Abstract
Background
Carotid endarterectomy (CEA) for asymptomatic patients with limited life expectancy may not be beneficial or cost-effective. The purpose of this study was to examine relationships between survival, outcomes and costs within two years following CEA among asymptomatic patients.
Methods
Prospectively collected data from 3,097 patients undergoing CEA for asymptomatic disease from Vascular Quality Initiative (VQI) Registry were linked to Medicare. Models were used to identify predictors of 2-year mortality following CEA. Patients were classified as low, medium or high risk of death based on this model. Next, we examined costs related to cerebrovascular care, occurrence of stroke, rehospitalization and reintervention within 2 years following CEA across risk strata.
Result
Overall 2-year mortality was 6.7%. Age, diabetes, smoking, CHF, COPD, renal insufficiency, absence of statin use and contralateral internal carotid artery stenosis were independently associated with a higher risk of death following CEA. In-hospital costs averaged $7,500 among patients defined as low risk for death, and exceeded $10,800 among high risk patients. While long-term costs related to cerebrovascular disease were two times higher in patients deemed high risk for death compared to low-risk patents ($17,800 vs $8,800, P=.001), high risk of death was not independently associated with a high probability of high cost. Predictors of high cost at 2-years were severe contralateral ICA stenosis, dialysis dependence, and ASA Class 4. Both statin use and CHF were protective of high cost.
Conclusions
Greater than 90% of patients undergoing CEA live long enough to realize the benefits of their procedure. Moreover, the long-term costs are supported by the effectiveness of this procedure at all levels of patient risk.
INTRODUCTION
Controversy exists regarding the need for carotid revascularization in patients with asymptomatic carotid stenosis 1–8. Asymptomatic patients have a much lower annual risk of stroke than those who have experienced neurologic sequelae related to their carotid stenosis -- approximately 3% per year in each year of the patient’s remaining life. This makes the absolute benefit of revascularization uncertain for many patients, especially those who may not live long enough to reap the prophylactic benefits of revascularization6. Decision-making surrounding carotid revascularization must include consideration of the up-front risks of a procedure, the long-term risk of stroke, and the patient’s life expectancy 9–11. Further, patients, payers and policymakers alike are anxious to avoid “unnecessary” procedures, as well as procedures where complications and their associated expense are likely to occur without the potential to achieve a clinical benefit.
However, while avoiding unnecessary carotid revascularization seems simple and plausible, two gaps in knowledge exist. First, despite several studies that describe factors associated with short-term risks of stroke or death, it is difficult for physicians to recognize when patients are likely to have poor long-term survival following carotid endarterectomy (CEA). Second, while many have studied factors associated with adverse clinical outcomes following CEA, little is known about the patient and procedural factors associated with higher long-term costs after CEA for asymptomatic carotid stenosis.
Therefore, we use data from the Vascular Quality Initiative, linked to Medicare claims, to examine relationships between survival, outcomes and costs related to cerebrovascular care within the first two years following CEA among asymptomatic patients. Our primary aim was to identify spending related to “unnecessary” carotid revascularization. We sought to define a cohort of high-risk patients who were unlikely to survive two years following CEA and to examine spending among this cohort of patients. We hypothesized that the majority of excess spending in carotid revascularization was attributable to care provided to these high-risk patients.
METHODS
Datasets and Cohort Construction
We identified all asymptomatic patients (those without prior stroke or transient ischemic attack) who underwent CEA between January 1st, 2003 and December 31st, 2011 in each the Vascular Quality Initiative (VQI) for the Society of Vascular Surgery Registry and in Medicare claims datasets. Then, using date of surgery, location of surgery (zip code), and gender the datasets were matched to one another on a patient level using a probabilistic matching algorithm. This matching process was successful in matching 70% of patients. Addition of the matched Medicare dataset allows long-term follow-up, as well as examination of late outcomes that can be identified in Medicare claims. Further details regarding the matched clinical-claims dataset can be found at vascularqualityinitiative.org.
