Abstract
A significant proportion of islets are lost following transplantation due to hypoxia and inflammation. We hypothesize that adipose tissue-derived mesenchymal stem cells (AD-MSCs) can rescue a sub-therapeutic number of transplanted islets by helping them establish a new blood supply and reducing inflammation. Diabetic mice received syngeneic transplantation with 75 (minimal), 150 (sub-therapeutic), or 225 (therapeutic) islets, with or without 1×10 mouse AD-MSCs. Fasting blood glucose (FBG) values were measured over 6 weeks with tissue samples collected for islet structure and morphology (H&E, insulin/glucagon staining). Histological and immunohistochemical analyses of islets were also performed at 2 weeks in animals transplanted with a sub-therapeutic number of islets, with and without AD-MSCs, to determine new blood vessel formation, the presence of pro-angiogenic factors facilitating revascularization, and the degree of inflammation. AD-MSCs had no beneficial effect on FBG values when co-transplanted with a minimal or therapeutic number of islets. However, AD-MSCs significantly reduced FBG values and restored glycemic control in diabetic animals transplanted with a sub-therapeutic number of islets. Islets co-transplanted with AD-MSCs preserved their native morphology and organization and exhibited less aggregation when compared to islets transplanted alone. In the sub-therapeutic group, AD-MSCs significantly increased islet revascularization and the expression of angiogenic factors including hepatocyte growth factor (HGF) and angiopoietin-1 (Ang-1) while also reducing inflammation. AD-MSCs can rescue the function of islets when transplanted in a sub-therapeutic number, for at least 6 weeks, via their ability to maintain islet architecture while concurrently facilitating islet revascularization and reducing inflammation.
Keywords: Islet transplantation, Islet number, Mesenchymal stem cells, Angiogenesis, Diabetes
Introduction
Compared to the whole pancreas transplantation, islet transplantation does not require the creation of a surgical vascular anastomosis. Hence, for islets to survive following their engraftment, they need to rebuild a complex network of blood vessels between each islet and the host-derived microvascular bed to ensure they receive an adequate supply of nutrients and oxygen to meet the needs of the islet graft (Carlsson et al. 2001; Pepper et al. 2013). However, this process can take up to 3 weeks, during which time the islets must survive by relying on the diffusion of oxygen and nutrients into cells (Komatsu et al. 2017); this is not ideal given the high metabolic requirement of the cells within the islets, especially the insulin-producing beta cells (Sato et al. 2011). As a result, up to 60% of the islet graft is lost within 2 weeks following transplantation from islet hypoxia due to an underdeveloped vascular supply, as well as the instant blood-mediated inflammatory reaction (IBMIR) towards the islets (Biarnes et al. 2002; Kanak et al. 2014; Lau et al. 2009; Nilsson et al. 2011). This situation is made worse by the fact that islet loss actually begins before transplantation as a result of islets suffering hypoxic stress from the isolation process (Linn et al. 2006). If not enough islets survive the transplantation procedure, the remaining islets ultimately get “overworked” resulting in them eventually failing from exhaustion, which further jeopardizes the overall success of the transplant (Boland et al. 2017). To account for these issues, a large number of islets are therefore transplanted to ensure that enough survive. Hence, patients receiving an allogenic islet transplant will typically receive at least 5000 islet equivalents/kg (i.e., 300,000–400,000 islets) per transplantation (McCall and Shapiro 2012). Sometimes, this requires procurement of islets from at least two donors or patients requiring two islet transplantations, which is problematic given the limited donor pool (Bruni et al. 2014).
One strategy to improve islet engraftment and survival following transplantation is to co-transplant mesenchymal stem cells (MSCs) with islets. MSCs can be procured from different tissues (i.e., adipose tissue (AD-MSC), bone marrow (BM-MSC), peripheral blood, placenta, and umbilical cord (UC-MSC)) with studies showing that they can act as a “mobile drug store” to protect and regenerate damaged cells through the release of angiogenic, anti-inflammatory, anti-apoptotic, immunomodulatory, and anti-fibrotic factors (Caplan 2016, Caplan and Correa 2011, Le Le Blanc and Davies 2018, Tao et al. 2016, Watt et al. 2013). BM-MSCs have been shown to improve the survival and function of transplanted islets through their paracrine function, with studies demonstrating that they can accelerate islet revascularization (Figliuzzi et al. 2014; Ito et al. 2010; Sakata et al. 2010; Yoshimatsu et al. 2015). While BM-MSCs are the most frequently investigated MSC, and often designated as the gold standard, they have to be procured from bone marrow aspirates which requires a painful invasive procedure. AD-MSCs have emerged as a new and readily available source of MSCs that can be isolated with high yield from liposuction or lipoplasty procedures without the use of enzymes or the need for ex vivo expansion (Bianchi et al. 2013; Tremolada et al. 2016). Compared to BM-MSCs, AD-MSCs have also demonstrated an improved proliferative capability and enhanced paracrine actions (Ikegame et al. 2011; Im 2017; Omar and Aboulkhair 2017; Strioga et al. 2012). Indeed, recent studies are now also showing that AD-MSCs can also increase islet survival and function, both in vitro as well as in vivo following transplantation (Cavallari et al. 2012; Ohmura et al. 2010; Schive et al. 2017; Yamada et al. 2014).
In previous studies using ischemic disease models, AD-MSCs have been shown to have potent pro-angiogenic properties through the secretion of a variety of trophic factors (Kim et al. 2007; Moon et al. 2006; Rehman et al. 2004). These pro-angiogenic properties of AD-MSCs are largely attributed to the release of vascular endothelial growth factor (VEGF) (Song et al. 2010), as well as other factors which include hepatocyte growth factor (HGF), and basic fibroblast growth factor (bFGF) (De Francesco et al. 2009; Gómez-Mauricio et al. 2016). Although Ohmura et al. (Ohmura et al. 2010) confirmed an increase of vWF-positive cells following co-transplantation of AD-MSCs and islets, what still remains unknown is the mechanism by which AD-MSCs can facilitate islet revascularization. Furthermore, in this study, they investigated using different ratios of AD-MSCs and islets and found that normoglycemia could only be achieved when a high number of islets alone (i.e., 400) were transplanted (Ohmura et al. 2010). When a lower number of islets were transplanted, AD-MSCs were needed to ensure initial graft survival and achieve normoglycemia; this effect was dependent on the dose of AD-MSCs, with a lower number of AD-MSCs having no effect (1 × 105) and a higher number of AD-MSCs being able to temporarily restore normoglycemia (2–4 × 105). However, in either case (i.e., with or without AD-MSCs), normoglycemia could only be maintained for 13 days.
Hence, in order to fully test the ability of AD-MSCs to rescue islets, our study used a higher number of AD-MSCs (1 × 106), tested a much lower number of islets (75, 150, and 225 islets), and examined the effects of AD-MSCs over a much longer duration (up to 6 weeks). In addition, given that AD-MSCs are able to stimulate angiogenesis, we evaluated the mechanisms by which they can help a sub-optimal number of islets survive transplantation by facilitating their revascularization and engraftment.
