Abstract
Spices and herbs have antioxidant, anti-inflammatory, and anti-microbial properties, amongst others. These characteristics are attributable to their composition, such as high polyphenol and flavonoid contents that are responsible for their antioxidative properties. Methanolic extracts of Ocimum basilicum (OB), Xylopia aethiopica (XA), and Piper guineensis (PG) were evaluated to profile their phenolic compounds and in vitro antioxidant properties. High performance liquid chromatography with diode-array detection phenolic compounds profiling revealed that quercetin, quercitrin, and isoquercitrin are the most prevalent phenolic compound in OB, XA, and PG, respectively. All the extracts possessed good antioxidant activity. XA showed the highest total phenolic content of 29.50 mg gallic acid equivalents/g, a total flavonoid content of 21.17 mg quercetin equivalents/g, 2,2-diphenyl-1-picrylhydrazyl and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) radical scavenging abilities of 29% and 88.23%, respectively, and a nitric oxide scavenging activity of 44.13 mg/g. Thus, the XA methanolic extract demonstrated a high content of phenolic compounds and significant antioxidative properties, with prospective potency to prevent oxidative damage and promote better cardiovascular health.
Keywords: HPLC-DAD, spices, antioxidant, phenolic fingerprinting
INTRODUCTION
Spices are herbs which contain complex mixtures of phytochemicals with characteristic odours and flavours. Spices are rich sources of phytochemicals, including phenolic compounds, which confer numerous health benefits including antioxidant and anti-diabetic properties, as well as anti-inflammatory activity (Re et al., 1999). Free radicals are a consequence of normal metabolic activity; they play an important role in the development of metabolic disorders and adversely affect quality of life. Spices can protect against the effects of reactive oxygen species (Shan et al., 2005). Phytochemicals found in spices produce a definite physiological non-nutritive action on the human body, which act as protective barriers against disease (Wang et al., 2013).
Ocimum basilicum (OB) popularly known as sweet basil or basil is one of the most common herbs in Nigeria, belonging to the Lamiaceae family; OB is rich in phenolic compounds, flavonol-glycosides, and anthocyanin (Halliwell and Gutteridge, 1989). Basil is used in industrial processing as a flavouring or preservative agent. Although different parts of the basil plant have various uses, the leaves are the most utilized part of the plant, and are often used as a spice in both the fresh and dried forms.
Piper guineensis (PG), also known as African black pepper, is another common spice in Nigeria. PG belongs to the Piperaceae family and is commonly consumed for its nutritional and therapeutic properties. PG is also useful in traditional medicine for the treatment of various disease conditions (Adefegha et al., 2017), and in the beverage and pharmaceutical industries as a flavouring and preservative agent (Agarwal et al., 2013).
Xylopia aethiopica (XA), also known as Negro pepper, is used for both culinary and medicinal purposes. Ethanolic and acetone extracts of XA have been reported to have antioxidant, immunomodulatory, hypolipidemic, and anti-diabetic properties (Lee and Scagel, 2009; Adefegha and Oboh, 2012; Oso et al., 2018).
The importance of phytochemicals derived from plant materials in drug synthesis and alternative medicine is growing globally. World Health Organization reported that plant medicine is recognized as an important healthcare delivery system for a large number of the world’s population (Sasidharan et al., 2009). Extraction is the main step for recovering and isolating phytochemicals from plant materials. This can be achieved using aqueous, organic, or aqueous-organic solvents. Previous studies have investigated spice extracts extracted using ethanol, methanol, water, or a combination of water and organic solvent. However, limited information is available regarding phenolic profiling of acidified methanol extract from the spices using high performance liquid chromatography with diode-array detection (HPLC-DAD). This study sought to investigate the phytochemicals of medicinal and nutritional importance from three prominent Nigerian spices, extracted using acidified methanol.
MATERIALS AND METHODS
Chemicals and reagents
Analytical grade chemicals were used for both HPLC and spectrophotometeric analysis. Deoxy-D-ribose, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) diammonium salt, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Methanol, formic acid, caffeic acid, catechin, and epicatechin were purchased from Merck KGaA (Darmstadt, Germany). Naringin (4H-1-benzopyran-4-one, 7-[[2-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl] oxy]-2,3-dihydro-5-hydroxy-2-(4-hydroxyphenyl)-, (2S)-), quercetin (3,5,7,3′,4′-pentahydroxyflavone), quercitrin (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-4H-chromen-4-one), kaempferol (3,4′,5,7-tetrahydroxyflavone), luteolin (3′,4′,5,7-tetrahydroxyflavone), and rutin (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 6-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranoside) were acquired from Sigma-Aldrich Co.. All other chemicals were obtained from standard chemical suppliers.