Identifying Factors Associated with 2-Year Survival
First, we sought to define patient characteristics associated with reduced 2-year survival. To do this, we identified patients who died from any cause within two years following CEA. Next, Kaplan-Meier survival analyses with logrank test (for categorical variables) and Cox proportional hazard regression (for continuous variables) were used to examine univariate associations between 2-year mortality and a variety of patient-related characteristics. All variables that were associated with mortality with p<0.2 were entered into a multivariate model and backwards stepwise Cox proportional hazard regression with nested likelihood ratios was performed to generate a final model for predicting mortality at 2 years. Following this, we created three risk strata for mortality -- low, medium and high. To do this, scores to predict risk-of-death within two years were assigned to each patient in our cohort. These scores were calculated by summing the beta coefficients for each covariate in our Cox model for each individual. Cut-points for defining patients as low, medium or high risk were selected based on the distribution of risk scores. CEA in high-risk patients was deemed potentially “unnecessary”, as these patients are most likely to die before experiencing the potential benefit of CEA. The 2-year timeframe was chosen based on recent national guidelines and multispecialty society expert recommendations 10, 12–14
Identifying Factors Associated with High Cost (highest 10th percentile of costs, >$18,000)
To examine in-hospital and 2-year costs for patients undergoing CEA we used price-adjusted Medicare spending beginning on the date of CEA. Price-adjusted Medicare spending is a regionally and inflation-adjusted measure of actual Medicare payments (not Medicare charges). Further information about price-adjustment has been reported in prior work 15. Reported costs include the cost of the index procedure as well as any costs related to readmission, re-intervention, or subsequent admission for stroke. Readmission was deemed to be related to the index procedure if it occurred within 30 days of discharge. Re-intervention was defined as a revisional procedure (either CEA or carotid artery stent) or progression of contralateral carotid stenosis requiring revascularization (CEA or CAS), and was determined using CPT codes for these interventions. Due to limitations within the dataset, we are unable to further delineate what proportion represent ipsilateral revision or contralateral primary interventions. However, prior work suggests a 20% ipsilateral re-intervention rate16. Subsequent admissions for stroke were determined by examining discharge ICD-9 codes indicative of stroke in Medicare claims (Supplemental Table 1). Each of these events occurred in the period after the individual index operation, and therefore can represent ipsilateral or contralateral events.
Once we understood the distribution of each in-hospital and 2-year costs we defined high-cost as the highest 10th percentile of cost, or greater than $18,000. We then used backwards stepwise logistic regression to examine patient, operative and hospital level characteristics associated with high-cost.
Outcomes by Risk Strata for Mortality within 2 years
Once we established low, medium and high-risk strata for mortality at 2 years we then examined the following outcomes across these risk strata: stroke, need for re-intervention, in-hospital costs, and overall costs at two years. Stroke outcomes were established using both variables indicative of stroke within the VQI dataset, as well as variables indicative of a post-operative hospitalization where ICD-9 codes for stroke were identified in Medicare claims (Supplemental Table 1). Determination of need for re-intervention, in-hospital and 2-year costs are described above. Events measured at 2 years were reported using life table analysis.
Outcomes by Cost Strata
In a similar fashion, using life table analysis, we also examined the following outcomes across low and high cost strata: in-hospital death, 2-year mortality, stroke and need for re-intervention. Operational definitions for each of these outcomes are described above.
All analyses were performed using SAS (Cary, NC) and STATA 12 MP (College Station, TX). Dartmouth’s Committee for the Protection of Human Subjects approved our research protocol and approved the waiver of need for informed consent.
RESULTS
We studied 3,097 patients who underwent CEA for asymptomatic disease within the linked dataset between January 1, 2003 and December 31, 2011. Overall two-year mortality was 6.7%. Mean spending at 2 years following CEA was $9,375 (SD $8,817), however it ranged greatly, from $5,539 to $232,099.
Factors Associated with Reduced 2-year Survival
Overall two-year mortality was 6.7%. The strongest predictors of death at 2 years were age >80 years (HR 2.9), congestive heart failure (CHF, HR 2.2), dialysis dependence (HR 4.0) and occluded contralateral internal carotid artery (HR 2.2) (Table 1). Additional risk factors included insulin-requiring diabetes, smoking, COPD, renal insufficiency, and lack of statin use (Table 1).
Table 1.
Cox Model for predicting death at 2 years following carotid endarterectomy in asymptomatic patients.
