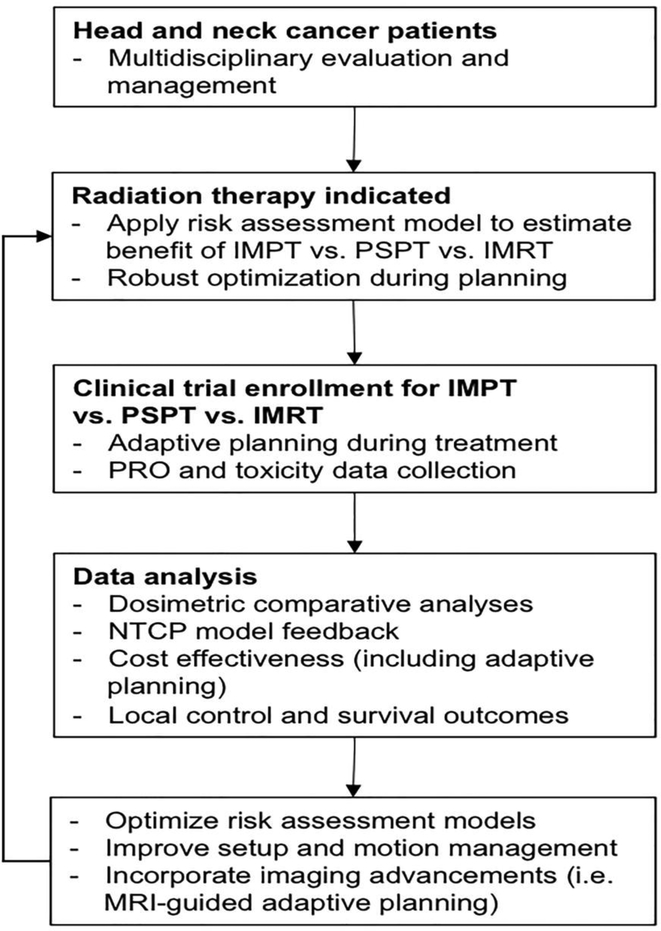
Figure 4 outlines a proposed workflow towards efficiently studying, validating, and refining what we know about and how we use IMPT in oncologic care. First and foremost, all patients presenting with a suspected HNCs should be properly assessed and staged in a multidisciplinary setting.87,88 Pre-treatment data collection on comorbidities, functional deficits, and disease extent should be performed and integrated into an evolving risk assessment model used to estimate the expected therapeutic ratio using the various RT modalities. Enrollment on clinical trials should be encouraged to prospectively collect and analyze radiographic data (CT, MRI, PET, etc.), quality of life, functional and survival outcomes. Radiomic texture analysis methods are currently being investigated for HNC applications89 and can be incorporated into developing risk assessment models for future patient stratification and treatments. Overall, having a structured methodology for investigating the value of IMPT, both in the primary and reirradiation settings, is crucial towards providing unbiased information and should be promoted on a national scale.
Figure 4. Proposed methodology to investigate the clinical benefits of IMPT for the management of head and neck cancers.
Abbreviations: IMPT, intensity modulated proton therapy; IMRT, intensity modulated radiation therapy; NTCP, normal tissue complication probability; PRO, patient reported outcomes; PSPT, passive scatter proton therapy.
Conclusions
Proton therapy offers a dosimetric advantage over IMRT in the management of HNCs. IMPT is a highly sophisticated, novel form of proton therapy that is promising for treatment-related toxicity reduction and potential dose escalation while respecting normal tissue dose constraints. The greatest limitation to IMPT is its high sensitivity to radiologic density changes due to setup errors or anatomical changes during or in between fractions. However, advancements with on-board image guidance resources, robust optimization algorithms, and standardization of patient-specific quality assurance programs and CT verification protocols will aid in the establishing of IMPT as a standard of care for HNC treatment. Additional prospective studies are necessary to quantify the clinical benefit of IMPT over IMRT or PSPT, and to provide dynamic feedback on improving current methods used to plan and treat patients with IMPT.
Highlights.
Intensity modulated proton therapy (IMPT) is a sophisticated mode of proton therapy that is analogous to IMRT and is also associated with a lower integral dose bath.
Several dosimetric studies have demonstrated the superiority of IMPT over IMRT to improve dose sparing of organs such as the larynx, salivary glands, and esophagus.
There is clinical evidence that the dosimetric advantages of IMPT can translate to acute and chronic toxicity reductions for patients with HNCs.
This review will summarize existing literature and discuss the future directions of IMPT use for HNCs.