Materials and methods
Animals
All experiments were approved by the Administrative Panel on Laboratory Animal Care (APLAC) at Stanford University. Male C57BL/6 mice, at 6–8 weeks of age, were purchased from Charles River Laboratories (Wilmington, MA) and used for either pancreatic islet isolation or islet transplantation. All animals were maintained in pathogen-free conditions at the Research Animal Facility at Stanford University and had free access to food and water with a 12 h:12 h light/dark cycle. Fasting blood glucose (FBG) levels were measured using a Bayer Contour blood glucose monitoring system following tail vein clipping and each animal was fasted for 6 h before any measurement was taken. Animals were randomly allocated either into six groups (75, 150, and 225 islets alone and 75, 150, and 225 islets + AD-MSCs) for 6 weeks or into two groups (150 islets alone and 150 islets + AD-MSCs) for 2 weeks. All groups contained 4–6 animals.
Induction of diabetes
To induce diabetes, each animal received 180 mg/kg Streptozotocin (STZ; Sigma-Aldrich) via intraperitoneal (i.p.) injection. For any animal which did not demonstrate a rise in blood glucose after 72 h following i.p. injection of STZ, a second dose was administered. Mice which did not develop hyperglycemia following a second dose of STZ were excluded from the study. Mice were considered diabetic once they demonstrated two consecutive FBG values >19.4 mmol/l, at which point they were randomly allocated into an experimental group for islet transplantation.
AD-MSCs isolation, culture, and characterization
Mouse adipose tissue was obtained from the lower abdomen in male C57BL/6 mice at 6–8 weeks of age, as previously described (Sung et al. 2008). In brief, procured adipose tissue was washed with sterile phosphate buffered saline (PBS), minced with scissors, and then digested with 1 mg/ml type I collagenase (Sigma-Aldrich) in serum-free medium at 37 °C for 3 h. The digestion was then inactivated with an equal volume of DMEM (Gibco) supplemented with 10% fetal bovine serum (FBS; Invitrogen). All samples were then filtered through a 100-μm mesh filter to remove any debris. The cellular pellets were collected and then re-suspended in DMEM containing 10% FBS in a humidified incubator at 37 °C with 5% carbon dioxide. Spindle-shaped cells appeared on day 3, with cell reaching 70–90% confluence within 4–5 days. Cells were then split and sub-cultured.
AD-MSCs from passage number 3–5 were used for all transplantation studies with cells examined using a Zeiss LSM710 Confocal Microscope. For cell surface marker expression, adherent AD-MSCs were detached, disaggregated into single cells, and then stained with the following antibodies for 40 min at 4 °C: phycoerythrin (PE)-conjugated mouse monoclonal antibodies against CD34, CD90, and CD105 and allophycocyanin (APC)-conjugated mouse monoclonal antibody against CD45 (Biolegend). Following incubation, AD-MSCs were washed twice with PBS before being re-suspended with 0.5 ml PBS at which point their surface marker expression compared to unstained AD-MSCs (as control) was determined using the Guava® easyCyte system (Millipore, Darmstadt, Germany).
Islet isolation
Pancreatic islets were isolated from C57BL/6 mice by means of collagenase digestion and histopaque gradients, as previously described (Neuman et al. 2014). In brief, the pancreas was surgically exposed in mice and the pancreatic duct isolated and cannulated. The pancreas was then distended using an infusion of 2–3 ml of Hank’s balanced salt solution (HBSS, Sigma-Aldrich) supplemented with 0.1% bovine serum albumin (BSA; Sigma-Aldrich) containing 1 mg/ml of collagenase VI (Sigma-Aldrich). Following distension, the pancreas was carefully dissected and incubated for 10 min in a 37 °C water bath. Islets were purified by gradient centrifugation on Histopaque-1119 and 1077 (Sigma-Aldrich), and then individually handpicked and cultured in p60 culture dish containing RPMI 1640 medium supplemented with 10% FBS. Islets were visually inspected, manually counted, and their purity determined using dithizone (DTZ) staining (Sigma-Aldrich). Islets were also stained with fluorescein diacetate (FDA) and propidium iodide (PI) (Sigma-Aldrich) and then examined under both rhodamine and FITC filters equipped on a Leica fluorescence microscope (Leica microsystem, Wetzlar, Germany) to determine viability.
Islet transplantation
Experimental groups were group 1: 75 islets alone; group 2: 150 islets alone; group 3: 225 islets alone; group 4: 75 islets + 1 × 106 AD-MSCs; group 5: 150 islets + 1 × 106 AD-MSCs; and group 6: 225 islets + 1 × 106. After islets were removed from culture, they were washed once with PBS and then either re-suspended alone, or with AD-MSCs, in 1:1 mixture of PBS and Matrigel (BD Bioscience). This mixture was then injected, using a micropipette, beneath the right kidney capsule. Animals were then followed with FBG measurements taken twice a week. All animals were humanly sacrificed at either 2 or 6 weeks following islet transplantation depending on the experimental group. The kidney containing the transplanted islet graft was then carefully removed for histological and/or immunohistochemical evaluation.
Histology and immunohistochemistry (IHC)
Graft-bearing kidneys were fixed in 10% (vol/vol) neutral buffered formalin (NBF) and embedded in paraffin at 2 or 6 weeks post-transplantation, sectioned (5 μm thick) using a HM 355S automatic microtome (ThermoFisher Scientific). Samples were either stained with hematoxylin and eosin (H&E) or different primary antibodies (AbCam) including guinea pig polyclonal antibodies to insulin (1:200), mouse monoclonal antibodies to glucagon (1:1000), tumor necrosis factor-α (TNF-α; 1:100), rabbit monoclonal antibodies to vWF (1:400), rabbit polyclonal antibodies to cluster of differentiation 31 (CD31; 1:100), VEGF (1:100), HGF (1:100), or angiopoietin-1 (Ang-1; 1:100). All stained sections were scanned for analyses using a NanoZoomer (Hamamatsu Photonics, Hamamatsu, Japan).
On H&E staining, transplanted islets were assessed for their overall individual morphology as well as for any infiltration of inflammatory cells (i.e., number of inflammatory cells per mm2). For each animal, three histological sections from different areas of the islet graft were evaluated for individual endocrine aggregates (i.e., areas of positive staining separated from each other by > 50 μm of non-endocrine tissue). The endocrine area refers to the sum of the area of all endocrine aggregates within a histological section. As previously described (Rackham et al. 2013), the vascular density (i.e., positive staining for vWF and CD31), and presence of pro-angiogenic factors (i.e., positive staining for VEGF, HGF, and Ang-1) or an inflammatory factor (i.e., positive staining for TNF-α) were determined using islets from five to nine different sections from at least four animals within experimental groups. All positive staining within islets was then quantified using FIJI ImageJ software (Jensen 2013).
Statistical analysis
FBG values following transplantation were assessed using two-way ANOVA with repeated measures comparing the effect of time, group, and interactions between time and group. A Student’s unpaired t test was also used to determine differences in the staining results between the transplantation groups. For all comparisons, statistical significance was accepted when P <0.05.
Results
AD-MSC characterization
AD-MSCs had a long and thin morphology with widely dispersed filopodia and flattened polygonal extensions. Guava analysis demonstrated AD-MSCs to express CD105 (90.53 ±5.45), and CD90 (92.41 ±3.62) (positive) surface antigen markers, with no expression of hematopoietic markers such as CD34 (1.92 ± 0.43) and CD45 (2.43 ± 0.72) (negative).
