Abstract
Background/Aims
To evaluate teleophthalmological assessment of choroidal and iris nevi (tele-oncology) compared to traditional in-person clinical evaluation for detection of either axial or basal growth.
Methods
This is a validation study. All 97 eyes of 99 patients were evaluated with an in-person ocular oncology visit utilizing standard testing, and subsequently had a tele-oncology evaluation with the standardized tests. The tele-oncology reviewer was blinded to the in-person examination findings. The primary study outcome was detection of nevus growth on tele-oncology compared to in-person clinical examination.
Results
Patients had a mean age of 61 years and the majority had nevi located in the choroid (n = 87; 88%). The most common diagnosis was a low-risk nevus (n = 38; 44%). By tele-oncology assessment, 11 eyes showed growth. Ten of these patients had growth confirmed on in-person clinical examination. Resultantly, tele-oncology assessment of choroidal and iris nevi growth had a sensitivity of 100%, specificity of 99%, positive predictive value (PPV) of 91%, and negative predictive value (NPV) of 100%.
Conclusions
The results of this study suggest that tele-oncology is a safe platform for monitoring choroidal and iris nevi for growth, with excellent sensitivity, specificity, PPV, and NPV.
Keywords: Telemedicine, Ocular oncology, Choroidal nevus, Iris nevus
Introduction
Technological advances over the past two decades have allowed for innovative health care delivery platforms to be developed. Telemedicine is defined as “the use of electronic information and communications technologies to provide and support health care when distance separates the participants” [1]. As many clinical decisions and diagnosis in ophthalmology are rooted in visual observations, it is a specialty that lends itself particularly well to telemedicine. Resultantly, several studies have evaluated the role of teleophthalmology for screening of various ophthalmic conditions, including diabetic retinopathy [2, 3, 4], retinopathy of prematurity [5], glaucoma screening [6], and more. The subspecialized training for ocular oncology and the rarity of the diseases has resulted in relatively few care centres with patients often traveling long distances to receive an assessment. Telemedicine, if safe, may therefore provide a useful tool to assist in the care of these distant patients.
Recently, our group published a pilot study investigating the validity and safety of teleophthalmology assessment of choroidal and iris nevi. We found that when performed by a trained ocular oncologist, this tool had 100% sensitivity and negative predictive value (NPV) to evaluate growth [7]. This validation study was conducted to more rigorously assess the reliability, accuracy, and safety of a tele-oncology platform for the assessment and monitoring of iris and choroidal nevi.
Methods
Health Research Ethics Board of Alberta Cancer Committee granted ethics approval. Following this, 100 eyes of 98 consecutive patients seen for an in-person ocular oncology evaluation with a diagnosis of either choroidal or iris nevi were identified. Patients were identified by reviewing clinic schedules in reverse chronological order until 100 eyes were enrolled. One patient was subsequently excluded for being incorrectly coded on the clinic schedule as a choroidal nevus, as further review of imaging and the patients chart revealed the actual diagnosis to be choroidal melanoma. The remaining 99 eyes of 97 patients were included in this validation study and their tele-oncology images were reviewed in a standardized way by one blinded author (K.R.). Similarly, all patients had standardized follow-up schedule and testing that was decided upon prospectively based on the risk level of their lesion and the length of time that they had already been followed. All patients seen for an in-person assessment at our ocular oncology clinic have the same imaging protocol as patients who are followed by teleophthalmology. This protocol consists of standard fundus or slit-lamp photos, B-scan ultrasound (for choroidal lesions) or ultrasound biomicroscopy (UBM) (for iris lesions), and OCT through the macula and over the choroidal lesion. All imaging was performed by accredited ophthalmic technicians and ultrasonographers. These images were then uploaded to a centralized software platform (Topcon Synergy), from which they were reviewed.
The reviewing author (K.R.) remained blinded to the in-person ocular oncology visit (which was conducted by the senior author, E.W.) by reviewing all imaging from that date as though it were a teleophthalmology visit, prior to opening the chart. Images and ultrasounds were assessed for growth (either basal dimensions or apical height) or development of a new risk factor for growth, such as subretinal fluid or orange pigment. For choroidal nevi, growth was further divided into “definite” or “equivocal.” Definite growth was defined as an increase in basal dimensions by ≥250 μm over a 5-year period. This cutoff is in keeping with the median rate of nevus enlargement of 0.06 mm/year reported in a long-term follow-up study by Mashayekhi et al. [8]. Equivocal growth was defined as an increase in basal dimensions by < 250 μm over a 5-year period. Growth on ultrasound was also defined as definite (≥0.5 mm increase in thickness) or equivocal (< 0.5 mm increase in thickness) over a 5-year period. As not all patients were followed for 5 years, clinical interpretation of trends in increasing basal diameter or apical height consistent with a rate of change that would satisfy the above criteria were also categorized as definite or equivocal growth. For iris nevi, an increase in basal diameter of ≥250 μm over a 5-year period or an increase in thickness on UBM ≥0.3 mm was defined as definite growth.
