Abstract
Background
Patient Navigation (PN) originated in Harlem as an intervention to help poor women overcome access barriers to timely breast cancer treatment. Despite rapid, nationally widespread adoption of PN, empirical evidence on its effectiveness is lacking. In 2005, NCI initiated a multi-center PN Research Program (PNRP) to measure PN effectiveness for several cancers. The GW Cancer Institute, a project participant, established DC-PNRP to determine PN’s ability to reduce breast cancer diagnostic time (number of days from abnormal screening to definitive diagnosis).
Methods
2,601 women (1,047 navigated; 1,554 concurrent records-based non-navigated) were examined for breast cancer from 2006-2010 at nine hospitals/clinics in DC. Analyses included only women who reached complete diagnostic resolution. Differences in diagnostic time between navigation groups were tested with ANOVA models including categorical demographic and treatment variables. Log transformations normalized diagnostic time. Geometric means were estimated and compared using Tukey-Kramer p-value adjustments.
Results
Average—geometric mean (95% CI)—diagnostic time (days) was significantly shorter for navigated, 25.1 (21.7, 29.0), than non-navigated women, 42.1 (35.8, 49.6). Sub-analyses revealed significantly shorter average diagnostic time for biopsied navigated women, 26.6 (21.8, 32.5) than biopsied non-navigated women, 57.5 (46.3, 71.5). Among non-biopsied women, diagnostic time was shorter for navigated, 27.2 (22.8, 32.4), than non-navigated women, 34.9 (29.2, 41.7), but not statistically significant.
Conclusions
Navigated women, especially those requiring biopsy, reached their diagnostic resolution significantly faster than non-navigated women.
Impact
Results support previous findings of PN’s positive influence on health care. PN should be a reimbursable expense to assure continuation of PN programs.
Keywords: disparities, race, ethnicity, health insurance, breast cancer
INTRODUCTION
Following release of the first Patient Navigation (PN) program report (1), patient navigation efforts have been initiated in many health care facilities throughout the United States. Precise estimates of the actual impact of these programs have not been available; therefore, in 2005, the Center to Reduce Cancer Health Disparities (CRCHD) of the National Cancer Institute (NCI) initiated a multicenter study to compare the effectiveness of navigation in reducing the time to diagnosis and time to treatment for breast, prostate, colon and cervical cancer. The George Washington Cancer Institute (GWCI) focused on breast cancer in the District of Columbia (DC), as DC has one of the higher breast cancer mortality rates in the country (22.9/100,000 for non-Hispanic whites (NHW); 31.8/100,000 for non-Hispanic blacks (NHB)), a particularly challenging problem because of the large disparity between whites and blacks (2). Nationally, mortality rates ranged from 21.5-28.0 for whites and 19.9-38.0 for blacks (2).
While increased screenings and advances in treatment have had a significant impact on reducing mortality rates of black women living in DC from 49.8 (per 100,000) in 1995 to 31.8 (per 100,000) in 2007, disparities between population groups persisted (2). In 2007, mortality rates from breast cancer among white women remained markedly lower than those of their black counterparts despite the fact that incidence was higher in whites than blacks (140.4/100,000 versus 122.4/100,000, respectively) and the proportion of women over age 40 who had a mammogram in the past year was very similar (66% versus 63%, respectively) (2).
In 2001, a publicly funded safety-net insurance program for low-income DC residents was implemented and has helped to lower the number of uninsured (10.6% compared to 15.5% nationally); however, among the uninsured individuals, the burden falls disproportionately on blacks (18%) and Hispanics (31%) (3-4). Furthermore, in a baseline study of women in DC with abnormal breast findings, we found that having health insurance did not reduce the diagnostic delay in NHB; insured minorities waited >2 times longer to reach diagnostic resolution than their NHW counterparts (5).
This study investigates the effect of navigation by comparing the delay between initial identification of a breast abnormality and diagnostic resolution in a multi-center program with navigated patients and proportionally matched (age, race/ethnicity) records-based non-navigated patients who did not have access to patient navigation. We hypothesized that navigated patients would have shorter diagnostic times than patients without navigation. We examined whether navigation would have the same impact on different subgroups of patients according to race/ethnicity, age and insurance status.
METHODS
Study Design
We prospectively evaluated 2,601 women (1,047 navigated; 1,554 non-navigated) identified with a suspicious breast abnormality between 2006 and 2010 at nine hospitals/clinics in DC. Navigated women were examined at seven hospitals/clinics in DC, including Howard University Hospital, Providence Hospital, Washington Hospital Center’s (WHC) Center for Breast Health, WHC Preventorium, Capital Breast Care Center (CBCC), Nueva Vida, and Unity Health Care, Inc. At any of these recruitment intake sites (RIS), women identified with a suspicious breast finding were encouraged to enroll in the study provided they did not fit into one of the exclusion categories established by the PNRP: age under 18 years, institutionalized, cognitively impaired, currently pregnant, previously navigated for cancer, or prior cancer diagnosis within the past five years. Patients who agreed to enroll in the study were asked to sign a consent form. Several RIS received permission from their respective IRBs to use verbal consent over the telephone. All navigated patients in this study signed informed consent documents approved by one or more IRBs.
