Abstract
The antioxidative effects of the bioactive compounds enriched sesame oil (e.g. lignans and tocopherols) are well established. This study aims to elucidate whether sesame oil could reduce renal oxidative stress induced by a high fat diet (HFD). Mice received HFD for 12 weeks (n=7 per group), which was prepared by adding 20% (w/w) lard (lard group) or sesame oil (sesame group) to the chow diet, respectively. Compared with mice in the lard group, renal lipid levels of those in the sesame group were reduced, shown by decreases in protein expression of transcription factors and enzymes involved in fatty acid synthesis (sterol regulatory element-binding protein-1 and acetyl coenzyme A carboxylase α) and an increase in β-oxidation (peroxisome proliferator-activated receptor α and carnitine palmitoyltransferase I) (P<0.05). In the sesame group, levels of peroxynitrite and thiobarbituric acid reactive substances were also reduced, whereas the level of glutathione was increased. In addition, there was elevated protein expression levels of antioxidant enzymes regulated by nuclear factor-like 2, such as superoxide dismutase, glutathione peroxidase, and glutathione S-transferase (P<0.05), and decreased expression for nuclear factor kappa B and cyclooxygenase 2 (P<0.05). These results suggest that sesame oil could ameliorate HFD-induced renal damage by suppressing oxidative stress and inflammation.
Keywords: sesame oil, high fat diet, kidney, oxidative stress, lipid metabolism
INTRODUCTION
Sesame oil obtained from seeds of Sesamum indicum L. is widely used in cooking in Asian countries. Recent studies have demonstrated antioxidative properties of sesame oil (Wan et al., 2015). Sesame oil prevents lipid peroxidation and decreases levels of hydroxyl radical and nitrites through elevating activities of antioxidant enzyme (Hsu et al., 2004). In addition, sesame oil inhibits lipid accumulation in adipocytes through suppression of lipogenesis, and elevates lipolysis (Pan et al., 2015). Sesame oil also shows preventive effects for development of atherosclerosis (Bhaskaran et al., 2006) and hypertension (Sankar et al., 2005). These health benefits are attributed to the antioxidant and anti-inflammatory activities (Monteiro et al., 2014) of lignans (sesamin, sesamol, and sesamolin) and tocopherols. Lignans in sesame oil have been extensively studied with respect to their antioxidant (Baluchnejadmojarad et al., 2013), anti-inflammatory (Monteiro et al., 2014), antihypertensive (Zhao et al., 2015), anticancer (Kanimozhi et al., 2016), and antihyperlipidemic effects (Zhang et al., 2016). In our previous study, the amounts of sesamin, sesamolin, and tocopherols in sesame oil was found to be 6.02, 3.84, and 1.45 g/kg, which is higher than in soybean oil (Kim et al., 2017). Furthermore, polyunsaturated fatty acids that have antioxidant activity are high in sesame oil. Linoleic acid (46.26%), oleic acid (38.84%), and arachidonic acid (0.9%) are abundantly present in sessame oil (Nzikou et al., 2009).
Oxidative stress and inflammation are well-known pathological features of obesity. Reactive oxygen species (ROS) are generated under the oxidative stress, which subsequently provokes inflammatory signaling cascades through the nuclear factor kappa B (NF-κB) pathway (Ratliff et al., 2016). The antioxidant status in the body plays a critical role in alleviating oxidative stress. Glutathione (GSH), an endogenous antioxidant, scavenges free radicals through its cysteine residues. In addition, nuclear factor-like 2 (Nrf2) transcribes various antioxidant enzyme genes such as superoxide dismutase (SOD), heme oxygenase-1 (HO-1), glutathione S-transferase (GST), and glutathione peroxidase (GSH-Px) (Choi et al., 2014). Animal studies have shown that concentrations of antioxidants in both plasma and tissue are low in high fat diet (HFD)-induced obesity (Noeman et al., 2011; Yang et al., 2006). HFD is a well-known dietary factor in obesity development through inducing lipid accumulation in various organs. Lipotoxicity, a critical condition for development of diseases, increases systemic inflammation, and oxidative stress (Ratliff et al., 2016; Kume et al., 2007).