Choroidal nevi were also stratified into low (≤1 clinical risk factor for growth), medium (2 risk factors), or high (≥3 risk factors) based on the previously published clinical factors predictive of growth of small choroidal melanocytic tumors by Shields et al. [9]. Once all patients had been reviewed, positive predictive value (PPV), NPV, specificity, and sensitivity for detection of growth by tele-oncology assessment were calculated.
Results
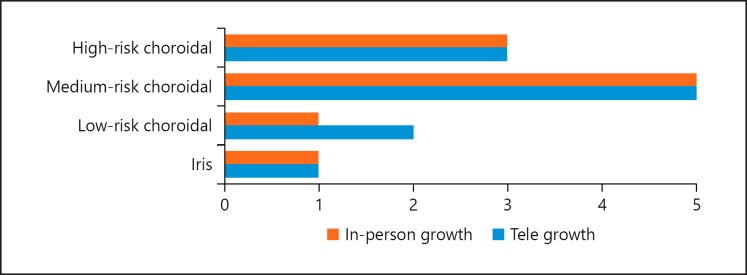
A total of 99 eyes of 97 patients with a mean age of 61 years (±SD 18 years) were included in this study. Forty-nine patients were women and 48 were men. A relatively small proportion of the nevi were located in the iris (n = 12) and the majority were located in the choroid (n = 87). Of the choroidal nevi, nearly half (n = 38; 44%) were classified as low risk, with the remainder being either medium (n = 33; 15%) or high risk (n = 16; 18%) (Table 1).
Table 1.
Diagnosis of nevi subdivided by location and risk stratification
| Diagnosis | Cases (n = 99), n (%) | Patients who showed growth on tele-oncology assessment | Patients who showed growth on in-person assessment |
|---|---|---|---|
| Iris nevi | 12 (12) | 1 | 1 |
| Choroidal nevi | 87 (88) (n = 87) | 11 | 10 |
| Low risk | 38 (44) | 2 | 1 |
| Medium risk | 33 (15) | 5 | 5 |
| High risk | 16 (18) | 3 | 3 |
A total of 11 patients were classified as having shown growth (either axial and/or basal) on tele-oncology assessment, given that some patients demonstrated growth in both parameters. Ten of these patients had growth (either axial and/or basal) confirmed on in-person clinical evaluation. Of these, 3 (30%) were categorized as high risk, 5 (50%) were medium risk, 1 (10%) was low risk, and 1 (10%) was an iris nevus. The one patient who was felt to have shown growth on tele-oncology assessment but not on in-person clinical exam was diagnosed as a low-risk choroidal nevus (Fig. 1). None of the patients included in this series developed new orange pigment or subretinal fluid (Table 2). There were no false negatives for either kind of growth and a total of one false positive for any (either axial and/or basal) growth (patient 8) (Table 3). One patient was felt to show growth in both axial and basal dimensions on tele-oncology assessment (patient 4); however, only basal growth (not axial) was confirmed on in-person clinical examination (Table 4). Resultantly, tele-oncology had a 100% sensitivity and 100% NPV for the detection of either axial and/or basal growth. Similarly, tele-oncology had a very high specificity (99%) and PPV (91%) for detection of any growth (axial and/or basal), respectively (Table 5). When only the iris lesions were included, tele-oncology had a sensitivity and specificity of 100% for detecting growth. Taking only the choroidal lesions into account, the sensitivity was 100% and specificity was 99%.
Fig. 1.
Comparison of growth detected on tele-oncology versus in-person evaluation.
Table 2.
