We report a response to fourth-line nivolumab in a patient with metastatic clear cell renal cell carcinoma (RCC) on dialysis. A 77-yr-old man with hypertension, congestive heart failure, diabetes, and chronic kidney disease under-went partial right nephrectomy in February 2011 for an 8-cm, grade 4 clear cell RCC with extensive necrosis. For local recurrence, a completion right nephrectomy was performed in January 2012. Nevertheless, by October 2012, he developed lung and peritoneal metastases.
Shortly after beginning treatment with pazopanib in November 2012, his kidney disease progressed to end-stage renal disease (ESRD), requiring dialysis. He achieved an initial response to pazopanib followed by slow progression, with treatment ultimately discontinued in October 2014 after he developed acute hepatic injury, thrombocytopenia, and systolic dysfunction. Following recovery, he received everolimus with poor tolerability and then received sorafenib. However, he quickly experienced marked progression in the peritoneum, liver, lungs, and pleura with decline in Karnofsky performance status to 60%.
With rapid progression and concerns about the effectiveness and tolerability of standard options, treatment with fourth-line nivolumab was initiated in June 2015. Two weeks later, the patient developed hypercarbic respiratory failure requiring intubation. Computed tomography (CT) of the chest showed further progression in the lungs and pleura without ground glass opacity. His respiratory failure was considered multifactorial, related to pneumonia, heart failure, and right diaphragmatic paralysis from pleural tumor deposits. He improved with antibiotics and diuresis, was extubated after 2 d, and was discharged 12 d later.
Subsequently, his respiratory symptoms and Karnofsky performance status rapidly improved. Repeated chest CT at 35 d after the single nivolumab dose showed significant disease regression. Treatment with nivolumab was continued, and imaging after five doses demonstrated further improvement (Fig. 1). He continues to benefit from nivolumab, now 8 mo from treatment initiation with no further respiratory complications.
Fig. 1.
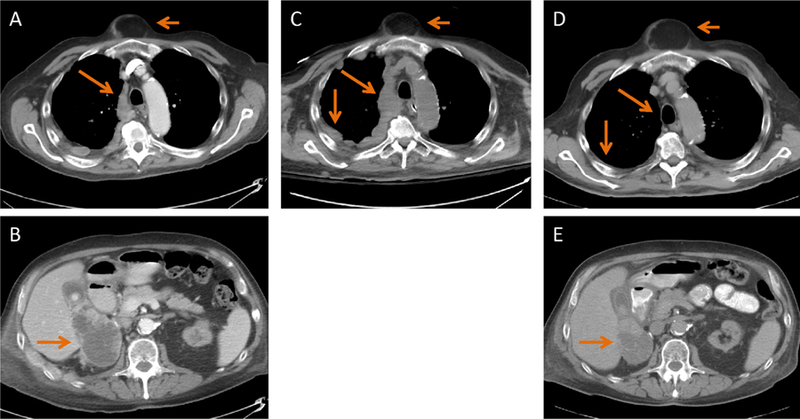
Computed tomography (CT) images (a, b) before and (c) 15 d after one dose of nivolumab show an increase in pleural thickening. (d, e) CT images after five doses of nivolumab show near resolution of pleural thickening (long arrows in top panels) as well as a significant decrease in the size of the nephrectomy fossa mass (long arrows in the bottom panels). Figures A, C, and D show an incidental sternal lipoma (short arrows).
To our knowledge, there are no published data on the safety and efficacy of programmed cell death 1 (PD-1) inhibitors in patients on dialysis. Although our patient experienced hypercarbic respiratory failure, we do not believe this was from nivolumab. While drug-induced pneumonitis is a known rare toxic effect of nivolumab [1], the CT appearance and timing of symptoms differ from other reports of anti–PD-1–related pneumonitis and our patient improved without steroids [2]. Furthermore, no additional respiratory complications occurred with further nivolumab treatment. Interestingly, he had radiographic progression 2 wk after his first nivolumab dose but then dramatic improvement after 1 mo, despite no additional doses. Pseudoprogression has been well documented with checkpoint inhibitors, and we hypothesize that this may have been the case in our patient [3].
In population pharmacokinetic studies, renal function did not affect nivolumab clearance, and the package insert does not recommend dose adjustments for chronic kidney disease [4]. Considering its high molecular weight, nivolumab is also probably not cleared by dialysis. Nevertheless, without documented safety or efficacy in dialysis patients, oncologists might be reluctant to use nivolumab in this population. This is particularly important because ESRD is not uncommon among patients with RCC. Our case demonstrates the safety and efficacy of nivolumab in this setting and exemplifies the pseudoprogression sometimes observed with checkpoint blockade.
Acknowledgments:
We thank Dr. Joshua Chaim for his help in selecting the computed tomography images displayed in Fig. 1.
Footnotes
Conflicts of interest: Dr. Feldman served as consultant for Bayer and Seattle Genetics within the past 2 yr and has also accepted research funding from Novartis. Dr. Carlo has nothing to disclose.
References
- [1].Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nishino M, Sholl LM, Hodi FS, et al. Anti-PD-1-related pneumonitis during cancer immunotherapy. N Engl J Med 2015;373:288–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol 2015;33:3541–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Opdivo [package insert] New York, NY: Bristol-Myers Squibb; 2016. [Google Scholar]