Non-navigated women were selected randomly and retrospectively at the George Washington University (GW) Hospital, Howard University Hospital, Providence Hospital, WHC’s Center for Breast Health, Unity Health Care, Inc., and the GW Mammovan (an outreach program focusing on underserved women in DC). The Mammovan served as a source of non-navigated women for the WHC Preventorium, CBCC, and Nueva Vida—sites that had patient navigation since their inception. A waiver was received from the GW IRB to eliminate the consent process for non-navigated women since they were all records-based using de-identified data.
Some sites supplied both navigated and non-navigated subjects, but CBCC, Nueva Vida, and WHC Preventorium had been established as navigation centers from their inception and insisted on navigating all women identified with a suspicious finding. Since CBCC has a predominantly African-American population and the other two sites focused on Latinas, we were able to choose fairly accurate race and ethnicity-matched control sites, particularly sites visited by the mobile mammography program, for these specific populations.
All non-navigated women had a suspicious breast abnormality, but did not receive the study intervention, patient navigation, because of the non-availability of navigation at the time. Non-navigated women concurrently matched as closely as possible the demographics of navigated women and the presence or absence of confirmed malignancy. Non-navigated patients were selected using frequency matching on the characteristics of race/ethnicity, time of first detection of abnormality, and diagnosis. Non-navigated women were identified over the same 5-year period (2006-2010) as navigated patients. Every effort was made to include only patients who never received patient navigation in the non-navigated group; however, some non-navigated women may have had access to navigation but it was never documented in their medical records.
The majority of suspicious findings were detected in 2007 (22%), 2008 (38%) and 2009 (32%), with fewer women examined in 2006 (5%) and 2010 (3%).
Our specific study design, “network, navigation,” including identification of relevant patients, enrollment procedures, and training methods were described in detail in a prior publication (6). During the study, 26 navigators were employed and six of these women had previously worked as navigators for several years. An additional 9 women had worked closely with patients as nurses, case managers, or community health workers. Our navigators received training in navigation and data collection through DC-PNRP. All navigators in the nationwide PNRPs, including our navigators, also received training at several ACS-sponsored conferences. GWCI provided additional navigator training through its Center for the Advancement of Cancer Survivorship, Navigation, and Policy.
Definition of Outcome and Covariates
The goal of this study was to identify the effect of patient navigation on diagnostic time (defined as the number of days from suspicious finding to diagnostic resolution) among those women who had a diagnostic resolution. This study included only women who reached diagnostic resolution and had a known diagnostic time between 0 and 365 days. The data for non-navigated women were obtained by retrospective record abstraction; therefore, dates of suspicious finding or diagnostic resolution were sometimes missing. These incomplete medical records for some non-navigated women made it impossible to know whether they achieved diagnostic resolution. Therefore, to make our comparison groups equivalent, we included only navigated and non-navigated women who had reached diagnostic resolution in all analyses. Including only non-navigated women who achieved diagnostic resolutions eliminated the potential for any bias from using non-navigated women with incomplete information. The diagnostic resolutions were primarily either (i) no evidence of malignancy on diagnostic mammogram or (ii) definitive diagnosis by biopsy (benign or malignant).
Suspicious finding was defined as any breast abnormality identified by a clinician during the physical exam, mammography, or ultrasound/MRI. Diagnostic resolution represents the definitive diagnosis for that patient, i.e., the result obtained after diagnostic studies were completed in order to resolve a suspicious finding. The primary dependent variable of interest was diagnostic time, reported as a continuous variable in days. The primary independent variable of interest was navigation group (navigated, non-navigated). Covariates included race/ethnicity (NHW, NHB, Hispanic), type of insurance coverage (private, government, none), age (<40, 40-49, 50-59, ≥60), and biopsy as the definitive test (yes, no). Government insurance included federal (Medicaid and Medicare), state, and local government health insurance programs, including the DC government safety-net insurance “Alliance”. If a woman had both private and government insurance, she was assigned to the private insurance group. Additional race/ethnicity groups were considered in preliminary analyses as an “other” category but removed from the final analysis as they were too diverse and too small when examining interactions to provide reliable estimates, e.g., Asian (n=48), Native Hawaiian/Pacific Islander (n=5), American Indian/Alaska Native (n=7), and multi-racial (n=17).
Data Abstraction for Non-Navigated Patients
All non-navigated patient data were abstracted from medical records and de-identified before being entered into a central database. All abstracted records were reviewed by a physician with more than 40 years of experience in oncology and pathology. If there were any potential inconsistencies or irregularities, the physician reviewed the original medical record. The exclusion criteria were identical to the criteria used for the navigated patients. Data on marital status, employment, education, income, and primary language— generally obtained via interviews with navigated patients—were not usually provided in the medical records. Consequently, these data were missing for non-navigated women.
For 427 women seen at the GW Mammovan, race and ethnicity were imputed based on the known demographics of the screening sites, which were predominantly African-American, Hispanic, and Asian churches known to be homogeneous with regard to race and ethnicity. Therefore, the imputed values were selected with a high degree of confidence based on the specific site location of the GW Mammovan on the date of initial abnormal screening. Imputation was applied only for those women seen at the GWU Mammovan.