The kidneys are excretory organs that are essential for sustaining life. Improper function, such as renal failure, can be fatal. Several studies have demonstrated that renal injury during obesity may be explained by lipid accumulation in the kidneys (Deji et al., 2009; Praga, 2002). HFDs induce lipid accumulation through elevating lipogenesis and inhibiting lipolysis (Kume et al., 2007). In this study, reno-protective effects of sesame oil were examined in HFD-fed mice. Lipid lowering, antioxidant, and anti-inflammatory effects of sesame oil as its mechanism of action were elucidated.
MATERIALS AND METHODS
Materials
Sesame oil (Dubio Co., Eumsung, Korea) and lard (Doo-Yeol Biotech., Seoul, Korea) were purchased. According to the information provided by the manufacturer, the sesame oil was prepared through pressed extraction using sesame seeds that were roasted for 20 min at three different temperatures: 250°C, 40°C, and 150~170°C.
Animals and diets
The HFD was prepared by adding lard or sesame oil, respectively, to the chow diet (2018S Teklad Global; Envigo, Madison, WI, USA). Calories from fat or oil in the HFD contributed 50% of the total calorie count. Male C57BL/6 mice (4 weeks old; DooYeol Biotech.) were acclimatized for 1 week and then divided into 3 groups based on body weight. The experimental groups were mice fed a chow diet alone (normal group, NOR group), a HFD containing either lard (Lard group) or sesame oil (SSO group). The duration of the animal study was 12 weeks. On the last day of experiment, mice were fasted for 12 h and then anesthetized by intraperitoneal injection of 30 mg/kg of Zoletil (Virbac Laboratories, Carros, France) and 10 mg/kg of xylazine (Rompun; Bayer Korea, Seoul, Korea). The organs were perfused with ice-cold phosphate-buffered saline (PBS, 10 mM, pH 7.2) and the kidneys were excised, frozen in liquid nitrogen, and stored at −80°C. The animal study was conducted in accordance with the Guidelines for Animal Experiments approved by the University Institutional Animal Care and Use Committee (PNU-IACUC, Approval Number PNU-2015-0823).
Total cholesterol and triacylglycerol concentrations
The renal tissue was homogenized with 10 volumes (w/v) of PBS (pH 7.4) using a polytron homogenizer (PT-MR 3100; Kinematica AG, Lucerne, Switzerland). Lipids were extracted from renal homogenates by the method of Folch et al. (1957). The triacylglycerol (TG) and total cholesterol (TC) concentrations were determined using commercial kits (AM157S-K and AM202-K, respectively; Asan Pharmaceuticals Co., Ltd., Seoul, Korea).
Thiobarbituric acid reactive substances (TBARS) and glutathione concentrations
The concentrations of TBARS (Ohkawa et al., 1979) and GSH (Teare et al., 1993) were determined using malondialdehyde and GSH standards, respectively. In brief, the TBARS reaction mixture containing the renal homogenate and TBARS solution (0.67% thiobarbituric acid and 0.05 N HCl) was prepared, and lipid oxidation was carried out at 95°C for 30 min. The GSH concentration was measured using a disulfide reagent comprising 0.1 M sodium phosphate buffer (pH 8) and 0.01 M 5,5′-dithiobis (2-nitrobenzoic acid).
ROS and peroxynitrite levels
For ROS and peroxynitrite (ONOO−) assays, the post-mitochondrial fraction (PMF) was obtained from the renal homogenate centrifugation at 3,012 g for 15 min followed by 18,627 g for 15 min at 4°C. The upper layer was used as the PMF. ROS and ONOO− concentrations were determined using 2′,7′-dichlorofluorescein diacetate and rhodamine solution (50 mM sodium phosphate buffer, 90 mM sodium chloride, 5 mM diethylenetriaminepentaacetic acid, and dihydrorhodamine 123), respectively. Changes in the fluorescence of the reaction samples for ROS or ONOO− were measured for 30 min at an excitation wavelength of 480 nm emission wavelength of 530 nm using a fluorescence plate reader (FLUOstar OPTIMA; BMG Labtech, Offenburg, Germany).
