Abstract
Objectives.—
We examined the cross-sectional association of sleep apnea and indices of sleep quality with both episodic migraine (EM) and chronic migraine (CM).
Background.—
Sleep apnea and abnormal patterns of sleep, such as insomnia, were associated with migraine onset, severity, and progression in previous research.
Methods.—
The Chronic Migraine Epidemiology & Outcomes Study, a longitudinal study, used a series of web-based surveys to assess migraine symptoms, burden, and patterns of health care utilization. Quota sampling was used from September 2012 to November 2013 to generate a representative sample of the US population. Persons who screened positive for sleep apnea on the Berlin Questionnaire are said to be at “high risk” for sleep apnea. Respondents indicated if they believed that they had sleep apnea, if a physician had diagnosed it, and if and how they were treated. Other aspects of sleep quality were assessed using the Medical Outcomes Study (MOS) Sleep Measures.
Results.—
Of 12,810 eligible respondents with migraine and data on sleep, 11,699 with EM (91.3%) and 1111 with CM (8.7%) provided valid data for this analyses. According to the Berlin Questionnaire, 4739/12,810 (37.0%) were at “high risk” for sleep apnea, particularly persons with CM vs EM (575/1111 [51.8%] vs 4164/11,699 [35.6%]), men vs women (1431/3220 [44.4%] vs 3308/9590 [34.5%]), people with higher body mass index, and older people (all P < .001). Among respondents to the MOS Sleep Measures, persons with CM were more likely to report poor sleep quality than those with EM, including sleep disturbance (mean [SD] values: 53.2 [26.9] vs 37.9 [24.3]), snoring (38.0 [33.9] vs 31.0 [32.1]), shortness of breath (34.9 [29.8] vs 15.3 [20.6]), somnolence (44.1 [23.4] vs 32.2 [21.2]), and less likely to report sleep adequacy (34.0 [24.2] vs 39.2 [22.1]).
Conclusions.—
Compared with respondents with EM, a larger proportion of those with CM were at “high risk” for sleep apnea and reported poor sleep quality. This reflects an association between CM vs EM and sleep apnea and poor sleep quality; the potential relationships are discussed.
Keywords: CaMEO, migraine, sleep quality, sleep disorders, sleep apnea, snoring
INTRODUCTION
Migraine is a common, potentially disabling disease,1 with an estimated United States and global prevalence of 11.72 and 14.7%,1 respectively. Migraine ranks second on the Global Burden of Disease Study list of causes of disability in 2016.3 Diagnostic criteria divide migraine primarily based on the number of headache days per month into episodic migraine (EM; <15 headache days/month) and chronic migraine (CM; ≥15 headache days/month for >3 months, with migraine features on ≥8 days/month).4 Both forms of migraine can present with medical, neurologic, and psychiatric comorbidities that may exacerbate the disease, complicate treatment, and reduce health-related quality of life. Sleep disorders are often discussed5,6 but less commonly studied comorbidities of migraine and incorporate a wide range of conditions, including but not limited to sleep apnea, insomnia, circadian rhythm (ie, sleep-wake) disorders, and sleep movement disorders.
Symptoms of these disorders, such as insufficient sleep, disturbed sleep, oversleeping and inconsistent sleep cycles (eg, shiftwork, lifestyle), are often reported as triggers for a migraine attack, but more research is needed to fully understand them as comorbidities of migraine.6–8 Evidence and clinical experience suggest that poor sleep quality could be a risk factor for migraine progression.9 Descriptive studies have demonstrated that sleep disturbances are prevalent in people with migraine, with approximately one-half reporting occasional sleep disturbances and one-third reporting frequent sleep disturbances.7 A recent meta-analysis of 40 self-reported headache triggers found that sleep was second only to stress in reported frequency.10 Short sleep duration was associated with the severity of migraine attack based on a prospective time-series analysis.11
The relationship between sleep and migraine is complex in part because there are a number of distinct sleep disorders, including respiratory and non-respiratory conditions. Causality and directionality are unclear. Migraine may disrupt sleep, sleep disorders may lower pain threshold and exacerbate migraine, or they may be linked by exacerbating factors, such as stress or caffeine consumption/overconsumption, or underlying factors such as obesity, as a manifestation of metabolic syndrome. Sleep is essential in the regulation of a wide range of homeostatic functions, including the newly discovered glymphatic system, which functions as a CNS waste clearing system and aids in the clearance of abnormal proteins like beta-amyloid. Because glymphatic activity is primarily a sleep-related activity, dysregulation of sleep processes may lead to accumulation of CNS waste with nociceptive properties, which may explain how sleep triggers migraine, relieves migraine, and contributes to migraine chronification.12 Several prospective longitudinal studies have shown that poor sleep quality, including sleep disturbances, may predict the onset or exacerbation of migraine.13–16 Interestingly, at least one report found that migraine and non-migraine headache were risk factors for later development of insomnia.16 Bidirectional influences are likely though additional exploration is necessary.
One common sleep disorder is sleep apnea. There are 2 types of sleep apnea: obstructive and central.Obstructive sleep apnea is caused by a blockage of the airway whereas in central sleep apnea the brain fails to signal the respiratory muscles. Prevalence of obstructive sleep apnea in general population studies ranged from 9 to 38%, and varied by gender, age, and body mass index (BMI), with prevalence rates as high as 90% reported in elderly men.17 Epidemiologic research reviewed elsewhere has associated snoring (as a marker for obstructive sleep apnea) with more frequent and severe migraine,18 and this evidence may be strengthened through improved methods using medical history and validated instruments along with symptoms to investigate sleep and obstructive sleep apnea.
In this cross-sectional analysis of data from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study,19 we assessed sleep apnea and poor sleep quality as comorbidities of migraine in a large sample of people with migraine, stratified by EM and CM and by BMI. We hypothesized that sleep apnea and poor sleep quality would be more common among people with migraine than the general population and that the rates of sleep apnea and poor sleep quality would increase with older age and higher BMI, among men, in those with migraine, and among those with CM.
METHODS
Study Design.—
The CaMEO Study has a longitudinal design that incorporates web-based cross-sectional surveys to assess migraine symptoms and severity, headache-day frequency, headache-related disability, consulting and health care utilization, medication use, comorbid health conditions, and family-related burden associated with headache, among other data. Complete details regarding the CaMEO Study have been previously published.19 The study was approved by the institutional review board of the Albert Einstein College of Medicine which waived written informed consent for study volunteers.
Participants.—
The CaMEO Study data were collected from a web-based panel (Research Now; Plano, TX, USA) designed to be representative of US demography. Quota sampling was used from September 2012 to November 2013 to generate a sample of 489,537 panel members, representative of the US population. Of these individuals, 80,783 (16.5%) responded and 58,418 (72.3% of respondents) provided usable surveys for analysis; 16,789 (28.7% of respondents with usable data) met the inclusion criteria. The Comorbidity/Endophenotype module from the CaMEO Study was developed to assess overall physical burden beyond migraine, including common comorbidities and other noncephalic pain signs and symptoms, using validated instruments when available. A total of 16,763 (99.8%) CaMEO Study respondents received the Comorbidity/Endophenotype module, of whom 12,810 (76.4%; EM, 11,699; CM, 1111) provided valid data (Fig. 1).