Statistical Methods
ANOVA models were used to examine the relationships both individually (two-way) and collectively (multi-way) between diagnostic time and the aforementioned categorical independent variables: navigation group, race/ethnicity, type of health insurance, age, and biopsy as the definitive test. Study site was included as a fixed effect in all models to control for the different purposively selected sites from where the subjects were obtained; and so, two-way as opposed to one-way ANOVA was used for the individual analyses. Two-way interactions between navigation group and each of the categorical variables (i.e., race/ethnicity, type of health insurance, age, and biopsy) were considered in the multivariable analysis. Only those interactions significant at the 0.05 level were included in the final multivariable models. Log transformations were taken on the dependent variable, diagnostic time, to satisfy the model assumption of normality. The average diagnostic time for women having a same day diagnosis was estimated to be approximately 2.5 hours. Therefore, diagnostic times of 0 were replaced with 0.1 in transforming the data. Residual plots showed the log-transformed data satisfied assumptions of normality and homogeneous variance. The Tukey-Kramer method was used for p-value adjustment in performing multiple comparisons.
The Centers for Disease Control and Prevention (CDC) recommended—as part of their National Breast and Cervical Cancer Early Detection Program (NBCCEDP)—that abnormal screens should reach diagnostic confirmation within 60 days (7). The odds of having a diagnostic delay >60 days for the navigation groups were examined by categorizing diagnostic time into two groups (<60 and >60 days) and fitting multiple logistic regression models that included study site as a fixed effect. For comparison, additional models were fit to the data using a cutoff of 30 days—one-half of the number of days recommended by CDC (7).
All statistical tests were two-sided and the level of significance was set at 0.05. All statistical analyses were performed using SAS® software, Version 9.2 (SAS Institute, Cary, NC).
RESULTS
The sample in this study consisted of 2,601 women (1,047 navigated; 1,554 non-navigated) ranging in age from 18 to 98 years (navigated patient median=49; non-navigated patient median=51). Excluded from all analyses were 36 women (18 navigated, 18 non-navigated) missing an abnormal screening date, 149 women (112 navigated, 37 non-navigated) missing a definitive diagnosis date, and 78 women (53 navigated, 25 non-navigated) with diagnostic times exceeding 365 days (most likely women who traveled out of our network for diagnosis). PNRP made the decision to censor anyone with a diagnostic time exceeding 365 days at one year. We conducted a sensitivity analysis including and excluding these women and did not see dramatic differences in the results.
Descriptive statistics for the remaining 2,338 women with valid diagnostic times are provided in Table 1. Among navigated women, 29% (n=17) of NHW and 12% (n=54) of NHB were known to be uninsured, whereas 78% (n=206) of Hispanics were known to be uninsured. Similarly, among non-navigated women, 67% (n=259) of Hispanics were known to be uninsured, while only 6% (n=8) of NHWs and 8% (n=52) of NHBs were known to be uninsured.
Table 1.
Patient characteristics
| Navigated N=864 (37.0%) |
Non-navigated N=1474 (63.0%) |
Total N=2338 (100%) |
|
|---|---|---|---|
|
| |||
| Variable | N (%) | N (%) | N (%) |
| Race/Ethnicity | |||
| Non-Hispanic White | 58 (6.7) | 130 (8.8) | 188 (8.0) |
| Non-Hispanic Black | 466 (53.9) | 663 (45.0) | 1129 (48.3) |
| Hispanic | 265 (30.7) | 386 (26.2) | 651 (27.8) |
| Other | 12 (1.4) | 65 (4.4) | 77 (3.3) |
| Unknown | 63 (7.3) | 230 (15.6) | 293 (12.5) |
| Type of Insurance | |||
| Private | 328 (38.0) | 624 (42.3) | 952 (40.7) |
| Government | 170 (19.7) | 340 (23.1) | 510 (21.8) |
| None | 318 (36.8) | 407 (27.6) | 725 (31.0) |
| Unknown | 48 (5.6) | 103 (7.0) | 151 (6.5) |
| Age Group | |||
| <40 | 177 (20.5) | 133 (9.0) | 310 (13.3) |
| 40-49 | 280 (32.4) | 566 (38.4) | 846 (36.2) |
| 50-59 | 210 (24.3) | 407 (27.6) | 617 (26.4) |
| ≥60 | 189 (21.9) | 361 (24.5) | 550 (23.5) |
| Unknown | 8 (0.9) | 7 (0.5) | 15 (0.6) |
| Biopsy | |||
| Yes | 283 (32.8) | 546 (37.0) | 829 (35.5) |
| No | 488 (56.5) | 926 (62.8) | 1414 (60.5) |
| Unknown | 93 (10.8) | 2 (0.1) | 95 (4.1) |
Summary statistics for diagnostic time are provided in Supplementary Table S1. Histograms and QQ plots revealed that diagnostic time was positively skewed. Log-transformations were taken to normalize the data, and the transformed variable was used in our ANOVA models.
