Abstract
Burn patients over the age of 60 are at a greater risk for developing pulmonary complications than younger patients. The mechanisms for this, however, have yet to be elucidated. The objective of this study was to determine whether increased chemoattraction plays a role in the age-related differences in pulmonary inflammation after burn injury. At 6 or 24 h after receiving sham or 15% total body surface area scald injury, lungs from young and aged mice were analyzed for leukocyte content by histological examination and immunostaining. Lungs were then homogenized, and levels of neutrophil chemokines, MIP-2 and KC, were measured. At 6 h after burn, the number of neutrophils was four times higher in the lungs of both burn groups compared with aged-matched controls (P<0.05), but no age difference was evident. At 24 h, in contrast, neutrophils returned to sham levels in the lungs of young, burn-injured mice (P<0.05) but did not change in the lungs of aged, burn-injured mice. Pulmonary levels of the neutrophil chemokine KC but not MIP-2 were consistently three times higher in aged, burn-injured mice compared with young, burn-injured mice at both time-points analyzed. Administration with anti-CXCR2 antibody completely abrogated the excessive pulmonary neutrophil content by 24 h (P<0.05), while not affecting the inflammatory response of the wounds. These studies show that CXCR2-mediated chemoattraction is involved in the pulmonary inflammatory response after burn and suggest that aged individuals sustaining a burn injury may benefit from treatment strategies that target neutrophil chemokines.
Keywords: aging, acute lung injury, KC, macrophage inflammatory protein-2, neutrophil
INTRODUCTION
Individuals over the age of 60 are at a greater risk of developing serious complications after burn injury than younger, otherwise healthy adults [1–3]. A main reason is that ~70% of elderly patients are admitted to the emergency room with one or more pre-existing conditions, such as cardiovascular disease or diabetes [4, 5]. However, simply being over the age of 60 has been found to be an independent risk factor for the development of multiple organ failure, sepsis, and acute respiratory distress syndrome after traumatic injury [6, 7]. Improvements in treatment strategies for burn patients have provided great benefit for younger individuals [8]. Unfortunately, the current advancements have made little progress for elderly burn patients [1, 9].
It has long been recognized that one of the most serious threats to the burn patient is the development of respiratory complications [10, 11]. Moreover, when pulmonary dysfunction develops in elderly patients, the risk of death is even higher than that of a younger burn patient [12, 13]. It is currently thought that with a moderate-to-severe burn injury, an extensive amount of proinflammatory mediators enters into circulation, incites a systemic inflammatory response, and affects organs other than the skin [14]. As the inciting injury is not at these remote sites, the accompanying tissue damage and edema formation can significantly compromise normal function [15, 16]. As the lungs receive 100% of the cardiac output, their risk of being affected by a systemic inflammatory response is considerable. Other characteristics of the lung that make it one of the most susceptible organs to damage following injury include its delicate architecture and the presence of numerous alveolar macrophages that can independently respond to systemic cytokines [17, 18]. The reasons why the lungs of aged individuals are even more susceptible to damage following injury than those of younger individuals, however, are not completely understood [4, 19, 20].
One of the acute markers of remote organ damage after burn injury is the infiltration of neutrophils [21, 22]. When activated, neutrophils release numerous proteases and reactive oxygen species, which can result in destruction of the surrounding tissue [23, 24]. There are three main mechanisms involved in neutrophil recruitment to the site of inflammation: chemoattraction, endothelial cell adhesion, and vascular permeability [25, 26]. In this study, we will only focus on chemokines. Neutrophil chemokines—mostly KC and MIP-2, which are orthologs of human growth-related oncogene α and β, respectively—are released from a number of cell types in the lung in response to inflammatory stimuli [27, 28]. Upon their release, chemokines bind to their cognate receptors on circulating leukocytes and induce cytoskeletal changes that allow the cells to migrate into the tissue [25, 29]. For neutrophils, the main receptor for MIP-2 and KC is CXCR2, which is up-regulated in response to various proinflammatory mediators [25, 30].
To date, most of the animal models that explore the mechanisms of pulmonary inflammation after injury use young adult animals [31–34]. Although many have shown that pulmonary sequelae have a higher incidence in elderly burn patients and are more detrimental, few have investigated the cause [2, 4,19]. The main objective of this study was to examine pathologic differences in the lungs of young and aged animals in a murine model of burn injury and to determine whether the neutrophil chemokines, MIP-2 and KC, play a role in this process. We have used a murine model in which animals receive only a moderate-sized burn injury (15% of the total body surface area). In the human population, only ~1% of individuals from 2 to 60 years old would succumb to this size burn, whereas 15% of those over the age of 60 would die from a similar-sized injury [1]. Here, we show for the first time that a moderatesized burn in aged mice parallels what is observed in humans and that a greater neutrophil accumulation is related to a protracted expression of KC in the lungs of aged mice. Blocking neutrophil chemoattraction through administration of anti-CXCR2 antibody effectively reduces pulmonary inflammation in the lungs of aged mice in the first 24 h after burn.
MATERIALS AND METHODS
Animals
Young (3–6 months) and aged (18–22 months) BALB/c female mice were obtained from the National Institute of Aging colony at Harlan Laboratories (Indianapolis, IN, USA) and maintained on a 12-h light/dark cycle with standard laboratory rodent chow and water ad libitum. All experimental procedures were performed according to the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals, National Institutes of Health (Bethesda, MD, USA), and approved by the Animal Care and Use Committee at Loyola University Medical Center (Maywood, IL, USA).
Induction of burn injury
Mice were anesthetized with Nembutal (50 mg/kg i.p.), shaved, and placed into a plastic template designed to give a 15% total body surface area, full-thickness dorsal scald injury when immersed in a boiling water bath for 8 s, according to a modified protocol of Walker and Mason [35, 36]. As a control, a separate group of mice received a sham injury, which entailed administration of anesthesia and shaving, but a room temperature water bath was used instead. Immediately following injury, the mice received warm saline resuscitation (1 ml per 20 g body weight), and their cages were placed on heating pads to prevent circulatory collapse and cardiovascular shock. After recovering from anesthesia, this procedure leaves young, healthy mice able to eat, drink, groom, and ambulate to their pre-injury capacity. The mice were killed using CO2 inhalation and cervical dislocation. No other therapeutic intervention was provided, as administration of anti-inflammatory or analgesic medication may introduce confounding factors into the assessment of inflammatory responses. To eliminate the complication of hormones regulated by circadian rhythms, all burn-injury procedures were administered between 8 and 10 a.m. In addition, all mice—including those that died before the time of sacrifice—were examined for visible tumors and if found, were removed from the study.
Histologic examination of the lungs
Lungs were removed and inflated with formalin immediately after sacrifice, as described previously [37]. After overnight fixation, the lungs were embedded in paraffin, sectioned, and stained with H&E. Lung sections were examined by light microscopy for pathologic changes and neutrophil content [37]. The total number of neutrophils in the lungs of each animal was determined in 10 400× fields.