Fig. 1.—
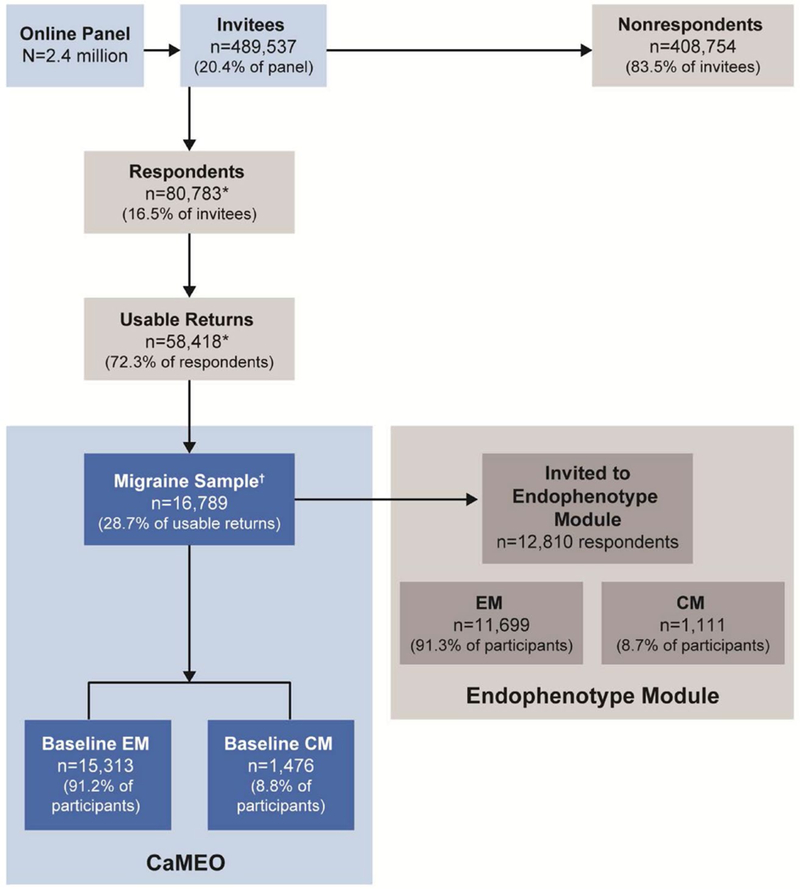
CaMEO respondent disposition. CaMEO = Chronic Migraine Epidemiology and Outcomes; CM = chronic migraine; EM = episodic migraine. *22,365 respondents abandoned the survey, were over quota, or had invalid (unusable) data and were removed during data cleaning. †Met inclusion criteria: agreed to participate, screened positive for modified International Classification of Headache Disorders, 3rd edition, migraine criteria, were ≥18 years old, and had ≥1 headache in the previous 12 months.
In this analysis, participants met American Migraine Study/American Migraine Prevalence and Prevention (AMPP) Study diagnostic module criteria for migraine,20,21 a modification of the International Classification of Headache Disorders, 3rd edition (ICHD-3) migraine criteria.4 Classification of CM was derived from Silberstein-Lipton criteria22,23 and ICHD-3 CM criteria (CM defined as ≥15 headache days/month averaged over the past 3 months). EM was defined as <15 headache days/month averaged over the past 3 months.4
Main Sleep Variables.—
Sleep apnea was assessed by 2 measures: the Berlin Questionnaire for Sleep Apnea and by patient self-report history. The Berlin Questionnaire for Sleep Apnea24 estimates the risk of sleep apnea, has been shown to accurately identify individuals at high risk for obstructive sleep apnea, and is used extensively in epidemiologic research.25,26 This questionnaire assesses 3 domains: snoring behavior, wake time sleepiness or fatigue, and history of obesity or hypertension. Individuals were classified as “high risk” or “low risk” for sleep apnea based on their responses to individual items and sum scores. Respondents with frequent or persistent symptoms in any 2 categories are considered at “high risk” for sleep apnea.
Respondents were also asked if they had sleep apnea. Those who responded positively (ie, self-reported sleep apnea) were subsequently asked whether the sleep apnea had been diagnosed by a physician and if they had received treatment in the preceding 90 days (if they had used medication and if they had used a continuous positive airway pressure [CPAP] device). Despite there being no FDA-approved medication for the treatment of sleep apnea per se, respondents may have considered a treatment such as modafinil, approved for treating excessive daytime sleepiness associated with sleep apnea,27 as a medication for sleep apnea.
The Medical Outcomes Study (MOS) Sleep Measures28 were applied to examine sleep quality. This method uses 12 items to measure 6 sleep dimensions: initiation (time to fall asleep), quantity (assessed as average hours of sleep each night), maintenance, respiratory problems, perceived adequacy and somnolence. With the exception of “quantity,” items are scored on a 0–100 scale, with higher scores reflecting more of the attribute implied by the scale name (eg, longer time to fall asleep). The time frame for the response is the past 4 weeks. Two indices have been derived from MOS: Sleep Problems Index I (short form) and Sleep Problems Index II (long form). Additional subscales include sleep disturbance, snoring, awakening with shortness of breath, sleep adequacy, daytime somnolence, sleep quantity, and optimal sleep.
Sample Size Determination.—
The CaMEO Study aimed to have ≥315 individuals with CM completing survey modules over the course of the study. Based on an assumed prevalence of migraine of 12% in the web-based panel, with 7% of those with migraine having CM, and accounting for anticipated response and attrition rates, 489,537 panelists were invited to complete the CaMEO screening survey.19
Statistical Analysis.—
Risk of sleep apnea from the Berlin Questionnaire and indicators of sleep quality from the MOS Sleep Measures were contrasted in persons with CM vs EM, by BMI category, and by demographics. The chi-square test was used to identify statistically significant differences for dichotomous or categorical variables between respondents with EM and CM and between men and women and assess risks by age and BMI. Independent-group t tests were used to evaluate significant differences for continuous variables. P values <.05 were considered statistically significant. Whereas no formal correction was employed for the large number of comparisons in this analysis, a Bonferroni correction (α/n, assuming 50 comparisons: 0.05/50 = 0.001) is consistent with our findings. All analyses were conducted with IBM SPSS Statistics, version 20.0 (IBM, Armonk, NY, USA).
RESULTS
Demographics and Baseline Characteristics.—
Of the 12,810 respondents with valid data, most were women (n = 9590; 74.9%) and white (n = 10,810; 84.4%) and had a mean (SD) age of 41.3 (14.5) years (Table 1). Compared with those with EM, respondents with CM had a similar mean (SD) age (41.9 [13.7] vs 41.3 [14.6] years; P = .188), were more likely to be women (906/1111 [81.5%] vs 8684/11,699 [74.2%]; P < .001) and white (985/1111 [88.7%] vs 9825/11,699 [84.0%]; P < .001), had a higher mean (SD) BMI (28.7 [7.9] vs 27.7 [7.4] kg/m2; P < .001), and were less likely to be employed either full-time, part-time, or selfemployed (684/1111 [61.6%] vs 8166/11,699 [69.8%]; P < .001; Table 1).