Western blot analysis
Renal tissue was homogenized in lysis buffer (1:9, v/w) (50 mM Tris, pH 8.0, 5 mM ethylenediaminetetraacetic acid, 150 mM NaCl, and 1% nonidet-P40) containing a protease inhibitor cocktail (10 μL/mL protease inhibitor cocktail; Sigma-Aldrich Co., St. Louis, MO, USA), 1 mM dithiothreitol, and 1 mM phenylmethylsulfonyl fluoride for 90 s using a polytron homogenizer (PT-MR 3100; Kinematica AG). The upper layer was obtained by centrifugation of the homogenate at 18,627 g for 20 min at 4°C. The protein concentration was measured using a Bio-Rad protein assay kit (500-0002; Bio-Rad Laboratories, Hercules, CA, USA). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed (Jung et al., 2015). Protein expression was visualized by the enhanced chemiluminescence, detected with CAS-400 (Core Bio, Seoul, Korea), and calculated using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Protein expression was normalized to that of α-tubulin. The primary antibodies used were α-tubulin (EP1332Y; ab52866; Abcam Inc., Cambridge, UK), and the following from Santa Cruz Biotechnology (Santa Cruz, CA, USA): sterol regulatory element-binding protein-1 (SREBP-1; H-160; sc-8984), peroxisome proliferator-activated receptor α (PPARα; H-98; sc-9000), acetyl coenzyme A carboxylase α (ACCα; T-18; sc-26817), carnitine palmitoyltransferase I (CPT-1; S-17; sc-139482), SOD (FL-154; sc-11407), HO-1 (H-105; sc-10789), GSH-Px (B-6; sc-133160), Nrf2 (H-300; sc-13032), GST (B-14; sc-138), NF-κB (p65 A; sc-109), cyclooxygenase 2 (COX-2; M-19; sc-1747), and inhibitor of NF-κB (IκB; C-21; sc-371). The secondary horseradish peroxidase-conjugated antibodies were donkey anti-rabbit IgG H&L (ab6802), rabbit anti-goat IgG H&L (ab6741), and rabbit anti-mouse IgG H&L (ab6728) (all from Abcam Inc.).
Statistical analysis
Statistical analysis was performed using SPSS version 23 (SPSS Inc., Chicago, IL, USA). Results were expressed as mean±standard deviations (SD). One-way analysis of variance (ANOVA) was carried out, followed by Duncan’s multiple range test for post hoc analysis. P-values <0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Effects of sesame oil on renal triglyceride and cholesterol concentrations
Kidney weight and renal lipid concentrations were higher in mice in the Lard group than those of the NOR group mice (Table 1). However, kidney weight and TG concentrations in the SSO group were 12.67% and 37.66% lower, respectively, than those in the Lard group (both P< 0.05). The renal TC concentration was also reduced in the SSO group compared with the Lard group but the difference was not significant.
Table 1.
Kidney weight and renal lipid concentrations of mice fed a high fat diet containing roasted sesame oil for 12 weeks
| Group | Kidney (g/100 g body weight) | TG (mg/g tissue) | TC (mg/g tissue) |
|---|---|---|---|
| NOR | 1.36±0.09b | 7.98±2.35b | 2.99±0.12NS |
| Lard | 1.50±0.07a | 11.72±3.46a | 3.07±0.31 |
| SSO | 1.31±0.05b | 7.30±2.07b | 2.81±0.34 |
Data show mean±SD (n=7 each group).
Data with different letters (a,b) are significantly different based on one-way ANOVA followed by Duncan’s multiple-range test at P<0.05.