Immunofluorescence of leukocytes in the lungs and skin
The lungs were removed at the time of sacrifice, inflated with 25% optimal cutting temperature (O.C.T.) freezing medium, and embedded for frozen sectioning. To compare the changes in the lung to the wound itself, the skin from the edge of the burn wound was also removed at the time of sacrifice and embedded in O.C.T. freezing medium for sectioning. Skin was not taken from the center of burn wounds, as most of this tissue is necrotic [38]. The lung and skin sections were fixed in acetone and blocked with normal goat serum. Sections were first incubated with 1 μg/mL rat anti-Gr-1 antibody (Invitrogen, Carlsbad, CA, USA), followed by 4 μg/ml goat anti-rat IgG conjugated to Alexa Fluor 488 (Invitrogen). As Gr-1 can also be found on certain macrophage populations [39, 40], the sections were dual-stained with 0.2 μg/mL biotinylated anti-monocyte and macrophage antibody 2 (MOMA-2) antibody (BMA Biomedicals, Augst, Switzerland), a pan-macrophage marker, and detected with 2 μg/ml Cy3 Streptavidin (Invitrogen). Using fluorescent microscopy, the total number of neutrophils (designated as Gr-1+ MOMA-2– cells) was counted across 10 high-power fields for each animal in lungs and skin [41]. The total tissue area across which cells were counted was quantified and determined to be consistent between animals in all treatment groups (data not shown).
KC and MIP-2 levels in lung and skin homogenates
One lobe per mouse or one 5 mm punch from the edge of the wound was homogenized in protease inhibitor cocktail (Roche Applied Sciences, Indianapolis, IN, USA) [38, 42]. Samples were analyzed for MIP-2 and KC content by ELISA (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s specifications [37]. Total protein content of the same aliquot of lung homogenate used for ELISA was determined by BioRad protein assay (BioRad Laboratories, Hercules, CA, USA). Final concentrations of each chemokine are in pg/mg protein.
Blocking CXCR2
An initial set of dose-response experiments was performed to determine the lowest dose of anti-CXCR2 antibody that would block neutrophil accumulation in lungs of young mice after burn injury without disturbing the inflammatory process in the skin. With these experiments, we determined that an i.p. dose of 20 μg per animal was sufficient to reduce neutrophil content in lungs of young, burn-injured mice to that of sham animals (data not shown). In a separate set of experiments, young and aged mice receiving a sham or 15% total body surface area burn injury were injected i.p. with 20 μg control IgG (R&D Systems) or 20 μg CXCR2 neutralizing antibody (R&D Systems). Mice were then killed at 24 h, and the lungs and skin were collected for further analysis, as described above.
Statistical analysis
Data were analyzed using GraphPad Prism 4 (GraphPad Software, Inc., San Diego, CA, USA) and are expressed as mean ±SEM. For comparisons of two groups, an unpaired Student’s t-test was used. For time-course experiments, a three-way ANOVA was used. Groups were considered significantly different at P values less than 0.05.
RESULTS
Histological changes in the lungs after burn
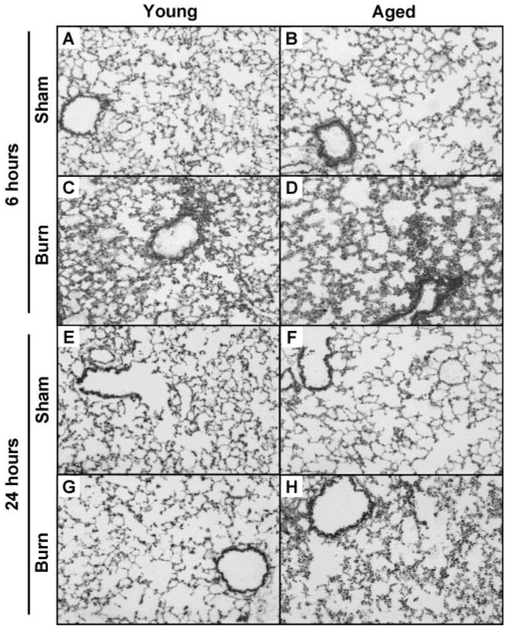
To first examine whether pathologic differences exist in the lungs of young and aged mice at 6 and 24 h after burn injury, frozen sections were stained with H&E. As shown by other laboratories [18, 21, 34], the lungs of young mice were found to have a greater accumulation of inflammatory cells, increased edema formation, and thickened alveolar walls at 6 h after injury compared with young sham controls (Fig. 1, A and C). At this time-point, similar pathological changes were found in lungs of aged, burn-injured mice, which were not apparent in lungs of aged, control animals (Fig. 1, B and D). By 24 h, the inflammatory cell accumulation in the lungs of young, burn-injured animals diminished, making them indistinguishable from sham controls (Fig. 1, E and G). In the lungs of aged animals that sustained injury, however, the inflammatory infiltrate did not decrease at 24 h compared with 6 h (Fig. 1, F and H). To note, the lungs of young and aged, sham-injured mice did not appear different from young and aged, unmanipulated animals (data not shown).
Fig. 1.
Representative micrographs of H&E-stained lung sections are shown from young (A, C, E, and G) and aged (B, D, F, and H) animals at 6 h after sham injury (A and B), 6 h after burn injury (C and D), 24 h after sham injury (E and F), and 24 h after burn injury (G and H). All images are at 100× original magnification.
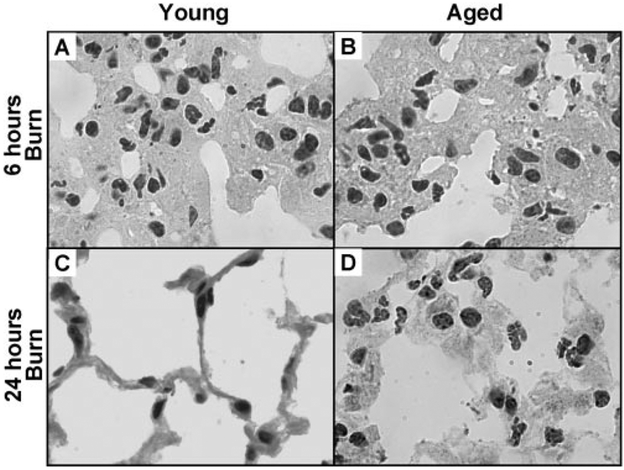
Upon closer examination, the vast majority of the inflamma-tory cells in the lungs after injury were neutrophils. To determine whether these neutrophils migrated into the tissue or remained in the circulation, lung sections from all burn-injured animals were examined at higher power (1000×). High-power images of lungs from young mice 24 h after burn looked identical to those of sham-injured mice; in these groups, the alveolar walls were thin and not very cellular. In lungs of young and aged mice at 6 h after burn, as well as in those of aged mice at 24 h after burn, the opposite was the case. Although many neutrophils were also observed within the vasculature, the majority appeared to have extravasated and localized within the alveolar walls leading to increased wall thickness (Fig. 2).
Fig. 2.