Preparation of plant extract
Leaves of OB were harvested from a research garden in Akure. XA seeds and PG were purchased from Oba market, Akure, Ondo State, Nigeria. The three spices were oven-dried separately at 30±2°C to constant weight, and milled to coarse powder. Acidified methanolic (10 mL glacial acetic acid+90 mL methanol) extraction (1:10 w/v) was then carried out, powdered samples (3 g) were added to 30 mL acidified methanol and stirred on the magnetic stirrer for 2 h. The resulting mixture was centrifuged at 4,000 g for 5 min. Residue extraction was carried out three times using 30 mL of acidified methanol, through stirring and centrifugation. The supernatants were pooled, concentrated using a rotary evaporator, and dried prior to further analysis.
HPLC-DAD analysis
HPLC-DAD was performed using a Shimadzu Prominence Auto Sampler HPLC (SIL-20A; Shimadzu, Kyoto, Japan), equipped with Shimadzu LC-20AT reciprocating pumps connected to a DGU 20A5 degasser with a CBM 20A integrator, SPD-M20A diode array detector, and LC solution 1.22 SP1 software (Shimadzu).
Reverse phase chromatographic analyses were carried out under gradient conditions using a C18 column (4.6 mm×150 mm, 5 μm diameter particles, Dikma Technologies Inc., Beijing, China) according to the modified method of Boligon et al. (2012). The mobile phase consisted of double distilled water containing 1% acetic acid (buffer A) and acetonitrile (buffer B). The gradient program was started with 13% of buffer B for 10 min, and was then changed to obtain 20%, 30%, 50%, 70%, and 100% buffer B at 20, 30, 40, 50, and 60 min, respectively. Sample extracts were analysed at a concentration of 20 mg/mL. The flow rate was 0.6 mL/min, the injection volume was 40 μL and the analysis wavelength was 254 nm to 327 nm. The buffers and extract were filtered through 0.45 μm membrane filter (Millipore, Burlington, MA, USA), and were degassed using an ultrasonic bath (25°C; 10 min) prior to use. Stock solutions of standards references were prepared in the HPLC mobile phase at a concentration range of 0.030~0.250 mg/mL for flavonoids and 0.050~0.350 mg/mL for phenolic acids. Identification of these compounds was performed by comparing retention time and UV absorption spectra with those of commercial standards. The chromatography peaks were confirmed by comparing the retention times with those of reference standards and by the DAD spectra (200 to 500 nm). All chromatography operations were carried out at ambient temperature and in triplicate. The limit of detection (LOD) and limit of quantification (LOQ) were calculated based on the standard deviation of the responses and the slope using three independent analytical curves, as defined by Sabir et al. (2012). LOD and LOQ were calculated as 3.3 and 10 σ/S, respectively, where σ is the standard deviation of the response and S is the slope of the calibration curve.
Determination of antioxidant properties
Total phenolic content (TPC)
TPCs were estimated by Folin-Ciocalteu method (Sun et al., 2007). Aliquots (125 μL) of extract (of known concentration) were incubated in test tubes with 500 μL distilled water and 125 μL of Folin-Ciocalteu reagent for 6 min, before addition of 1.25 mL of 7% sodium carbonate. The final volumes of the mixtures was made to 3 mL by adding 1 mL distilled water. Samples were incubated for 90 min for completion of the reactions. The absorbance was then taken in triplicate at 750 nm by using a UV-Vis spectrophotometer (Cary 4000 UV-Vis, Agilent, Abingdon, UK), controlled by Agilent scan software (Agilent). Gallic acid (GA) was used to generate the standard plot. GA solution was prepared by dissolving 25 mg GA in 25 mL distilled water; concentrations ranging from 0 to 450 μg/mL were used to generate standard curves to calculate the TPCs of the samples. Results were expressed as mg of GA equivalents (mg GAE/g).
Total flavonoid content (TFC)
TFC of the extract was determined using a slightly modified version of the method reported by Kim et al. (2005). Briefly, 0.5 mL of appropriately diluted sample was mixed with 0.5 mL methanol, 50 μL of 10% AlCl3, 50 μL of 1 mol/L potassium acetate, and 1.4 mL distilled water, and incubated at room temperature for 30 min. The absorbance of the reaction mixture was then measured at 510 nm using an UV-Vis spectrophotometer (Agilent). A calibration curve was prepared using quercetin as a standard.