Fasting blood glucose response following islet transplantation
Following STZ treatment, all animals became hyperglycemic with their FBG values increasing from an overall average of 6.6 ±0.4 mmol/l (baseline) to 25.6 ± 1.5 mmol/l (post-STZ treatment) (Fig. 1a–c). Following transplantation of a therapeutic number of islets alone (i.e., 225 islets), there was a significant reduction in FBG values in all animals by 6 weeks (21.3 ±1.5 vs. 6.5 ±0.3 mmol/l; P< 0.05) with re-establishment of glycemic control seen from as early as 1 week following islet transplantation. This effect was similar when a therapeutic number of islets were co-transplanted with AD-MSCs with no overall difference observed between groups (Fig. 1c). When a sub-therapeutic number of islets alone (i.e., 150 islets) were transplanted, there was an initial trend for the FBG values to decrease by 3 weeks (19.1 ±3.3 mmol/l), but this was reversed by 6 weeks so animals remained hyperglycemic (27.5±2.7 vs. 26.1 ±2.5 mmol/l; P>0.05; Fig. 1b). However, when a sub-therapeutic number of islets were co-transplanted with AD-MSCs, there was a reduction in FBG values over 6 weeks (22.2 ±1.0 vs. 9.4 ± 0.8 mmol/l; P < 0.05) with animals demonstrating re-establishment of glycemic control from 2.5 weeks following islet transplantation (Fig. 1b). When a minimal number of islets alone (i.e., 75 islets) were transplanted into diabetic animals, there was no change in the FBG values by 6 weeks (24.9 ±0.8 vs. 28.1 ±1.9 mmol/l; P >0.05). Furthermore, no additional beneficial effect was seen when a minimal number of islets were co-transplanted with AD-MSCs with animals remaining hyperglycemic at 6 weeks following transplantation (29.3 ±0.8 vs. 30.8 ±1.5 mmol/l; P> 0.05; Fig. 1a).
Fig. 1.
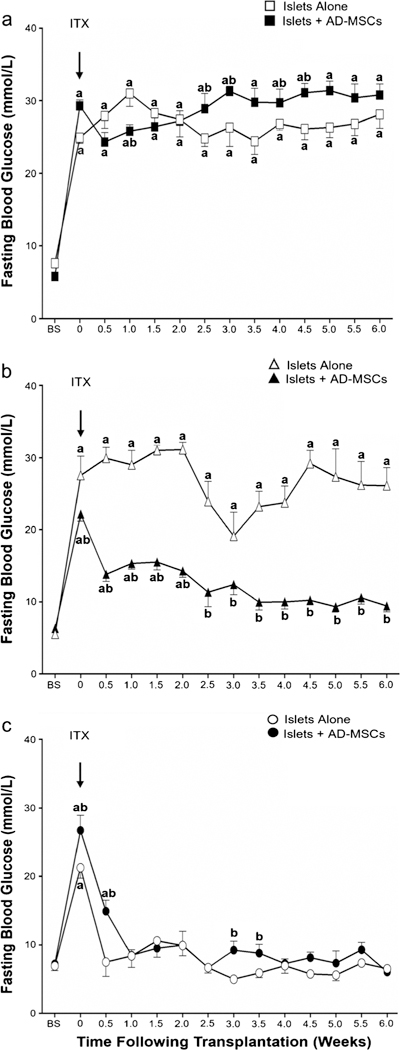
Fasting blood glucose levels in mice following islet transplantation. Values represent the mean ± S.E.M. at baseline (BS), islet transplantation (ITX arrow), and at week (W) 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, and 6.0 following islet transplantation. Animals received either 75 islets alone (white square), 75 islets with AD-MSCs (black square), 150 islets alone (white up-pointing triangle), 150 islets with AD-MSCs (black up-pointing triangle), 225 islets alone (white circle), and 225 islets with AD-MSCs (black circle). Significant differences: aP < 0.05, baseline vs. all other time points; bP < 0.05, islets alone vs. islets + AD-MSCs (two-way RM ANOVA with post hoc Tukey test)
Pancreatic islet morphology following transplantation
At 6 weeks post-transplantation, islets could only be consistently identified in the experimental groups which had been transplanted with 150 or 225 islets (Fig. 2a–f). In the experimental groups which received 75 islets, we could not reliably identify islets within tissue samples. In all groups, when islets were co-transplanted with AD-MSCs, they retained their native size and spherical morphology, clustered less, and maintained their intrinsic architecture with beta cells (positive insulin staining) located in the center and alpha cells (positive glucagon staining) located in the periphery of the islet (Figs. 2e–f and 3a–h). In contrast, when islets were transplanted without AD-MSCs, they lost their spherical morphology and tended to cluster more, thereby making it difficult to readily identify single islets (Fig. 2b, c). In addition, many of these islets had a more disorganized architecture as demonstrated by loss of their spatial organization of beta and alpha cells, as seen by positive glucagon staining located throughout the islets rather than being specifically localized in the periphery (Fig. 3a–h).
Fig. 2.
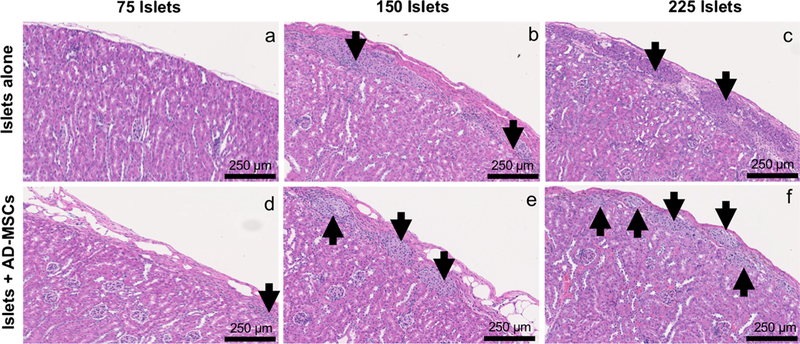
The morphology of transplanted islets. Representative H&E images of 75, 150, or 225 islets that were transplanted under the kidney capsule, either alone (a–c) or with AD-MSCs (d–f), at 6 weeks following transplantation. Arrows indicate islets present within the representative images
Fig. 3.
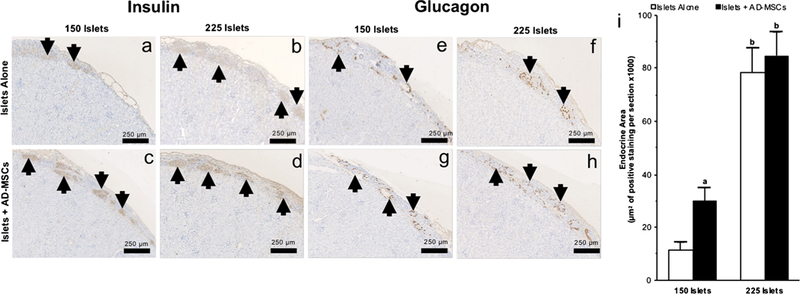
The endocrine index of transplanted islets. a–h Representative images following insulin (a–d) and glucagon (e–h) immunohistochemical staining of 150 or 225 islets that were transplanted under the kidney capsule, either alone or with AD-MSCs, at 6 weeks following transplantation. Arrows indicate islets present within the representative images. i Quantification of the positive staining endocrine area in samples where 150 or 225 islets were either transplanted alone (white square) or with AD-MSCs (black square). Significant differences: aP <0.05 for islets alone vs. islets + AD-MSCs; bP <0.05, 150 vs. 250 islets (Student’s unpaired t test)
The endocrine index of islets following transplantation
At 6 weeks post-transplantation, the total endocrine area was greater when more islets were transplanted; this was evident by there being more staining for insulin and glucagon in groups which had 225 islets transplanted compared to those which only received 150 islets. When 225 islets were transplanted, the total endocrine area was similar without or with co-transplantation of AD-MSCs (78,449 ±9379 vs. 84,867 ± 8835 μm2 of positive staining per section; P>0.05; Fig. 3i). However, when 150 islets were transplanted, there was a higher total endocrine area in the group which was co-transplanted with AD-MSCs compared to the group in which islets were transplanted alone (29,772 ±5160 vs. 11,377 ± 2958 μm2 of positive staining per section; P<0.05; Fig. 3i).