Comparison of tele-oncology versus in-person visit determinations of growth and development of new risk factors
| Tele-oncology visit, % | In-person visit, % | |
|---|---|---|
| Basal growth | ||
| No growth | 93 (94) | 94 (95) |
| Equivocal | 3 (3) | 2 (2) |
| Definite | 3 (3) | 3 (3) |
| Axial growth | ||
| No growth | 92 (93) | 93 (94) |
| Equivocal | 4 (4) | 4 (4) |
| Definite | 3 (3) | 2 (2) |
| Any growth | ||
| No growth | 88 (89) | 89 (90) |
| Growth* | 11 (11) | 10 (10) |
| New OP | 0 (0) | 0 (0) |
| New SRF | 0 (0) | 0 (0) |
Growth includes axial and/or basal, definite or equivocal growth. OP, orange pigment; SRF, subretinal fluid.
Table 3.
Two-by-two table for any (either axial and/or basal) growth
| Tele-oncology visit | In-person visit |
||
|---|---|---|---|
| any growth | no growth | total number | |
| Any growth | 10 | 1 | 11 |
| No growth | 0 | 88 | 88 |
| Total number | 10 | 89 | 99 |
Table 4.
Breakdown of patients showing axial, basal, or any growth on tele-oncology assessment correlated with in-person clinical conclusions
| Patient | Tele-oncology assessment |
In-person clinical assessment |
||||
|---|---|---|---|---|---|---|
| axial growth | basal growth | any growth | axial growth | basal growth | any growth | |
| 1 | Definite | Definite | YES | Definite | Definite | YES |
| 2 | Equivocal | No growth | YES | Equivocal | No growth | YES |
| 3 | No growth | Definite | YES | No growth | Definite | YES |
| 4 | Definite | Definite | YES | No growth | Definite | YES |
| 5 | Definite | No growth | YES | Definite | No growth | YES |
| 6 | No growth | Equivocal | YES | No growth | Equivocal | YES |
| 7 | Equivocal | No growth | YES | Equivocal | No growth | YES |
| 8 | No growth | Equivocal | YES | No growth | No growth | NO |
| 9 | No growth | Equivocal | YES | No growth | Equivocal | YES |
| 10 | Equivocal | No growth | YES | Equivocal | No growth | YES |
| 11 | Equivocal | No growth | YES | Equivocal | No growth | YES |
Table 5.
Prevalence, sensitivity, specificity, and positive and negative predictive values for tele-oncology assessment of axial or basal growth for iris and choroidal nevi
| Axial growth | Basal growth | Any growth | |
|---|---|---|---|
| Prevalence | 6% | 5% | 10% |
| Sensitivity | 100% | 100% | 100% |
| Specificity | 99% | 99% | 99% |
| Positive predictive value | 86% | 83% | 91% |
| Negative predictive value | 100% | 100% | 100% |
Discussion
Recently, our group published a pilot study on the use of teleophthalmology for the monitoring of iris and choroidal nevi [7]. This validation study has confirmed that teleophthalmology assessment of choroidal and iris nevi has a sensitivity and NPV of 100% for detection of growth. While our study sample size was relatively small, the results suggest that tele-oncology performed by an ophthalmologist may be an effective modality by which to monitor patients with iris or choroidal nevi. One other group has previously utilized teleophthalmology as a virtual platform to assess new referrals for melanocytic lesions. Balaskas et al. [10] evaluated a virtual service for rapid assessment of these patients and concluded that this platform may be feasible, safe, and cost-effective.
A recent review of the current state of teleophthalmology in the United States noted that teleophthalmology may allow for earlier detection and more reliable monitoring of ocular conditions and has the potential to improve access to care and adherence to evidence-based protocols [11]. Additionally, teleophthalmology has the ability to increase access to subspecialty care, especially for people living in remote locations. In 2012, Resnikoff et al. [12] published a report stating that despite 18 countries having 100 ophthalmologists per million population, 23 countries had less than 1 ophthalmologist per million population. In fact, even within countries such as Canada, in certain areas such as the territories, the ratio of ophthalmologists drops from the national average of 3.35 to 0.89 ophthalmologists per 100,000 population [13].
Several studies have reported that the incidence of choroidal nevi in the general population ranges from 1.4 to 6.5% [14, 15, 16]. However, in a large series of patients followed at a tertiary care center, nevus growth into melanoma occurred in 2, 9, and 13% of eyes at 1, 5, and 10 years, respectively [17]. Despite many advances in our ability to locally treat uveal melanoma, we have yet to develop an effective treatment to reduce the risk of metastasis, or effectively treat it when it occurs [18, 19, 20]. It has been well established that larger tumor size is associated with a greater risk of metastasis and melanoma-specific mortality [21, 22, 23, 24, 25, 26]. Perhaps improved monitoring of nevi for growth suggestive of malignant behavior via teleophthalmology screening platforms will allow for earlier treatment of smaller choroidal melanomas than has historically been possible.