ABTS radical scavenging activity
The reaction was carried out according to the method previously described by Re et al. (1999) with slight modifications. Briefly, ABTS radical cation (ABTS•+) was prepared by dissolving 7 mM ABTS and 2.45 mM potassium persulphate in phosphate buffered saline (PBS, pH 7.4). The solution was incubated in the dark for 16 h to generate the ABTS•+. For analysis, the ABTS•+ stock was diluted using PBS buffer and equilibrated at 30°C to an absorbance of 0.7±0.02 at 734 nm. Trolox was used to create a standard curve (6.25~200 μM). The antioxidant capacity was measured by mixing 0.2 mL of samples with 2 mL of ABTS•+ solution, and the decline in absorbance over a 5 min period was observed. Appropriate blanks were run for each sample and the radical scavenging capacity was compared to that of Trolox. The results were expressed as μM Trolox equivalent (TE) per gram of sample. The percentage ABTS•+ scavenged was calculated using the following equation:
where Ai and Af represents initial and final absorbance’s of sample, respectively.
DPPH radical scavenging activity
The free radical scavenging ability of the extract against DPPH radical (DPPH•) was carried out as described by Mansouri et al. (2005). Aliquots (60 μL) of sample extract were mixed with 1,500 μL of DPPH solution and incubated in the dark for 30 min. Absorbance was measured at 517 nm using a spectrophotometer and the percentage DPPH radical scavenging activity was determined using the following equation:
where Ab and As represents absorbance of blank and sample, respectively.
Ferric reducing antioxidant activity
The reducing properties of the extracts were determined by assessing the ability of the extracts to reduce FeCl3 solution as described by Oyaizu (1986). Aliquots (500 μL) were mixed with 2.5 mL of 200 mmol/L sodium phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide. The mixtures were incubated at 50°C for 20 min, before addition of 2.5 mL of 10% trichloroacetic acid. The mixture was then centrifuged at 650 rpm for 10 min. Aliquots (5 mL) of the supernatant was mixed with an equal volume of distilled water and 1 mL of 0.1% FeCl3. The absorbance was measured at 700 nm, and ferric reducing power was subsequently calculated and expressed as ascorbic acid equivalent (AAE).
Hydroxyl radical scavenging ability
The ability of the extract to prevent Fe2+/H2O2 induced decomposition of deoxyribose was carried out using the method described by Halliwell and Gutteridge (1989). Briefly, freshly prepared extract (0~100 μL) was added to a reaction mixture containing 120 μL of 20 mM deoxyribose, 400 μL of 0.1 M phosphate buffer (pH 7.4), 40 μL of 20 mM hydrogen peroxide, and 40 μL of 500 μM FeSO4, made to 800 μL total volume with distilled water. The reaction mixture was incubated at 37°C for 30 min, and the reaction was stopped by addition of 0.5 mL of 2.8% trichloroacetic acid, followed by addition of 0.4 mL of 0.6% thiobarbituric acid solution. Mixtures were subsequently incubated in boiling water for 20 min. The absorbance was measured at 532 nm in a spectrophotometer, and the percentage hydroxyl radical scavenging activity was calculated as follows:
where Absref represents absorbance of reference and Abssample represents absorbance of sample.
Nitric oxide scavenging activity
Nitric oxide radical scavenging assay was carried out as described by Balakrishnan et al. (2009). The spice extracts and GA standards were prepared from 10 mg/mL methanolic crude extract, which were serially diluted with distilled water to create concentrations ranging from 100~1,000 μg/mL; dilutions were stored at 4°C for later use. Griess reagent was prepared by mixing equal amounts of 1% sulphanilamide in 2.5% phosphoric acid with 0.1% naphthylethylene diamine dihydrochloride in 2.5% phosphoric acid immediately before use. A volume of 0.5 mL of 10 mM sodium nitroprusside in phosphate buffered saline was mixed with 1 mL of each concentration of methanol extract (100~1,000 μg/mL) and incubated at 25°C for 180 min. The extract was mixed with an equal volume of freshly prepared Griess reagent. Control samples without extracts but with an equivalent volume of buffer were prepared in a similar manner as the test samples. The colour tubes contained methanol extracts at the same concentrations but without sodium nitroprusside. A volume of 150 μL of the reaction mixture was transferred into a 96-well plate. The absorbance was measured at 546 nm using a SpectraMax Plus UV-Vis microplate reader (Molecular Devices, San Jose, CA, USA). GA was used as a positive control. The percentage inhibition of the extracts and standards were calculated. The percentage nitrite radical scavenging activity of the ethanol extracts and GA were calculated using the following formula:
where Abscontrol represents absorbance of control sample and Abstest represents absorbance in the presence of the samples of extracts or standards.