The vascular index of islets following transplantation
At 2 weeks post-transplantation, islets demonstrated no significant change in CD31 when they were transplanted either alone or with AD-MSCs (94 ± 20 vs. 94 ±3 positive cells per mm2; P >0.05; Fig. 4a–e). However, islets co-transplanted with AD-MSCs demonstrated a significantly higher expression of vWF compared to those transplanted alone (1193 ±208 vs. 444 ±73 positive cells per mm2; P <0.05; Fig. 4c–f). In keeping with the increase in microvascular density seen in islets co-transplanted with AD-MSCs, islets also demonstrated an increase in HGF (1081 ±234 vs. 126 ± 20 positive cells per mm2; P<0.05; Fig. 5a–g) and Ang-1 (925 ±45 vs. 81 ±15 positive cells per mm2; P <0.05; Fig. 5c–g) staining compared to the control group in which 150 islets were transplanted alone. There was no significant difference in VEGF staining within the islets, regardless of whether or not they were co-transplanted with AD-MSCs (530 ±73 vs. 468 ± 60 positive cells per mm2; P >0.05; Fig. 5b–g).
Fig. 4.
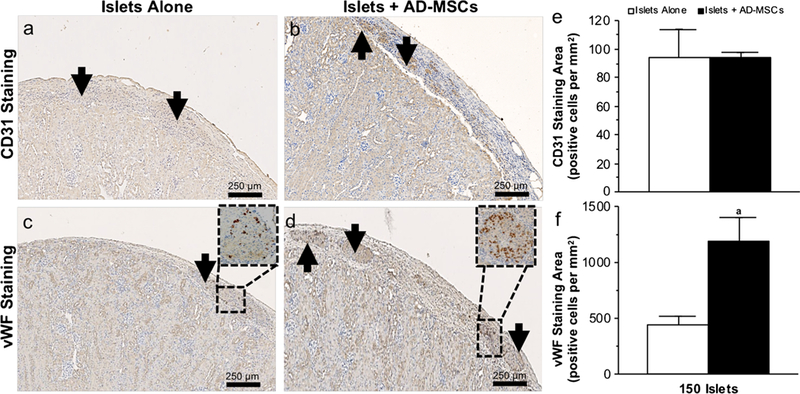
The vascular index of transplanted islets. a–d Representative images following CD31 (a, b) and vWF (c, d) immunohistochemical staining of 150 islets that were transplanted under the kidney capsule, either alone or with AD-MSCs, at 2 weeks following transplantation. Arrows indicate islets present within the representative images. Magnified views of the box region were shown for vWF. e, f Quantification of the positive CD31 and vWF staining in samples where 150 islets were either transplanted alone (white square) or with AD-MSCs (black square). Significant differences: aP < 0.05 for islets alone vs. islets + AD-MSCs (Student’s unpaired t test)
Fig. 5.
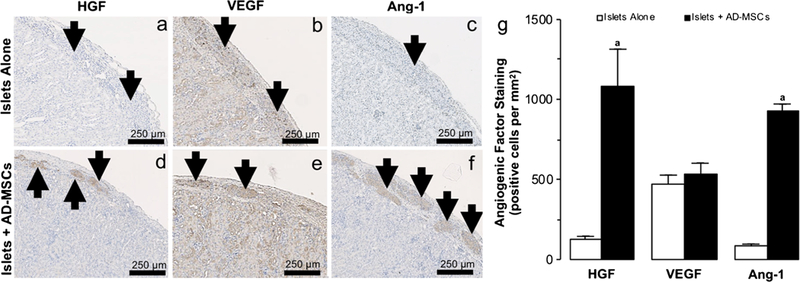
Angiogenic factors within transplanted islets. a–f Representative images following hepatocyte growth factor: HGF (a, d), vascular endothelial growth factor: VEGF (b, e), and angiopoetin-1: Ang-1 (c, f) immunohistochemical staining of 150 islets that were transplanted under the kidney capsule, either alone or with AD-MSCs, at 2 weeks following transplantation. Arrows indicate islets present within the representative images. g Quantification of the positive HGF, VEGF, and Ang-1 staining in samples where 150 islets were either transplanted alone (white square) or with AD-MSCs (black square). Significant differences: aP < 0.05 for islets alone vs. islets + AD-MSCs (Student’s unpaired t test)
The inflammatory response in islets following transplantation
At 2 weeks post-transplantation, islets that were cotransplanted with AD-MSCs demonstrated a lesser degree of inflammation as demonstrated by a reduction in the amount of inflammatory cell infiltrate when compared to islets that were transplanted alone (146 ±31 vs. 278 ±52 positive cells per mm2; P <0.05; Fig. 6a–e). In addition, there was also a corresponding reduction in the presence of TNF-a within the islets that were co-transplanted with AD-MSCs compared to those that were transplanted alone (74 ±14 vs. 449 ±52 positive cells per mm2; P <0.05; Fig. 6c–e). Of note, islets which were transplanted alone also subjectively demonstrated areas of hemorrhage within islets (Fig. 6).
Fig. 6.
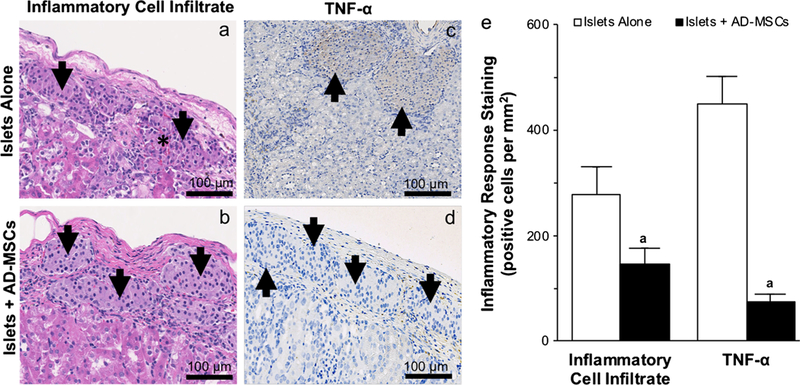
The inflammatory response within transplanted islets. a–d Representative images of the inflammatory cell infiltrate on H&E staining (a, b) and following tumor necrosis factor-α: TNF-α immunohistochemical staining (c, d) of 150 islets that were transplanted under the kidney capsule, either alone or with AD-MSCs, at 2 weeks following transplantation. Arrows indicate islets present within the representative images. Asterisk (*) indicates area of hemorrhage within the representative images. e Quantification of the inflammatory cell infiltrate and TNF-a staining in samples where 150 islets were either transplanted alone (white square) or with AD-MSCs (black square). Significant differences: aP < 0.05 for islets alone vs. islets + AD-MSCs (Student’s unpaired t test)
Discussion
In this study, we used an STZ-induced diabetic mouse model to investigate the effects of pancreatic islet co-transplantation with AD-MSCs. The kidney capsule was chosen as our site of transplantation given that it is a well-established site of islet transplantation in small animals which is easily accessible. Our results show when pancreatic islets were transplanted alone, only a therapeutic (225 islets) number of islets resulted in restoration of glucose homeostasis over 6 weeks, with neither a sub-therapeutic (150 islets) nor minimal (75 islets) number of islets alone having the capacity to reverse hyperglycemia in diabetic animals. Although there was no additional biological benefit when AD-MSC were co-transplanted with a therapeutic number of islets (i.e., the speed of hyperglycemia reversal and the maintenance of this response over 6 weeks), AD-MSCs reversed hyperglycemia and restored glucose homeostasis when co-transplanted with a sub-therapeutic number of islets for up to 6 weeks. Furthermore, we demonstrated that this effect was due to AD-MSCs promoting angiogenesis to facilitate islet revascularization and hence their engraftment and survival. As previously noted, we also found that AD-MSCs were able to reduce the inflammatory response in the transplanted islets.