| Variable | HR | 95% CI | P-Value |
|---|---|---|---|
| Age | |||
| <70 | Reference | - | - |
| 70–79 | 1.5 | 1.2–1.9 | <0.001 |
| ≥ 80 | 2.9 | 2.2–3.8 | <0.001 |
| Insulin Requiring Diabetes | 1.7 | 1.3–2.2 | <0.001 |
| Past or Current Smoking | 1.3 | 1.02–1.7 | 0.037 |
| Congestive Heart Failure | 2.2 | 1.7–2.8 | <0.001 |
| COPD* | 1.8 | 1.5–2.3 | <0.001 |
| Renal Function (%) | |||
| eGFR†≥60 | Reference | - | - |
| eGFR<60 | 1.5 | 1.2–1.9 | <0.001 |
| Dialysis Dependent | 4.0 | 2.2–7.2 | <0.001 |
| Contralateral ICA‡ stenosis | |||
| <50% | Reference | - | - |
| ≥50% | 1.4 | 1.2–1.7 | 0.001 |
| Occluded | 2.2 | 1.5–3.1 | <0.001 |
| Lack of Statin | 1.3 | 1.05–1.6 | 0.019 |
COPD, chronic obstructive pulmonary disease
eGFR, estimated glomerular filtration rate
ICA, internal carotid artery
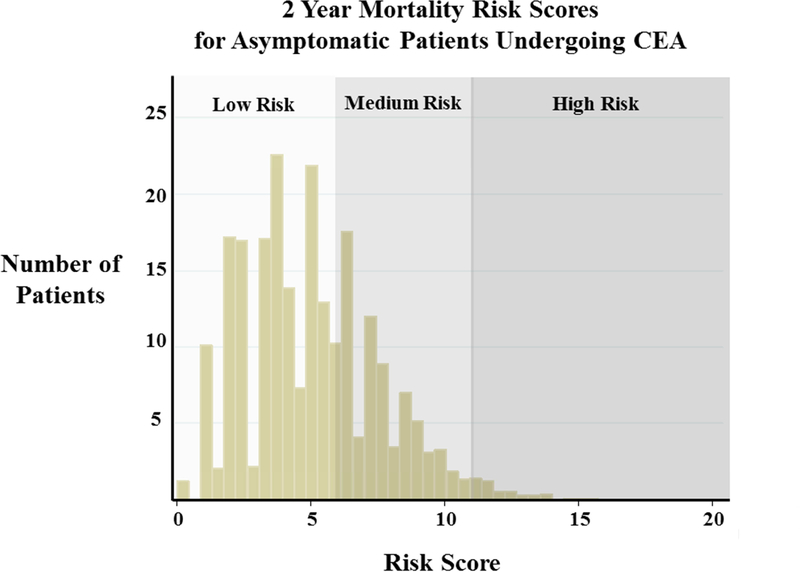
Scores for predicting risk of death at 2 years following CEA were calculated for each individual in the cohort and ranged from 0 to 20. Based on the distribution of assigned risk scores (Figure 1) 1,894 patients were classified as low risk of death at 2 years (score <6), 1,164 patients were designated medium risk of death (score 6–12) and an additional 39 were high-risk (score >12). Two-year mortality for low-risk patients was 4% (N=75). Medium risk patients had an average 2-year mortality of 10% (N=116), and 44% (N=17) of patients designated as high-risk were dead within 2 years following CEA. Patient characteristics for each risk strata are shown in Table 2. As expected, low risk patients were, on average, younger with fewer comorbidities such as hypertension, coronary artery disease (CAD), CHF, diabetes and COPD when compared to medium and high risk patients.
Figure 1:
Distribution of risk scores for predicting 2 year mortality among asymptomatic patients undergoing carotid endarterectomy (CEA).
Table 2.
Patient characteristics by risk strata for death (low, medium, high) at 2 years following carotid endarterectomy.