Table 1.—
Sociodemographic Characteristics of Respondents With Episodic Migraine and Chronic Migraine
| Event | EM (n = 11,699) | CM (n = 1111) | Total (N = 12,810) | Chi-square | P Value |
|---|---|---|---|---|---|
| Age, years, mean (SD) | 41.3 (14.6) | 41.9 (13.7) | 41.3 (14.5) | 1.3 | .188 |
| Gender, n (%) | 28.9 | <.001 | |||
| Women | 8684 (74.2) | 906 (81.5) | 9590 (74.9) | ||
| Men | 3015 (25.8) | 205 (18.5) | 3220 (25.1) | ||
| Race, n (%) | 16.8 | <.001 | |||
| White | 9825 (84.0) | 985 (88.7) | 10,810 (84.4) | ||
| Other | 1874 (16.0) | 126 (11.3) | 2000 (15.6) | ||
| Employment, n (%) | 32.2 | <0.001 | |||
| No | 3533 (30.2) | 427 (38.4) | 3960 (30.9) | ||
| Yes | 8166 (69.8) | 684 (61.6) | 8850 (69.1) | ||
| BMI, kg/m2, mean (SD) | 27.7 (7.4) | 28.7 (7.9) | 27.8 (7.4) | 4.3 | <.001 |
| BMI categories, n (%) | 22.7 | <0.001 | |||
| Underweight | 446 (3.8) | 43 (3.9) | 489 (3.8) | ||
| Normal | 4444 (38.0) | 369 (33.2) | 4813 (37.6) | ||
| Overweight | 3250 (27.8) | 284 (25.6) | 3534 (27.6) | ||
| Obese | 3559 (30.4) | 415 (37.4) | 3974 (31) | ||
BMI = body mass index; CM = chronic migraine; EM = episodic migraine.
Full-time, part-time, or self-employed.
Sleep Apnea.—
Among 12,810 respondents, 37.0% (n = 4739) were at “high risk” for sleep apnea based on the Berlin Questionnaire. Respondents with CM were more likely to be at “high risk” for sleep apnea than respondents with EM (575/1111 [51.8%] vs 4164/11,699 [35.6%]; P < .001; Table 2). Rates of being at “high-risk” for sleep apnea were higher in men than in women (1431/3220 [44.4%] vs 3308/9590 [34.5%]; P < .001; Table 3), and in the obese and in the older age group (P < .001 for both; Table 3). Notably, a dose-response relationship was observed for weight and sleep apnea risk (Table 3). Respondents who were obese (BMI >30 kg/m2) were 5 times more likely to be “high risk,” while those who were overweight (BMI 25.0–<30 kg/m2) were twice as likely to be “high risk” for sleep apnea compared with respondents of normal weight (obese, 2943/3974 [74.1%]; overweight, 1054/3534 [29.8%]; normal, 692/4813 [14.4%]; Table 2).
Table 2.—
Results for Berlin Questionnaire and Self-Reported History and Diagnosis of Sleep Apnea Among EM and CM Study Respondents
| EM (n = 11,699) n (%) |
CM (n= 1111) n (%) |
Total (N= 12,810) n (%) |
Chi-square | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| Berlin Questionnaire | ||||||||
| “Low risk” for sleep apnea | 7535 (64.4) | 536(48.2) | 8071 (63.0) | 113.7 | <.001 | |||
| “High risk” for sleep apnea | 4164 (35.6) | 575 (51.8) | 4739 (37.0) | |||||
| Self-Reported History | ||||||||
| Have you ever had sleep apnea? | ||||||||
| No | 10,563 (90.3) | 954 (85.9) | 11,517 (89.9) | 21.9 | <.001 | |||
| Yes | 1136 (9.7) | 157(14.1) | 1293 (10.1) | |||||
| Given self-reported sleep apnea, “Has this condition been diagnosed or confirmed by an HCP?” | ||||||||
| No | 287 (25.3) | 27(17.2) | 314(24.3) | 4.9 | .027 | |||
| Yes | 849 (74.7) | 130(82.8) | 979(75.7) | |||||
| Berlin Questionnaire Sleep Apnea Risk by BMI, kg/m2 | ||||||||
| Underweight <18.5 (n = 489) | Normal 18.5−<25.0 (n = 4813) | Overweight 25.0−<30 (n = 3534) | Obese >30.0 (n = 3974) | Chi-square | P Value | |||
| “Low risk” for sleep apnea | 439 (89.8) | 4121 (85.6) | 2480 (70.2) | 1031 (25.9) | 3626 | <.001 | ||
| “High risk” for sleep apnea | 50 (10.2) | 692 (14.4) | 1054 (29.8) | 2943 (74.1) | ||||
| Berlin Questionnaire by Age, years | ||||||||
| 18–24 (n= 1968) | 25–34 (n = 2847) | 35–44 (n = 2682) | 45–54 (n = 2694) | 55–64 (n = 1647) | ≥65 (n = 972) | Chi-square | P Value | |
| “Low risk” for sleep apnea | 1641 (83.4) | 1997(70.1) | 1563 (58.3) | 1447 (53.7) | 900 (54.6) | 523 (53.8) | 623.1 | <.001 |
| “High risk” for sleep apnea | 327 (16.6) | 850 (29.9) | 1119(41.7) | 1247 (46.3 ) | 747 (45.4) | 449 (46.2) | ||
BMI = body mass index; CM = chronic migraine; EM = episodic migraine; HCP = health care professional.
Table 3.—
Respondents Who Were “High Risk” on the Berlin Questionnaire by Migraine Subtype, Gender, Age, and BMI
| Underweight <18.5 kg/m2 (n = 50) |
Normal 18.5–<25.0 kg/m2 (n = 692) |
Overweight 25.0–<30 kg/m2 (n = 1054) |
Obese >30.0 kg/m2 (n = 2943) |
Total (N = 4739) |
|
|---|---|---|---|---|---|
| Episodic migraine, n (%) | |||||
| Gender | |||||
| Men | 6 (11.5) | 176 (18.8) | 387 (35.1) | 732 (78.9) | 1301 (43.2) |
| Women | 31 (7.9) | 427 (12.2) | 547 (25.5) | 1858 (70.6) | 2863 (33.0) |
| Age, years | |||||
| 18–24 | 11 (5.8) | 59 (5.8) | 49 (14.5) | 158 (58.7) | 277 (15.2) |
| 25–34 | 10 (8.5) | 110 (9.7) | 120 (18.9) | 516 (69.3) | 756 (28.7) |
| 35–44 | 4 (7.8) | 115 (15.1) | 177 (26.3) | 685 (74) | 981 (40.7) |
| 45–54 | 5 (11.9) | 134 (19) | 257 (32.4) | 675 (76.5) | 1071 (44.2) |
| 55–64 | 3 (9.4) | 113 (22.1) | 180 (38.1) | 374 (76.3) | 670 (44.5) |
| ≥65 | 4 (26.7) | 72 (23.8) | 151 (44.5) | 182 (73.7) | 409 (45.3) |
| Chronic migraine, n (%) | |||||
| Gender | |||||
| Men | 0 (0) | 24 (43.6) | 38 (54.3) | 68 (89.5) | 130 (63.4) |
| Women | 13 (33.3) | 65 (20.7) | 82 (38.3) | 285 (84.1) | 445 (49.1) |
| Age, years | |||||
| 18–24 | 1 (9.1) | 13 (17.3) | 7 (26.9) | 29 (78.4) | 50 (33.6) |
| 25–34 | 0 (0) | 12 (14.6) | 17 (36.2) | 65 (84.4) | 94 (44.1) |
| 35–44 | 1 (14.3) | 17 (24.3) | 26 (32.5) | 94 (83.9) | 138 (51.3) |
| 45–54 | 3 (42.9) | 23 (29.9) | 39 (59.1) | 111 (92.5) | 176 (65.2) |
| 55–64 | 8 (72.7) | 16 (35.6) | 18 (43.9) | 35 (79.5) | 77 (54.6) |
| ≥65 | 0 (0) | 8 (40.0) | 13 (54.2) | 19 (76.0) | 40 (58.0) |
BMI = body mass index.