High-power view of H&E lung sections illustrating neutrophils within thickened alveolar walls of young and aged, burn-injured mice at 6 h (A andB) and at 24 h (C and D). High-power images of young, burn-injured mice at 24 h did not appear different from those of sham-injured mice (not shown). All images are at 1000 × original magnification.
Inflammatory cell accumulation in lungs of aged mice after burn
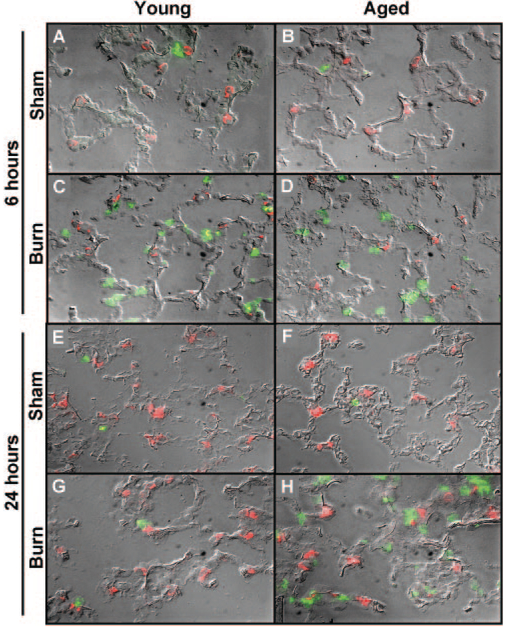
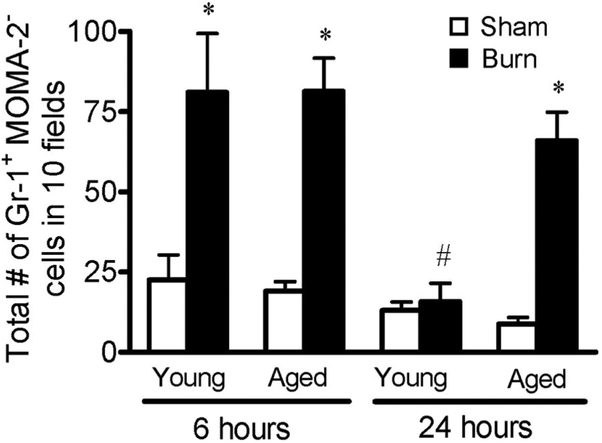
To confirm that the injury-associated inflammatory cells seen in the lungs after burn injury were indeed neutrophils, frozen sections of lung were immunostained with anti-Gr-1 [40]. As anti-Gr-1 can also detect certain macrophage populations, lungs were simultaneously stained with anti-MOMA-2—a pan-macrophage marker [39, 40]. Thus, cells that were Gr-1-positive but were negative for MOMA-2 were considered neutrophils. Representative images of immunostained lungs from all treatment groups are shown in Figure 3, and quantification of neutrophils is shown in Figure 4. At 6 h after injury, the number of neutrophils was more than four times higher in lungs of young mice when compared with sham-injured controls (P<0.05; Figs. 3, A and C, and 4). Similar increases in pulmonary neutrophils were found at 6 h in the lungs of aged, burn-injured mice (Figs. 3, B and D, and 4). By 24 h after injury, the number of neutrophils in the lungs of young, burn-injured mice decreased to sham levels (Figs. 3, E and G, and 4). Parallel to the H&E analysis in Figures 1 and 2, the neutrophils remained elevated in the lungs of aged, burn-injured animals at 24 h compared with sham controls (P<0.05; Figs. 3, F and H, and 4). Neither age nor burn injury affected the number of MOMA-2+cells or Gr-1+ MOMA-2+cells observed in the lungs at either time-point analyzed (data not shown). In addition, the number of neutrophils in the lungs of young and aged, sham-injured mice was not different from those of young and aged, unmanipulated mice (data not shown).
Fig. 3.
Sections of lungs from young (A, C, E, and G) and aged (B, D, F, andH) mice at 6 and 24 h after receiving a burn injury were stained with anti-Gr-1 (green) and anti-MOMA-2 (red) antibodies. Representative fluorescent images are shown with a differential interference contrast overlay to indicate the alveolar architecture in lungs from animals 6 h after sham injury (A and B), 6 h after burn injury (C and D), 24 h after sham injury (E and F), and 24 h after burn injury (G and H). All images are at 400× original magnification.
Fig. 4.
Total numbers of Gr-1+MOMA-2– cells from lungs of young and aged animals at 6 and 24 h after sham (open bars) or burn (solid bars) injury were counted in sections of lung tissue. Data are represented as the average number of cells counted in 10 400×fields for each group ± SEM; n =4–7 mice per group; *, P < 0.05, compared with age- and time-matched sham groups; #, P< 0.05, compared with the young burn group at 6 h.
Chemokines in lungs of aged mice after burn injury
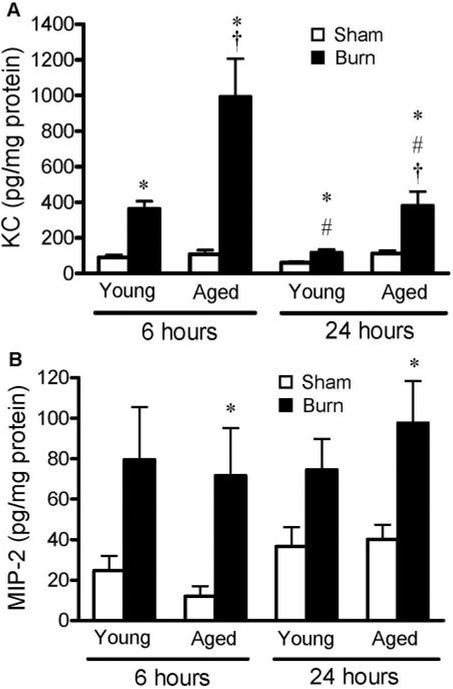
To determine whether increased numbers of neutrophils seen in the lungs of aged mice after injury correlated with enhanced levels of chemokines, lung homogenates obtained from young and aged mice were analyzed for neutrophil chemokines, MIP-2 and KC, by ELISA. At 6 h after burn injury, young and aged mice had significantly higher pulmonary KC levels than their sham controls, but levels were three times higher in the lungs of aged mice compared with young mice after burn (Fig. 5A). At 24 h, KC levels remained elevated in the lungs of young and aged, burn-injured mice compared with shams, but levels were still three times higher in the lungs of aged mice receiving a burn injury. In contrast, although pulmonary levels of MIP-2 were two to six times higher than sham levels in all animals receiving a burn, there were no differences between age groups or time-points after injury (P<0.05; Fig. 5B). These data imply that although KC and MIP-2 are elevated in the lungs in the acute phases of burn injury, only levels of KC can account for the age-related differences in pulmonary neutrophil recruitment.
Fig. 5.
Levels of (A) KC and (B) MIP-2 were measured in lung homogenates of young and aged mice at 6 and 24 h after sham (open bars) or burn (solid bars) injury. Data are represented as average concentration in pg/mg protein ± SEM; n =8–13 mice per group; *, P < 0.05, compared with age- and time-matched sham groups; #, P < 0.05, compared with burn animals at 6 h;†, P < 0.05, compared with the young burn group at the same time-point.