Although teleophthalmology appears to be a cost-effective method of screening for both diabetic retinopathy and retinopathy of prematurity, there is often a large initial cost associated with setting up this platform [2], which may serve as a barrier to its implementation. Additionally, it should be noted that all of the imaging in this study was performed by accredited ophthalmic technicians and ultrasonographers and read by an ophthalmologist specializing in ocular oncology. The follow-up schedule for patients included in this study was not universally standardized; thus, it is possible that the reviewing author could have been biased to concluding a lesion had grown if the patient had received closer follow-up. Yet, the follow-up interval would be available for the clinician utilizing telemedicine, and thus, these findings should represent real-world accuracy. While barriers to implementation may exist, embracing this technology, which we have proven to be a safe and sensitive modality for monitoring iris and choroidal nevi, will improve access to subspecialist care. Moreover, it may increase adherence to evidence-based monitoring of iris and choroidal nevi for growth. This will potentially allow for earlier treatment of smaller uveal melanomas, which may improve patient outcomes with regard to metastasis and melanoma-specific mortality.
Disclosure Statement
No funding was received for this study. None of the authors have any financial disclosures to make or proprietary interests/conflicts of interest to declare.
Statement of Ethics
Health Research Ethics Board of Alberta Cancer Committee granted ethics approval.
References
- 1.Field M. Institute of Medicine (US) Committee on Evaluating Clinical Applications of Telemedicine: A Guide to Assessing Telecommunications in Health Care. 1996 http://www.nap.edu/catalog/5296.html. [PubMed] [Google Scholar]
- 2.Au A, Gupta O. The economics of telemedicine for vitreoretinal diseases. Curr Opin Ophthalmol. 2011 May;22((3)):194–8. doi: 10.1097/ICU.0b013e3283459508. [DOI] [PubMed] [Google Scholar]
- 3.Jones S, Edwards RT. Diabetic retinopathy screening: a systematic review of the economic evidence. Diabet Med. 2010 Mar;27((3)):249–56. doi: 10.1111/j.1464-5491.2009.02870.x. [DOI] [PubMed] [Google Scholar]
- 4.Kirkizlar E, Serban N, Sisson JA, Swann JL, Barnes CS, Williams MD. Evaluation of telemedicine for screening of diabetic retinopathy in the Veterans Health Administration. Ophthalmology. 2013 Dec;120((12)):2604–10. doi: 10.1016/j.ophtha.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 5.Quinn GE, Ying GS, Daniel E, Hildebrand PL, Ells A, Baumritter A, et al. e-ROP Cooperative Group Validity of a telemedicine system for the evaluation of acute-phase retinopathy of prematurity. JAMA Ophthalmol. 2014 Oct;132((10)):1178–84. doi: 10.1001/jamaophthalmol.2014.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas SM, Jeyaraman MM, Hodge WG, Hutnik C, Costella J, Malvankar-Mehta MS. The effectiveness of teleglaucoma versus in-patient examination for glaucoma screening: a systematic review and meta-analysis. PLoS One. 2014 Dec;9((12)):e113779. doi: 10.1371/journal.pone.0113779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lapere S, Weis E. Tele -ophthalmology for the monitoring of choroidal and iris nevi: a pilot study. Can J Ophthalmol Forthcoming. 2017 doi: 10.1016/j.jcjo.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 8.Mashayekhi A, Siu S, Shields CL, Shields JA. Slow enlargement of choroidal nevi: a long-term follow-up study. Ophthalmology. 2011 Feb;118((2)):382–8. doi: 10.1016/j.ophtha.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Shields CL, Cater J, Shields JA, Singh AD, Santos MC, Carvalho C. Combination of clinical factors predictive of growth of small choroidal melanocytic tumors. Arch Ophthalmol. 2000 Mar;118((3)):360–4. doi: 10.1001/archopht.118.3.360. [DOI] [PubMed] [Google Scholar]
- 10.Balaskas K, Gray J, Blows P, Rajai A, Flaye D, Peto T, et al. Management of choroidal naevomelanocytic lesions: feasibility and safety of a virtual clinic model. Br J Ophthalmol. 2016 May;100((5)):665–70. doi: 10.1136/bjophthalmol-2015-307168. [DOI] [PubMed] [Google Scholar]