A total of 10.1% of respondents (n = 1293) self-reported sleep apnea. Respondents with CM were more likely to self-report sleep apnea than respondents with EM (157/1111 [14.1%] vs 1136/11,699 [9.7%]; P < .001). Among those self-reporting sleep apnea (n = 1293), a diagnosis of sleep apnea by a health care professional (HCP) was reported by 75.7% of respondents (n = 979). Report of an HCP diagnosis was slightly more common among those with CM than EM (130/157 [82.8%] vs 849/1136 [74.7%]; P = .027; Table 2). Among those self-reporting sleep apnea, there were no significant differences in HCP diagnoses of sleep apnea between EM and CM groups among respondents who were “high risk” on the Berlin Questionnaire (Supplementary Table 1). Men were also more likely to self-report sleep apnea than women (580/3220 [18.0%] vs 713/9590 [7.4%]; P < .001). Among those self-reporting sleep apnea, men and women reported a similar likelihood of an HCP diagnosis (men, 440/580 [75.9%]; women, 539/713 [75.6%]). Of all who self-reported sleep apnea (n = 1293), 35.3% (n = 456) had used a CPAP device in the previous 90 days (CM, 51/157 [32.5%]; EM, 405/1136 [35.7%]). An additional 17.7% (n = 229) had a CPAP device available but were not currently using it (CM, 26/157 [16.6%]; EM, 203/1136 [17.9%]).
Sleep Quality.—
Among all respondents, the mean MOS sleep scale component values ranged from 17.0 (shortness of breath subscale) to 39.2 (sleep disturbance subscale; Table 4). All mean (SD) MOS sleep scale component values differed significantly between respondents with CM and those with EM, with better sleep quality among those with EM: sleep disturbance (CM, 53.2 [26.9] vs EM, 37.9 [24.3]; P < .001), snoring (38.0 [33.9] vs 31.0 [32.1]; P < .001), shortness of breath (34.9 [29.8] vs 15.3 [20.6]; P < .001), somnolence (44.1 vs 32.2 [21.2]; P < .001), and sleep adequacy (34.0 [24.2] vs 39.2 [22.1]; P < .001). Significant differences were identified between men and women in the mean (SD) values of sleep disturbance (men, 35.4 [23.9] vs women, 40.5 [25.1]; P < .001), snoring (39.2 [33.5] vs 29.1 [31.4]; P < .001), shortness of breath (15.0 [21.2] vs 17.7 [22.5]; P < .001), and sleep adequacy (39.7 [22.6] vs 38.4 [22.2]; P = .003; Table 4).
Table 4.—
Sleep Quality Results from the Medical Outcomes Study Sleep Measures for EM and CM Study Respondents*
| Sleep Subscale | EM Mean (SD) (n = 11,699) |
CM Mean (SD) (n = 1111) |
Total Mean (SD) (N = 12,810) |
T Value | P Value† |
|---|---|---|---|---|---|
| Sleep Index II | 40.2 (17.1) | 53.5 (18.7) | 41.3 (17.6) | 24.6 | <.001 |
| Sleep disturbance scale | 37.9 (24.3) | 53.2 (26.9) | 39.2 (24.9) | 19.8 | <.001 |
| Snoring scale | 31.0 (32.1) | 38.0 (33.9) | 31.6 (32.3) | 6.9 | <.001 |
| Short of breath scale | 15.3 (20.6) | 34.9 (29.8) | 17.0 (22.2) | 28.9 | <.001 |
| Somnolence scale | 32.2 (21.2) | 44.1 (23.4) | 33.2 (21.7) | 17.7 | <.001 |
| Sleep adequacy scale | 39.2 (22.1) | 34.0 (24.2) | 38.7 (22.3) | 7.4 | <.001 |
| Average number of hours slept | 6.8 (1.4) | 6.4 (1.7) | 6.7 (1.4) | 8.9 | <.001 |
| Optimal hours slept, n (% yes) | 6,403 (54.7) | 452 (40.7) | 6855 (53.5) | 80.5 | <.001 |
| Sleep Subscale | Men Mean (SD) (n = 3220) |
Women Mean (SD) (n = 9590) |
Total Mean (SD) (N = 12,810) |
T Value | P Value‡ |
| Sleep Index II | 38.7 (17.2) | 42.2 (17.7) | 41.3 (17.6) | 9.9 | <.001 |
| Sleep disturbance scale | 35.4 (23.9) | 40.5 (25.1) | 39.2 (24.9) | 10.1 | <.001 |
| Snoring scale | 39.2 (33.5) | 29.1 (31.4) | 31.6 (32.3) | 15.6 | <.001 |
| Short of breath scale | 15.0 (21.2) | 17.7 (22.5) | 17.0 (22.2) | 5.9 | <.001 |
| Somnolence scale | 32.6 (21.5) | 33.5 (21.7) | 33.2 (21.7) | 1.9 | .058 |
| Sleep adequacy scale | 39.7 (22.6) | 38.4 (22.2) | 38.7 (22.3) | 2.9 | <.01 |
| Average number of hours slept | 6.6 (1.4) | 6.8 (1.4) | 6.7 (1.4) | 5.4 | <.001 |
| Optimal hours slept, n (% yes) | 1701 (52.8) | 5154 (53.7) | 6855 (53.5) | 0.8 | .366 |
CM = chronic migraine; EM = episodic migraine.
Medical Outcomes Study consists of 12 items to measure 6 sleep dimensions: initiation (time to fall asleep), quantity (hours of sleep each night), maintenance, respiratory problems, perceived adequacy, somnolence (the last 4 items are reported using a 6-item Likert scale ranging from “all of the time” to “none of the time”). The time frame for the responses is the past 4 weeks. Two indexes can be derived: Sleep Problems Index I (short form), which is not presented, and Sleep Problems Index II (long form). Additional subscales are derived: sleep disturbance, snoring, sleep shortness of breath or headache, sleep adequacy, sleep somnolence, sleep quantity, and optimal sleep. Higher scores reflect more of the attribute implied by scale name.
P for comparison between EM and CM.
P for comparison between men and women.