Navigated women had a significantly shorter average diagnostic time, 30.1 days (27.2, 33.3), than non-navigated women, 38.8 days (35.9, 41.9) (p<0.0001). A more dramatic difference in average diagnostic times was estimated between navigated and non-navigated women when controlling for study site (29.8 days (26.3, 33.7) versus 51.0 days (44.8, 58.1), respectively; p<0.0001). Results for all two-way ANOVA models are summarized in Table 2, along with results for the multi-way ANOVA model. Navigated women had a significantly shorter adjusted average diagnostic time, 25.1 days (21.7, 29.0), than non-navigated women, 42.1 days (35.8, 49.6), after controlling for race/ethnicity, type of insurance, age, and study site (p<0.0001).
Table 2.
Two-way and multi-way ANOVA of the time from abnormal screening result to diagnostic resolution among patients receiving and not receiving patient navigation
| Two-way ANOVAa (N=2338) |
Multi-way ANOVAb (N=1842) |
|||
|---|---|---|---|---|
|
| ||||
| Variable | N | Geometric Mean (95% CI) |
N | Geometric Mean (95% CI) |
| Navigation Group | 2338 | --- | 1842 | --- |
| Navigated | 864 | 29.8 (26.3, 33.7) |
743 | 25.1 (21.7, 29.0) |
| Non-navigated | 1474 | 51.0 (44.8, 58.1) |
1099 | 42.1 (35.8, 49.6) |
| Race/Ethnicity | 1968 | --- | 1842 | --- |
| Non-Hispanic White | 188 | 19.4 (15.3, 24.6) |
184 | 20.3 (15.9, 25.9) |
| Non-Hispanic Black | 1129 | 40.2 (35.3, 45.7) |
1042 | 38.8 (33.8, 44.5) |
| Hispanic | 651 | 44.2 (37.4, 52.2) |
616 | 43.7 (36.3, 52.6) |
| Type of Insurance | 2187 | --- | 1842 | --- |
| Private | 952 | 33.9 (29.5, 38.9) |
780 | 30.9 (26.2, 36.4) |
| Government | 510 | 40.1 (34.3, 46.9) |
466 | 34.7 (29.0, 41.5) |
| None | 725 | 40.1 (34.6, 46.4) |
596 | 32.1 (26.8, 38.4) |
| Age Group | 2323 | --- | 1842 | --- |
| <40 | 310 | 26.3 (22.0, 31.4) |
220 | 25.0 (20.1, 31.1) |
| 40-49 | 846 | 43.8 (38.8, 49.5) |
655 | 37.4 (32.2, 43.3) |
| 50-59 | 617 | 39.8 (34.8, 45.6) |
494 | 37.2 (31.7, 43.8) |
| ≥60 | 550 | 38.1 (33.0, 44.1) |
473 | 32.1 (27.1, 38.0) |
Each two-way ANOVA model includes one variable listed and study site as fixed-effects.
The multi-way ANOVA model includes all variables listed and study site as fixed-effects.
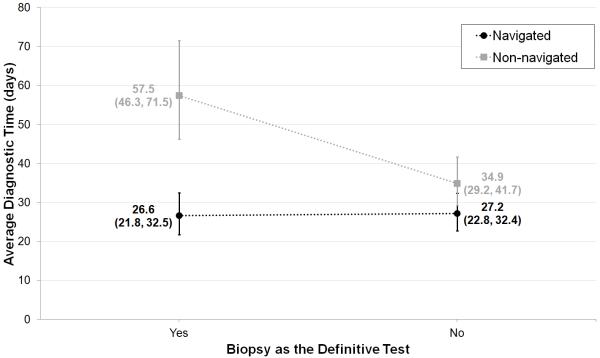
In addition to this major finding, a subset analysis was conducted on 1,760 women (662 navigated; 1098 non-navigated) for whom type of definitive test was recorded to examine the effect of having a biopsy as the definitive test on diagnostic time. In the two-way ANOVA model (with study site), average diagnostic time was significantly shorter for women who did not have a biopsy, 36.8 days (33.0, 41.0), than women who did have a biopsy as the definitive test, 44.3 days (38.9, 50.5) (p=0.015). Adding this variable to our multi-way ANOVA model revealed a significant interaction between group and biopsy as the definitive test (p=0.0011). Applying the Tukey-Kramer method for p-value adjustment in performing multiple comparisons, adjusted average diagnostic times were significantly shorter for biopsied navigated women, 26.6 days (21.8, 32.5) than for biopsied non-navigated women, 57.5 days (46.3, 71.5) (p<0.0001). Among non-biopsied women, diagnostic time was shorter for navigated, 27.2 days (22.8, 32.4), than non-navigated, 34.9 days (29.2, 41.7), but the difference was not statistically significant (p=0.15). Results are summarized in Figure 1.
Figure 1.
Interaction plot of the average—geometric means (95% confidence intervals)—diagnostic times (in days) estimated from multi-way ANOVA
Among identified cancer patients, 93% (n=416) had a biopsy and 7% (n=30) had fine-needle aspiration, while 19% (n=244) of non-cancer patients had a biopsy. Navigated women with cancer had a significantly shorter average diagnostic time, 9.8 days (7.5, 12.8), than non-navigated women with cancer, 39.9 days (28.0, 57.0) (p<0.0001). Navigated women without cancer had a non-significantly shorter average diagnostic time, 32.5 days (27.5, 38.4), than non-navigated women without cancer, 37.4 days (30.9, 45.2) (p=0.61). While there was no significant difference in the average diagnostic time between non-navigated women with and without cancer (p=0.99), navigated women identified with cancer had a significantly shorter average diagnostic time than navigated women without cancer (p<0.0001).