Footnotes
Conflict of Interest Statement
All authors hereby confirm that none of the authors have any personal or financial relationships to disclose when pertaining to the special issue manuscript titled, “Intensity Modulated Proton Therapy (IMPT)- The Future of IMRT for Head and Neck Cancer” which was recently submitted to Oral Oncology for publication.
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Verbakel WFAR Cuijpers JP, Hoffmans D,Bieker M,Slotman BJ,Senan S Volumetric intensity-modulated arc therapy vs. conventional IMRT in head-and-neck cancer: a comparative planning and dosimetric study. Int J Radiat Oncol Biol Phys. 2009;74(1):252–259. http://linkinghub.elsevier.com/retrieve/pii/S0360301609000303. Accessed October 14, 2018. [DOI] [PubMed] [Google Scholar]
- 2.Puri DR, Chou W, Lee N. Intensity-modulated radiation therapy in head and neck cancers: dosimetric advantages and update of clinical results. Am J Clin Oncol. 2005;28(4):415–423. http://www.ncbi.nlm.nih.gov/pubmed/16062086. Accessed October 14, 2018. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Eisbruch A. IMRT for head and neck cancer: reducing xerostomia and dysphagia. J Radiat Res. 2016;57 Suppl 1(S1):i69–i75. https://academic.oup.com/jrr/article-lookup/doi/10.1093/jrr/rrw047. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Endo M Robert R. Wilson (1914−−2000): the first scientist to propose particle therapy---use of particle beam for cancer treatment. Radiol Phys Technol. 2018;11(1):1–6. 10.1007/s12194-017-0428-z. [DOI] [PubMed] [Google Scholar]
- 5.Cozzi L, Fogliata A, Lomax A, Bolsi A. A treatment planning comparison of 3D conformal therapy, intensity modulated photon therapy and proton therapy for treatment of advanced head and neck tumours. Radiother Oncol. 2001;61(3):287–297. http://www.ncbi.nlm.nih.gov/pubmed/11730999. Accessed October 14, 2018. [DOI] [PubMed] [Google Scholar]
- 6.Hu Q X-Ray Physics-with Emphasis on Attenuation of X-Rays in Matter.; 2005. http://web.mit. Accessed October 14, 2018.
- 7.Mohan R, Grosshans D. Proton therapy - Present and future. Adv Drug Deliv Rev. 2017;109:26–44. http://www.ncbi.nlm.nih.gov/pubmed/27919760. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald MW, Liu Y, Moore MG, Johnstone PAS. Acute toxicity in comprehensive head and neck radiation for nasopharynx and paranasal sinus cancers: cohort comparison of 3D conformal proton therapy and intensity modulated radiation therapy. Radiat Oncol. 2016;11(1):32 http://www.ro-journal.com/content/11/1/32. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zenda S, Akimoto T, Mizumoto M, Hayashi R, Arahira S, Okumura T, et al. Phase II study of proton beam therapy as a nonsurgical approach for mucosal melanoma of the nasal cavity or para-nasal sinuses. Radiother Oncol 2016;118(2):267–271. https://linkinghub.elsevier.com/retrieve/pii/S0167814015005745. Accessed October 14, 2018. [DOI] [PubMed] [Google Scholar]
- 10.Romesser PB, Cahlon O, Scher ED, Hug EB, Sine K, DeSelm C, et al. Proton Beam Reirradiation for Recurrent Head and Neck Cancer: Multi-institutional Report on Feasibility and Early Outcomes. Int J Radiat Oncol Biol Phys. 2016;95(1):386–395. https://linkinghub.elsevier.com/retrieve/pii/S0360301616001565. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phan J, Sio TT, Nguyen TP, Takiar V, Gunn GB, Garden AS, et al. Reirradiation of Head and Neck Cancers With Proton Therapy: Outcomes and Analyses. Int J Radiat Oncol Biol Phys. 2016;96(1):30–41. https://linkinghub.elsevier.com/retrieve/pii/S0360301616300694. Accessed October 14, 2018. [DOI] [PubMed] [Google Scholar]
- 12.Chan A, Adams JA, Weyman E, Parambi R, Goldsmith T, Holman A, et al. A Phase II Trial of Proton Radiation Therapy With Chemotherapy for Nasopharyngeal Carcinoma. Int J Radiat Oncol 2012;84(3):S151–S152. http://linkinghub.elsevier.com/retrieve/pii/S0360301612013351. Accessed October 14, 2018. [Google Scholar]