Among all respondents (n = 12,810), 53.5% (n = 6855) reported having slept 7 to 8 hours (ie, optimal hours). Respondents with CM were less likely to report having slept optimal hours than those with EM (452/1111 [40.7%] vs 6403/11,699 [54.7%]; P < .001; Table 4). No significant differences were noted between men and women in the percentage of individuals reporting 7 to 8 hours slept (men, 1701/3220 [52.8%]; women, 5154/9590 [53.7%]; Table 4).
DISCUSSION
We used data from the CaMEO Study to explore the relationship of migraine (EM and CM) with both sleep apnea, and measures of sleep quality. These results are important to HCPs managing people with migraine, as our results are the first to report the risk and rate of sleep apnea and measures of sleep quality, assessed using validated instruments, in a large sample representative of the US population, stratified by demographics as well as EM and CM. We will first discuss the findings for sleep apnea and then contextualize our results on sleep quality. Based on the Berlin Questionnaire, “high risk” of sleep apnea was more common among respondents with CM (51.8%) than those with EM (35.6%), across all age groups and all BMI categories (Table 3). The rates of “high risk” of sleep apnea in the EM group were similar to estimates from the US general population (35.8%).29 In both CM and EM the rates of “high risk” of sleep apnea varied considerably based on BMI, age and gender; HCPs should consider all of these factors when considering the risk profile of their individual patients. Being at “high risk” for sleep apnea was associated with higher BMI category, older age, and male gender in those with migraine in our study, as was typically also found in general population studies.29 The association with obesity may be expected as a BMI >30 kg/m2 contributes 1 of the 2 categories required for an individual to be considered at “high risk” for sleep apnea.24 Of respondents who were “high risk” for sleep apnea, 84% were overweight or obese as measured by BMI. As expected, older men with CM and high BMI had a very high risk for sleep apnea in these analyses.
The risk and rate of obstructive sleep apnea in people with migraine, particularly in those with CM, have not been studied in detail. Much early research evaluating headache in relation to sleep apnea lacked diagnostic precision; rather than diagnosing migraine, tension-type, or other formal headache diagnoses, the headache was often described by its relationship to sleep (eg, morning, awakening, or chronic daily headache).30,31
Previous evidence suggests that breathing-related sleep disorders, such as obstructive sleep apnea and/or sleep pattern disorders, negatively influence headache severity (intensity) in people with chronic headache11 and that there is a positive relationship with migraine incidence32 and with onset of CM.33 Compelling evidence exists to support a relationship between sleep disorders and headache, including a strong association between sleep apnea and headache.34,35 Despite the relationship between obstructive sleep apnea and headache intensity,11 only approximately one-third of respondents with self-reported sleep apnea had used a CPAP device in the 90 days before assessment. The acceptance of and adherence to CPAP treatment is problematic;36 for example, the treatment has the potential to interfere in intimate relationships between couples.37 As such, low overall adherence rates to CPAP treatment similar to those observed in our study have been reported,36–38 leading to the suggestion that cognitive behavioral training and peer group support be implemented alongside CPAP treatment to improve adherence.37 In addition to future research exploring mechanisms to improve adherence to CPAP, the benefit of CPAP in people with CM and EM is also worthy of further investigation, and, in particular, the role of CPAP in preventing new onset CM in people with EM and obstructive sleep apnea.
Our findings from this sample of people with migraine, demonstrating “high risk” for sleep apnea in CM, is consistent with evidence that sleep apnea influences headache severity (intensity). However, some literature has been contradictory. Some studies have found no relationship or have concluded that the trends fail to reach statistical significance, but the contradiction in findings may be related to design or lack of distinction between EM and CM.39,40 A recent Norwegian population-based survey identified individuals at high vs low risk for obstructive sleep apnea and brought participants to the hospital for clinical evaluation and polysomnography; results found no difference between those with and without sleep apnea in the number of individuals with migraine, with or without aura, although they did find an increased risk of daytime sleepiness.41 Notably, Stark and Stark42 raised the issue that methods used in the Norwegian study that looked at headache in the population with and without sleep apnea did not address the frequency or severity of migraine, and thus could not rule out the possibility that sleep apnea may aggravate headache occurring in a person with migraine.42 Differences in sampling, diagnostic groups of interest, and methods may account for some of the contradictory findings in earlier research.
Poor sleep quality is reported in 16% of a US general population sample (mean age, 46 years).28 Our results suggest that sleep quality, as assessed by the MOS Sleep Measures, was generally poorer in people with migraine than in a sample representative of the US general population.28 Scores were higher (ie, worse) in this analysis in respondents with migraine than those reported for the general US population for sleep disturbance (39.2 vs 24.5), snoring (31.6 vs 28.3), shortness of breath (17.0 vs 9.5), and daytime somnolence (33.2 vs 21.9); sleep adequacy was lower (38.7 vs 60.5), meaning that people with migraine were more likely to have inadequate sleep. Furthermore, sleep quality was typically poorer among respondents with CM than those with EM. Although all differences may not have reached the threshold for clinical significance, respondents with CM were more likely to report snoring, shortness of breath during sleep, daytime somnolence and sleep inadequacy (all P < .001) than those with EM. Consequently, respondents with EM were more likely to report having slept optimal hours than those with CM. Indeed, average hours slept per night for respondents with EM (6.8) were the same as reported in the general population (6.8)28 and statistically more than reported by respondents with CM (6.6; P < .001).