Blocking CXCR2
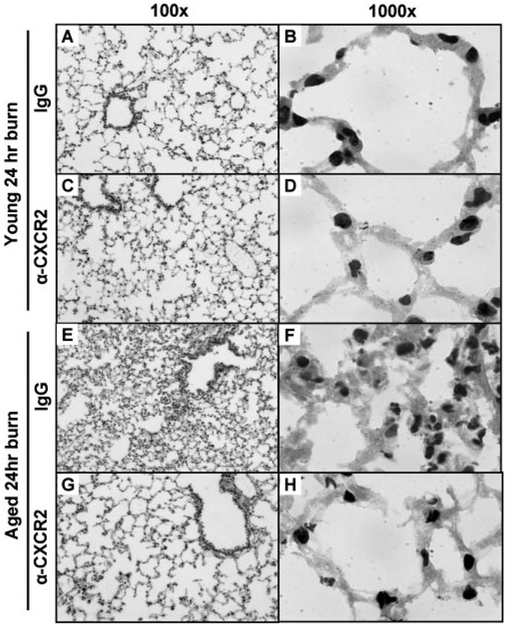
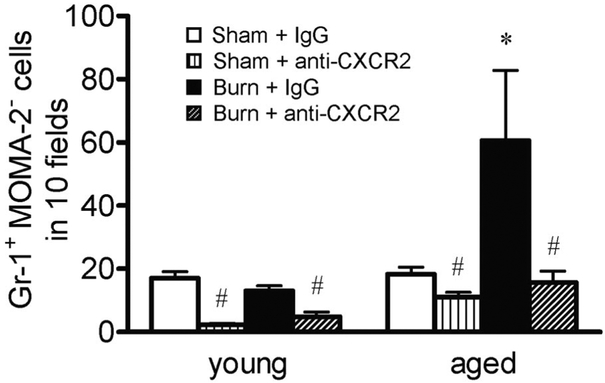
To test the hypothesis that neutrophil chemokines are directly involved in the accumulation of pulmonary neutrophils after burn injury, mice were administered with control IgG or a neutralizing antibody against CXCR2 i.p., 30 min after receiving a burn or a sham injury. Animals were then killed at 24 h after injury. As shown above, this time-point correlated with neutrophil clearance in the lungs of young mice, and the lungs of aged mice still had a considerable degree of inflammation (Figs. 1–4). As expected, the lungs of young mice 24 h after burn did not appear different from the lungs of sham animals following injection of control IgG or anti-CXCR2 antibody (Fig. 6, A–D, compared with Fig. 1). Administration of the anti-CXCR2 antibody to aged mice after burn injury significantly reduced the pulmonary pathology seen with the injection of control antibody (Fig. 6, E–H). This effect correlated with a reduction in pulmonary neutrophils to that of sham levels (Fig. 7). Interestingly, blocking CXCR2 significantly lowered the pulmonary levels of neutrophils in all treatment groups, including young, sham-injured mice, suggesting that CXCR2 also has a role in the normal, homeostatic maintenance of neutrophil numbers in the lungs. To note, KC, MIP-2, and IL-1β levels in the lungs were not different between control IgG and anti-CXCR2 administration in any of the treatment groups (data not shown), indicating that antibody treatment did not decrease neutrophil accumulation in the lungs through diminishing the proinflammatory response itself.
Fig. 6.
Representative micrographs of H&E-stained lung sections are shown from young (A–D) and aged (E–H) animals receiving control IgG i.p. (A, B, E, and F) or anti-CXCR2 i.p. (C, D, G, and H) at 6 h after burn injury. Images in the left column are at 100× original magnification, and images shown on the right are at 1000X original magnification.
Fig. 7.
Total numbers of Gr-1+ MOMA-2− cells from lungs of young and aged animals at 24 h after sham or burn injury, receiving 20 μg i.p. control IgG or 20 μg i.p. anti-CXCR2 antibody, were counted in sections of lung tissue as described above. Data are represented as the average number of cells counted in 10 400x fields for each group ± SEM. The average tissue area over which cells were counted did not differ between groups; n =3–9 mice per group; *, P < 0.05, compared with all other groups; #, P < 0.05, compared with IgG controls.
Wound analysis
With the observation that chemokines are higher in the lungs of aged mice after burn leading to greater neutrophil accumulation, we sought to determine whether this held true for the burn wound as well. As shown in Table 1, levels of KC were significantly elevated in the wounds of burn-injured mice at 24 h, but age differences seen in the lungs at this time-point were not apparent. MIP-2 levels at this time-point were below the minimum level of detection by ELISA in the wounds of all treatment groups (data not shown). Neutrophils in the skin at 24 h after burn were also elevated compared with shams (P<0.05) but were not significantly different between the age groups (Table 2). These data suggest that the exaggerated, inflammatory response to burn injury in aged mice is specific for the lung.
TABLE 1.
Chemokine Levels in Wounds 24 h after Burn
Levels of KC and MIP-2 were measured by ELISA in wound homogenates of young and aged mice at 24 h after sham or burn injury. Data are represented as average concentration in pg/mg protein ± SEM; N = 8–12 mice per group.
P < 0.05 compared to sham controls. MIP-2 was below the minimum detection level for all groups.
TABLE 2.
Neutrophil Counts in Wounds 24 h after Burn
| IgG (i.p.) | Anti-CXCR2 (i.p.) | |||
|---|---|---|---|---|
| Sham | Burn | Sham | Burn | |
| Young | 1.3 ± 0.9 | 74.0 ± 8.3a | 2.3 ± 1.1 | 46.8 ± 11.1a |
| Aged | 9.5 ± 3.1 | 76.9 ± 9.2a | 7.3 ± 2.8 | 75.0 ± 7.0a |
Tissue from the edge of burn wounds of young and aged animals at 24 h after sham or burn injury, receiving either 20 μg i.p. of control IgG or 20 μg i.p. of anti-CXCR2 antibody, was collected. Total numbers of Gr-1+MOMA-2— cells in paraffin sections were counted as described above for the lungs. Data are represented as the average number of cells counted in ten 400× fields for each group ±SEM; n = 7–14 mice per group;
P < 0.05 compared to sham controls.
As the anti-CXCR2 antibody was given systemically, there was a concern that neutrophil migration to the wound itself would be compromised, potentially leading to abnormal wound healing or a risk of infection. For this reason, the dose of anti-CXCR2 antibody used (20 μg per mouse) was intention ally kept at a level that would effectively reduce the neutrophil accumulation in the lung and preserve the inflammatory response in the burn wound. The results in Table 2 show that, in fact, the dose of the anti-CXCR2 antibody given did not significantly affect the neutrophil accumulation in the wounds of mice at either age at 24 h after burn injury. As anti-CXCR2 antibody treatment did not affect levels of KC or MIP-2 in the lungs, these chemokines were not measured in the wounds of this treatment group.