| Low Risk of Death at 2Y (riskscore <6) (n=1894) | Medium Risk of Death at 2Y (riskscore 6–12) (n=1164) | High Risk of Death at 2Y (riskscore >12) (n=39) | P-Value | |
|---|---|---|---|---|
| Age (mean in years (SD)) | 72.2 (4.9) | 77.4 (6.1) | 78.8 (5.7) | <0.0001 |
| Female (%) | 43.0 | 43.9 | 38.5 | 0.74 |
| Past or Current Smoking (%) | 69.2 | 83.4 | 94.9 | <0.0001 |
| Race (%) | 0.25 | |||
| White | 97.3 | 97.4 | 100.0 | |
| Black | 0.9 | 1.5 | 0.0 | |
| Other | 1.8 | 1.1 | 0.0 | |
| Hypertension (%) | 89.2 | 90.8 | 94.9 | 0. 2 |
| Coronary Artery Disease (%) | 25.0 | 38.3 | 66.7 | <0.0001 |
| Congestive Heart Failure (%) | 1.1 | 17.5 | 79.5 | <0.0001 |
| Diabetes (%) | 27.4 | 35.8 | 61.5 | <0.0001 |
| COPD* (%) | 11.7 | 32.0 | 74.4 | <0.0001 |
| Renal Function (%) | <0.0001 | |||
| eGFR†≥60 | 68.3 | 30.7 | 12.8 | |
| eGFR<60 | 31.7 | 68.3 | 71.8 | |
| Dialysis Dependent | 0.0 | 1.0 | 15.4 | |
| Statin (%) | 74.3 | 88.4 | 94.9 | <0.0001 |
| Contralateral ICA‡ Stenosis (%) | <0.0001 | |||
| ≤ 50% | 65.9 | 39.6 | 38.5 | |
| 51–79% | 27.8 | 44.8 | 35.9 | |
| ≥ 80% | 4.8 | 5.8 | 12.8 | |
| Occluded | 1.5 | 9.8 | 12.8 | |
| ASA§ class (%) | <0.0001 | |||
| Class 1 | 0.7 | 0.2 | 3.7 | |
| Class 2 | 13.3 | 7.7 | 0.0 | |
| Class 3 | 78.9 | 78.0 | 55.6 | |
| Class 4 | 7.2 | 14.1 | 40.7 | |
| Stress test (%) | 0.0 17 | |||
| None Performed | 59.6 | 60.5 | 59.0 | |
| Normal | 31.1 | 28.6 | 17.9 | |
| Abnormal | 9.3 | 10.9 | 23.1 | |
| Use of Shunt (%) | 48.5 | 49.5 | 51.3 | 0.83 |
COPD, chronic obstructive pulmonary disease
eGFR, estimated glomerular filtration rate
ICA, internal carotid artery
ASA, American Society for Anesthesia
Factors Associated with High Cost (highest 10% of costs, >$18,000)
We examined all spending in the two year period following CEA, including spending on the operative procedure, as well as spending on post-operative admissions related to reinterventions or stroke. Costs related to admissions for other diagnoses (such as for management of CAD, CHF, etc) were not included in this analysis. Overall costs averaged a mean of $9,375 (SD $8,817), and ranged from $5,539 to $232,099. The rate of readmission for stroke within 2 years following CEA was 11.9%. The incidence of reintervention by CEA or carotid artery stenting was generally low, with a mean of 6.3%.
Patients were designated as high cost if they were within the highest 10% of costs (>$18,000). Compared to those patients designated as low cost, these high cost patients were significantly more likely to smoke and to have comorbidities such as CAD, CHF, renal insufficiency and contralateral ICA stenosis. These patients were also, on average, classified as more ill based on ASA scoring and were less likely to be taking a statin (Table 3). After adjusting for differences in baseline comorbidities, our multivariate model indicated several independent predictors of high-cost at 2 years including degree of contralateral ICA stenosis (OR for >80% stenosis 19.5) and dialysis dependence (OR 33.4, Table 4). Increasing ASA Class was protective of high cost (compared to ASA Class I, OR for Class 2, 3 and 4 were 0.23, 0.37 and 1.0 respectively). Likewise, statin use and CHF were each protective of high cost (OR 0.7 each). However, this was not statistically significant (Table 4).
Table 3.
Patient characteristics by cost strata at 2 years following carotid endarterectomy (CEA). High cost is defined as >90th percentile of cost ($>18,000).
| Not High Cost (≤$18,000) at 2Y (n=2749) | High Cost (>$18,000) at 2Y (n=348) | P-Value | |
|---|---|---|---|
| Age (mean in years (SD)) | 74.3 (6.0) | 74.0 (5.9) | 0.38 |
| Female (%) | 43.3 | 43.7 | 0.88 |
| Any Smoke (%) | 74.3 | 79.8 | 0.024 |
| Race (%) | 0.77 | ||
| White | 97.5 | 96.8 | |
| Black | 1.1 | 1.4 | |
| Other | 1.5 | 1.7 | |
| Hypertension (%) | 89.6 | 92.0 | 0.17 |
| Coronary Artery Disease (%) | 29.7 | 37.4 | 0.003 |
| Congestive Heart Failure (%) | 7.5 | 14.1 | <0.0001 |
| Diabetes (%) | 30.6 | 34.5 | 0.14 |
| COPD* (%) | 19.7 | 23.6 | 0.089 |
| Renal Function (%) | <0.0001 | ||
| eGFR†≥60 | 54.1 | 48.9 | |
| eGFR<60 | 45.8 | 47.1 | |
| Dialysis Dependent | 0.1 | 4.0 | |
| Statin (%) | 80.4 | 75.6 | 0.03 |
| Contralateral ICA‡ Stenosis (%) | <0.0001 | ||
| ≤ 50% | 58.5 | 33.3 | |
| 51–79% | 33.9 | 37.4 | |
| ≥ 80% | 2.8 | 24.7 | |
| Occluded | 4.8 | 4.6 | |
| ASA§ class (%) | <0.0001 | ||
| Class 1 | 0.5 | 0.7 | |
| Class 2 | 11.8 | 5.7 | |
| Class 3 | 79.1 | 72.3 | |
| Class 4 | 8.5 | 21.3 | |
| Stress test (%) | 0.21 | ||
| None Performed | 60.3 | 57.3 | |
| Normal | 30.0 | 30.0 | |
| Abnormal | 9.7 | 12.7 | |
| CEA type (%) | 0.78 | ||
| Conventional | 88.3 | 88.8 | |
| Eversion | 11.7 | 11.2 | |
| Use of Shunt (%) | 48.4 | 52.9 | 0.12 |
COPD, chronic obstructive pulmonary disease
eGFR, estimated glomerular filtration rate
ICA, internal carotid arterys
ASA, American Society for Anesthesia
Table 4.