NOR, C57BL/6 mice fed a chow diet; Lard, C57BL/6 mice fed a high fat diet (HFD); SSO, C57BL/6 mice fed a HFD containing roasted sesame oil; TC, total cholesterol; TG, triglyceride.
NS, data are not significantly different.
Lipid accumulation in the kidney is a characteristic feature of obesity-induced renal injury (Kume et al., 2007; Praga, 2002). This pathological condition elevates oxidative stress and inflammation. A mouse model for HFD-related renal lipid injury has already been established (Deji et al., 2009; Jiang et al., 2005). In the present study, the kidney weight of lard-fed mice was found to be significantly higher than that of the chow diet-fed mice, indicating that significant lipid accumulation occurred in mice receiving lard. In addition, sesame oil significantly lowered renal TG concentrations, which were elevated by lard. Reductions in kidney lipid levels may be attributed to the unsaturated fatty acids and the lipid-lowering effects of bioactive compounds in sesame oil, such as lignans (Zhang et al., 2016). Lipid lowering effects of polyunsaturated fatty acid (PUFA) are well established (Sekiya et al., 2003). Sesame oil contains a significantly higher amount of PUFA than lard (Che Man et al., 2011).
Inhibition of sesame oil on renal oxidative stress
Levels of oxidative stress were significantly higher in the Lard group compared with the NOR group (Table 2). However, the elevated oxidative stress induced by lard was alleviated by sesame oil (P<0.05). ROS concentration of mice in the SSO group was 55.95% lower than that in the Lard group (P<0.05). ONOO− and TBARS concentrations were 27.10% and 53.15% lower in the SSO group than the Lard group, respectively (P<0.05). In contrast, the renal GSH concentration for mice in the SSO group was 111.60% higher than that in the Lard group (P<0.05).
Table 2.
Renal ROS, ONOO−, TBARS, and GSH concentrations in mice fed a high fat diet containing roasted sesame oil for 12 weeks
| Group | ROS (Flu/min/mg protein) | ONOO− (Flu/min/mg protein) | TBARS (mmol/g tissue) | GSH (mmol/g tissue) |
|---|---|---|---|---|
| NOR | 3,587±1,220c | 4,791±1,925b | 14.10±1.74b | 2.75±1.58ab |
| Lard | 41,631±3,488a | 6,652±916a | 26.02±2.53a | 1.55±1.00b |
| SSO | 18,337±13,917b | 4,849±989b | 12.19±1.71b | 3.29±0.79a |
Data show mean±SD (n=7 each group).
Data with different letters (a–c) are significantly different based on one-way ANOVA followed by Duncan’s multiple range test at P<0.05.
See the legend of Table 1 for the experimental groups.
ROS, reactive oxygen species; ONOO−, peroxynitrite; TBARS, thiobarbituric acid reactive substances; GSH, glutathione.
It is well known that lipid accumulation elevates oxidative stress, which subsequently leads to organ disorders. ROS production and lipid peroxidation are highly noticeable (Noeman et al., 2011). In the present study, renal ROS, ONOO−, and TBARS levels were significantly reduced and GSH concentration was significantly increased in the SSO group compared with the Lard group, indicating that bioactive compounds in the sesame oil might act as antioxidants. In our previous study, we recorded higher levels of tocopherol and lignans, such as sesamin and sesamolin, in sesame oil than in soybean oil (Kim et al., 2017). The antioxidant activities of these bioactive compounds may protect against lipid-peroxidation damage (Baluchnejadmojarad et al., 2013). In particular, sesamin inhibits lipid peroxidation in the liver (Lv et al., 2015) and kidneys (Zhang et al., 2016), which is induced by either carbon tetrachloride or HFD. Sesamol, a metabolite of sesamolin in the body or a product of the roasting process, exhibits antioxidant activity and radical scavenging activity (Lv et al., 2015) and shows scavenging effect against superoxide anions, nitric oxide, hydroxyl radicals, and 2,2-diphenyl-1-picrylhydrazyl radicals (Kanimozhi and Prasad, 2009). Moreover, DNA damage in lymphocytes of mice treated with γ-radiation can be prevented by intraperitoneal administration of sesamol (Kanimozhi and Prasad, 2009).