Overall, our findings related to poorer sleep quality in people with migraine were consistent with previous migraine studies.18,43–49 An analysis of 2695 participants in the Korean Headache-Sleep Study demonstrated that people with migraine had increased odds of insufficient sleep after adjusting for sociodemographics, psychiatric comorbidities, and other variables.45 Similarly, a study of 143 people with migraine found that headache frequency was 2-fold higher among participants with short sleep duration and poor sleep quality than among those without. However, headache intensity among people with migraine with short sleep duration and poor sleep quality was not significantly different from those without these sleep disorders.46 Most recently, it has been demonstrated that poor sleep quality may mediate sensitivity to lack of sleep as a headache trigger in people with headache, including migraine headache.49 Although poor sleep quality, as measured by the Pittsburgh Sleep Quality Index, in those with migraine is a common finding,45,47–49 not all studies have found increased daytime somnolence.47 Others, however, have reported excessive daytime sleepiness to be 3-fold more likely in those with migraine44 and 4-fold more likely in those with CM50 than in those without headache.47 Sleep disturbances were 5-fold more likely in those with migraine; the likelihood of sleep disturbance increased as headache frequency increased,44 with some investigators reporting that this effect reaches a plateau when migraine headaches are ≥9 days per month.43
Although the relationship between migraine and poor sleep is well established, further research with objective sleep data is needed to fully understand the relationship between sleep disturbance and migraine. Discrepancies in earlier literature may stem at least in part from the limits of subjective (self-report) measures. There is a well-documented but poorly understood discrepancy between subjective and objective sleep that is unique to insomnia compared to normal sleepers and those with sleep disorders like sleep apnea.51 Across studies, people with insomnia, with or without a wide range of medical and psychiatric conditions, overestimate the time taken to fall asleep and underestimate total sleep time relative to objective sleep estimates as provided by polysomnography and actigraphy, and become more inaccurate in retrospective reports relative to daily diary reports.52 Objective sleep data such as polysomnography are often not feasible for research due to cost and reactivity to test conditions or “first night effect.” Actigraphy was utilized recently in an outcome study of Cognitive behavioral therapy for insomnia (CBTi) in migraine.53 Wearable accelerometers are inexpensive and increasingly widely available, and can represent sleep/wake cycles in normal sleepers with an accuracy that approximates actigraphy; however, they lack accuracy in determining sleep/wake cycles in clinical populations and correlate poorly with sleep stages documented in polysomnography.54 Thus, the current standard of care in diagnosis of insomnia in the clinical setting is prospective sleep diaries and validated questionnaires.55
Given the likely bidirectional relationship between sleep and pain, particularly migraine and sleep apnea and poor sleep quality, a validated questionnaire approach, the MOS Sleep Measures, was used to measure sleep quality and sleep disturbances. Further use of validated measures in studies in people with migraine would be helpful. In addition, the presence of sleep apnea and poor sleep quality should be considered when treating migraine.56 There have been reports that treatment of sleep disorders improved or resolved headache. For example, a retrospective analysis of patients with sleep apnea headache who were adherent to CPAP showed headache improvement compared with those who were untreated or nonad-herent to CPAP; all 33 treated patients reported improvement in either headache or sleep.57 CBTi is the recommended preferred treatment for insomnia;58 a meta-analysis of 87 randomized controlled trials found CBTi to be useful in patients with and without comorbid disease.59 Specifically in CM, a small pilot study, not included in the meta-analysis, showed that in women, behavioral sleep modification resulted in reduced headache frequency and reversion to EM.60 Similarly, in individuals with CM, behavioral treatment of comorbid insomnia yielded reductions in headache frequency.53 These findings are interesting, albeit limited in number and scope, and underscore a need for additional, larger studies for confirmation.
The CaMEO study has some limitations that must be considered when interpreting the data. As previously reported, there are limitations with data collected via self-report from a web-based panel, including issues such as nonresponse bias, which have been discussed in detail elsewhere.19 Despite these potential limitations, the baseline demographics of the CaMEO population were similar to that of the AMPP Study population, a population that is representative of the US population.61 As such, it has been suggested that the results of the CaMEO Study could also be generalizable to the US population with migraine.61
For this study, it should be noted that for a population as large as the CaMEO study population small differences in outcomes between subgroups can be observed to be statistically significant. Although no formal correction was used for the multiple comparisons in this study, the P values associated with most of the comparisons reported here are sufficiently low as to be significant had such corrections been employed. It is important to note that statistical significance can be achieved without necessarily reaching a threshold to be clinically meaningful. As such, the likely clinical consequence of any differences reported herein should be considered carefully. Furthermore, the sensitivity and specificity of the Berlin Questionnaire has not been established specifically within a migraine population, and our estimates of the rates of sleep apnea risk may, as a result, be inflated. Obesity is an important risk factor for sleep apnea and a variable in the Berlin Questionnaire algorithm.24 Obesity is also a recognized risk factor for migraine disease progression to CM,62 and thus would be expected to be higher in subpopulations with headache than among the general population. Obesity rates were, indeed, high in our sample, especially among people with CM. This may lead to an overestimation of “high risk” of sleep apnea in our sample that may not be observed in CM in other regions. In addition, other variables assessed by the Berlin Questionnaire, such as daytime fatigue, are known to be associated with migraine.63 For example, daytime fatigue may be a prodromal or post-dromal symptom of migraine and may not be directly due to the effects of sleep apnea. Secondly, all data are self-reported and retrospective and have not been verified by other sources, such as physician diagnoses or medical records. Consequently, the data are subject to recall and reporting error. Validated instruments were used when available, including for the primary out-come variables and for diagnosis and categorization of migraine. In addition, we were unable to determine the type of sleep apnea (obstructive vs central) based on the questionnaires and items included in the study; therefore, we were unable to adjust for any underlying relationship between obstructive sleep apnea and obesity or any other variable. The likelihood of central sleep apnea in this population is low, and central sleep apnea has not been particularly associated with migraine, except perhaps in the uncommon case of Arnold-Chiari malformation. Sleep studies that provide objective measures of respiration should be conducted to confirm these results. In this cross-sectional, observational analysis it is impossible to determine causality or directionality. In the case of sleep apnea and migraine, there may be a bidirectional relationship, with each triggering or exacerbating the other, or they may share a common underlying mechanism.
CONCLUSIONS
General prevalence estimates of sleep apnea are wide, ranging from 9 to 38%17; typically rates are higher in men than in women and increase with age and BMI.17,64 Results from the CaMEO Study suggest that persons with CM have a “high risk” of sleep apnea and high rates of poor sleep quality. The increased risk of sleep apnea and breathing-related sleep problems was particularly high among men vs women and those with CM, higher BMI, and older age. Women reported higher rates of sleep disturbance, although reports of sleep disturbance were high across both genders. These data follow the patterns and prevalence rates seen in large epidemiologic studies that reported rates by those variables, but appear to be higher than many population estimates, especially among people with CM. Future analyses may include sleep studies with the goal of confirming the present findings, having participants with a clinician-determined sleep apnea diagnosis use wearable devices to precisely and prospectively collect respiratory and sleep-related data, and studies aimed at determining whether treatment of sleep apnea and sleep disorders reduces the frequency or intensity of migraine attacks and associated symptoms. Clinical wisdom suggests that assessing sleep quality and screening for sleep apnea is valuable, especially among men and people with CM, older age, and higher BMI. All people with migraine, particularly those with sleep disturbances such as insomnia, could benefit from being educated about behavioral sleep regulation and its likely value in the management of migraine. People with migraine who screen positive for sleep apnea should be referred for additional evaluation and possible CPAP treatment, including supportive education to ensure adequate adherence to CPAP treatment.
Supplementary Material
Acknowledgments:
Writing and editorial assistance was provided to the authors by Scarlett Geunes-Boyer, PhD, and Dana Franznick, PharmD, of Complete Healthcare Communications, LLC (West Chester, PA, USA), and funded by Allerganplc.
Funding: This study was sponsored by Allergan plc (Dublin, Ireland).