In contrast to previous studies which have shown that AD-MSCs co-transplanted with islets can only maintain normoglycemia for 13 days (Ohmura et al. 2010), we have shown that with a higher amount of AD-MSCs, we can maintain normoglycemia in both sub-therapeutic and therapeutic number of transplanted islets for up to 6 weeks. One possible explanation for this could be due to the release of more trophic factors from AD-MSCs which can further support islet engraftment. Also, while there is no information available on passage number of AD-MSCs used in previous studies, we used AD-MSCs from early passage numbers (i.e., 2–4) which have been shown to have a greater regenerative effect compared to those isolated from a higher passage number (Bogdanova et al. 2014). Future research will therefore aim to study the effect of AD-MSC passage number on transplanted islet survival and function.
Although islet transplantation can maintain glycated hemoglobin levels ≤ 7% while offering freedom from life-threatening severe hyperglycemia in T1DM patients with problematic hypoglycemia (Hering et al. 2016), there is a substantial loss of islets following transplantation. Our results show that when AD-MSCs are co-transplanted with islets, they improve their function and viability especially when given with a sub-therapeutic number of islets; here, they are able to help restore glycemic control in diabetic animals by promoting islet revascularization and reducing the inflammatory response towards islets. These results would suggest that AD-MSCs could potentially rescue sub-optimal transplants thereby reducing the number or additional transplants required for any one individual. Although no difference was seen in the biological response (i.e., return to normoglycemia) when a therapeutic number of islets was transplanted, the islets cotransplanted with AD-MSCs demonstrated a healthier morphology and preserved alpha and beta spatial organization with reduced aggregation/clustering; combined, this will enable these islets to have a better long-term outcome and improved revascularization. In keeping with this, studies have shown that the architecture, organization, and morphology of islets play an important role in their function and outcome following transplantation and that these can be maintained by co-transplanting with MSCs (Rackham et al. 2011). This can be attributed to the fact that when islets aggregate, the diffusion of oxygen and nutrients to cells within the center of larger aggregates will be limited compared to smaller aggregates or separated islets, thereby affecting their function and ultimately their survival (Carlsson et al. 1998; Dionne et al. 1989). Furthermore, the revascularization of smaller islets (and hence aggregates) has also been shown to be more efficient when compared to larger islets (Kampf et al. 2006). Also, the presence of a collagen matrix between islets, which is eliminated when islets aggregate together, has been shown to be beneficial to islet graft function following transplantation (Jalili et al. 2011). In our study, we used Matrigel® as an extracellular matrix (ECM) to provide a physical scaffold for transplanted islets (Stendahl et al. 2009). The presence of an ECM can help support transplanted islets via its ability to bind, store, and protect the degradation of growth factors, cytokines, and other soluble signaling molecules (Flaumenhaft and Rifkin 1992; Folkman et al. 1988), especially those that are released from AD-MSCs that have been co-transplanted with islets. These effects are likely to be further enhanced given that the ECM can help regulate islet survival, insulin secretion, and proliferation, in addition to preserving and restoring the spherical morphology of islets (Beattie et al. 2002; Bosco et al. 2000; Hayeketal. 1995; Lucas-Clerc etal. 1993;Nagataetal. 2002; Navarro-Alvarez et al. 2008; Wang and Rosenberg 1999). Taken together, these beneficial effects of Matrigel will contribute to the improved responses seen when AD-MSCs are co-transplanted with a sub-therapeutic number of islets.
Following islet transplantation, islets need to rapidly re-establish their vascular network to ensure they receive an adequate supply of nutrients and oxygen for their survival. Within 3–5 days following their transplantation, islets start to form new blood vessels and by 7–14 days, blood flow to the transplanted islets is established with further vascular development, organization, and functional maturation continuing over several months (Jansson and Carlsson 2002; Patan 2000; Vajkoczy et al. 1995). For these reasons, we chose 14 days as our time point to examine the differences in vascular development between our experimental groups when a sub-therapeutic number of islets were transplanted. In our study, transplanted islets demonstrated positive staining for both CD31 as well as vWF at 14 days post-transplantation. While CD31 is thought to be one of the earliest markers of endothelial cell differentiation and hence specific for early angiogenesis (Baldwin et al. 1994; Rongish et al. 1996), vWF acts as a regulator of angiogenesis, controlling vessel proliferation and maturation following initial formation through multiple pathways (Randi and Laffan 2017). Our data is supported by previous studies which have also shown that over the first few weeks following islet transplantation, vWF immunoreactivity becomes more homogenous and less speckled reflecting an increase in microvasculature density (Watanabe et al. 2000). When AD-MSCs were co-transplanted with islets, we observed no significant difference in CD31 expression and this can be attributed to the fact that majority of the endothelial cell differentiation required to form the new blood vessels supplying the transplanted islets has already happened by 14 days. However, there was a significant increase in vWF expression within islets suggesting that AD-MSCs are helping to promote vessel proliferation and maturation, in order to form a more functional microcirculation. To explain the increased vascularity observed within islets co-transplanted with AD-MSCs at 14 days, we investigated three pro-angiogenic factors (i.e., VEGF, HGF, Ang-1); these factors have not only been shown to play a role and islet revascularization following transplantation, but have also been shown to be secreted by AD-MSCs (Kalinina et al. 2015).