- 13.McDonald MW, Zolali-Meybodi O, Lehnert SJ, Estabrook NC, Liu Y, Cohen-Gadol AA, et al. Reirradiation of Recurrent and Second Primary Head and Neck Cancer With Proton Therapy. Int J Radiat Oncol. 2016;96(4):808–819. http://www.ncbi.nlm.nih.gov/pubmed/27788954. Accessed October 14, 2018. [DOI] [PubMed] [Google Scholar]
- 14.Holliday EB, Frank SJ. Proton radiation therapy for head and neck cancer: a review of the clinical experience to date. Int J Radiat Oncol Biol Phys. 2014;89(2):292–302. https://linkinghub.elsevier.com/retrieve/pii/S0360301614002417. Accessed October 15, 2018. [DOI] [PubMed] [Google Scholar]
- 15.Patel SH, Wang Z, Wong WW, Murad MH, Buckey CR, Mohammed K, et al. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol. 2014;15(9):1027–1038. http://www.ncbi.nlm.nih.gov/pubmed/24980873. Accessed October 14, 2018. [DOI] [PubMed] [Google Scholar]
- 16.Kooy HM, Grassberger C. Intensity modulated proton therapy. Br J Radiol. 2015;88(1051):20150195 http://www.birpublications.org/doi/10.1259/bjr.20150195. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith A, Gillin M, Bues M, Zhu XR, Suzuki K, Mohan R, et al. The M. D. Anderson proton therapy system. Med Phys. 2009;36(9Part1):4068–4083. http://www.ncbi.nlm.nih.gov/pubmed/19810479. Accessed October 14, 2018. [DOI] [PubMed] [Google Scholar]
- 18.Ding X, Li X, Qin A, Zhou J, Yan D, Chen P, et al. Redefine the role of range shifter in treating bilateral head and neck cancer in the era of Intensity Modulated Proton Therapy. J Appl Clin Med Phys. 2018;19(5):749–755. http://doi.wiley.com/10.1002/acm2.12416. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frank SJ, Cox JD, Gillin M, Mohan R, Garden AS, Rosenthal DI, et al. Multifield Optimization Intensity Modulated Proton Therapy for Head and Neck Tumors: A Translation to Practice. Int J Radiat Oncol 2014;89(4):846–853. http://www.ncbi.nlm.nih.gov/pubmed/24867532. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lomax A Intensity modulation methods for proton radiotherapy. Phys Med Biol. 1999;44(1):185–205. http://www.ncbi.nlm.nih.gov/pubmed/10071883. Accessed August 19, 2018. [DOI] [PubMed] [Google Scholar]
- 21.Michiels S, Barragán AM, Souris K, Poels K, Crijns W, Lee JA, et al. Patient-specific bolus for range shifter air gap reduction in intensity-modulated proton therapy of head-and-neck cancer studied with Monte Carlo based plan optimization. Radiother Oncol. 2018;128(1):161–166. https://linkinghub.elsevier.com/retrieve/pii/S0167814017325689. Accessed October 14, 2018. [DOI] [PubMed] [Google Scholar]
- 22.Cubillos-Mesías M, Baumann M, Troost EGC, Lohaus F, Löck S, Richter C, et al. Impact of robust treatment planning on single- and multi-field optimized plans for proton beam therapy of unilateral head and neck target volumes. Radiat Oncol. 2017;12(1):190 https://ro-journal.biomedcentral.com/articles/10.1186/s13014-017-0931-8. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quan EM, Liu W, Wu R, Li Y, Frank SJ, Zhang X, et al. Preliminary evaluation of multifield and single-field optimization for the treatment planning of spot-scanning proton therapy of head and neck cancer. Med Phys. 2013;40(8):081709 http://doi.wiley.com/10.1118/1.4813900. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yasui K, Toshito T, Omachi C, Hayashi K, Tanaka K, Asai K, et al. Evaluation of dosimetric advantages of using patient-specific aperture system with intensity-modulated proton therapy for the shallow depth tumor. J Appl Clin Med Phys. 2018;19(1):132–137. http://doi.wiley.com/10.1002/acm2.12231. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraan AC, van de Water S, Teguh DN, Al-Mamgani A, Madden T, Kooy HM, et al. Dose uncertainties in IMPT for oropharyngeal cancer in the presence of anatomical, range, and setup errors. Int J Radiat Oncol Biol Phys. 2013;87(5):888–896. https://linkinghub.elsevier.com/retrieve/pii/S0360301613031015. Accessed October 14, 2018. [DOI] [PubMed] [Google Scholar]