Multivariate model for predicting high cost (>$18,000) at 2 years following carotid endarterectomy.
| Variable | OR | 95% CI | P-Value |
|---|---|---|---|
| Contralateral ICA* Stenosis (%) | <0.0001 | ||
| <50% | Reference | ||
| 51–79% | 2.2 | 1.6–2.9 | |
| ≥ 80% | 19.5 | 13.2–28.8 | |
| Occluded | 1.8 | 1.04–3.3 | - |
| ASA† Class | <0.0001 | ||
| Class I | Reference | ||
| Class 2 | 0.23 | 0.04–1.2 | |
| Class 3 | 0.37 | 0.1–1.7 | |
| Class 4 | 1.0 | 0.2–4.9 | - |
| Renal Function (%) | |||
| eGFR‡≥60 | Reference | <0.0001 | |
| eGFR<60 | 1.2 | 0.9–1.5 | |
| Dialysis Dependent | 33.4 | 10.1–110.6 | - |
| Statin | 0.7 | 0.6–1.0 | 0.0546 |
| Congestive Heart Failure | 0.7 | 0.5–1.0 | 0.0514 |
C-index 0.77, H-L goodness of fit: 0.79
ICA, internal carotid artery
ASA, American Society for Anesthesia
eGFR, estimated glomerular filtration rate
Relationships between risk of death, high costs, and outcomes
We examined the relationships between patient-level risk of death within two years following CEA and costs. The results are shown in Table 5. Overall, in-hospital price adjusted Medicare spending was similar among all patients regardless of their risk of death (low risk $7,500; medium risk $8,276; high risk $10,868). However, long-term costs were markedly different across risk strata. Costs accrued by high risk patients were, on average, two times those of low risk patients at 2 years ($17,815 vs $8,801, P=.001). Further, while only 9.5% of low risk patients were designated high cost, nearly one quarter (24.6%) of high risk patients met our criteria for high cost.
Table 5.
Incidence of in-hospital death, death at 2 years, stroke at 2 years and reintervention at 2 years, as well as in-hospital and longterm costs for asymptomatic patients undergoing carotid endarterectomy stratified by risk of death.
| Low Risk of Death at 2Y (riskscore <6) | Medium Risk of Death at 2Y (riskscore 6–12) | High Risk of Death at 2Y (riskscore >12) | |
|---|---|---|---|
| MORTALITY | |||
| In-Hospital Death (n (%)) | 2 (0.1%) | 4 (0.3%) | 1 (2.6% |
| Death at 2 years (n (%)) | 75 (4%) | 116 (10%) | 17 (43.6%) |
| COST | |||
| Mean In-Hospital Cost (SD) | $7,500 (5,654) | $8,276 (6,583) | $10,868 (7,685) |
| Mean Cost at 2 years (SD) | $8,801 (7,550) | $10,025 (8,252) | $17,815 (36,113) |
| Low Cost, ≤$18,000 (n (% in risk strata)) | 1715 (90.5%) | 1005 (86.3%) | 29 (74.4%) |
| High Cost, >$18,000 (n (% in risk strata)) | 179 (9.5%) | 159 (13.7%) | 10 (24.6%) |
| STROKE AND RE-INTERVENTION at 2 YEARS | |||
| Rehospitalization for Stroke (n (%)) | 194 (10.2%) | 168 (14.4%) | 5 (12.8%) |
| Re-Intervention (n (%)) | 109 (5.8%) | 82 (7.0%) | 3 (7.7%) |
Lastly, we examined incidence of readmission for stroke or reintervention by risk strata for death at 2 years (Table 5). Overall, the rate of readmission for stroke within 2 years following CEA was 11.9%. This was not strongly associated with increasing risk strata for mortality (low risk 10.2%, medium 14.4%, and high risk 12.8%). The incidence of reintervention by CEA or carotid artery stenting was generally low, with a mean of 6.3%. Unlike rehospitalization for stoke, however, rates of reintervention increased by risk strata, with high risk patients more likely to undergo reintervention than medium or low-risk patients (7.7% vs 7.0% and 5.8%, respectively).