Effects of sesame oil on fatty acid synthesis and β-oxidation
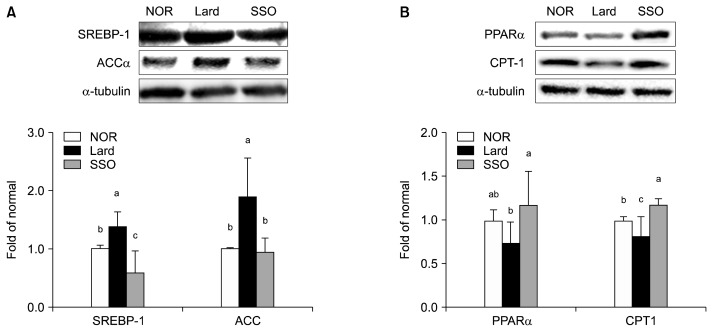
In the SSO group, protein expression levels of SREBP-1 and ACCα were 57.14% and 50.29% lower, respectively, than those in the Lard group (both P<0.05; Fig. 1A). In contrast, protein expression levels of PPARα and CPT-1 in the SSO group were 158.21% and 144.28% higher compared with those in the Lard group, respectively (both P<0.05; Fig. 1B).
Fig. 1.
Protein expression levels of (A) SREBP-1 and ACCα, and (B) PPARα and CPT-1 in the kidneys of mice fed a high fat diet containing roasted sesame oil for 12 weeks. Data show mean±SD (n=7 each group). Data with different letters (a–c) are significantly different based on one-way ANOVA followed by Duncan’s multiple range test at P <0.05. See the legend of Table 1 for the experimental groups. SREBP-1, sterol regulatory element-binding protein-1; ACCα, acetyl coenzyme A carboxylase alpha; PPARα, peroxisome proliferator-activated receptor alpha; CPT-1, carnitine palmitoyltransferase I.
Several studies have demonstrated that HFD induces fat deposition in kidneys through elevating lipogenesis with a concomitant decrease of fatty acid oxidation (Kume et al., 2007), which leads to lipotoxicity-induced renal damage. In this study, dysregulation of lipid metabolism caused by HFD was alleviated in the sesame oil-treated group. Sesame oil shows lipid lowering effects through downregulating SREBP-1 and ACCα, both responsible for fatty acid synthesis in adipose and hepatic tissues of rodents (Pan et al., 2015). In addition, the study showed that sesame oil increases fatty acid oxidation via upregulating PPARα (Bhaskaran et al., 2006), which is in line with our results. In particular, sesamin reduces hepatic lipid levels in the rodents via downregulation of SREBP-1 and upregulation of PPARα (Ide et al., 2004; Anilakumar et al., 2010). Our results show that sesame oil is beneficial against HFD-induced renal damage through suppressing lipogenesis and activating fatty acid oxidation.
Effects of sesame oil on antioxidant enzyme expression
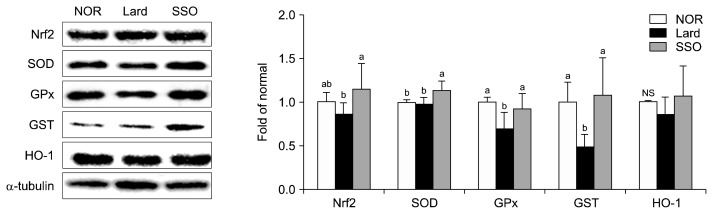
Protein expression levels of Nrf2 and antioxidant enzymes in the SSO group were upregulated in mice compared with those in the Lard group (P<0.05, Fig. 2). Nrf2, SOD, GSH-Px, and GST expression in the SSO group were 133.17%, 115.88%, 131.13%, and 220.06% higher, respectively, than those in the Lard group (all P<0.05). Moreover, higher expression of HO-1 was observed in the SSO group, however there were no significant differences between the experimental groups.