Among patients whose diagnosis was resolved within one year, 42% and 63% of navigated women were respectively diagnosed within 30 and 60 days, and 30% and 63% of non-navigated women were respectively diagnosed within 30 and 60 days. The odds of having a diagnostic delay >60 days were examined in a multiple logistic regression model with the independent variables: navigation group, race/ethnicity, type of insurance, age, and study site (Table 3). Among patients whose diagnosis was resolved within one year, the odds of having a diagnostic time >60 days for navigated women were not significantly different from non-navigated women in simple (OR=0.98, 95% CI: 0.76, 1.27) or multiple (OR=0.96, 95% CI: 0.72, 1.30) logistic regression models.
Table 3.
Simple and multiple logistic regression of the time from abnormal screening result to diagnostic resolution among patients receiving and not receiving patient navigation
| 30-day cutoff | 60-day cutoff | |||
|---|---|---|---|---|
|
| ||||
| Variable | Simplea (n=2338) OR (95% CI) |
Multipleb (n=1842) OR (95% CI) |
Simplea (n=2338) OR (95% CI) |
Multipleb (n=1842) OR (95% CI) |
| Navigation Group | --- | --- | --- | --- |
| Navigated | REF | --- | REF | REF |
| Non-navigated | 1.78 (1.39, 2.29) | --- | 0.98 (0.76, 1.27) | 0.96 (0.72, 1.30) |
| Race/Ethnicity | --- | --- | --- | --- |
| Non-Hispanic White | REF | REF | REF | REF |
| Non-Hispanic Black | 2.52 (1.76, 3.60) | 2.30 (1.59, 3.32) | 1.59(1.06, 2.40) | 1.47 (0.97, 2.24) |
| Hispanic | 4.39 (2.73, 7.06) | 3.41 (2.04, 5.69) | 1.88 (1.19, 2.98) | 1.73 (1.06, 2.83) |
| Type of Insurance | --- | --- | --- | --- |
| Private | REF | --- | REF | REF |
| Government | 1.48 (1.16, 1.88) | --- | 1.25 (0.98, 1.60) | 1.17 (0.89, 1.52) |
| None | 1.73 (1.23, 2.43) | --- | 1.65 (1.25, 2.18) | 1.34 (0.95, 1.89) |
| Age Group | --- | --- | --- | --- |
| <40 | REF | REF | REF | REF |
| 40-49 | 1.96 (1.44, 2.66) | 1.56(1.08, 2.26) | 1.61 (1.19, 2.16) | 1.45 (1.01, 2.06) |
| 50-59 | 1.56 (1.13, 2.14) | 1.42 (0.97, 2.08) | 1.46 (1.07, 2.00) | 1.43 (0.99, 2.07) |
| ≥60 | 1.59 (1.15, 2.20) | 1.37 (0.93, 2.01) | 1.16(0.83, 1.61) | 1.09 (0.74, 1.60) |
| Group*Insurance | --- | --- | --- | --- |
| Private | ||||
| Non-navigated vs Navigated | NA | 1.65 (1.17, 2.33) | NA | NA |
| Government | ||||
| Non-navigated vs Navigated | NA | 1.25 (0.81, 1.94) | NA | NA |
| None | ||||
| Non-navigated vs Navigated | NA | 5.41 (2.32, 12.61) | NA | NA |
Each simple logistic regression model includes one variable listed and study site as fixed-effects.
The multiple logistic regression model includes all variables listed and study site as fixed-effects.
Using a 30-day cutoff, the multiple logistic regression model with the aforementioned covariates also included a significant interaction between navigation group and type of insurance (p=0.0056). The odds of having a diagnostic time >30 days for uninsured, non-navigated women were >5 times the odds for uninsured navigated women (p<0.0001). The odds of having a diagnostic time >30 days for privately insured non-navigated women were 1.7 times the odds for privately insured navigated women (p=0.0042). The odds of having a diagnostic time >30 days for government insured non-navigated women were 1.3 times the odds for government insured navigated women, but not significant (p=0.32).
DISCUSSION
This study showed navigation was successful in reducing the time from abnormal screening to diagnostic resolution among NHW, NHB, and Hispanic women of all ages, especially for women who had a biopsy as the definitive test. A nearly 4-fold reduction in time to diagnostic resolution was seen when comparing navigated to non-navigated women who resolved with cancer.
Navigation significantly reduced the odds of having diagnostic delays for both uninsured and privately insured women, with a similar non-significant trend seen for women with government insurance. Navigation was most effective in reducing diagnostic times within the first 60 days following abnormal screening.
If we assume that women excluded from the analysis due to missing definitive diagnosis dates never received a definitive diagnosis or took a longer time to obtain a definitive diagnosis, then the disproportionate number of missing diagnosis dates—112 navigated versus 37 non-navigated—may have biased the results towards seeing a positive effect of navigation. Most likely, however, the majority of these women received a definitive diagnosis outside of the DC-PNRP network so the dates were just not captured in the study dataset. This can be inferred from internal business-based, health care systems-related analyses of distribution of cancer care in the DC area.