DISCUSSION
Carotid revascularization is a major cost for Medicare patients. Many have examined the cost-effectiveness of carotid revascularization 17–19 and undoubtedly improved patient selection for CEA could result in significant savings for healthcare payers. In our analysis of more than 3,000 patients undergoing carotid revascularization in the VQI, more than 90% were still alive within two years after surgery, suggesting that most patients selected for carotid revascularization by surgeons in the VQI have been chosen appropriately. However, approximately 8% of patients died within 2 years of surgery, suggesting that these patients may not have benefited from the procedure, which intends to protect against future stroke. Monies spent on these procedures, and subsequent related expenses, may represent costs that are better spent elsewhere.
Factors associated with poor survival following carotid revascularization
The current study established several risk factors for poor long-term survival following carotid revascularization. Specifically, age, diabetes, smoking, CHF, COPD, renal insufficiency, lack of statin therapy, and contralateral ICA stenosis all impact survival among patients undergoing CEA. These findings echo those of our prior work examining 5-year survival following CEA among those registered in the Vascular Study Group of New England 20, and are similar to those of previous studies. For example, Kragsterman et al examined outcomes following over 6,000 CEAs performed for asymptomatic disease in a population based study using the Swedish Vascular Registry and identified four major predictors of decreased 5-year survival: prior vascular surgery (OR 1.8), cardiac disease (OR 1.7), diabetes (OR 2.3), and age (OR 1.5 per 10 years) 21. Other studies also suggest that diabetes 20,22, cardiac disease 20,22 and widespread arterial disease (as indicated by claudication 22, contralateral ICA stenosis 20, 22, and intracranial obstructive lesions 23) affect long-term survival in those patients undergoing CEA.
Pre-operatively identifying patients who will be more costly in the period after surgery
Our data suggest that progression of cerebrovascular disease, especially contralateral stenosis is a central opportunity for reducing complications and cost. These findings also echo those of previous researchers, who have noted significantly higher risks of stroke or death among asymptomatic patients with contralateral stenosis undergoing CEA or CAS. For example, Bennet et al used NSQIP data to show that contralateral ICA stenosis was an independent predictor of stroke or death (OR 3.1) in patients undergoing CEA for asymptomatic disease 24 Our own findings have noted contralateral internal carotid artery stenosis independently associated with restenosis, leading to reintervention or stroke. Notably, this association holds true even in high risk populations: Salomon du Mont in his analysis of 118 octogenarians undergoing CEA noted that even within this high risk population, contralateral ICA stenosis imparted a near five-fold risk of stroke or death (20% vs 4.88%) 25.
Further, one might assume that characteristics that make a patient high risk for death following carotid revascularization, such as CHF, would be associated with higher cost. However, the current study found that CHF and higher ASA class were actually protective of high cost. While these findings are limited by a small number of observations in the high-cost cohort, they may potentially represent the fact that patients with advanced comorbidities, such as advanced CHF, did not live long enough to accrue a significant cost burden.
Pathways forward
Which option is likely to help health systems be most cost-effective in caring for patients with carotid artery disease: avoiding surgery in patients unlikely to survive in the long term after surgery, or avoiding surgery in patients likely to suffer recurrent disease and incur high costs? Our results suggest that better patient selection – at least in terms of survival - is not likely to result in dramatic cost savings, for two reasons. First, only a small proportion – less than 10% - of carotid revascularizations were performed in patients who did not live long enough to benefit from revascularization. Eliminating the costs of these procedures is unlikely to dramatically impact the overall spending on carotid revascularization among Medicare patients. Second, the highest differences in costs were not evident at the time of the initial operation, but instead accumulated later in follow-up, where certain patients’ higher costs were driven by late events such as the need for revisional surgery or treatment for subsequent stroke. These findings suggest that cost savings are likely to be best achieved by better long-term management of risk factors for stroke, rather than eliminating “unnecessary” carotid revascularization.