Fig. 2.
Protein expression levels of antioxidant enzymes regulated by Nrf2 in the kidneys of mice fed a high fat diet containing roasted sesame oil for 12 weeks. Data show mean±SD (n=7 each group). Data with different letters (a,b) are significantly different based on one-way ANOVA followed by Duncan’s multiple range test at P <0.05. See the legend of Table 1 for the experimental groups. Nrf2, nuclear factor-like 2; SOD, superoxide dismutase; HO-1, heme oxygenase-1; GST, glutathione S-transferase; GSH-Px, glutathione peroxidase; NS, data are not significantly different.
The antioxidant status of the body is the most important factor contributing to oxidative damage. Nrf2, transcription factor, regulates antioxidant enzymes including SOD, GSH-Px, and GST (Choi et al., 2014). In a model of renal injury, Nrf2 activity and expression of its target antioxidant enzymes were significantly diminished (Choi et al., 2014). Sesame oil rich in tocopherols and lignans has demonstrated preventive effects against oxidative stress (Kanu et al., 2010). Sesame oil and sesamin augment Nrf2 signaling, thereby increasing the amount of antioxidant enzyme expression in tissues (including the kidney) damaged by oxidative stress (Hamada et al., 2011; Liu et al., 2015), which are in line with our current results. Moreover, the antioxidant effects of lignin in sesamin and sesamol are well documented (Hamada et al., 2011; Kanimozhi and Prasad, 2009; Lv et al., 2015). It is apparent that bioactive compounds with antioxidative activity in sesame oil could reduce renal oxidative stress through increasing Nrf2 signaling and expression of its target antioxidant enzymes.
Anti-inflammatory effects of sesame oil
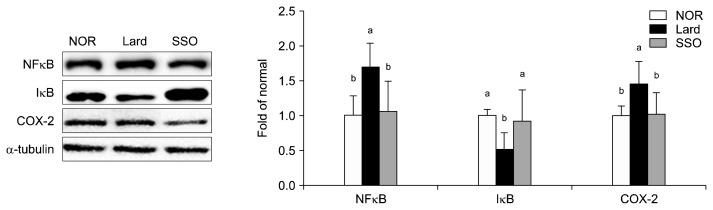
In the SSO group, protein expression levels of NF-κB and COX-2 were decreased by 37.17% and 30.48%, respectively, compared with those in the Lard group (both P< 0.05; Fig. 3). In contrast, IκB expression was increased 179.05% in the SSO group compared with that in the Lard group (P<0.05).
Fig. 3.
Protein expression levels of NFκB, IκB, and COX-2 in the kidneys of mice fed a high fat diet containing roasted sesame oil for 12 weeks. Data show mean±SD (n=7 each group). Data with different letters (a,b) are significantly different based on one-way ANOVA followed by Duncan’s multiple range test at P <0.05. See the legend of Table 1 for the experimental groups. NFκB, nuclear factor kappa B; IκB, inhibitor of nuclear factor kappa B; COX-2, cyclooxygenase 2.
Oxidative stress commonly accompanies inflammation. ROS stimulates translocation of NF-κB to the nucleus, where it upregulates inflammation-related enzymes (Ratliffet al., 2016). NF-κB activation has been observed in kidneys with elevated oxidative stress (Harikumar et al., 2010). Sesamin and sesamolin suppress NF-κB activation (Geetha et al., 2009) via inhibition of IκB dissociation through decreasing IκB kinase activity (Harikumar et al., 2010). Moreover, γ-tocopherol inhibits COX-2 and inducible nitric oxide synthase expression in lipopolysaccharide-treated macrophages (Jiang et al., 2000), suggesting that bioactive compounds with antioxidative activity in sesame oil may reduce inflammation. In the present study, sesame oil significantly inhibited NF-κB activation by increasing IκB expression (P<0.05), which is in agreement with the previous reports.