Data analysis
All experiments were performed in triplicate. Results were expressed as mean±standard deviation (SD). Data were analysed by analysis of variance using statistical package for social sciences SPSS v.17.0 (SPSS Inc., Chicago, IL, USA). Differences between means were calculated using Duncan’s new multiple range test at 95% confidence level.
RESULTS AND DISCUSSION
HPLC-DAD phenolic profiling of the spices
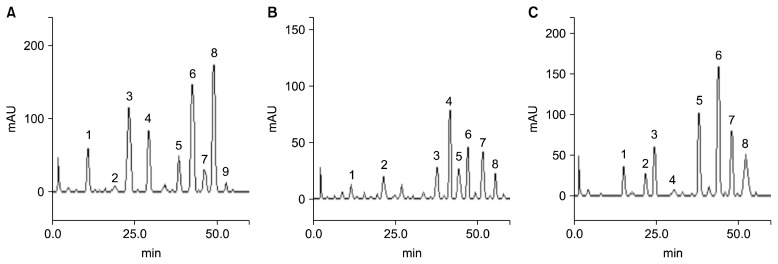
HPLC-DAD fingerprinting showed the presence of many phenolic compounds in the three spices, namely gallic acid, chlorogenic acid, rosmarinic acid, xylopic acid, elliagic acid, caffeic acid, rutin, quercitrin, quercetin, isoquercitrin, kaempferol, capsaicin, and dihydrocapsaicin (Table 1). A total of nine dominant peaks (Fig. 1A) were observed for OB, grouped as four phenolic acids (gallic acid, chlorogenic acid, caffeic acid, and rosmarinic acid) and five flavonoids (rutin, isoquercitrin, quercitrin, quercetin, and kaempferol). XA and PG each showed eight dominant peaks respectively (Fig. 1B and 1C). The phenolic compounds chlorogenic acid, rutin, quercitrin, and quercetin were common to the three spices. For each spice, the phenolic compound with the highest concentration was as follows: isoquercetin in PG (46.70 mg/g), rutin and quercitrin in XA (54.93 mg/g, and 78.35 mg/g, repectively), and quercetin in OB (92.31 mg/g). Quercetin and rutin had significantly higher concentrations in XA than the other spices. Rutin is a glycoside comprising of flavonolic aglycone quercetin along with disaccharide rutinose, which possess anti-oxidative, neuroprotective, cardioprotective, and hypercholesterolemic activities (Trumbeckaite et al., 2006; Kanashiro et al., 2009; Javed et al., 2012). Rutin and quercetin are widely used as important materials in food and the pharmaceutical industry (Wang et al., 2011). The dominance of these compounds in XA suggests that XA may possess these associated properties. Contrary to our finding, Oso et al. (2017) reported quercetin to be the prevalent compound in XA ethanolic extracts. Adefegha et al. (2018) also reported rutin and quercetin (at values close to those in our study) as the most abundant phenolic compounds present in phenolic extracts of XA. The differences in the dominating phenolic compound may be attributed to the different solvent used for extraction. Identification of quercetin as the most prevalent compound in OB methanolic extracts corroborates the reports by Kaurinovic et al. (2011) in OB solvent extracts (ethanol, butanol, and water). However, our results contrast other reports showing rosmarinic acid to be the most biologically active compound in OB (Lee and Scagel, 2009; Shiga et al., 2009). Moreover, a further study by Güez et al. (2017) suggested caffeic acid to be the most predominant compound in OB hydroalcoholic extracts. Variations in phenolic significance may be attributed to environmental factors (including planting conditions, soil, and location variability), extraction conditions and sensitivity of phenolic fingerprinting methods. Quercetin is a class of flavonol which cannot be produced in the human body, and has been associated with the treatment of metabolic and inflammatory disorders. The abundance of quercetin in OB suggests that OB may be useful for preventing oxidative stress and improving cardiovascular health.
Table 1.
Phenolic composition of methanolic extracts of three spices from Nigeria
| Compounds | Composition (mg/g) | ||
|---|---|---|---|
|
| |||
| Ocimum basilicum | Xylopia aethiopica | Piper guineensis | |
| Gallic acid | 26.75±0.02a | ND | 8.53±0.01b |
| Chlorogenic acid | 4.19±0.03c | 11.50±0.03b | 13.91±0.02a |
| Caffeic acid | 51.30±0.01a | 23.61±0.01b | ND |
| Rosmarinic acid | 40.65±0.01 | ND | ND |
| Xylopic acid | ND | 12.93±0.01 | ND |
| Ellagic acid | ND | 1.78±0.02 | ND |
| Rutin | 25.93±0.03b | 54.93±0.01a | 20.47±0.02c |
| Isoquercitrin | 78.24±0.02a | ND | 46.70±0.01b |
| Quercitrin | 22.18±0.02b | 78.35±0.01a | 19.85±0.03c |
| Quercetin | 92.31±0.01a | 39.42±0.03b | 24.13±0.01c |
| Kaempferol | 4.50±0.01b | 25.81±0.01a | ND |
| Dihydrocapsaicin | ND | ND | 23.82±0.03 |
| Capsaicin | ND | ND | 13.50±0.01 |
Values are mean±SD of three determinations.