Given the poor clinical outcomes and high cost associated with patients who have bilateral disease undergoing carotid revascularization, our findings suggest that risk factor modification is central to both primary and secondary prevention of stroke, in both the short and long-term. In a recent review, Constantinou et al summed up the evidence in favor for combination medication therapy with antiplatelet, antihypertensive and anti-diabetic medication, together with smoking cessation to reduce stroke rates among patients with carotid stenosis 26 These findings have been echoed in medical best practice guidelines by several professional organizations. The SAMMPRIS trial (examined patients with intracranial artery stenosis) systematically implemented strict medical guidelines in high-risk symptomatic patients to show superiority of medical therapy alone to carotid artery stenting 27, and it is likely that future trials may show similar benefit among asymptomatic patients.
These findings can help health systems prioritize both medical and surgical treatments for patients with carotid atherosclerosis. Many have hypothesized that eliminating “overuse” of asymptomatic carotid artery surgery in elderly patients who are unlikely to benefit from surgery would result in significant cost savings. While this may be true, our study suggests that optimal medical management of all patients at risk for primary or recurrent carotid artery disease may offer similar, if not greater, savings for health care systems.
Limitations
Our study has several limitations. First, our matching algorithms successfully matched 70% of patients to their respective Medicare claims, meaning that 30% of eligible VQI patients were not matched to their respective Medicare claims. While this match success rate is similar to other efforts, future efforts will seek to increase the success of matching algorithms using identifiers within the structure of our patient safety organization. Second, our long-term outcomes suffer from the ability to discern laterality - whether a late event refers to a second carotid intervention on the same side as the original revascularization, or whether it represents a second procedure on the contralateral side. While ICD-10 coding changes, which incorporate laterality, will help eliminate this limitation in future work 29, our estimates here describing patients with recurrent disease represent a heterogeneous population that includes both recurrence (about 20% of all endarterectomies from previous estimates 16) and progression of contralateral disease. While these are obviously different events, their final result – contribution to the cost of caring for patients with aggressive cerebrovascular disease – is similar in that they both raise the total costs in our cohort. Along these same lines, the current study is limited by inability to account for underlying etiology of stroke following CEA, recognizing that 87% of strokes are ischemic in origin, and roughly 20% are due to extracranial carotid artery disease30. Finally, our study considers costs in Medicare patients alone, although this is a commonly used proxy for overall costs of care 15.
Conclusions
More than 90% of patients selected for carotid revascularization live well beyond 2 years after surgery, although factors associated with poor survival can be used to improve patient selection and limit unnecessary carotid revascularization. While eliminating these procedures is important to keep patients from undergoing unneeded treatments, our work suggests that policymakers interested in achieving cost savings are more likely to reach this goal by focusing on better long-term management of risk factors for stroke rather than focusing on eliminating “unnecessary” carotid revascularization.
Supplementary Material
Acknowledgments
Funding Sources: Dr. Goodney was supported by a K08 from NHLBI (1K08HL05676-01), and an R21 from AHRQ (HS021581-01A1).
Footnotes
Disclosures: We have no disclosures relevant to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wallaert JB, Cronenwett JL, Bertges DJ, et al. Optimal selection of asymptomatic patients for carotid endarterectomy based on predicted 5-year survival. J Vasc Surg 2013; 58(1):112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallaert JB, De Martino RR, Finlayson SR, et al. Carotid endarterectomy in asymptomatic patients with limited life expectancy. Stroke 2012; 43(7): 1781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gould DA, Birkmeyer JD. Efficacy versus effectiveness of carotid endarterectomy. Eff Clin Pract 1999; 2(1):30–6. [PubMed] [Google Scholar]
- 4.Moore WS, Barnett HJ, Beebe HG, et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the ad hoc Committee, American Heart Association. Stroke 1995; 26(1):188–201. [DOI] [PubMed] [Google Scholar]
- 5.Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal- carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators [see comments]. New England Journal of Medicine 2000; 342(23):1693–700. [DOI] [PubMed] [Google Scholar]