This study shows that sesame oil exerts several mechanisms of action to ameliorate HFD-induced renal damage; sesame oil lowers renal lipid concentrations by decreasing expression of genes involved in lipogenesis and increases those involved in fatty acid oxidation. Sesame oil exhibits antioxidative and anti-inflammatory effects through upregulating Nrf2 and its target genes, and downregulating the NF-κB pathway, respectively. The present study provides substantial evidence for the beneficial effects of sesame oil against HFD-induced renal damage.
ACKNOWLEDGEMENTS
This work was supported by a 2-year Research Grant of Pusan National University.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- Anilakumar KR, Pal A, Khanum F, Bawa AS. Nutritional, medicinal and industrial uses of sesame (Sesamum indicum L.) seedsan overview. Agric Conspec Sci. 2010;75:159–168. [Google Scholar]
- Baluchnejadmojarad T, Roghani M, Jalali Nadoushan MR, Vaez Mahdavi MR, Kalalian-Moghaddam H, Roghani-Dehkordi F, et al. The sesame lignan sesamin attenuates vascular dysfunction in streptozotocin diabetic rats: involvement of nitric oxide and oxidative stress. Eur J Pharmacol. 2013;698:316–321. doi: 10.1016/j.ejphar.2012.09.031. [DOI] [PubMed] [Google Scholar]
- Bhaskaran S, Santanam N, Penumetcha M, Parthasarathy S. Inhibition of atherosclerosis in low-density lipoprotein receptornegative mice by sesame oil. J Med Food. 2006;9:487–490. doi: 10.1089/jmf.2006.9.487. [DOI] [PubMed] [Google Scholar]
- Che Man YB, Rohman A, Mansor TST. Differentiation of lard from other edible fats and oils by means of fourier transform infrared spectroscopy and chemometrics. J Am Oil Chem Soc. 2011;88:187–192. doi: 10.1007/s11746-010-1659-x. [DOI] [Google Scholar]
- Choi BH, Kang KS, Kwak MK. Effect of redox modulating NRF2 activators on chronic kidney disease. Molecules. 2014;19:12727–12759. doi: 10.3390/molecules190812727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deji N, Kume S, Araki S, Soumura M, Sugimoto T, Isshiki K, et al. Structural and functional changes in the kidneys of high-fat diet-induced obese mice. Am J Physiol Renal Physiol. 2009;296:F118–F126. doi: 10.1152/ajprenal.00110.2008. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Geetha T, Rohit B, Pal KI. Sesamol: an efficient antioxidant with potential therapeutic benefits. Med Chem. 2009;5:367–371. doi: 10.2174/157340609788681476. [DOI] [PubMed] [Google Scholar]
- Hamada N, Tanaka A, Fujita Y, Itoh T, Ono Y, Kitagawa Y, et al. Involvement of heme oxygenase-1 induction via Nrf2/ARE activation in protection against H2O2-induced PC12 cell death by a metabolite of sesamin contained in sesame seeds. Bioorg Med Chem. 2011;19:1959–1965. doi: 10.1016/j.bmc.2011.01.059. [DOI] [PubMed] [Google Scholar]
- Harikumar KB, Sung B, Tharakan ST, Pandey MK, Joy B, Guha S, et al. Sesamin manifests chemopreventive effects through the suppression of NF-κB-regulated cell survival, proliferation, invasion, and angiogenic gene products. Mol Cancer Res. 2010;8:1541–7786. doi: 10.1158/1541-7786.MCR-09-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DZ, Chiang PJ, Chien SP, Huang BM, Liu MY. Parenteral sesame oil attenuates oxidative stress after endotoxin intoxication in rats. Toxicology. 2004;196:147–153. doi: 10.1016/j.tox.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Ide T, Hong DD, Ranasinghe P, Takahashi Y, Kushiro M, Sugano M. Interaction of dietary fat types and sesamin on hepatic fatty acid oxidation in rats. Biochim Biophys Acta. 2004;1682:80–91. doi: 10.1016/j.bbalip.