- 26.Engelsman M, Rietzel E, Kooy HM. Four-dimensional proton treatment planning for lung tumors. Int J Radiat Oncol. 2006;64(5):1589–1595. http://www.ncbi.nlm.nih.gov/pubmed/16580508. Accessed October 14, 2018. [DOI] [PubMed] [Google Scholar]
- 27.Liu W, Zhang X, Li Y, Mohan R. Robust optimization of intensity modulated proton therapy. Med Phys. 2012;39(2):1079–1091. http://www.ncbi.nlm.nih.gov/pubmed/22320818. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Müller BS, Duma MN, Kampfer S, Nill S, Oelfke U, Geinitz H, et al. Impact of interfractional changes in head and neck cancer patients on the delivered dose in intensity modulated radiotherapy with protons and photons. Phys Med. 2015;31(3):266–272. https://linkinghub.elsevier.com/retrieve/pii/S1120179715000411. Accessed October 14, 2018. [DOI] [PubMed] [Google Scholar]
- 29.Stuschke M, Kaiser A, Abu Jawad J, Pöttgen C, Levegrün S, Farr J. Multi-scenario based robust intensity-modulated proton therapy (IMPT) plans can account for set-up errors more effectively in terms of normal tissue sparing than planning target volume (PTV) based intensity-modulated photon plans in the head and neck region. Radiat Oncol. 2013;8(1):145 http://ro-journal.biomedcentral.com/articles/10.1186/1748-717X-8-145. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W, Frank SJ, Li X, Li Y, Park PC, Dong L, et al. Effectiveness of robust optimization in intensity-modulated proton therapy planning for head and neck cancers. Med Phys 2013;40(5):051711 http://doi.wiley.com/10.1118/1.4801899. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu W, Frank SJ, Li X, Li Y, Zhu RX, Mohan R. PTV-based IMPT optimization incorporating planning risk volumes vs robust optimization. Med Phys 2013;40(2):021709 http://doi.wiley.com/10.1118/1.4774363. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu W, Zhu X, Li X, Li Y, Zhang X, Frank S, et al. SU-E-T-624: Comparison of PTV+PRV-Based Optimization and Robust Optimization in Intensity-Modulated Proton Therapy. Med Phys 2012;39(6Part20):3849–3850. http://doi.wiley.com/10.1118/1.4735714. Accessed October 14, 2018. [DOI] [PubMed] [Google Scholar]
- 33.Stützer K, Jakobi A, Bandurska-Luque A, Barczyk S, Arnsmeyer C, Löck S, et al. Potential proton and photon dose degradation in advanced head and neck cancer patients by intratherapy changes. J Appl Clin Med Phys. 2017;18(6):104–113. http://doi.wiley.com/10.1002/acm2.12189. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Placidi L, Bolsi A, Lomax AJ, Schneider RA, Malyapa R, Weber DC, et al. Effect of Anatomic Changes on Pencil Beam Scanned Proton Dose Distributions for Cranial and Extracranial Tumors. Int J Radiat Oncol Biol Phys. 2017;97(3):616–623. https://linkinghub.elsevier.com/retrieve/pii/S0360301616334368. Accessed October 14, 2018. [DOI] [PubMed] [Google Scholar]
- 35.Gunn GB, Blanchard P, Garden AS, Zhu XR, Fuller CD, Mohamed AS, et al. Clinical Outcomes and Patterns of Disease Recurrence After Intensity Modulated Proton Therapy for Oropharyngeal Squamous Carcinoma. Int J Radiat Oncol 2016;95(1):360–367. http://www.ncbi.nlm.nih.gov/pubmed/27084653. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paganetti H Dose to water versus dose to medium in proton beam therapy. Phys Med Biol. 2009;54(14):4399–4421. http://stacks.iop.org/0031-9155/54/i=14/a=004?key=crossref.1df042584d5814dcd3b81a848caf45fb. Accessed October 14, 2018. [DOI] [PubMed] [Google Scholar]