Abbreviations:
- BMI
body mass index
- CaMEO
Chronic Migraine Epidemiology and Outcomes
- CM
chronic migraine
- CPAP
continuous positive airway pressure
- EM
episodic migraine
- HCP
health care professional
- ICHD-3
International Classification of Headache Disorders, 3rd edition
- MOS
Medical Outcomes Study
Footnotes
Conflict of Interest: Dawn C. Buse has received grant support and honoraria from Allergan, Amgen, Avanir, Dr. Reddy’s Laboratories, Eli Lilly, MAP Pharmaceuticals, Novartis, Teva, and Zogenix. She is on the editorial board of Current Pain and Headache Reports, Journal of Headache and Pain, Pain Medicine News, and PainPathways. Jeanetta C. Rains holds stock in ResMed, Inc. Jelena M. Pavlovic has received honoraria from Alder Biopharmaceuticals, Allergan, Dr. Reddy’s Laboratories, and the American Headache Society. Kristina M. Fanning is an employee of Vedanta Research, which has received support funded by Allergan, Amgen, Dr. Reddy’s Laboratories, Eli Lilly, GlaxoSmithKline, Merck & Co., and Novartis via grants to the National Headache Foundation. Michael L. Reed is Managing Director of Vedanta Research, which has received research funding from Allergan, Amgen, Dr. Reddy’s Laboratories, Eli Lilly, GlaxoSmithKline, Merck & Co., and Novartis via grants to the National Headache Foundation. Vedanta Research has received funding directly from Allergan for work on the CaMEO Study. Aubrey Manack Adams is a full-time employee of Allergan plc and owns stock in the company. Richard B. Lipton serves on the editorial boards of Neurology and Cephalalgia and as senior advisor to Headache. He has received research support from the NIH. He also receives support from the Migraine Research Foundation and the National Headache Foundation. He has reviewed for the NIA and NINDS and serves as consultant or advisory board member or has received honoraria from Alder, Allergan, Amgen, Autonomic Technologies, Avanir, Boston Scientific, Dr. Reddy’s Laboratories, Electrocore, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, Novartis, Teva, and Vedanta. He receives royalties from Wolff’s Headache (8th edition, Oxford University Press) and Informa. He holds stock options in eNeura Therapeutics and Biohaven.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web site.
Contributor Information
Dawn C. Buse, Albert Einstein College of Medicine, Bronx, NY, USA.
Jeanetta C. Rains, Elliot Hospital, Center for Sleep Evaluation, Manchester, NH, USA.
Jelena M. Pavlovic, Albert Einstein College of Medicine, Bronx, NY, USA; Montefiore Headache Center, Bronx, NY, USA.
Kristina M. Fanning, Vedanta Research, Chapel Hill, NC, USA.
Michael L. Reed, Vedanta Research, Chapel Hill, NC, USA.
Aubrey Manack Adams, Allergan plc, Irvine, CA, USA.
Richard B. Lipton, Albert Einstein College of Medicine, Bronx, NY, USA; Montefiore Headache Center, Bronx, NY, USA.
REFERENCES
- 1.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- 5.Singh NN, Sahota P. Sleep-related headache and its management. Curr Treat Options Neurol. 2013;15:704–722. [DOI] [PubMed] [Google Scholar]
- 6.Aguggia M, Cavallini M, Divito N, et al. Sleep and primary headaches. Neurol Sci. 2011;32(suppl 1):S51–S54. [DOI] [PubMed] [Google Scholar]
- 7.Kelman L, Rains JC. Headache and sleep: Examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache. 2005;45:904–910. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Z, Fan X, Li X, Tan G, Chen L, Zhou J . Prevalence and predictive factors for poor sleep quality among migraineurs in a tertiary hospital headache clinic. Acta Neurol Belg. 2013;113:229–235. [DOI] [PubMed] [Google Scholar]
- 9.Bigal ME, Lipton RB. What predicts the change from episodic to chronic migraine? Curr Opin Neurol. 2009;22:269–276. [DOI] [PubMed] [Google Scholar]
- 10.Pellegrino ABW, Davis-Martin RE, Houle TT, Turner DP, Smitherman TA. Perceived triggers of primary headache disorders: A meta-analysis. Cephalalgia. 2017. doi: 10.1177/0333102417727535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houle TT, Butschek RA, Turner DP, Smitherman TA, Rains JC, Penzien DB. Stress and sleep duration predict headache severity in chronic headache sufferers. Pain. 2012;153:2432–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vgontzas A, Pavlovic JM. Sleep disorders and migraine: Review of literature and potential pathophysiology mechanisms. Headache. 2018;58:1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boardman HF, Thomas E, Millson DS, Croft PR. One-year follow-up of headache in an adult general population. Headache. 2005;45:337–345. [DOI] [PubMed] [Google Scholar]
- 14.Boardman HF, Thomas E, Millson DS, Croft PR. The natural history of headache: Predictors of onset and recovery. Cephalalgia. 2006;26:1080–1088. [DOI] [PubMed] [Google Scholar]
- 15.Odegard SS, Sand T, Engstrom M, Stovner LJ, Zwart JA, Hagen K. The long-term effect of insomnia on primary headaches: A prospective population-based cohort study (HUNT-2 and HUNT-3). Headache. 2011;51:570–580. [DOI] [PubMed] [Google Scholar]
- 16.Odegard SS, Sand T, Engstrom M, Zwart JA, Hagen K. The impact of headache and chronic musculoskeletal complaints on the risk of insomnia: Longitudinal data from the Nord-Trondelag health study. J Headache Pain. 2013;14:24. doi: 10.1186/1129-2377-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med Rev. 2017;34:70–81. [DOI] [PubMed] [Google Scholar]
- 18.Rains JC, Poceta JS. Sleep-related headaches. Neurol Clin. 2012;30:1285–1298. [DOI] [PubMed] [Google Scholar]
- 19.Manack Adams A, Serrano D, Buse DC, et al. The impact of chronic migraine: The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study methods and baseline results. Cephalalgia. 2015;35:563–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipton RB, Diamond S, Reed M, Diamond ML, Stewart WF. Migraine diagnosis and treatment: Results from the American Migraine Study II. Headache. 2001;41:638–645. [DOI] [PubMed] [Google Scholar]
- 21.Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267:64–69. [PubMed] [Google Scholar]
- 22.Silberstein SD, Lipton RB, Sliwinski M. Classification of daily and near-daily headaches: Field trial of revised IHS criteria. Neurology. 1996;47:871–875. [DOI] [PubMed] [Google Scholar]
- 23.Silberstein SD, Lipton RB, Solomon S, Mathew N. Classification of daily and near-daily headaches in the headache clinic Proposed revisions to the International Headache Society criteria. In: Olesen J, ed. Frontiers in Headache Research; New York, NY: Raven Press, Ltd; 1994:117–126. [Google Scholar]
- 24.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. [DOI] [PubMed] [Google Scholar]
- 25.Senaratna CV, Perret JL, Matheson MC, et al. Validity of the Berlin questionnaire in detecting obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med Rev. 2017;36:116–124. [DOI] [PubMed] [Google Scholar]
- 26.Tan A, Yin JD, Tan LW, van Dam RM, Cheung YY, Lee CH. Using the berlin questionnaire to predict obstructive sleep apnea in the general population. J Clin Sleep Med. 2017;13:427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cephalon I PROVIGIL® (modafinil) Tablets [C-IV], FDA Approved Labeling. Frazer, PA; 2007:19355. [Google Scholar]