During islet development, VEGF-A is secreted by beta cells and directly stimulates the proliferation of vascular endothelial cells as well as their release of vWF, which in turn promotes endothelial cell proliferation, migration, and sprouting of tip cells in the early stages of angiogenesis (Gerhardt 2008; Johansson et al. 2006; Konstantinova and Lammert 2004; Leung et al. 1989). Following islet transplantation, VEGF-A is expressed in islets from as early as 1 day with expression still seen up to 28 days, albeit much reduced (Watanabe et al. 2003). The driving force behind VEGF-A expression is likely due to the increased expression of hypoxia-inducible factor-1α (HIF-1α) which is strongly expressed in islets at day 1 following transplantation, peaking around day 3, and then gradually diminishing by day 14 (Miao et al. 2006). Although our results show that VEGF-A is expressed within transplanted islets at day 14, there was only slight increase in its expression when islets were cotransplanted with AD-MSCs which failed to reach statistical significance. This is somewhat interesting given that AD-MSCs have been shown to upregulate VEGF in porcine islets when they were co-cultured with AD-MSCs. However, our result could be explained by either a difference in the species being tested (i.e., porcine vs. mouse), experimental conditions (i.e., in vitro vs. in vivo), or timing. In the latter case, while we may have expected islets co-transplanted with AD-MSCs to exhibit an increased expression of VEGF via its release from either AD-MSCs (as a result of inflammatory signals and/or hypoxia) or beta cells themselves, this may have occurred at earlier time points with any difference at 14 days now less evident. Our results also showed no significant difference in CD31 between groups in keeping with previous studies which have shown a temporal correlation between VEGF-A and CD31 expression (Watanabe et al. 2003). Other important pro-angiogenic factors which have been shown to be involved in the neovascularization of transplanted islets include HGF and Ang-1 (Golocheikine et al. 2010; Su et al. 2007). In contrast to VEGF, we observed that both of these factors were significantly increased in islets that were co-transplanted with AD-MSCs. Although HGF and Ang-1 can both stimulate angiogenesis and islet microvascular density, they both have additional roles after blood vessels have been formed—HGF increasing endothelial permeability and inhibiting endothelial cell apoptosis (Book et al. 1999; Bussolino et al. 1992; Morishita et al. 1997), while Ang-1 binds to the receptor tyrosine kinase Tie-2 to promote the survival and integrity of blood vessels (Brindle et al. 2006). Hence, given that islets cotransplanted with AD-MSCs have more blood vessels at day 14, it is plausible that AD-MSCs could either be working directly (via releasing these angiogenic factors) or indirectly (via their paracrine action on beta cells which then release these angiogenic factors) to help the maturation of blood vessels which have already been formed. In turn, this results in improved glucose profiles and increased glucose-stimulated insulin secretion in islets co-transplanted with AD-MSCs. Furthermore, while both VEGF-A and HGF act synergistically to enhance angiogenesis after islet transplantation (Golocheikine et al. 2010), previous studies have shown that the expression of HGF actually becomes stronger in the transplanted islets over time (Watanabe et al. 2003) and hence this effect is likely amplified in the presence of AD-MSCs based on our data. Given that Ang-1 can also confer cytoprotective effects on islets, as well enhance their engraftment and preserve their functional mass (Su et al. 2007), it is plausible that the transplantation conditions favor the release of this cytokine directly from AD-MSCs.
Our results also showed that when AD-MSCs are cotransplanted with islets, they can protect them by reducing the inflammatory response within the graft (Angaswamy et al. 2012). This is supported by data from our study which showed that AD-MSCs could reduce the degree of inflammatory cells and TNF-α (i.e., potent pro-inflammatory cytokine) present within transplanted islets. One potential mechanism responsible for this finding is the ability of MSCs to adopting an immunosuppressive phenotype where they then release indoleamine 2,3-dioxygenase (IDO), prostaglandin-E2 (PGE2), nitric oxide, transforming growth factor β (TGF-β), hepatocyte growth factor (HGF), and interleukins 6 and 10; all of which can reduce the inflammatory response to transplanted islets (Yeung et al. 2012).
In summary, AD-MSCs can rescue the function of transplanted islets for up to 6 weeks, especially when they are transplanted in sub-therapeutic numbers, via their ability to maintain islet architecture while concurrently facilitating islet revascularization through their pro-angiogenic properties and reducing inflammation.
Acknowledgments
This work was supported by the NIDDK/NIH award to the Stanford Diabetes Research Center (P30DK116074).
Abbreviations
- AD
Adipose tissue
- Ang-1
Angiopoietin-1
- APLAC
Administrative Panel on Laboratory Animal Care
- bFGF
Basic fibroblast growth factor
- BM
Bone marrow
- DTZ
Dithizone
- FBG
Fasting blood glucose
- FDA
Fluorescein diacetate
- HBSS
Hank’s balanced salt solution
- HGF
Hepatocyte growth factor
- HIF-1α
Hypoxia-inducible factor-1α
- IBMIR
Instant blood-mediated inflammatory reaction
- IDO
Indoleamine 2,3-dioxygenase
- ITX
Islet transplantation
- MSC
Mesenchymal stem cell
- NBF
Neutral buffered formalin
- PGE2
Prostaglandin E2
- PI
Propidium iodide
- STZ
Streptozotocin
- TNF-α
Tumor necrosis factor-α
- T1DM
Diabetes mellitus type 1
- UC
Umbilical cord
- VEGF
Vascular endothelial growth factor
- vWF
Von Willebrand factor
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Statement of animal welfare All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Angaswamy N, Fukami N, Tiriveedhi V, Cianciolo GJ, Mohanakumar T (2012) LMP-420, a small molecular inhibitor of TNF-alpha, prolongs islet allograft survival by induction of suppressor of cytokine signaling-1: synergistic effect with cyclosporin-A. Cell Transplant 21:1285–1296 [DOI] [PubMed] [Google Scholar]
- Baldwin HS, Shen HM, Yan HC, DeLisser HM, Chung A, Mickanin C, Trask T, Kirschbaum NE, Newman PJ, Albelda SM et al. (1994) Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development 120:2539–2553 [DOI] [PubMed] [Google Scholar]
- Beattie GM, Montgomery AM, Lopez AD, Hao E, Perez B, Just ML, Lakey JR Hart ME, Hayek A (2002) A novel approach to increase human islet cell mass while preserving β-cell function. Diabetes 51: 3435–3439 [DOI] [PubMed] [Google Scholar]
- Bianchi F, Maioli M, Leonardi E, Olivi E, Pasquinelli G, Valente S, Mendez AJ, Ricordi C, Raffaini M, Tremolada C, Ventura C(2013) A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant 22: 2063–2077 [DOI] [PubMed] [Google Scholar]
- Biarnes M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E (2002) Beta-cell death and mass in syngeneically transplanted islets exposed to short-and long-term hyperglycemia. Diabetes 51:66–72 [DOI] [PubMed] [Google Scholar]
- Bogdanova A, Berzins U, Nikulshin S, Skrastina D, Ezerta A, Legzdina D, Kozlovska T (2014) Characterization of human adipose-derived stem cells cultured in autologous serum after subsequent passaging and long term cryopreservation. J Stem Cells 9:135–148 [PubMed] [Google Scholar]
- Boland BB, Rhodes CJ, Grimsby JS (2017) The dynamic plasticity of insulin production in β-cells. Mol Metab 6:958–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Book AA, Ranganathan S, Abounader R, Rosen E, Laterra J (1999) Scatter factor/hepatocyte growth factor gene transfer increases rat blood-glioma barrier permeability. Brain Res 833:173–180 [DOI] [PubMed] [Google Scholar]