For both navigated and non-navigated women, diagnostic time differed significantly based on the type of exam used to determine eligibility for the study. Specifically, women with an abnormal screening mammogram waited >2 times longer to reach diagnostic resolution as women with an abnormal clinical breast exam or an abnormal ultrasound/MRI (data not shown).
We recently published an analysis examining only non-navigated women from DC PNRP (n=1538) (5). We found insured minorities waited >2 times longer to reach diagnostic resolution than insured NHWs (5). Having private health insurance did increase the speed of diagnosis in NHBs but privately insured Hispanics had the longest time to diagnosis of any of our race/ethnicity groups: 51 days. While private insurance helped reduce diagnostic times, number of days until diagnosis remained significantly longer for minorities than for privately insured NHWs suggesting diagnostic delays in minorities are more likely caused by other barriers associated with race/ethnicity than by insurance status. This makes the results obtained when comparing our navigated and non-navigated patients much more compelling in terms of the benefits of navigation.
A number of studies found results similar to ours in terms of the effectiveness of patient navigation versus usual care using different outcome variables. One study used a design similar to ours in terms of their focus on urban minority women, but examined delay in follow-up after an abnormal mammogram by randomly assigning patients with similar demographics to usual care (n=50) or usual care plus patient navigation (n=55) (8). Navigated women had shorter times to diagnostic resolution than non-navigated women (25.0 days versus 42.7 days, p=0.001). At 60 days, 22% of non-navigated women still did not have a diagnosis versus 6% in the navigated group. Navigated women also had significantly lower anxiety scores and higher satisfaction scores than non-navigated women. The authors concluded that navigation is effective in improving time to diagnostic resolution, decreasing anxiety, and increasing patient satisfaction (8). Another study found that navigation reduced time between diagnostic biopsy to first consultation with a specialist from 14.6 days to 12.8 days and time between diagnostic biopsy to first treatment from 30 days to 26.2 days (9). The majority of patient barriers (71%) were resolved by the time treatment was initiated (9).
While we did not assess patient satisfaction or perspectives in our study, a number of other studies examined patients’ feelings and opinions as outcome variables and they also found positive results for PN. In comparing 72 navigated patients and 181 non-navigated patients referred to a hospital due to an abnormal mammogram, navigated patients were significantly more likely to definitely understand what to expect at their visit (79% vs 60%), to receive a reminder letter or phone call, and to feel welcome (10). Assessing patient perspectives of patient navigation is needed to evaluate navigation programs. An analysis of navigation effectiveness for low-income African Americans noted that barriers to diagnostic follow-up or treatment were identified by the navigators and this helped the navigators to guide patients through the healthcare system as evidenced by the fact that 93% of patients kept their appointments (11). Among 176 Korean women randomly assigned to usual care or patient navigation, self-reported completion of follow-up procedures was 97% for navigated and 67% for non-navigated (p<0.001) providing more evidence for the effectiveness of navigation in another minority group, Asians (12). These studies provide support for PN in general, along with our study results.
Several other studies found patient navigation to be a significantly effective intervention in patient care using different study designs and outcome variables (13-21); however, two studies did not find that patient navigation was any better than usual care (22-23). A review of the results to-date on navigation effectiveness found that most studies had significant limitations including no control group, small sample size, and contamination with other interventions (24).
The DC site is unique compared to all other national PNRP sites in its “network navigation” approach. Navigators from a city-wide partnership of unaffiliated clinical and community sites were trained to work collaboratively with open communication across sites, within a city-wide network to enroll patients in the study and assure each patient received timely, quality care (6). Three techniques helped enhance care coordination and assure appropriate referral strategies between sites: 1) conducting frequent staff trainings, 2) promoting increased communication between navigators, and 3) sharing information about community resources.
We instituted a longitudinal navigation program that follows the patient from outreach through survivorship (6). In a review paper discussing navigation generally, our program was perfectly defined: “patient navigation is a system, as opposed to a person, comprised primarily of navigators and directors that work together to remove barriers and facilitate access in a well-defined course of care” (25).
A strength of our study is the sizeable sample in both the navigated and non-navigated groups representative of all demographics in the DC metropolitan area. Larger sample sizes help to increase statistical power and add to the validity of the findings. In addition, our subjects were identified at a variety of facilities including major medical centers, a storefront mammography unit, a clinic geared specifically to Hispanics, a non-clinical community site focused on assisting Hispanic women with cancer and obtaining cancer screening, and clinics specifically available to the poor. This helped to assure a representative sample of the underserved—the population of greatest concern in terms of cancer morbidity and mortality.
A limitation of this study is that it was based on a sample selected from a single metropolitan area that was not probabilistic, i.e., random selection was not utilized. In addition, all our patients had reached diagnostic resolution; hence, there was no opportunity to assess differences in diagnostic resolution rates between navigated and non-navigated women. Also, race and ethnicity had to be imputed for 427 non-navigated women seen by the GWU Mammovan. As stated previously, the imputed values were selected with a high degree of confidence based on specific locations of the GWU Mammovan on the date of initial abnormal screening, which were primarily African-American, Hispanic, and Asian churches.