- 28.Hays RD, Martin SA, Sesti AM, Spritzer KL. Psychometric properties of the Medical Outcomes Study Sleep measure. Sleep Med. 2005;6:41–44. [DOI] [PubMed] [Google Scholar]
- 29.Netzer NC, Hoegel JJ, Loube D, et al. Prevalence of symptoms and risk of sleep apnea in primary care. Chest. 2003;124:1406–1414. [DOI] [PubMed] [Google Scholar]
- 30.Cevoli S, Giannini G, Favoni V, Pierangeli G, Cortelli P. Migraine and sleep disorders. Neurol Sci. 2012;33(suppl 1):S43–S46. [DOI] [PubMed] [Google Scholar]
- 31.Provini F, Vetrugno R, Lugaresi E, Montagna P. Sleep-related breathing disorders and headache. Neurol Sci. 2006;27(suppl 2):S149–S152. [DOI] [PubMed] [Google Scholar]
- 32.Harnod T, Wang YC, Kao CH. Association of migraine and sleep-related breathing disorder: A population-based cohort study. Medicine (Baltimore). 2015;94:e1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scher AI, Lipton RB, Stewart WF. Habitual snoring as a risk factor for chronic daily headache. Neurology. 2003;60:1366–1368. [DOI] [PubMed] [Google Scholar]
- 34.Aldrich MS, Chauncey JB. Are morning headaches part of obstructive sleep apnea syndrome? Arch Intern Med. 1990;150:1265–1267. [PubMed] [Google Scholar]
- 35.Sand T, Hagen K, Schrader H. Sleep apnoea and chronic headache. Cephalalgia. 2003;23:90–95. [DOI] [PubMed] [Google Scholar]
- 36.Yang MC, Huang YC, Lan CC, Wu YK, Huang KF. Beneficial effects of long-term CPAP treatment on sleep quality and blood pressure in adherent subjects with obstructive sleep apnea. Respir Care. 2015;60:1810–1818. [DOI] [PubMed] [Google Scholar]
- 37.Libman E, Bailes S, Fichten CS, et al. CPAP treatment adherence in women with obstructive sleep apnea. Sleep Disord. 2017;2017:2760650. doi: 10.1155/2017/2760650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salepci B, Caglayan B, Kiral N, et al. CPAP adherence of patients with obstructive sleep apnea. Respir Care. 2013;58:1467–1473. [DOI] [PubMed] [Google Scholar]
- 39.Jensen R, Olsborg C, Salvesen R, Torbergsen T, Bekkelund SI. Is obstructive sleep apnea syndrome associated with headache? Acta Neurol Scand. 2004;109:180–184. [DOI] [PubMed] [Google Scholar]
- 40.Neau JP, Paquereau J, Bailbe M, Meurice JC, Ingrand P, Gil R. Relationship between sleep apnoea syndrome, snoring and headaches. Cephalalgia. 2002;22:333–339. [DOI] [PubMed] [Google Scholar]
- 41.Kristiansen HA, Kvaerner KJ, Akre H, Overland B, Russell MB. Migraine and sleep apnea in the general population. J Headache Pain. 2011;12:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stark CD, Stark RJ. Sleep and chronic daily headache. Curr Pain Headache Rep. 2015;19:468. doi: 10.1007/s11916-014-0468-6. [DOI] [PubMed] [Google Scholar]
- 43.Lin YK, Lin GY, Lee JT, et al. Associations between sleep quality and migraine frequency: A cross-sectional case-control study. Medicine (Baltimore). 2016;95:e3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Odegard SS, Engstrom M, Sand T, Stovner LJ, Zwart JA, Hagen K. Associations between sleep disturbance and primary headaches: The third Nord-Trondelag Health Study. J Headache Pain. 2010;11:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim J, Cho SJ, Kim WJ, Yang KI, Yun CH, Chu MK. Insufficient sleep is prevalent among migraineurs: A population-based study. J Headache Pain. 2017;18:50. doi: 10.1186/s10194-017-0756-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song TJ, Yun CH, Cho SJ, Kim WJ, Yang KI, Chu MK. Short sleep duration and poor sleep quality among migraineurs: A population-based study. Cephalalgia. 2017. doi: 10.1177/0333102417716936. [DOI] [PubMed] [Google Scholar]
- 47.Seidel S, Hartl T, Weber M, et al. Quality of sleep, fatigue and daytime sleepiness in migraine—A controlled study. Cephalalgia. 2009;29:662–669. [DOI] [PubMed] [Google Scholar]
- 48.Karthik N, Kulkarni GB, Taly AB, Rao S, Sinha S. Sleep disturbances in ‘migraine without aura’—A questionnaire based study. J Neurol Sci. 2012;321:73–76. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan DP, Martin PR. Sleep and headaches: Relationships between migraine and non-migraine headaches and sleep duration, sleep quality, chronotype, and obstructive sleep apnoea risk. Aust J Psychol. 2017;69:210–217. [Google Scholar]
- 50.Barbanti P, Aurilia C, Egeo G, Fofi L, Vanacore N. A case-control study on excessive daytime sleepiness in chronic migraine. Sleep Med. 2013;14:278–281. [DOI] [PubMed] [Google Scholar]
- 51.Bianchi MT, Williams KL, McKinney S, Ellenbogen JM. The subjective-objective mismatch in sleep perception among those with insomnia and sleep apnea. J Sleep Res. 2013;22:557–568. [DOI] [PubMed] [Google Scholar]
- 52.Harvey AG, Tang NK. (Mis)perception of sleep in insomnia: A puzzle and a resolution. Psychol Bull. 2012;138:77–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smitherman TA, Walters AB, Davis RE, et al. Randomized controlled pilot trial of behavioral insomnia treatment for chronic migraine with comorbid insomnia. Headache. 2016;56:276–291. [DOI] [PubMed] [Google Scholar]
- 54.Fino E, Mazzetti M. Monitoring healthy and disturbed sleep through smartphone applications: A review of experimental evidence. Sleep Breath. 2018. doi: 10.1007/s11325-018-1661-3. [DOI] [PubMed] [Google Scholar]
- 55.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- 56.Edwards RR, Almeida DM, Klick B, Haythornthwaite JA, Smith MT. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson KG, Ziemba AM, Garb JL. Improvement in headaches with continuous positive airway pressure for obstructive sleep apnea: A retrospective analysis. Headache. 2013;53:333–343. [DOI] [PubMed] [Google Scholar]
- 58.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125–133. [DOI] [PubMed] [Google Scholar]
- 59.van Straten A, van der Zweerde T, Kleiboer A, Cuijpers P, Morin CM, Lancee J. Cognitive and behavioral therapies in the treatment of insomnia: A meta-analysis. Sleep Med Rev. 2018;38:3–16. [DOI] [PubMed] [Google Scholar]
- 60.Calhoun AH, Ford S. Behavioral sleep modification may revert transformed migraine to episodic migraine. Headache. 2007;47:1178–1183. [DOI] [PubMed] [Google Scholar]
- 61.Lipton RB, Manack Adams A, Buse DC, Fanning KM, Reed ML. A comparison of the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study and American Migraine Prevalence and Prevention (AMPP) Study: Demographics and headache-related disability. Headache. 2016;56:1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bigal ME, Rapoport AM. Obesity and chronic daily headache. Curr Pain Headache Rep. 2012;16:101–109. [DOI] [PubMed] [Google Scholar]
- 63.Rossi P, Ambrosini A, Buzzi MG. Prodromes and predictors of migraine attack. Funct Neurol. 2005;20:185–191. [PubMed] [Google Scholar]
- 64.2005. Sleep in America Poll. National Sleep Foundation; Available at: https://sleepfoundation.org/sites/default/files/2005_summary_of_findings.pdf. Accessed January 29, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