DISCUSSION
It is widely recognized that advanced age is a significant risk factor for increased pulmonary complications after burn injury [10, 12]. However, few studies have directly examined the mechanisms that could contribute to this age-associated susceptibility. In studies using young animals, neutrophils are a main mediator of pulmonary inflammation and tissue damage in the acute stages of injury [18, 34, 43]. Here, we have shown that chemokines acting through CXCR2 play a role in causing acute inflammation in the lungs after burn injury. We have also shown that aged mice exhibit an exacerbated pulmonary response to burn as a result of an increased and sustained level of KC in the lungs. Most importantly, these data indicate that in this model of burn injury, blocking CXCR2 is an effective way to reduce acute pulmonary inflammation, especially in aged mice.
Many have previously shown that chemokines are instrumental in neutrophil-mediated pulmonary damage after injury in young animals [28–30, 44, 45]. Here, we demonstrate that levels of KC and MIP-2 are elevated in the lungs within the first 24 h after injury but that only pulmonary KC levels are affected by age. Experiments aimed at blocking CXCR2 show that inhibiting the neutrophil response to KC completely abrogates pulmonary inflammation after burn in young and aged mice, indicating that chemoattraction is indeed part of this mechanism for both age groups. These results are consistent with those from other laboratories studying the effects of inhibiting CXCR2-mediated chemoattraction in various models of systemic inflammation, such as hemorrhagic shock and sepsis, as well as in wound healing [29, 33, 46, 47]. Whether using CXCR2 knockout mice, neutralizing antibody against the receptor, small molecule inhibitors of the receptor, or antibodies against KC and MIP-2 themselves, studies show that blocking CXCR2-mediated chemoattraction sufficiently attenuates acute inflammatory responses following a systemic challenge.
As a caveat, in vitro experiments conducted by other laboratories have indicated that neutrophils from aged animals actually display decreased chemotaxis in response to various inflammatory stimuli [48–50]. The contradiction between these in vitro experiments and those conducted in the current study reveals the importance of a cellular environment when analyzing defects associated with aging. It is well known that in vivo, aging is associated with increased circulating, proinflammatory cytokines, such as IL-6, TNF-α,IL-1, and IL-8 [51–54]. Interestingly, we have also found that IL-1β levels are significantly higher in the lungs of aged mice (V. Nomellini and E. J. Kovacs, unpublished observations). A significant increase in the levels of IL-1β in the lungs of elderly humans in the absence of clinically detectable disease has also been observed[55]. This increase in pulmonary IL-1β, however, does not correlate with higher levels of KC and MIP-2—both of which can be induced by IL-1β [44, 56]—in the lungs of uninjured, aged mice (Fig. 5). After burn injury, though, KC levels are threefold greater in the lungs of aged mice compared with those of young mice at 6 and 24 h. In contrast, IL-1β is not different in the skin of young and aged, uninjured mice (V. Nomellini and E. J. Kovacs, unpublished observations). Following this, neither KC nor MIP-2 shows age differences in the skin after burn (Table 1). Differential levels of IL-1β in the absence of injury may help explain why, following an inflammatory challenge, the lungs of aged mice show an exacerbated response compared with those of young mice, and the wounds themselves do not. To note, levels of IL-6, another key player in the inflammatory response, are not different in the lungs of young and aged, uninjured mice (data not shown).
With the observation that aging is associated with an increased proinflammatory state, the results of the current study are similar to those seen in “two hit” models of injury. In these models, the clinically important situation, whereby two inflammatory challenges occur simultaneously or as subsequent challenges, results in an exaggerated response beyond that of either injury alone. Examples of this include hemorrhagic shock plus sepsis or burn injury plus infection [57, 58]. We propose that burn injury in aged individuals parallels these two hit models. Advanced age acts as the first hit by increasing the inflammatory milieu of the lungs. Once receiving a burn injury (the second hit), aged mice exhibit an augmented response beyond that of young mice receiving a comparable injury. We have shown that blocking this excessive inflammation effectively reduces the pulmonary consequences in the aged mice after burn. In the current studies, though, the anti-CXCR2 treatment did not decrease the mortality rate of the aged mice (data not shown). Regardless of whether they received anti-CXCR2 antibody, ~20% of the aged mice succumb to the burn injury within the first 24 h, and all of the young mice survive. In other words, a burn size that is normally manageable in young mice leaves aged mice at a greater risk for complications and death; this is very similar to what is seen in the human population [12, 59, 60]. In humans, on the other hand, mortality rates are four times higher in elderly patients with pulmonary failure compared with those without [12]. Therefore, although anti-CXCR2 treatment does not affect mortality within the first 24 h after burn, perhaps it will prove to be a valuable tool to prevent or limit pulmonary failure and death at later time-points.
Although we have shown that CXCR2 chemokines are mechanistically important in mediating pulmonary neutrophil accumulation after burn, the therapeutic implications of these data are also intriguing. As described above, the intended use of the anti-CXCR2 antibody was to effectively reduce the systemic component to burn injury while preserving the inflammatory response of the wound itself. Using only 20 μg per mouse, we were able to accomplish this. The reasons for this are unknown, but we believe that it is related to the degree of tissue injury and the number and types of proinflammatory mediators involved. At the primary site of injury in the skin, there is a great deal of cellular damage and necrosis that is not seen in the lungs [38]. The persistence of this necrotic tissue acts as a nidus for a protracted inflammatory response. In addition, chemokine signaling via CXC receptors is not the only mechanism that contributes to neutrophil migration to the wound. Other candidate chemoattractants not examined in this study include C5a, platelet-activating factor, and leukotriene B4, all of which are potent mediators of neutrophil migration [25, 28,61]. C5a, in particular, has been shown to play a role in burn injury [28, 62].
Interestingly, these data also show that blocking CXCR2 decreases the number of neutrophils in the lungs of uninjured animals. It is well known that there is a highly controlled regulation of neutrophil numbers in the peripheral blood and in tissues. The proposed mechanisms for neutrophil homeostasis are related to the β-integrin, CD18 [63], as well as G-CSF and IL-23 [64]. Some have also suggested that there is an important interplay between neutrophil responses to the CXCR4/stromal-derived factor-1 axis and the CXCR2/KC axis [65]. Upon down-regulation or cleavage of CXCR4, neutrophils have an increased propensity to leave the bone marrow and migrate to the periphery via CXCR2 ligands. Our results support this hypothesis, indicating that blockage of CXCR2 attenuates neutrophil numbers in the peripheral blood (data not shown) as well as in the lungs of uninjured mice (Fig. 7). Although not a primary goal for the current study, these data provide further insight into normal maintenance of neutrophil homeostasis.