Values with different letters (a–c) in the same row are significantly different at P<0.05.
ND, not detected.
Fig. 1.
Representative high performance liquid chromatography profiles. (A) Extract of Ocimum basilicum: peak 1, gallic acid; peak 2, chlorogenic acid; peak 3, caffeic acid; peak 4, rosmarinic acid; peak 5, rutin; peak 6, isoquercitrin; peak 7, quercitrin; peak 8, quercetin; peak 9, kaempferol. (B) Extract of Piper guineensis: peak 1, gallic acid; peak 2, chlorogenic acid; peak 3, rutin; peak 4, isoquercitrin; peak 5, quercitrin; peak 6, quercetin; peak 7, dihydrocapsaicin; peak 8, capsaicin. (C) Extract of Xylopia aethiopica: peak 1, xylopic acid; peak 2, chlorogenic acid; peak 3, caffeic acid; peak 4, ellagic acid; peak 5, rutin; peak 6, quercitrin; peak 7, quercetin; peak 8, kaempferol.
The flavonoid isoquercitrin was the most prevalent phenolic compound in the methanolic extract of PG. In contrast to our results, Diaz et al. (2012) reported quercetin to be the most prevalent flavonol in Piper imperiale ethanolic extracts, and Adefegha et al. (2017) reported quercitrin to be the most predominant phenolic in extracts from PG. The various phytochemicals in the methanolic extract of these spices suggests their potential usefulness in phyto-medicine.
Antioxidant properties of spices
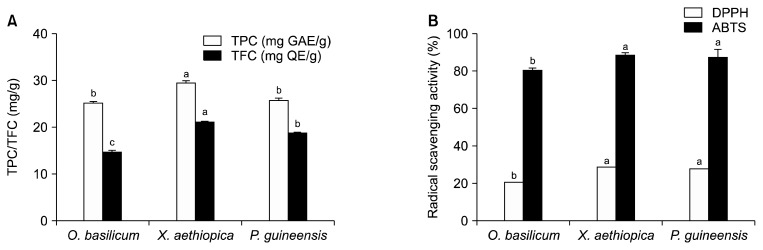
The TPC of the methanolic extracts from the three different spices is presented in Fig. 2A. TPCs ranged from 25.32 to 29.50 mg GAE/g; there was no significant difference (P>0.05) between OB (25.32 mg GAE/g) and PG (25.83 mg GAE/g). However, XA extract exhibited the highest TPC (29.50 mg GAE/g). The TPC of XA extract was found to be superior to that in extracts of Piper nigrum and Ellattaria cardamom (5.04 and 15.67 mg GAE/g, respectively), as reported by Asha Devi et al. (2012). However, Parikh and Kothari (2016) reported a lower total phenol content of only 7.15 mg GAE/g in OB seed methanolic extracts. Our findings reveal that for methanolic extracts the TPC is significantly higher than the TFC, despite the fact that phenolic compounds are broadly distributed in plants. Consequently, in this study we observed TFCs ranging from 14.89 to 21.17 mg QE/g, and showed XA extracts possess the highest TFC. Contrary to the present result, Adefegha and Oboh (2012) observed lower TFCs for XA methanolic extracts, whereas higher TFCs (86.09 mg RE/g) were reported by Adeniyi et al. (2017); however, the latter study used different standards (rutin vs. quercetin) and therefore may not be comparable. We showed that OB extracts contained the lowest TFCs (14.89 mg QE/g), which is consistent with findings by Güez et al. (2017). The TPC and TFC were higher in the present study than those reported by Kaurinovic et al. (2011) using other extraction solvents.
Fig. 2.
(A) Total phenolic content (TPC) and total flavonoid content (TFC) and (B) 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azinobis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical scavenging activities of methanolic extract of Ocimum basilicum, Xylopia aethiopica, and Piper guineensis. Values with different letters (a–c) are significantly different at P <0.05. GAE, gallic acid equivalents; QE, quercetin equivalents.