- 6.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study [see comments]. JAMA 1995; 273(18):1421–8. [PubMed] [Google Scholar]
- 7.Clinical advisory: carotid endarterectomy for patients with asymptomatic internal carotid artery stenosis. Stroke 1994; 25(12):2523–4. [DOI] [PubMed] [Google Scholar]
- 8.Role of carotid endarterectomy in asymptomatic carotid stenosis. A Veterans Administration Cooperative Study. Stroke 1986; 17(3):534–9. [DOI] [PubMed] [Google Scholar]
- 9.Yuo TH, Goodney PP, Powell RJ, et al. “Medical high risk” designation is not associated with survival after carotid artery stenting. J Vasc Surg 2008; 47(2):356–62. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke 2006; 37(6):1583–633. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2006; 113(24):e873–923. [DOI] [PubMed] [Google Scholar]
- 12.Albers GW, Hart RG, Lutsep HL, et al. AHA Scientific Statement. Supplement to the guidelines for the management of transient ischemic attacks: A statement from the Ad Hoc Committee on Guidelines for the Management of Transient Ischemic Attacks, Stroke Council, American Heart Association. Stroke 1999; 30(11):2502–11. [DOI] [PubMed] [Google Scholar]
- 13.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56(25):e50–103. [DOI] [PubMed] [Google Scholar]
- 14.Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM /SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation 2011; 124(4):489–532. [DOI] [PubMed] [Google Scholar]
- 15.Gottlieb DJ, Zhou W, Song Y, et al. Prices don’t drive regional Medicare spending variations. Health Aff (Millwood) 2010; 29(3):537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimakakos PB, Kotsis TE, Tsiligiris B, et al. Comparative results of staged and simultaneous bilateral carotid endarterectomy: a clinical study and surgical treatment. Cardiovasc Surg 2000; 8(1):10–7. [DOI] [PubMed] [Google Scholar]
- 17.Cronenwett JL, Birkmeyer JD, Nackman GB, et al. Cost-effectiveness of carotid endarterectomy in asymptomatic patients. J Vasc Surg 1997; 25(2):298–309; discussion 310–1. [DOI] [PubMed] [Google Scholar]
- 18.Benade MM, Warlow CP. Cost of identifying patients for carotid endarterectomy. Stroke 2002; 33(2):435–9. [DOI] [PubMed] [Google Scholar]
- 19.McDonald RJ, Kallmes DF, Cloft HJ. Comparison of hospitalization costs and Medicare payments for carotid endarterectomy and carotid stenting in asymptomatic patients. AJNR Am J Neuroradiol 2012; 33(3):420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallaert JB, Cronenwett JL, Bertges DJ, et al. Optimal selection of asymptomatic patients for carotid endarterectomy based on predicted 5-year survival. J Vasc Surg 2013; 58(1): 112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kragsterman B, Bjorck M, Lindback J, et al. Long-term survival after carotid endarterectomy for asymptomatic stenosis. Stroke 2006; 37(12):2886–91. [DOI] [PubMed] [Google Scholar]
- 22.Cohen SN, Hobson RW 2nd, Weiss DG, et al. Death associated with asymptomatic carotid artery stenosis: long-term clinical evaluation. VA Cooperative Study 167 Group. J Vasc Surg 1993; 18(6):1002–9; discussion 1009–11. [PubMed] [Google Scholar]
- 23.Branchereau A, Ede B, Magnan PE, et al. Surgery for asymptomatic carotid stenosis: a study of three patient subgroups. Ann Vasc Surg 1998; 12(6):572–8. [DOI] [PubMed] [Google Scholar]
- 24.Bennett KM, Scarborough JE, Shortell CK. Predictors of 30-day postoperative stroke or death after carotid endarterectomy using the 2012 carotid endarterectomy-targeted American College of Surgeons National Surgical Quality Improvement Program database. J Vasc Surg 2014. [DOI] [PubMed] [Google Scholar]
- 25.Salomon du Mont L, Ravelojaona M, Puyraveau M, et al. Carotid endarterectomy in octogenarian: short- and midterm results. Ann Vasc Surg 2014; 28(4):917–23. [DOI] [PubMed] [Google Scholar]
- 26.Constantinou J, Jayia P, Hamilton G. Best evidence for medical therapy for carotid artery stenosis. J Vasc Surg 2013; 58(4): 1129–39. [DOI] [PubMed] [Google Scholar]
- 27.Derdeyn CP, Chimowitz MI, Lynn MJ, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet 2014; 383(9914):333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs JP, Edwards FH, Shahian DM, et al. Successful linking of the Society of Thoracic Surgeons adult cardiac surgery database to Centers for Medicare and Medicaid Services Medicare data. Ann Thorac Surg 2010; 90(4): 1150–6; discussion 1156–7. [DOI] [PubMed] [Google Scholar]
- 29.Steindel SJ. International classification of diseases, 10th edition, clinical modification and procedure coding system: descriptive overview of the next generation HIPAA code sets. J Am Med Inform Assoc 2010; 17(3):274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cronenwett JaJ, KW. Rutherford’s Vascular Surgery. Vol. 2: Elsevier Saunders, 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.