From the results of this study alone, it is not possible to predict whether the neutrophil retention in lungs of aged, burn-injured mice was only a result of enhanced recruitment to the tissue or whether increased adhesion to the pulmonary vasculature and/or diminished clearance also play a role. There has been extensive research about the role of endothelial adhesion molecules on neutrophil accumulation in the lungs after burn injury [66, 67]. In young animals, expression of ICAM-1 is reportedly elevated in the lungs following various systemic inflammatory responses, including burn injury and sepsis [68, 69]. To date, there are no published reports that assess the cell adhesion molecule profile in lungs of aged animals after injury. However, many have reported an up-regulation of membrane and soluble forms of ICAM-1, VCAM-1, and selectins in the serum of aged humans and animals, whether in the absence or in the presence of injury [70–73]. Preliminary data from our laboratory also suggest that levels of ICAM-1 are elevated in the lungs of uninjured, aged mice compared with those of young mice (V. Nomellini andE. J. Kovacs, unpublished observations). In addition, as aging and burn injury are known to compromise the phagocytic capacity of macrophages, decreased removal of apoptotic neutrophils may provide an alternative explanation for the increased pulmonary inflammation seen in aged mice after burn [74, 75]. In our hands, histologic examination does not reveal an accumulation of apoptotic bodies. Specific staining for active caspase 3 and similar studies aimed at characterizing the state of neutrophil apoptosis, as well as macrophage phagocytosis, are required to define the role of neutrophil clearance in the lungs of aged animals in response to injury.
In summary, these data show that CXCR2-mediated neutrophil migration is important in the development of pulmonary inflammation in the acute stages of burn injury. In addition, increased age is associated with an exaggerated response in the lungs, but not in the wounds, following burn injury, possibly as a result of differential levels of IL-1β expression before injury. Regardless of age, prolonged neutrophil exposure can lead to excessive tissue destruction as a result of protease release and oxidative stress [14, 23, 24]. This may explain why the elderly are at an increased risk for pulmonary complications after burn injury. Low doses of CXCR2-neutralizing antibody are effective in attenuating acute pulmonary neutrophil accumulation in aged mice, while maintaining the inflammatory environment of the wound tissue. Importantly, these data imply that the development of more targeted therapies against neutrophil chemokines may be beneficial for preventing or diminishing remote organ damage after injury, especially in the aged population.
ACKNOWLEDGMENTS
The authors thank Luis Ramirez, Michelle Morgan, John Karavitis, Eva Murdoch, Dr. Melanie Bird, and Mary Kay Olsen for their technical assistance and Pamela Witte, Ph.D., head of the Immunology and Aging Program at Loyola University Medical Center, for thoughtful discussions.
REFERENCES
- 1.(2005) National Burn Repository Report. http://www.ameriburn.org/NBR2005.pdf.
- 2.Linn BS (1980) Age differences in the severity and outcome of burns. J. Am. Geriatr. Soc 28, 118–123. [DOI] [PubMed] [Google Scholar]
- 3.McGill V, Kowal-Vern A, Gamelli RL (2000) Outcome for older burn patients. Arch. Surg 135, 320–325. [DOI] [PubMed] [Google Scholar]
- 4.Lionelli GT, Pickus EJ, Beckum OK, Decoursey RL, Korent-ager RA (2005) A three decade analysis of factors affecting burn mortality in the elderly. Burns 31, 958–963. [DOI] [PubMed] [Google Scholar]
- 5.Tran DD, Groeneveld AB, van der Meulen J, Nauta JJ, Strack van Schijndel RJ, Thijs LG (1990) Age, chronic disease, sepsis, organ system failure, and mortality in a medical intensive care unit. Crit. Care Med 18, 474–479. [DOI] [PubMed] [Google Scholar]
- 6.Fitzwater J, Purdue GF, Hunt JL, O’Keefe GE (2003) The risk factors and time course of sepsis and organ dysfunction after burn trauma.J. Trauma 54, 959–966. [DOI] [PubMed] [Google Scholar]
- 7.Dancey DR, Hayes J, Gomez M, Schouten D, Fish J, Peters W, Slutsky AS, Stewart TE (1999) ARDS in patients with thermal injury. Intensive Care Med. 25, 1231–1236. [DOI] [PubMed] [Google Scholar]
- 8.(2002) Abstracts of the 34th Annual Meeting of the American Burn Association. April 24–27, 2002. Chicago, Illinois, USA. J. Burn Care Rehabil 23, S41–S183. [PubMed] [Google Scholar]
- 9.Griffiths RW, Laing JE (1981) A burn formula in clinical practice. Ann. R. Coll. Surg. Engl 63, 50–53. [PMC free article] [PubMed] [Google Scholar]
- 10.Achauer BM, Allyn PA, Furnas DW, Bartlett RH (1973) Pulmonary complications of burns: the major threat to the burn patient. Ann. Surg 177, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollingsed TC, Saffle JR, Barton RG, Craft WB, Morris SE (1993) Etiology and consequences of respiratory failure in thermally injured patients. Am. J. Surg 166, 592–596. [DOI] [PubMed] [Google Scholar]
- 12.Clayton MC, Solem LD, Ahrenholz DH (1995) Pulmonary failure in geriatric patients with burns: the need for a diagnosis-related group modifier. J. Burn Care Rehabil 16, 451–454. [DOI] [PubMed] [Google Scholar]
- 13.Ely EW, Wheeler AP, Thompson BT, Ancukiewicz M, Steinberg KP, Bernard GR (2002) Recovery rate and prognosis in older persons who develop acute lung injury and the acute respiratory distress syndrome. Ann. Intern. Med 136, 25–36. [PubMed] [Google Scholar]
- 14.Ward PA, Till GO (1990) Pathophysiologic events related to thermal injury of skin. J. Trauma 30, S75–S79. [DOI] [PubMed] [Google Scholar]
- 15.Lund T, Onarheim H, Reed RK (1992) Pathogenesis of edema formation in burn injuries. World J. Surg 16, 2–9. [DOI] [PubMed] [Google Scholar]
- 16.Iliopoulou E, Markaki S, Poulikakos L (1993) Autopsy findings in burn injuries. Arch. Anat. Cytol. Pathol 41, 5–8. [PubMed] [Google Scholar]
- 17.Williams JG, Bankey P, Minei JP, McIntyre K, Turbeville T (1994) Burn injury enhances alveolar macrophage endotoxin sensitivity. J. Burn Care Rehabil 15, 493–498. [DOI] [PubMed] [Google Scholar]