The radical scavenging activity showed that the spices possess good antioxidative properties. Two different assays were used to analyse the radical scavenging antioxidant activities, namely DPPH and ABTS assays. DPPH radical scavenging activity is evident by a change in colour from purple to yellow. Fig. 2B shows the DPPH radical scavenging activity of the extracts no significant difference were observed for the methanolic extracts of XA and PG (29 and 28%, respectively), but these were superior to that of OB (21%). All DPPH radical scavenging activities observed in this study were superior to those reported for P. nigrum and E. cardamom (11.77 and 12.61%) by Asha Devi et al. (2012). However, Nahak and Sahu (2011) reported higher DPPH radical scavenging activity (58.07 and 53.07%) for methanolic extracts of Piper spp. (P. cubeba and P. nigrum). The results of the ABTS radical scavenging activity assays showed similar trend but with a higher activity (range from 80.28 to 88.23%). This suggests that ABTS may be a more sensitive assay for evaluating radical scavenging activity than DPPH. This corroborates with Re et al. (1999) who suggested that ABTS is more versatile because both polar and non-polar components can be assessed at the measurement wavelength. Relative to other studies, Sasidharan et al. (2009) reported that methanolic extracts of Elaeis guineensis exhibit a good DPPH• activity of 50.14%. In addition, Womeni et al. (2013) reported superior DPPH radical scavenging activities for methanolic extracts of XA.
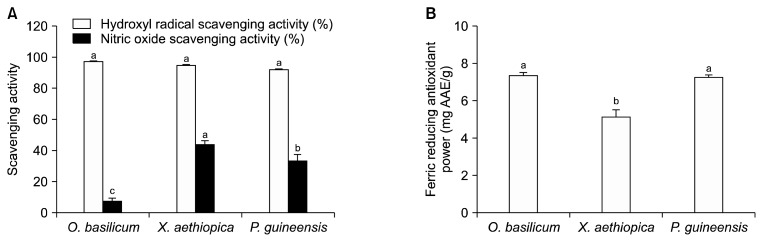
Hydroxyl radical scavenging capacity is directly related to its antioxidant activity. We did not observe any significant differences (P>0.05) in the hydroxyl radical scavenging activity between samples (Fig. 3A), suggesting that all these spice extracts may serve as practical hydroxyl radical scavengers in food systems. The scavenging activities observed in this study were significantly higher than those reported by Kim et al. (2011) for several aqueous spice extracts (turmeric, clove, and thyme, etc.), including of OB (25.25%). Excessive nitric oxide production has been implicated as a major causative agents in the development of several neurodegenerative diseases (Womeni et al., 2013). In this study, XA extracts showed the highest nitric oxide scavenging activities (44.13%) through the greatest reduction in nitrite concentrations in the assay medium. OB extracts exhibited the lowest activities, similar to those reported by Agarwal et al. (2013); in this study of three aqueous plant extracts, OB extracts exhibited the lowest nitric oxide scavenging activities, equivalent to 11.34 mM of sodium nitrite.
Fig. 3.
(A) Hydroxyl radical and nitric oxide scavenging activities and (B) ferric reducing antioxidant power of methanolic extract of Ocimum basilicum, Xylopia aethiopica, and Piper guineensis. Values with different letters (a–c) are significantly different at P <0.05.
The ferric reducing antioxidant powers of the spice extracts are represented in Fig. 3B. OB and PG (7.36 and 7.27 mg AAE/g, respectively) were readily able to reduce Fe3+ to Fe2+ and no significant differences were observed This suggests that these spices may have good reducing potential, and may therefore be useful in food systems to prevent oxidation. A lower reducing ability (1.49 mg AAE/g) was previously reported for OB aqueous-ethanolic extracts (Sailaja et al., 2010). George and Osioma (2011) reported that XA ethanolic extracts exhibit the lowest reducing power when compared ethanolic extracts of Aframomum sceptrum, Monodora myristica, and Allium sativum. The antioxidant activity of the spices used in the present study, in particular XA, may be attributed to the presence of phenolic compounds such as rutin, quercitrin, kaempferol, and chlorogenic acid, which are known to possess strong antioxidative properties.