- 11.Rathi S, Tsui E, Mehta N, Zahid S, Schuman JS. The Current State of Teleophthalmology in the United States. Ophthalmology. 2017 Dec;124((12)):1729–34. doi: 10.1016/j.ophtha.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Resnikoff S, Felch W, Gauthier TM, Spivey B. The number of ophthalmologists in practice and training worldwide: a growing gap despite more than 200,000 practitioners. Br J Ophthalmol. 2012 Jun;96((6)):783–7. doi: 10.1136/bjophthalmol-2011-301378. [DOI] [PubMed] [Google Scholar]
- 13.Bellan L, Buske L, Wang S, Buys YM. The landscape of ophthalmologists in Canada: present and future. Can J Ophthalmol. 2013 Jun;48((3)):160–6. doi: 10.1016/j.jcjo.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Sumich P, Mitchell P, Wang JJ. Choroidal nevi in a white population: the Blue Mountains Eye Study. Arch Ophthalmol. 1998 May;116((5)):645–50. doi: 10.1001/archopht.116.5.645. [DOI] [PubMed] [Google Scholar]
- 15.Greenstein MB, Myers CE, Meuer SM, Klein BE, Cotch MF, Wong TY, et al. Prevalence and characteristics of choroidal nevi: the multi-ethnic study of atherosclerosis. Ophthalmology. 2011 Dec;118((12)):2468–73. doi: 10.1016/j.ophtha.2011.05.007. 10.1016/j.ophtha.2011.05.007.Prevalence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng CH, Wang JJ, Mitchell P, Amirul Islam FM, Wong TY. Prevalence and characteristics of choroidal nevi in an Asian vs white population. Arch Ophthalmol. 2009 Mar;127((3)):314–9. doi: 10.1001/archophthalmol.2008.625. [DOI] [PubMed] [Google Scholar]
- 17.Shields CL, Furuta M, Berman EL, Zahler JD, Hoberman DM, Dinh DH, et al. Choroidal nevus transformation into melanoma: analysis of 2514 consecutive cases. Arch Ophthalmol. 2009 Aug;127((8)):981–7. doi: 10.1001/archophthalmol.2009.151. [DOI] [PubMed] [Google Scholar]
- 18.Harbour JW, Chao DL. A molecular revolution in uveal melanoma: implications for patient care and targeted therapy. Ophthalmology. 2014 Jun;121((6)):1281–8. doi: 10.1016/j.ophtha.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pereira PR, Odashiro AN, Lim LA, Miyamoto C, Blanco PL, Odashiro M, et al. Current and emerging treatment options for uveal melanoma. Clin Ophthalmol. 2013;7:1669–82. doi: 10.2147/OPTH.S28863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weis E, Salopek TG, McKinnon JG, Larocque MP, Temple-Oberle C, Cheng T, et al. Management of uveal melanoma: a consensus-based provincial clinical practice guideline. Curr Oncol. 2016 Feb;23((1)):e57–64. doi: 10.3747/co.23.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cancer Staging Manual AJ. Springer International Publishing; 2017. 8th ed. [Google Scholar]
- 22.Corrêa ZM, Augsburger JJ. Independent Prognostic Significance of Gene Expression Profile Class and Largest Basal Diameter of Posterior Uveal Melanomas. Am J Ophthalmol. 2016 Feb;162:20–27.e1. doi: 10.1016/j.ajo.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Walter SD, Chao DL, Feuer W, Schiffman J, Char DH, Harbour JW. Prognostic Implications of Tumor Diameter in Association With Gene Expression Profile for Uveal Melanoma. JAMA Ophthalmol. 2016 Jul;134((7)):734–40. doi: 10.1001/jamaophthalmol.2016.0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shields CL, Furuta M, Thangappan A, Nagori S, Mashayekhi A, Lally DR, et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009 Aug;127((8)):989–98. doi: 10.1001/archophthalmol.2009.208. [DOI] [PubMed] [Google Scholar]
- 25.Augsburger JJ, Gamel JW. Clinical prognostic factors in patients with posterior uveal malignant melanoma. Cancer. 1990 Oct;66((7)):1596–600. doi: 10.1002/1097-0142(19901001)66:7<1596::aid-cncr2820660726>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 26.Walter S, Caho D, Schiffman J, Feuer W, Harbour J. Tumor diameter contributes prognostic information that enhances the accuracy of gene expression profile molecular classification in uveal melanoma. Invest Ophthalmol Vis Sci. Abstract. 2015;4:56. [Google Scholar]