- 18.Arbak S, Ercan F, Hurdag CG, Karabulut O, Gurbuz V, Corak A, Alican I (1999) Acute lung injury following thermal insult to the skin: a light and transmission electron microscopial study. Acta Histochem. 101, 255–262. [DOI] [PubMed] [Google Scholar]
- 19.Slater H, Gaisford JC (1981) Burns in older patients. J. Am. Geriatr. Soc 29, 74–76. [DOI] [PubMed] [Google Scholar]
- 20.Duchateau J (2003) [Immunosenescence and the lung]. Rev. Mal. Respir 20, 735–741. [PubMed] [Google Scholar]
- 21.Baskaran H, Yarmush ML, Berthiaume F (2000) Dynamics of tissue neutrophil sequestration after cutaneous burns in rats. J. Surg. Res 93, 88–96. [DOI] [PubMed] [Google Scholar]
- 22.Demling RH, LaLonde C, Liu YP, Zhu DG (1989) The lung inflammatory response to thermal injury: relationship between physiologic and histologic changes. Surgery 106, 52–59. [PubMed] [Google Scholar]
- 23.Hansbrough JF, Wikstrom T, Braide M, Tenenhaus M, Rennekampff OH, Kiessig V, Bjursten LM (1996) Neutrophil activation and tissue neutrophil sequestration in a rat model of thermal injury.J. Surg. Res 61, 17–22. [DOI] [PubMed] [Google Scholar]
- 24.Ravage ZB, Gomez HF, Czermak BJ, Watkins SA, Till GO (1998) Mediators of microvascular injury in dermal burn wounds. Inflammation 22, 619–629. [DOI] [PubMed] [Google Scholar]
- 25.Reutershan J, Ley K (2004) Bench-to-bedside review: acute respiratory distress syndrome–how neutrophils migrate into the lung. Crit. Care 8, 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hillyer P, Mordelet E, Flynn G, Male D (2003) Chemokines, chemokine receptors and adhesion molecules on different human endothelia: discriminating the tissue-specific functions that affect leucocyte migration. Clin. Exp. Immunol 134, 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lomas-Neira JL, Chung CS, Wesche DE, Perl M, Ayala A (2005) In vivo gene silencing (with siRNA) of pulmonary expression of MIP-2 versus KC results in divergent effects on hemorrhage-induced, neutrophil-mediated septic acute lung injury. J. Leukoc. Biol 77, 846–853. [DOI] [PubMed] [Google Scholar]
- 28.Piccolo MT, Wang Y, Sannomiya P, Piccolo NS, Piccolo MS, Hugli TE, Ward PA, Till GO (1999) Chemotactic mediator requirements in lung injury following skin burns in rats. Exp. Mol. Pathol 66, 220–226. [DOI] [PubMed] [Google Scholar]
- 29.Lomas-Neira JL, Chung CS, Grutkoski PS, Miller EJ, Ayala A (2004) CXCR2 inhibition suppresses hemorrhage-induced priming for acute lung injury in mice. J. Leukoc. Biol 76, 58–64. [DOI] [PubMed] [Google Scholar]
- 30.Goodman RB, Pugin J, Lee JS, Matthay MA (2003) Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 14, 523–535. [DOI] [PubMed] [Google Scholar]
- 31.Dries DJ, Lorenz K, Kovacs EJ (2001) Differential neutrophil traffic in gut and lung after scald injury. J. Burn Care Rehabil 22, 203–209. [DOI] [PubMed] [Google Scholar]
- 32.O’Dea KP, Young AJ, Yamamoto H, Robotham JL, Brennan FM, Takata M (2005) Lung-marginated monocytes modulate pulmonary microvascular injury during early endotoxemia. Am. J. Respir. Crit. Care Med 172, 1119–1127. [DOI] [PubMed] [Google Scholar]
- 33.Lomas-Neira J, Chung CS, Grutkoski PS, Dunican A, Simms HH, Cioffi WG, Ayala A (2005) Divergent roles of murine neutrophil chemokines in hemorrhage induced priming for acute lung injury. Cytokine 31, 169–179. [DOI] [PubMed] [Google Scholar]
- 34.Stengle J, Meyers R, Pyle J, Dries DJ (1996) Neutrophil recruitment after remote scald injury. J. Burn Care Rehabil 17, 14–18. [DOI] [PubMed] [Google Scholar]
- 35.Walker HL, Mason AD Jr. (1968) A standard animal burn. J. Trauma 8, 1049–1051. [DOI] [PubMed] [Google Scholar]
- 36.Faunce DE, Gregory MS, Kovacs EJ (1997) Effects of acute ethanol exposure on cellular immune responses in a murine model of thermal injury. J. Leukoc. Biol 62, 733–740. [DOI] [PubMed] [Google Scholar]
- 37.Patel PJ, Faunce DE, Gregory MS, Duffner LA, Kovacs EJ (1999) Elevation in pulmonary neutrophils and prolonged production of pulmonary macrophage inflammatory protein-2 after burn injury with prior alcohol exposure. Am. J. Respir. Cell Mol. Biol 20, 1229–1237. [DOI] [PubMed] [Google Scholar]
- 38.Faunce DE, Llanas JN, Patel PJ, Gregory MS, Duffner LA, Kovacs EJ (1999) Neutrophil chemokine production in the skin following scald injury. Burns 25, 403–410. [DOI] [PubMed] [Google Scholar]
- 39.Vermaelen K, Pauwels R (2004) Accurate and simple discrimination of mouse pulmonary dendritic cell and macrophage populations by flow cytometry: methodology and new insights. Cytometry A 61, 170–177. [DOI] [PubMed] [Google Scholar]
- 40.Sugimoto Y, Katayama N, Masuya M, Miyata E, Ueno M, Ohishi K, Nishii K, Takakura N, Shiku H (2006) Differential cell division history between neutrophils and macrophages in their development from granulocyte-macrophage progenitors. Br. J. Haematol 135, 725–731. [DOI] [PubMed] [Google Scholar]
- 41.Lam CF, Caterina P, Filion P, van Heerden PV, Ilett KF (2002) The ratio of polymorphonuclear leucocytes (PMN) to non-PMN cells–a novel method of assessing acute lung inflammation. Exp. Toxicol. Pathol 54, 187–191. [DOI] [PubMed] [Google Scholar]
- 42.Gomez CR, Hirano S, Cutro BT, Birjandi S, Baila H, Nomellini V, Kovacs EJ (2007) Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit. Care Med. 35, 246–251 [DOI] [PubMed] [Google Scholar]
- 43.Abraham E, Carmody A, Shenkar R, Arcaroli J (2000) Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol 279, L1137–L1145. [DOI] [PubMed] [Google Scholar]
- 44.Calkins CM, Bensard DD, Shames BD, Pulido EJ, Abraham E, Fernandez N, Meng X, Dinarello CA, McIntyre RC Jr. (2002) IL-1 regulates in vivo C-X-C chemokine induction and neutrophil sequestration following endotoxemia. J. Endotoxin Res 8, 59–67. [PubMed] [Google Scholar]
- 45.Mercer-Jones MA, Shrotri MS, Peyton JC, Remick DG, Cheadle WG (1999) Neutrophil sequestration in liver and lung is differentially regulated by C-X-C chemokines during experimental peritonitis. Inflammation 23, 305–319. [DOI] [PubMed] [Google Scholar]