- Bosco D, Meda P, Halban PA, Rouiller DG (2000) Importance of cell-matrix interactions in rat islet beta-cell secretion in vitro: role of alpha6beta1 integrin. Diabetes 49:233–243 [DOI] [PubMed] [Google Scholar]
- Brindle NP, Saharinen P, Alitalo K (2006) Signaling and functions of angiopoietin-1 in vascular protection. Circ Res 98:1014–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni A, Gala-Lopez B, Pepper AR, Abualhassan NS, Shapiro AJ (2014) Islet cell transplantation for the treatment of type 1 diabetes: recent advances and future challenges. Diabetes Metab Syndr Obes 7:211–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM (1992) Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol 119:629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI (2016) MSCs: the sentinel and safe-guards of injury. J Cell Physiol 231:1413–1416 [DOI] [PubMed] [Google Scholar]
- Caplan AI, Correa D (2011) The MSC: an injury drugstore. Cell Stem Cell 9:11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson PO, Liss P, Andersson A, Jansson L (1998) Measurements of oxygen tension in native and transplanted rat pancreatic islets. Diabetes 47:1027–1032 [DOI] [PubMed] [Google Scholar]
- Carlsson PO, Palm F, Andersson A, Liss P (2001) Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes 50:489–495 [DOI] [PubMed] [Google Scholar]
- Cavallari G, Olivi E, Bianchi F, Neri F, Foroni L, Valente S, La Manna G, Nardo B, Stefoni S, Ventura C (2012) Mesenchymal stem cells and islet cotransplantation in diabetic rats: improved islet graft revascularization and function by human adipose tissue-derived stem cells preconditioned with natural molecules. Cell Transplant 21:2771–2781 [DOI] [PubMed] [Google Scholar]
- De Francesco F, Tirino V, Desiderio V, Ferraro G, DAndrea F, Giuliano M, Libondi G, Pirozzi G, De Rosa A, Papaccio G (2009) Human CD34+/CD90+ ASCs are capable of growing as sphere clusters, producing high levels of VEGF and forming capillaries. PLoS One 4:e6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne KE, Colton CK, Yarmush ML (1989) Effect of oxygen on isolated pancreatic tissue. ASAIO Trans 35:739–741 [DOI] [PubMed] [Google Scholar]
- Figliuzzi M, Bonandrini B, Silvani S, Remuzzi A (2014) Mesenchymal stem cells help pancreatic islet transplantation to control type 1 diabetes. World J Stem Cells 6:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaumenhaft R, Rifkin DB (1992) The extracellular regulation of growth factor action. Mol Biol Cell 3:1057–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J, Klagsbrun M, Sasse J, Wadzinski M, Ingber D, Vlodavsky I (1988) A heparin-binding angiogenic protein—basic fibroblast growth factor—is stored within basement membrane. Am J Pathol 130:393. [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H (2008) VEGF and endothelial guidance in angiogenic sprouting. Organogenesis 4:241–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golocheikine A, Tiriveedhi V, Angaswamy N, Benshoff N, Sabarinathan R, Mohanakumar T (2010) Cooperative signaling for angiogenesis and neovascularization by VEGF and HGF following islet transplantation. Transplantation 90:725–731 [DOI] [PubMed] [Google Scholar]
- Gómez-Mauricio G, Moscoso I, Martín-Cancho M-F, Crisóstomo V, Prat-Vidal C, Báez-Díaz C, Sánchez-Margallo FM, Bernad A (2016) Combined administration of mesenchymal stem cells overexpressing IGF-1 and HGF enhances neovascularization but moderately improves cardiac regeneration in a porcine model. Stem Cell Res Ther 7:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayek A, Beattie GM, Cirulli V, Lopez AD, Ricordi C, Rubin JS (1995) Growth factor/matrix-induced proliferation of human adult β-cells. Diabetes 44:1458–1460 [DOI] [PubMed] [Google Scholar]
- Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, Kaufman DB, Korsgren O, Larsen CP, Luo X, Markmann JF, Naji A, Oberholzer J, Posselt AM, Rickels MR, Ricordi C, Robien MA, Senior PA, Shapiro AM, Stock PG, Turgeon NA, Clinical Islet Transplantation C (2016) Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 39:1230–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegame Y, Yamashita K, Hayashi S, Mizuno H, Tawada M, You F, Yamada K, Tanaka Y, Egashira Y, Nakashima S, Yoshimura S, Iwama T (2011) Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 13:675–685 [DOI] [PubMed] [Google Scholar]
- Im GI (2017) Bone marrow-derived stem/stromal cells and adipose tissue-derived stem/stromal cells: their comparative efficacies and synergistic effects. J Biomed Mater Res A 105:2640–2648 [DOI] [PubMed] [Google Scholar]
- Ito T, Itakura S, Todorov I, Rawson J, Asari S, Shintaku J, Nair I, Ferreri K, Kandeel F, Mullen Y (2010) Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation 89:1438–1445 [DOI] [PubMed] [Google Scholar]
- Jalili RB, Moeen Rezakhanlou A, Hosseini-Tabatabaei A, Ao Z, Warnock GL, Ghahary A (2011) Fibroblast populated collagen matrix promotes islet survival and reduces the number of islets required for diabetes reversal. J Cell Physiol 226:1813–1819 [DOI] [PubMed] [Google Scholar]
- Jansson L, Carlsson PO (2002) Graft vascular function after transplantation of pancreatic islets. Diabetologia 45:749–763 [DOI] [PubMed] [Google Scholar]
- Jensen EC (2013) Quantitative analysis of histological staining and fluorescence using ImageJ. Anat Rec 296:378–381 [DOI] [PubMed] [Google Scholar]
- Johansson M, Andersson A, Carlsson PO, Jansson L (2006) Perinatal development of the pancreatic islet microvasculature in rats. J Anat 208:191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinina N, Klink G, Glukhanyuk E, Lopatina T, Efimenko A, Akopyan Z, Tkachuk V (2015) miR-92a regulates angiogenic activity of adipose-derived mesenchymal stromal cells. Exp Cell Res 339:61–66 [DOI] [PubMed] [Google Scholar]
- Kampf C, Mattsson G, Carlsson PO (2006) Size-dependent revascularization of transplanted pancreatic islets. Cell Transplant 15:205–209 [DOI] [PubMed] [Google Scholar]
- Kanak MA, Takita M, Kunnathodi F, Lawrence MC, Levy MF, Naziruddin B (2014) Inflammatory response in islet transplantation. Int J Endocrinol 2014:451035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Kim HK, Cho HH, Bae YC, Suh KT, Jung JS (2007) Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol Biochem 20:867–876 [DOI] [PubMed] [Google Scholar]
- Komatsu H, Cook C, Wang CH, Medrano L, Lin H, Kandeel F, Tai YC, Mullen Y (2017) Oxygen environment and islet size are the primary limiting factors of isolated pancreatic islet survival. PLoS One 12: e0183780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinova I, Lammert E (2004) Microvascular development: learning from pancreatic islets. Bioessays 26:1069–1075 [DOI] [PubMed] [Google Scholar]
- Lau J, Henriksnas J, Svensson J, Carlsson PO (2009) Oxygenation of islets and its role in transplantation. Curr Opin Organ Transplant 14:688–693 [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Davies LC (2018) MSCs-cells with many sides. Cytotherapy 20(3):273–278 [DOI] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N (1989) Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246:1306–1309 [DOI] [PubMed] [Google Scholar]
- Linn T, Schmitz J, Hauck-Schmalenberger I, Lai Y, Bretzel RG, Brandhorst H, Brandhorst D (2006) Ischaemia is linked to inflammation and induction of angiogenesis in pancreatic islets. Clin Exp Immunol 144:179–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Clerc C, Massart C, Campion J, Launois B, Nicol M (1993) Long-term culture of human pancreatic islets in an extracellular matrix: morphological and metabolic effects. Mol Cell Endocrinol 94:9–20 [DOI] [PubMed] [Google Scholar]