Acidified methanolic spice extracts exhibited significant antioxidant activities and contained various useful phytochemicals. However, only chlorogenic acid, rutin, quercitrin, and quercetin were the only phenolic compounds common to all three spices investigated. All the extracts exhibited excellent antioxidant activities; however, extracts of XA possessed superior biological activities, which suggests it could be the most potent raw material of the three spices. XA may therefore be of great use in phytomedicine to prevent diseases associated with oxidative stress.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- Adefegha SA, Oboh G, Adefegha OM. Ashanti pepper (Piper guineense Schumach et Thonn) attenuates carbohydrate hydrolyzing, blood pressure regulating and cholinergic enzymes in experimental type 2 diabetes rat model. J Basic Clin Physiol Pharmacol. 2017;28:19–30. doi: 10.1515/jbcpp-2016-0001. [DOI] [PubMed] [Google Scholar]
- Adefegha SA, Oboh G, Olasehinde TA, Boligon AA. Dietary supplementation with Ethiopian pepper (Xylopia aethiopica) modulates angiotensin-I converting enzyme activity, antioxidant status and extenuates hypercholesterolemia in high cholesterol fed Wistar rats. PharmaNutrition. 2018;6:9–16. doi: 10.1016/j.phanu.2017.11.001. [DOI] [Google Scholar]
- Adefegha SA, Oboh G. Effect of diets supplemented with Ethiopian pepper [Xylopia aethiopica (Dun.) A. Rich (Annonaceae)] and Ashanti pepper [Piper guineense Schumach. et Thonn (Piperaceae)] on some biochemical parameters in normal rats. Asian Pac J Trop Biomed. 2012;2:S558–S566. doi: 10.1016/S2221-1691(12)60274-3. [DOI] [Google Scholar]
- Adeniyi FO, Wilson FO, Oluboade OO. Phytochemicals, antioxidant potentials and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of Piper guineense (Schumach & Thonn) seed. Afr J Plant Sci. 2017;11:99–104. doi: 10.5897/AJPS2016.1509. [DOI] [Google Scholar]
- Agarwal R, Gupta SK, Agarwal P, Srivastava S, Alyayutdin R. Anticholinesterase, antioxidant and nitric oxide scavenging activity of the aqueous extract of some medicinal plants. Br J Pharm Res. 2013;3:807–816. doi: 10.9734/BJPR/2013/4833. [DOI] [Google Scholar]
- Asha Devi S, Umasankar ME, Babu S. A comparative study of anti oxidant properties in common Indian spices. Int Res J Pharm. 2012;3:465–468. [Google Scholar]
- Balakrishnan N, Panda AB, Raj NR, Shrivastava A, Prathani R. The evaluation of nitric oxide scavenging activity of Acalypha indica Linn root. Asian J Res Chem. 2009;2:148–150. [Google Scholar]
- Boligon AA, Sagrillo MR, Machado LF, De Souza Filho O, Machado MM, Da Cruz IBM, et al. Protective effects of extracts and flavonoids isolated from Scutia buxifolia Reissek against chromosome damage in human lymphocytes exposed to hydrogen peroxide. Molecules. 2012;17:5757–5769. doi: 10.3390/molecules17055757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz LE, Munoz DR, Prieto RE, Cuervo SA, Gonzalez DL, Guzman JD, et al. Antioxidant, antitubercular and cytotoxic activities of Piper imperiale. Molecules. 2012;17:4142–4157. doi: 10.3390/molecules17044142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George BO, Osioma E. Phenolic content and total antioxidant capacity of local spices in Nigeria. Afr J Plant Sci. 2011;5:741–746. [Google Scholar]
- Güez CM, de Souza RO, Fischer P, de Moura Leão MF, Duarte JA, Boligon AA, et al. Evaluation of basil extract (Ocimum basilicum L.) on oxidative, anti-genotoxic and anti-inflammatory effects in human leukocytes cell cultures exposed to challenging agents. Braz J Pharm Sci. 2017;53:e15098. doi: 10.1590/s2175-97902017000115098. [DOI] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 2nd ed. Clarendon Press; Oxford, UK: 1989. p. 120. [Google Scholar]
- Javed H, Khan MM, Ahmad A, Vaibhav K, Ahmad ME, Khan A, et al. Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience. 2012;17:340–352. doi: 10.1016/j.neuroscience.2012.02.046. [DOI] [PubMed] [Google Scholar]
- Kanashiro A, Andrade DC, Kabeya LM, Turato WM, Faccioli LH, Uyemura SA, et al. Modulatory effects of rutin on biochemical and hematological parameters in hypercholesterolemic Golden Syrian hamsters. An Acad Bras Cienc. 2009;81:67–72. doi: 10.1590/S0001-37652009000100009. [DOI] [PubMed] [Google Scholar]