In addition, insurance status may have changed for some patients during the interval being assessed. Also, medical records were relied on to assess variables and outcomes for non-navigated patients rather than self-reports, though some studies found medical records provided similar results as self-reports, administrative records, and health department records (26-29). Since we used only one chart reviewer for all non-navigated patients, inter-rater reliability, an important assessment of the quality of data abstraction, could not be calculated.
Despite these limitations, this study clearly shows navigation is effective in decreasing time to diagnostic resolution particularly among women who have a biopsy and have a diagnostic resolution of cancer. PN should be implemented as a means to reduce diagnostic times. We are currently working on an analysis of the barriers faced by subjects in our study. A few other researchers have examined this issue (30-32) with varying results. Analyses of the national PNRP data are currently being conducted, including further analysis of navigation’s cost-effectiveness. These results will determine whether patient navigation will be viewed positively by national health policy makers and will become a usual part of the cancer care process with insurance reimbursement for patient navigation.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank all DC-PNRP Patient Navigators who worked so hard to serve our patients and enroll them in this study. We also thank Hong Nguyen (GWU Hospital Cancer Registrar) for her valuable help collecting data for the non-navigated group.
GRANT SUPPORT
Grant Number 1 U01 CA116937 to Steven R. Patierno; Patient Navigation Research Program (PNRP), Center for Research on Cancer Health Disparities (CRCHD), National Cancer Institute (NCI).
Footnotes
CONFLICTS OF INTEREST/DISCLOSURES: None
REFERENCES
- 1.Freeman HP, Muth BJ, Kerner JF. Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Pract. 1995;3(1):19–30. [PubMed] [Google Scholar]
- 2.DeSantis C, Siegel R, Bandi P, Jemal A. Breast Cancer Statistics, 2011. CA-A Cancer Journal for Clinicians. 2011;61:409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 3.DeNavas-Walt C, Proctor BD, Smith JC. Income, Poverty, and Health Insurance Coverage in the United States, 2007. Current Population Reports, P60-235, U.S. Census Bureau, U.S. Government Printing Office; Washington, DC: 2008. [Google Scholar]
- 4.Turner J, Boudreaux M, Lynch V. A preliminary evaluation of Elath insurance coverage in the 2008 American Community Survey. Final Report. U.S. Census Bureau; Washington, DC: 2009. [Google Scholar]
- 5.Hoffman HJ, LaVerda NL, Levine PH, Young HA, Alexander LM, Patierno SR, the DC Citywide Patient Navigator Research Group Having health insurance does not eliminate race/ethnicity-associated delays in breast cancer diagnosis in the District of Columbia. Cancer. 2011;117:3824–3832. doi: 10.1002/cncr.25970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patierno SR, LaVerda NL, Alexander LM, Levine PH, Young HA, Hoffman HJ. Oncology Issues March/April. 2010. Longitudinal Network Navigation: Development of a city-wide integrative model to reduce breast cancer disparities in Washington, DC; pp. 28–35. [Google Scholar]
- 7.Holden DJ. Final Report. RTI Project Number 0208235.033. Research Triangle Institute; Research Triangle Park, NC: 2006. Strategic management and evaluation plan of the National Breast and Cervical Cancer Early Detection Program. [Google Scholar]
- 8.Ferrante JM, Chen PH, Kim S. The effect of patient navigation on time to diagnosis, anxiety, and satisfaction in urban minority women with abnormal mammograms: a randomized controlled trial. J Urban Health. 2008;85(1):114–24. doi: 10.1007/s11524-007-9228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koh C, Nelson JM, Cook PF. Evaluation of a patient navigation program. Clin J Oncol Nurs. 2011;15(1):41–8. doi: 10.1188/11.CJON.41-48. [DOI] [PubMed] [Google Scholar]
- 10.Donelan K, Mailhot JR, Dutwin D, Barnicle K, Oo SA, Hobrecker K, et al. Patient perspectives of clinical care and patient navigation in follow-up of abnormal mammography. J Gen Intern Med. 2011;26(2):116–22. doi: 10.1007/s11606-010-1436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fouad M, Wynn T, Martin M, Partridge E. Patient navigation pilot project: results from the Community Health Advisors in Action Program (CHAAP) Ethn Dis. 2010;20(2):155–61. [PubMed] [Google Scholar]
- 12.Maxwell AE, Jo AM, Crespi CM, Sudan M, Bastani R. Peer navigation improves diagnostic follow-up after breast cancer screening among Korean American women: results of a randomized trial. Cancer Causes Control. 2010;21(11):1931–40. doi: 10.1007/s10552-010-9621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mason TA, Thompson WW, Allen D, Rogers D, Gabram-Mendola S, Jacob Arriola KR. Evaluation of the Avon Foundation Community Education and Outreach Initiative Community Patient Navigation Program. Health Promot Pract. 2011 doi: 10.1177/1524839911404229. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Schlueter DF, Thompson WW, Mason TA, Rayton M, Arriola KJ. A qualitative evaluation of the Avon Foundation Community Education and Outreach Initiative Patient Navigation Program. J Cancer Educ. 2010;25(4):571–6. doi: 10.1007/s13187-010-0073-2. [DOI] [PubMed] [Google Scholar]