- McCall M, Shapiro AM (2012) Update on islet transplantation. Cold Spring Harb Perspect Med 2:a007823. 10.1101/cshperspect.a007823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao G, Ostrowski RP, Mace J, Hough J, Hopper A, Peverini R, Chinnock R, Zhang J, Hathout E (2006) Dynamic production of hypoxia-inducible factor-1alpha in early transplanted islets. Am J Transplant 6:2636–2643 [DOI] [PubMed] [Google Scholar]
- Moon MH, Kim SY, Kim YJ, Kim SJ, Lee JB, Bae YC, Sung SM, Jung JS (2006) Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem 17:279–290 [DOI] [PubMed] [Google Scholar]
- Morishita R, Higaki J, Hayashi SI, Yo Y, Aoki M, Nakamura S, Moriguchi A, Matsushita H, Matsumoto K, Nakamura T, Ogihara T (1997) Role of hepatocyte growth factor in endothelial regulation: prevention of high D-glucose-induced endothelial cell death by prostaglandins and phosphodiesterase type 3 inhibitor. Diabetologia 40:1053–1061 [DOI] [PubMed] [Google Scholar]
- Nagata N, Iwanaga A, Inoue K, Tabata Y (2002) Co-culture of extracellular matrix suppresses the cell death of rat pancreatic islets. J Biomater Sci Polym Ed 13:579–590 [DOI] [PubMed] [Google Scholar]
- Navarro-Alvarez N, Rivas-Carrillo JD, Soto-Gutierrez A, Yuasa T, Okitsu T, Noguchi H, Matsumoto S, Takei J, Tanaka N, Kobayashi N (2008) Reestablishment of microenvironment is necessary to maintain in vitro and in vivo human islet function. Cell Transplant 17: 111–119 [DOI] [PubMed] [Google Scholar]
- Neuman JC, Truchan NA, Joseph JW, Kimple ME (2014) A method for mouse pancreatic islet isolation and intracellular cAMP determination. J Viss Exp (88):e50374. 10.3791/50374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B, Ekdahl KN, Korsgren O (2011) Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment. Curr Opin Organ Transplant 16:620–626 [DOI] [PubMed] [Google Scholar]
- Ohmura Y, Tanemura M, Kawaguchi N, Machida T, Tanida T, Deguchi T, Wada H, Kobayashi S, Marubashi S, Eguchi H (2010) Combined transplantation of pancreatic islets and adipose tissue-derived stem cells enhances the survival and insulin function of islet grafts in diabetic mice. Transplantation 90:1366–1373 [DOI] [PubMed] [Google Scholar]
- Omar AI, Aboulkhair AG (2017) Effect of bone marrow versus adipose tissue derived mesenchymal stem cells on the pancreas of Streptozotocin-induced diabetes mellitus type I in adult male rats (histological study). Egypt J Histol 40:12–24 [Google Scholar]
- Patan S (2000) Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J Neuro-Oncol 50:1–15 [DOI] [PubMed] [Google Scholar]
- Pepper AR, Gala-Lopez B, Ziff O, Shapiro AM (2013) Revascularization of transplanted pancreatic islets and role of the transplantation site. Clin Dev Immunol 2013:352315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackham CL, Chagastelles PC, Nardi NB, Hauge-Evans AC, Jones PM, King AJ (2011) Co-transplantation of mesenchymal stem cells maintains islet organisation and morphology in mice. Diabetologia 54:1127–1135 [DOI] [PubMed] [Google Scholar]
- Rackham CL, Jones PM, King AJ (2013) Maintenance of islet morphology is beneficial for transplantation outcome in diabetic mice. PLoS One 8:e57844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randi AM, Laffan MA (2017) Von Willebrand factor and angiogenesis: basic and applied issues. J Thromb Haemost 15:13–20 [DOI] [PubMed] [Google Scholar]
- Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL (2004) Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109:1292–1298 [DOI] [PubMed] [Google Scholar]
- Rongish BJ, Hinchman G, Doty MK, Baldwin HS, Tomanek RJ (1996) Relationship of the extracellular matrix to coronary neovascularization during development. J Mol Cell Cardiol 28:2203–2215 [DOI] [PubMed] [Google Scholar]
- Sakata N, Chan NK, Chrisler J, Obenaus A, Hathout E (2010) Bone marrow cell co-transplantation with islets improves their vascularization and function. Transplantation 89:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Endo H, Okuyama H, Takeda T, Iwahashi H, Imagawa A, Yamagata K, Shimomura I, Inoue M (2011) Cellular hypoxia of pancreatic β-cells due to high levels of oxygen consumption for insulin secretion in vitro. J Biol Chem JBC 286(14):12524–12532 M110 1 94738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schive SW, Mirlashari MR, Hasvold G, Wang M, Josefsen D, Gullestad HP, Korsgren O, Foss A, Kvalheim G, Scholz H (2017) Human adipose-derived mesenchymal stem cells respond to short-term hypoxia by secreting factors beneficial for human islets in vitro and potentiate antidiabetic effect in vivo. Cell Medicine 9:103–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S-Y, Chung H-M, Sung J-H (2010) The pivotal role of VEGF in adipose-derived-stem-cell-mediated regeneration. Expert Opin Biol Ther 10:1529–1537 [DOI] [PubMed] [Google Scholar]
- Stendahl JC, Kaufman DB, Stupp SI (2009) Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell Transplant 18:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J (2012) Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev 21:2724–2752 [DOI] [PubMed] [Google Scholar]
- Su D, Zhang N, He J, Qu S, Slusher S, Bottino R, Bertera S, Bromberg J, Dong HH (2007) Angiopoietin-1 production in islets improves islet engraftment and protects islets from cytokine-induced apoptosis. Diabetes 56:2274–2283 [DOI] [PubMed] [Google Scholar]
- Tao H, Han Z, Han ZC, Li Z (2016) Proangiogenic features of mesenchymal stem cells and their therapeutic applications. Stem Cells Int 2016:1314709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremolada C, Colombo V, Ventura C (2016) Adipose tissue and mesenchymal stem cells: state of the art and Lipogems(R) technology development. Curr Stem Cell Rep 2:304–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajkoczy P, Menger MD, Simpson E, Messmer K (1995) Angiogenesis and vascularization of murine pancreatic islet isografts. Transplantation 60:123–127 [PubMed] [Google Scholar]
- Wang R, Rosenberg L (1999) Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. J Endocrinol 163:181–190 [DOI] [PubMed] [Google Scholar]
- Watanabe H, Sumi S, Urushihata T, Kitamura Y, Iwasaki S, Xu G, Yano S, Nio Y, Tamura K (2000) Immunohistochemical studies on vascular endothelial growth factor and platelet endothelial cell adhesion molecule-1/CD-31 in islet transplantation. Pancreas 21:165–173 [DOI] [PubMed] [Google Scholar]
- Watanabe H, Sumi S, Kitamura Y, Nio Y, Higami T (2003) Immunohistochemical analysis of vascular endothelial growth factor and hepatocyte growth factor, and their receptors, in transplanted islets in rats. Surg Today 33:854–860 [DOI] [PubMed] [Google Scholar]
- Watt SM, Gullo F, van der Garde M, Markeson D, Camicia R, Khoo CP, Zwaginga JJ (2013) The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br Med Bull 108:25–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Shimada M, Utsunomiya T, Ikemoto T, Saito Y, Morine Y, Imura S, Mori H, Arakawa Y, Kanamoto M (2014) Trophic effect of adipose tissue-derived stem cells on porcine islet cells. J Surg Res 187:667–672 [DOI] [PubMed] [Google Scholar]
- Yeung TY, Seeberger KL, Kin T, Adesida A, Jomha N, Shapiro AM, Korbutt GS (2012) Human mesenchymal stem cells protect human islets from pro-inflammatory cytokines. PLoS One 7:e38189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimatsu G, Sakata N, Tsuchiya H, Minowa T, Takemura T, Morita H, Hata T, Fukase M, Aoki T, Ishida M (2015) The co-transplantation of bone marrow derived mesenchymal stem cells reduced inflammation in intramuscular islet transplantation. PLoS One 10: e0117561 [DOI] [PMC free article] [PubMed] [Google Scholar]