- 46.Gordon JR, Li F, Zhang X, Wang W, Zhao X, Nayyar A (2005) The combined CXCR1/CXCR2 antagonist CXCL8(3–74)K11R/G31P blocks neutrophil infiltration, pyrexia, and pulmonary vascular pathology in endotoxemic animals. J. Leukoc. Biol 78, 1265–1272. [DOI] [PubMed] [Google Scholar]
- 47.Ness TL, Hogaboam CM, Strieter RM, Kunkel SL (2003) Immunomodulatory role of CXCR2 during experimental septic peritonitis.J. Immunol 171, 3775–3784. [DOI] [PubMed] [Google Scholar]
- 48.Gomez CR, Boehmer ED, Kovacs EJ (2005) The aging innate immune system. Curr. Opin. Immunol 17, 457–462. [DOI] [PubMed] [Google Scholar]
- 49.Fulop T, Larbi A, Douziech N, Fortin C, Guerard KP, Lesur O, Khalil A, Dupuis G (2004) Signal transduction and functional changes in neutrophils with aging. Aging Cell 3, 217–226. [DOI] [PubMed] [Google Scholar]
- 50.Niwa Y, Kasama T, Miyachi Y, Kanoh T (1989) Neutrophil chemo-taxis, phagocytosis and parameters of reactive oxygen species in human aging: cross-sectional and longitudinal studies. Life Sci. 44, 1655–1664. [DOI] [PubMed] [Google Scholar]
- 51.Sarkar D, Fisher PB (2006) Molecular mechanisms of aging-associated inflammation. Cancer Lett. 236, 13–23. [DOI] [PubMed] [Google Scholar]
- 52.Ershler WB (1993) Interleukin-6: a cytokine for gerontologists. J. Am. Geriatr. Soc 41, 176–181. [DOI] [PubMed] [Google Scholar]
- 53.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci 908, 244–254. [DOI] [PubMed] [Google Scholar]
- 54.Bruunsgaard H, Andersen-Ranberg K, Hjelmborg JB, Pedersen BK, Jeune B (2003) Elevated levels of tumor necrosis factor α and mortality in centenarians. Am. J. Med 115, 278–283. [DOI] [PubMed] [Google Scholar]
- 55.Meyer KC, Ershler W, Rosenthal NS, Lu XG, Peterson K (1996) Immune dysregulation in the aging human lung. Am. J. Respir. Crit. Care Med 153, 1072–1079. [DOI] [PubMed] [Google Scholar]
- 56.Chen LW, Chang WJ, Wang JS, Hsu CM (2007) Interleukin-1 mediates thermal injury-induced lung damage through C-Jun NH2-terminal kinase signaling. Crit. Care Med 35, 1113–1122. [DOI] [PubMed] [Google Scholar]
- 57.Perl M, Chung CS, Lomas-Neira J, Rachel TM, Biffl WL, Cioffi WG, Ayala A (2005) Silencing of Fas, but not caspase-8, in lung epithelial cells ameliorates pulmonary apoptosis, inflammation, and neutrophil influx after hemorrhagic shock and sepsis. Am. J. Pathol 167, 1545–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davis KA, Santaniello JM, He LK, Muthu K, Sen S, Jones SB, Gamelli RL, Shankar R (2004) Burn injury and pulmonary sepsis: development of a clinically relevant model. J. Trauma 56, 272–278. [DOI] [PubMed] [Google Scholar]
- 59.Hammond J, Ward CG (1991) Burns in octogenarians. South. Med. J 84, 1316–1319. [DOI] [PubMed] [Google Scholar]
- 60.Suchyta MR, Clemmer TP, Elliott CG, Orme JF Jr., Morris AH, Jacobson J, Menlove R (1997) Increased mortality of older patients with acute respiratory distress syndrome. Chest 111, 1334–1339. [DOI] [PubMed] [Google Scholar]
- 61.Burns AR, Smith CW, Walker DC (2003) Unique structural features that influence neutrophil emigration into the lung. Physiol. Rev 83, 309–336. [DOI] [PubMed] [Google Scholar]
- 62.Schmid E, Piccolo MT, Friedl HP, Warner RL, Mulligan MS, Hugli TE, Till GO, Ward PA (1997) Requirement for C5a in lung vascular injury following thermal trauma to rat skin. Shock 8, 119–124. [DOI] [PubMed] [Google Scholar]
- 63.Weinmann P, Scharffetter-Kochanek K, Forlow SB, Peters T, Walzog B (2003) A role for apoptosis in the control of neutrophil homeostasis in the circulation: insights from CD18-deficient mice. Blood 101, 739–746. [DOI] [PubMed] [Google Scholar]
- 64.Christopher MJ, Link DC (2007) Regulation of neutrophil homeostasis. Curr. Opin. Hematol 14, 3–8. [DOI] [PubMed] [Google Scholar]
- 65.Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM (2003) Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity 19, 583–593. [DOI] [PubMed] [Google Scholar]
- 66.Muller AM, Cronen C, Muller KM, Kirkpatrick CJ (2002) Heterogeneous expression of cell adhesion molecules by endothelial cells in ARDS. J. Pathol 198, 270–275. [DOI] [PubMed] [Google Scholar]
- 67.Mulligan MS, Till GO, Smith CW, Anderson DC, Miyasaka M, Tamatani T, Todd RF III, Issekutz TB, Ward PA (1994) Role of leukocyte adhesion molecules in lung and dermal vascular injury after thermal trauma of skin. Am. J. Pathol 144, 1008–1015. [PMC free article] [PubMed] [Google Scholar]
- 68.Czermak BJ, Breckwoldt M, Ravage ZB, Huber-Lang M, Schmal H, Bless NM, Friedl HP, Ward PA (1999) Mechanisms of enhanced lung injury during sepsis. Am. J. Pathol 154, 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jin RB, Zhu PF, Wang ZG, Liu DW, Zhou JH (2003) Changes of pulmonary intercellular adhesion molecule-1 and CD11b/CD18 in peripheral polymorphonuclear neutrophils and their significance at the early stage of burns. Chin. J. Traumatol 6, 156–159. [PubMed] [Google Scholar]
- 70.Richter V, Rassoul F, Purschwitz K, Hentschel B, Reuter W, Kuntze T (2003) Circulating vascular cell adhesion molecules VCAM-1, ICAM-1, and E-selectin in dependence on aging. Gerontology 49, 293–300. [DOI] [PubMed] [Google Scholar]
- 71.Zou Y, Jung KJ, Kim JW, Yu BP, Chung HY (2004) Alteration of soluble adhesion molecules during aging and their modulation by calorie restriction. FASEB J. 18, 320–322. [DOI] [PubMed] [Google Scholar]
- 72.Laudes IJ, Guo RF, Riedemann NC, Speyer C, Craig R, Sarma JV, Ward PA (2004) Disturbed homeostasis of lung intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 during sepsis. Am. J. Pathol 164, 1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Forsey RJ, Thompson JM, Ernerudh J, Hurst TL, Strindhall J, Johansson B, Nilsson BO, Wikby A (2003) Plasma cytokine profiles in elderly humans. Mech. Ageing Dev 124, 487–493. [DOI] [PubMed] [Google Scholar]
- 74.Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S (2004) Innate immunity in aging: impact on macrophage function. Aging Cell 3, 161–167. [DOI] [PubMed] [Google Scholar]
- 75.Sebastian C, Espia M, Serra M, Celada A, Lloberas J (2005) MacrophAging: a cellular and molecular review. Immunobiology 210, 121–126. [DOI] [PubMed] [Google Scholar]