- 15.Clark CR, Baril N, Kunicki M, Johnson N, Soukup J, Ferguson K, et al. Addressing social determinants of health to improve access to early breast cancer detection: results of the Boston REACH 2010 Breast and Cervical Cancer Coalition Women’s Health Demonstration Project. J Womens Health (Larchmt) 2009;18(5):677–90. doi: 10.1089/jwh.2008.0972. [DOI] [PubMed] [Google Scholar]
- 16.Gabram SG, Lund MJ, Gardner J, Hatchett N, Bumpers HL, Okoli J, et al. Effects of an outreach and internal navigation program on breast cancer diagnosis in an urban cancer center with a large African-American population. Cancer. 2008;113(3):602–7. doi: 10.1002/cncr.23568. [DOI] [PubMed] [Google Scholar]
- 17.Han HR, Lee H, Kim MT, Kim KB. Tailored lay health worker intervention improves breast cancer screening outcomes in non-adherent Korean-American women. Health Educ Res. 2009;24(2):318–29. doi: 10.1093/her/cyn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ell K, Padgett D, Vourlekis B, Nissly J, Pineda D, Sarabia O, et al. Abnormal mammogram follow-up: a pilot study women with low income. Cancer Pract. 2002 May-Jun;10(3):130–8. doi: 10.1046/j.1523-5394.2002.103009.x. [DOI] [PubMed] [Google Scholar]
- 19.Ell K, Vourlekis B, Lee PJ, Xie B. Patient navigation and case management following an abnormal mammogram: a randomized clinical trial. Prev Med. 2007;44(1):26–33. doi: 10.1016/j.ypmed.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Ell K, Vourlekis B, Xie B, Nedjat-Haiem FR, Lee PJ, Muderspach L, et al. Cancer treatment adherence among low-income women with breast or gynecologic cancer: a randomized controlled trial of patient navigation. Cancer. 2009;115(19):4606–15. doi: 10.1002/cncr.24500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Psooy BJ, Schreuer D, Borgaonkar J, Caines JS. Patient navigation: improving timeliness in the diagnosis of breast abnormalities. Can Assoc Radiol J. 2004;55(3):145–50. [PubMed] [Google Scholar]
- 22.Haideri NA, Moormeier JA. Impact of patient navigation from diagnosis to treatment in an urban safety net breast cancer population. J Cancer. 2011;2:467–73. doi: 10.7150/jca.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bastani R, Mojica CM, Berman BA, Ganz PA. Low-income women with abnormal breast findings: results of a randomized trial to increase rates of diagnostic resolution. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1927–36. doi: 10.1158/1055-9965.EPI-09-0481. [DOI] [PubMed] [Google Scholar]
- 24.Wells KJ, Battaglia TA, Dudley DJ, Garcia R, Greene A, Calhoun E, et al. Patient navigation: state of the art or is it science? Cancer. 2008;113(8):1999–2010. doi: 10.1002/cncr.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vargas RB, Ryan GW, Jackson CA, Rodriguez R, Freeman HP. Characteristics of the original patient navigation programs to reduce disparities in the diagnosis and treatment of breast cancer. Cancer. 2008;113(2):426–33. doi: 10.1002/cncr.23547. [DOI] [PubMed] [Google Scholar]
- 26.Niccolai LM, Kershaw TS, Lewis JB, Cicchetti DV, Ethier KA, Ickovics JR. Data collection for sexually transmitted disease diagnoses: a comparison of medical record reviews and state health department records. Ann Epidemiol. 2005;15:236–242. doi: 10.1016/j.annepidem.2004.07.093. [DOI] [PubMed] [Google Scholar]
- 27.Gupta V, Gu K, Chen Z, Lu W, Shu XO, Zheng Y. Concordance of self-reported and medical chart information on cancer diagnosis and treatment. BMC Med Res Methodol. 2011;11:72. doi: 10.1186/1471-2288-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Diamant AL, Thind A, Maly RC. Validity of self-reports of breast cancer treatment in low-income, medically underserved women with breast cancer. Breast Cancer Res Treat. 2010;119:745–751. doi: 10.1007/s10549-009-0447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.To T, Dell S, Dick PT, Cicutto L, Harris JK, MacLusky IB, Tassoudji M. Case verification of children with asthma in Ontario. Pediatr Allergy Immunol. 2006;17:69–76. doi: 10.1111/j.1399-3038.2005.00346.x. [DOI] [PubMed] [Google Scholar]
- 30.Hendren S, Chin N, Fisher S, Winters P, Griggs J, Mohile S, Fiscella K. Patients’ barriers to receipt of cancer care, and factors associated with needing more assistance from a patient navigator. J Natl Med Assoc. 2011;103(8):701–10. doi: 10.1016/s0027-9684(15)30409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korber SF, Padula C, Gray J, Powell M. A breast navigator program: barriers, enhancers, and nursing interventions. Oncol Nurs Forum. 2011;38(1):44–50. doi: 10.1188/11.ONF.44-50. [DOI] [PubMed] [Google Scholar]
- 32.Davis C, Darby K, Likes W, Bell J. Social workers as patient navigators for breast cancer survivors: what do African-American medically underserved women think of this idea? Soc Work Health Care. 2009;48(6):561–78. doi: 10.1080/00981380902765212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.