- 37.Hong L, Goitein M, Bucciolini M, Comiskey R, Gottschalk B, Rosenthal S, et al. A pencil beam algorithm for proton dose calculations. Phys Med Biol. 1996;41(8):1305–1330. http://www.ncbi.nlm.nih.gov/pubmed/8858722. Accessed October 14, 2018. [DOI] [PubMed] [Google Scholar]
- 38.Kang JH, Wilkens JJ, Oelfke U. Non-uniform depth scanning for proton therapy systems employing active energy variation. Phys Med Biol. 2008;53(9):N149–55. http://stacks.iop.org/0031-9155/53/i=9/a=N01?key=crossref.2d88ce0e83a510b51bf434dba9bea60a. Accessed October 14, 2018. [DOI] [PubMed] [Google Scholar]
- 39.Gu W, O’Connor D, Nguyen D, Yu VY, Ruan D, Dong L, et al. Integrated beam orientation and scanning-spot optimization in intensity-modulated proton therapy for brain and unilateral head and neck tumors. Med Phys. 2018;45(4):1338–1350. http://doi.wiley.com/10.1002/mp.12788. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohan R, Das IJ, Ling CC. Empowering Intensity Modulated Proton Therapy Through Physics and Technology: An Overview. Int J Radiat Oncol. 2017;99(2):304–316. http://www.ncbi.nlm.nih.gov/pubmed/28871980. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toramatsu C, Inaniwa T. Beam angle selection incorporation of anatomical heterogeneities for pencil beam scanning charged-particle therapy. Phys Med Biol. 2016;61(24):8664–8675. http://stacks.iop.org/0031-9155/61/i=24/a=8664?key=crossref.ce59391080b1ea79ea8f744e070dc1dc. Accessed October 14, 2018. [DOI] [PubMed] [Google Scholar]
- 42.Giantsoudi D, Grassberger C, Craft D, Niemierko A, Trofimov A, Paganetti H. Linear energy transfer-guided optimization in intensity modulated proton therapy: feasibility study and clinical potential. Int J Radiat Oncol Biol Phys. 2013;87(1):216–222. http://linkinghub.elsevier.com/retrieve/pii/S0360301613005452. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu XR, Li Y, Mackin D, Li H, Poenisch F, Lee AK, et al. Towards effective and efficient patient-specific quality assurance for spot scanning proton therapy. Cancers (Basel). 2015;7(2):631–647. http://www.mdpi.com/2072-6694/7/2/631. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Comprehensive Cancer Network: Cancer of the Oropharynx.
- 45.Romesser PB, Cahlon O, Scher E, Zhou Y, Berry SL, Rybkin A, et al. Proton beam radiation therapy results in significantly reduced toxicity compared with intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiother Oncol 2016;118(2):286–292. https://linkinghub.elsevier.com/retrieve/pii/S0167814015006672. Accessed October 15, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lukens JN, Lin A, Hahn SM. Proton therapy for head and neck cancer. Curr Opin Oncol. 2015;27(3):165–171. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00001622-201505000-00004. Accessed October 15, 2018. [DOI] [PubMed] [Google Scholar]
- 47.McKeever MR, Sio TT, Gunn GB, Holliday EB, Blanchard P, Kies MS, et al. Reduced acute toxicity and improved efficacy from intensity-modulated proton therapy (IMPT) for the management of head and neck cancer. Chinese Clin Oncol. 2016;5(4):54 http://cco.amegroups.com/article/view/11172/11902. Accessed October 15, 2018. [DOI] [PubMed] [Google Scholar]
- 48.Chan AW, Liebsch NJ. Proton radiation therapy for head and neck cancer. J Surg Oncol. 2008;97(8):697–700. http://doi.wiley.com/10.1002/jso.21013. Accessed October 15, 2018. [DOI] [PubMed] [Google Scholar]
- 49.International Commission on Radiation Units and Measurements (ICRU). https://icru.org/home/reports/prescribing-recording-and-reporting-proton-beam-therapy-icru-report-78. Accessed November 11, 2018.
- 50.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68(1):7–30. http://www.ncbi.nlm.nih.gov/pubmed/29313949. Accessed October 14, 2018. [DOI] [PubMed] [Google Scholar]
- 51.Cancer Stat Facts: Oral Cavity and Pharynx Cancer. https://seer.cancer.gov/statfacts/html/oralcav.html. Published 2017. Accessed September 19, 2017.