- Kaurinovic B, Popovic M, Vlaisavljevic S, Trivic S. Antioxidant capacity of Ocimum basilicum L. and Origanum vulgare L. extracts. Molecules. 2011;16:7401–7414. doi: 10.3390/molecules16097401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EC, Min JK, Kim TY, Lee SJ, Yang HO, Han S, et al. [6]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis in vitro and in vivo. Biochem Biophys Res Commun. 2005;335:300–308. doi: 10.1016/j.bbrc.2005.07.076. [DOI] [PubMed] [Google Scholar]
- Kim IS, Yang MR, Lee OH, Kang SN. Antioxidant activities of hot water extracts from various spices. Int J Mol Sci. 2011;12:4120–4131. doi: 10.3390/ijms12064120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Scagel CF. Chicoric acid found in basil (Ocimum basilicum L.) leaves. Food Chem. 2009;115:650–656. doi: 10.1016/j.foodchem.2008.12.075. [DOI] [Google Scholar]
- Mansouri A, Embarek G, Kokkalou E, Kefalas P. Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera) Food Chem. 2005;89:411–420. doi: 10.1016/j.foodchem.2004.02.051. [DOI] [Google Scholar]
- Nahak G, Sahu RK. Phytochemical evaluation and antioxidant activity of Piper cubeba and Piper nigrum. J Appl Pharm Sci. 2011;1:153–157. [Google Scholar]
- Oso BJ, Boligon AA, Oladiji AT. Metabolomic profiling of ethanolic extracts of the fruit of Xylopia aethiopica (Dunal) a. rich using gas chromatography and high-performance liquid chromatography techniques. J Pharmacogn Phytochem. 2018;7:2083–2090. [Google Scholar]
- Oso BJ, Oyewo EB, Oladiji AT. Ethanolic, n-hexane and aqueous partitioned extracts of Xylopia aethiopica fruit modulated inflammatory responses in turpentine oil induced acute inflammation in male Wistar rats. Int J Res Health Sci. 2017;5:1–10. [Google Scholar]
- Oyaizu M. Studies on products of browning reaction–antioxidative activities of products of browning reaction prepared from glucosamine. Japan J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Parikh NH, Kothari CS. Phytochemical analysis and total phenolic and flavonoid contents determination of methanolic extract of Ocimum basilicum L seed. Int J PharmTech Res. 2016;9:215–219. [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Sabir SM, Ahmad SD, Hamid A, Khan MQ, Athayde ML, Santos DB, et al. Antioxidant and hepatoprotective activity of ethanolic extract of leaves of Solidago microglossa containing polyphenolic compounds. Food Chem. 2012;131:741–747. doi: 10.1016/j.foodchem.2011.09.026. [DOI] [Google Scholar]
- Sailaja I, Shaker IA, Ratna YK. Antioxidant activity and phenolic contents in Ocimum sanctum and Ocimum bascilicum. Asian J Bio Sci. 2010;5:1–5. [Google Scholar]
- Sasidharan S, Sharmini R, Vijayarathna S, Yoga LL, Vijenthi R, Amala R, et al. Antioxidant and hepatoprotective activity of methanolic extracts of Elaeis guineensis Jacq leaf. Pharmacology-online. 2009;3:84–90. [Google Scholar]
- Shan B, Cai YZ, Sun M, Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem. 2005;20:7749–7759. doi: 10.1021/jf051513y. [DOI] [PubMed] [Google Scholar]
- Shiga T, Shoji K, Shimada H, Hashida S, Goto F, Yoshihara T. Effect of light quality on rosmarinic acid content and antioxidant activity of sweet basil, Ocimum basilicum L. Plant Biotechnol. 2009;26:255–259. doi: 10.5511/plantbiotechnology.26.255. [DOI] [Google Scholar]
- Sun T, Powers JR, Tang J. Evaluation of the antioxidant activity of asparagus, broccoli and their juices. Food Chem. 2007;105:101–106. doi: 10.1016/j.foodchem.2007.03.048. [DOI] [Google Scholar]
- Trumbeckaite S, Bernatoniene J, Majiene D, Jakstas V, Savickas A, Toleikis A. The effect of flavonoids on rat heart mitochondrial function. Biomed Pharmacother. 2006;60:245–248. doi: 10.1016/j.biopha.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhao LL, Sun GX, Liang Y, Wu FA, Chen ZL, et al. A comparison of acidic and enzymatic hydrolysis of rutin. Afr J Biotechnol. 2011;10:1460–1466. [Google Scholar]
- Wang L, Chen J, Xie H, Ju X, Liu RH. Phytochemical profiles and antioxidant activity of adlay varieties. J Agric Food Chem. 2013;61:5103–5113. doi: 10.1021/jf400556s. [DOI] [PubMed] [Google Scholar]
- Womeni HM, Djikeng FT, Tiencheu B, Linder M. Antioxidant potential of methanolic extracts and powders of some Cameroonian spices during accelerated storage of soybean oil. Adv Biol Chem. 2013;3:304–313. doi: 10.4236/abc.2013.33034. [DOI] [Google Scholar]