- 52.Slater JD, Yonemoto LT, Mantik DW, Bush DA, Preston W, Grove RI, et al. Proton radiation for treatment of cancer of the oropharynx: early experience at Loma Linda University Medical Center using a concomitant boost technique. Int J Radiat Oncol Biol Phys. 2005;62(2):494–500. http://linkinghub.elsevier.com/retrieve/pii/S0360301604026987. Accessed October 26, 2018. [DOI] [PubMed] [Google Scholar]
- 53.Sio TT, Lin H-K, Shi Q, Gunn GB, Cleeland CS, Lee JJ, et al. Intensity Modulated Proton Therapy Versus Intensity Modulated Photon Radiation Therapy for Oropharyngeal Cancer: First Comparative Results of Patient-Reported Outcomes. Int J Radiat Oncol Biol Phys. 2016;95(4):1107–1114. https://linkinghub.elsevier.com/retrieve/pii/S0360301616001772. Accessed October 15, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blanchard P, Garden AS, Gunn GB, Rosenthal DI, Morrison WH, Hernandez M, et al. Intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer – A case matched analysis. Radiother Oncol. 2016;120(1):48–55. https://linkinghub.elsevier.com/retrieve/pii/S0167814016311367. Accessed October 15, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holliday EB, Kocak-Uzel E, Feng L, Thaker NG, Blanchard P, Rosenthal DI, et al. Dosimetric advantages of intensity-modulated proton therapy for oropharyngeal cancer compared with intensity-modulated radiation: A case-matched control analysis. Med Dosim. 2016;41(3):189–194. https://linkinghub.elsevier.com/retrieve/pii/S0958394716000169. Accessed October 15, 2018. [DOI] [PubMed] [Google Scholar]
- 56.van de Water TA, Lomax AJ, Bijl HP, de Jong ME, Schilstra C, Hug EB, et al. Potential benefits of scanned intensity-modulated proton therapy versus advanced photon therapy with regard to sparing of the salivary glands in oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2011;79(4):1216–1224. http://linkinghub.elsevier.com/retrieve/pii/S0360301610006887. Accessed October 16, 2018. [DOI] [PubMed] [Google Scholar]
- 57.van de Water TA, Lomax AJ, Bijl HP, Schilstra C, Hug EB, Langendijk JA. Using a reduced spot size for intensity-modulated proton therapy potentially improves salivary gland-sparing in oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2012;82(2):e313–9. http://linkinghub.elsevier.com/retrieve/pii/S0360301611006651. Accessed October 16, 2018. [DOI] [PubMed] [Google Scholar]
- 58.van der Laan HP, van de Water TA, van Herpt HE, Christianen MEMC, Bijl HP, Korevaar EW, et al. The potential of intensity-modulated proton radiotherapy to reduce swallowing dysfunction in the treatment of head and neck cancer: A planning comparative study. Acta Oncol. 2013;52(3):561–569. http://www.tandfonline.com/doi/full/10.3109/0284186X.2012.692885. Accessed October 16, 2018. [DOI] [PubMed] [Google Scholar]
- 59.Apinorasethkul O, Kirk M, Teo K, Swisher-McClure S, Lukens JN, Lin A. Pencil beam scanning proton therapy vs rotational arc radiation therapy: A treatment planning comparison for postoperative oropharyngeal cancer. Med Dosim. 2017;42(1):7–11. https://linkinghub.elsevier.com/retrieve/pii/S0958394716300826. Accessed October 16, 2018. [DOI] [PubMed] [Google Scholar]
- 60.Holliday EB, Frank SJ. Proton therapy for nasopharyngeal carcinoma. Chinese Clin Oncol. 2016;5(2):25 http://cco.amegroups.com/article/view/9551/10777. Accessed October 16, 2018. [DOI] [PubMed] [Google Scholar]
- 61.Taheri-Kadkhoda Z, Björk-Eriksson T, Nill S, Wilkens JJ, Oelfke U, Johansson K-A, et al. Intensity-modulated radiotherapy of nasopharyngeal carcinoma: a comparative treatment planning study of photons and protons. Radiat Oncol. 2008;3(1):4 http://ro-journal.biomedcentral.com/articles/10.1186/1748-717X-3-4. Accessed October 16, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Widesott L, Pierelli A, Fiorino C, Dell’oca I, Broggi S, Cattaneo GM, et al. Intensity-modulated proton therapy versus helical tomotherapy in nasopharynx cancer: planning comparison and NTCP evaluation. Int J Radiat Oncol Biol Phys. 2008;72(2):589–596. http://linkinghub.elsevier.com/retrieve/pii/S0360301608024711. Accessed October 16, 2018. [DOI] [PubMed] [Google Scholar]
- 63.Lewis GD, Holliday EB, Kocak-Uzel E, Hernandez M, Garden AS, Rosenthal DI, et al. Intensity-modulated proton therapy for nasopharyngeal carcinoma: Decreased radiation dose to normal structures and encouraging clinical outcomes. Head Neck. 2016;38 Suppl 1(S1):E1886–95. http://doi.wiley.com/10.1002/hed.24341. Accessed October 16, 2018. [DOI] [PubMed] [Google Scholar]
- 64.Holliday EB, Garden AS, Rosenthal DI, Fuller CD, Morrison WH, Gunn GB, et al. Proton Therapy Reduces Treatment-Related Toxicities for Patients with Nasopharyngeal Cancer: A Case-Match Control Study of Intensity-Modulated Proton Therapy and Intensity-Modulated Photon Therapy. Int J Part Ther. 2015;2(1):19–28. http://theijpt.org/doi/10.14338/IJPT-15-00011.1. Accessed October 16, 2018. [Google Scholar]
- 65.Resto VA, Chan AW, Deschler DG, Lin DT. Extent of surgery in the management of locally advanced sinonasal malignancies. Head Neck. 2008;30(2):222–229. http://doi.wiley.com/10.1002/hed.20681. Accessed October 19, 2018. [DOI] [PubMed] [Google Scholar]
- 66.Fuji H, Yoshikawa S, Kasami M, Murayama S, Onitsuka T, Kashiwagi H, et al. High-dose proton beam therapy for sinonasal mucosal malignant melanoma. Radiat Oncol. 2014;9(1):162 http://rojournal.biomedcentral.com/articles/10.1186/1748-717X-9-162. Accessed October 19, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakamura T, Azami Y, Ono T, Yamaguchi H, Hayashi Y, Suzuki M, et al. Preliminary results of proton beam therapy combined with weekly cisplatin intra-arterial infusion via a superficial temporal artery for treatment of maxillary sinus carcinoma. Jpn J Clin Oncol. 2016;46(1):46–50. https://academic.oup.com/jjco/article-lookup/doi/10.1093/jjco/hyv160. Accessed October 19, 2018. [DOI] [PubMed] [Google Scholar]
- 68.Current applications of proton beam radiation for the treatment of head and neck tumors - Otorinolaringologia 2014. March;64(1):1–11 - Minerva Medica - Journals. https://www.minervamedica.it/en/journals/otorinolaringologia/article.php?cod=R27Y2014N01A0001. Accessed October 16, 2018. [Google Scholar]
- 69.Guzzo M, Locati LD, Prott FJ, Gatta G, McGurk M, Licitra L. Major and minor salivary gland tumors. Crit Rev Oncol Hematol. 2010;74(2):134–148. http://www.ncbi.nlm.nih.gov/pubmed/19939701. Accessed October 19, 2018. [DOI] [PubMed] [Google Scholar]
- 70.El-Sawy T, Frank SJ, Hanna E, Sniegowski M, Lai SY, Nasser QJ, et al. Multidisciplinary management of lacrimal sac/nasolacrimal duct carcinomas. Ophthal Plast Reconstr Surg. 2013;29(6):454–457. https://insights.ovid.com/crossref?an=00002341-201311000-00008. Accessed October 19, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holliday EB, Esmaeli B, Pinckard J, Garden AS, Rosenthal DI, Morrison WH, et al. A Multidisciplinary Orbit-Sparing Treatment Approach That Includes Proton Therapy for Epithelial Tumors of the Orbit and Ocular Adnexa. Int J Radiat Oncol Biol Phys. 2016;95(1):344–352. https://linkinghub.elsevier.com/retrieve/pii/S0360301615031211. Accessed October 16, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee LN, Scott AR, Chan AW, Frankenthaler RA. Management of transitional cell carcinoma of the lacrimal sac: a multidisciplinary approach to orbit sparing treatment. Laryngoscope. 2010;120 Suppl 4(S4):S161 http://doi.wiley.com/10.1002/lary.21625. Accessed October 19, 2018. [DOI] [PubMed] [Google Scholar]
- 73.Leeman JE, Lee NY, Zhou Y, Neal B, Sine K, Tabar V, et al. Endoscopic Resection Followed by Proton Therapy With Pencil Beam Scanning for Skull Base Tumors. Laryngoscope. September 2018. http://doi.wiley.com/10.1002/lary.27512. Accessed October 14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ares C, Hug EB, Lomax AJ, Bolsi A, Timmermann B, Rutz HP, et al. Effectiveness and Safety of Spot Scanning Proton Radiation Therapy for Chordomas and Chondrosarcomas of the Skull Base: First Long-Term Report. Int J Radiat Oncol. 2009;75(4):1111–1118. http://www.ncbi.nlm.nih.gov/pubmed/19386442. Accessed October 19, 2018. [DOI] [PubMed] [Google Scholar]
- 75.Puthawala A, Nisar Syed AM, Gamie S, Chen YJ, Londrc A, Nixon V. Interstitial low-dose-rate brachytherapy as a salvage treatment for recurrent head-and-neck cancers: long-term results. Int J Radiat Oncol Biol Phys. 2001;51(2):354–362. http://www.ncbi.nlm.nih.gov/pubmed/11567809. Accessed October 15, 2018. [DOI] [PubMed] [Google Scholar]
- 76.Dawson LA, Myers LL, Bradford CR, Chepeha DB, Hogikyan ND, Teknos TN, et al. Conformal re-irradiation of recurrent and new primary head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50(2):377–385. http://www.ncbi.nlm.nih.gov/pubmed/11380224. Accessed October 15, 2018. [DOI] [PubMed] [Google Scholar]
- 77.Ho JC, Phan J. Reirradiation of head and neck cancer using modern highly conformal techniques. Head Neck. 2018;40(9):2078–2093. http://doi.wiley.com/10.1002/hed.25180. Accessed October 15, 2018. [DOI] [PubMed] [Google Scholar]
- 78.Blanchard P, Gunn GB, Lin A, Foote RL, Lee NY, Frank SJ. Proton Therapy for Head and Neck Cancers. Semin Radiat Oncol. 2018;28(1):53–63. https://linkinghub.elsevier.com/retrieve/pii/S1053429617300851. Accessed October 15, 2018. [DOI] [PubMed] [Google Scholar]
- 79.Eekers DBP, Roelofs E, Jelen U, Kirk M, Granzier M, Ammazzalorso F, et al. Benefit of particle therapy in re-irradiation of head and neck patients. Results of a multicentric in silico ROCOCO trial. Radiother Oncol 2016;121(3):387–394. https://linkinghub.elsevier.com/retrieve/pii/S0167814016342840. Accessed October 15, 2018. [DOI] [PubMed] [Google Scholar]
- 80.PROTON THERAPY - The National Association for Proton Therapy (NAPT) Proton Beam Therapy, Protons and Prostate Cancer. http://www.proton-therapy.org/. Accessed January 28, 2018.
- 81.Proton Radiation Therapy Treatment History | Proton Therapy Treatment Center. https://protons.com/proton-advantage/history-proton-radiation-therapy. Accessed January 29, 2018.
- 82.Jakobi A, Bandurska-Luque A, Stützer K, Haase R, Löck S, Wack L-J, et al. Identification of Patient Benefit From Proton Therapy for Advanced Head and Neck Cancer Patients Based on Individual and Subgroup Normal Tissue Complication Probability Analysis. Int J Radiat Oncol Biol Phys. 2015;92(5):1165–1174. https://linkinghub.elsevier.com/retrieve/pii/S0360301615004319. Accessed October 26, 2018. [DOI] [PubMed] [Google Scholar]
- 83.Jakobi A, Lühr A, Stützer K, Bandurska-Luque A, Löck S, Krause M, et al. Increase in Tumor Control and Normal Tissue Complication Probabilities in Advanced Head-and-Neck Cancer for Dose-Escalated Intensity-Modulated Photon and Proton Therapy. Front Oncol. 2015;5:256 http://journal.frontiersin.org/Article/10.3389/fonc.2015.00256/abstract. Accessed October 26, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jakobi A, Stützer K, Bandurska-Luque A, Löck S, Haase R, Wack L-J, et al. NTCP reduction for advanced head and neck cancer patients using proton therapy for complete or sequential boost treatment versus photon therapy. Acta Oncol. 2015;54(9):1658–1664. http://www.tandfonline.com/doi/full/10.3109/0284186X.2015.1071920. Accessed October 26, 2018. [DOI] [PubMed] [Google Scholar]
- 85.Blanchard P, Wong AJ, Gunn GB, Garden AS, Mohamed ASR, Rosenthal DI, et al. Toward a model-based patient selection strategy for proton therapy: External validation of photon-derived normal tissue complication probability models in a head and neck proton therapy cohort. Radiother Oncol 2016;121(3):381–386. https://linkinghub.elsevier.com/retrieve/pii/S0167814016342864. Accessed October 26, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arts T, Breedveld S, de Jong MA, Astreinidou E, Tans L, Keskin-Cambay F, et al. The impact of treatment accuracy on proton therapy patient selection for oropharyngeal cancer patients. Radiother Oncol. 2017;125(3):520–525. https://linkinghub.elsevier.com/retrieve/pii/S0167814017326142. Accessed October 26, 2018. [DOI] [PubMed] [Google Scholar]
- 87.Foote RL, Gilbert J, Gillison ML, Haddad RI, Hicks WL, Hitchcock YJ, et al. Continue NCCN Guidelines Panel Disclosures † Medical Oncology ¶ Surgery/Surgical Oncology § Radiation Oncology ξ Otolaryngology Þ Internal Medicine * Discussion Writing Committee Member NCCN Guidelines Version 2.2018 Head and Neck Cancers.; 2018. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed October 15, 2018.
- 88.Robson A, Sturman J, Williamson P, Conboy P, Penney S, Wood H. Pre-treatment clinical assessment in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130(S2):S13–S22. http://www.ncbi.nlm.nih.gov/pubmed/27841110. Accessed October 15, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wong AJ, Kanwar A, Mohamed AS, Fuller CD. Radiomics in head and neck cancer: from exploration to application. Transl Cancer Res. 2016;5(4):371–382. http://tcr.amegroups.com/article/view/8805/html. Accessed October 15, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]