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. γ-Tocopherol and its major metabolite, in contrast to α-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci USA. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Wang Z, Proctor G, Moskowitz S, Liebman SE, Rogers T, et al. Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem. 2005;280:32317–32325. doi: 10.1074/jbc.M500801200. [DOI] [PubMed] [Google Scholar]
- Jung K, Hong SH, Kim M, Han JS, Jang MS, Song YO. Antiatherogenic effects of Korean cabbage kimchi with added short arm octopus. Food Sci Biotechnol. 2015;24:249–255. doi: 10.1007/s10068-015-0033-z. [DOI] [Google Scholar]
- Kanimozhi P, Prasad NR. Antioxidant potential of sesamol and its role on radiation-induced DNA damage in whole-body irradiated Swiss albino mice. Environ Toxicol Pharmacol. 2009;28:192–197. doi: 10.1016/j.etap.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Kanu PJ, Bahsoon JZ, Kanu JB, Kandeh JBA. Nutraceutical importance of sesame seed and oil: a review of the contribution of their lignans. Sierra Leone J Biomed Res. 2010;2:4–16. doi: 10.4314/sljbr.v2i1.56583. [DOI] [Google Scholar]
- Kim M, Woo M, Noh JS, Choe E, Song YO. Sesame oil lignans inhibit hepatic endoplasmic reticulum stress and apoptosis in high-fat diet-fed mice. J. Funct Foods. 2017;37:658–665. doi: 10.1016/j.jff.2017.08.036. [DOI] [Google Scholar]
- Kume S, Uzu T, Araki S, Sugimoto T, Isshiki K, Chin-Kanasaki M, et al. Role of altered renal lipid metabolism in the development of renal injury induced by a high-fat diet. J Am Soc Nephrol. 2007;18:2715–2723. doi: 10.1681/ASN.2007010089. [DOI] [PubMed] [Google Scholar]
- Liu CT, Chien SP, Hsu DZ, Periasamy S, Liu MY. Curative effect of sesame oil in a rat model of chronic kidney disease. Nephrology. 2015;20:922–930. doi: 10.1111/nep.12524. [DOI] [PubMed] [Google Scholar]
- Lv D, Zhu CQ, Liu L. Sesamin ameliorates oxidative liver injury induced by carbon tetrachloride in rat. Int J Clin Exp Pathol. 2015;8:5733–5738. [PMC free article] [PubMed] [Google Scholar]
- Monteiro EM, Chibli LA, Yamamoto CH, Pereira MC, Vilela FM, Rodarte MP, et al. Antinociceptive and anti-inflammatory activities of the sesame oil and sesamin. Nutrients. 2014;6:1931–1944. doi: 10.3390/nu6051931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noeman SA, Hamooda HE, Baalash AA. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol Metab Syndr. 2011;3:17. doi: 10.1186/1758-5996-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nzikou JM, Matos L, Bouanga-Kalou G, Ndangui CB, Pambou-Tobi NPG, Kimbonguila A, et al. Chemical composition on the seeds and oil of sesame (Sesamum indicum L.) grown in Congo-Brazzaville. Adv J Food Sci Technol. 2009;1:6–11. [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Pan JH, Kim MJ, Kim JH, Cho YJ, Shin HS, Sung JS, et al. Inhibition of the lipogenesis in liver and adipose tissue of diet-induced obese C57BL/6 mice by feeding oleic acid-rich sesame oil. Food Sci Biotechnol. 2015;24:1115–1121. doi: 10.1007/s10068-015-0142-8. [DOI] [Google Scholar]
- Praga M. Obesity-a neglected culprit in renal disease. Nephrol Dial Transplant. 2002;17:1157–1159. doi: 10.1093/ndt/17.7.1157. [DOI] [PubMed] [Google Scholar]
- Ratliff BB, Abdulmahdi W, Pawar R, Wolin MS. Oxidant mechanisms in renal injury and disease. Antioxid Redox Signal. 2016;25:119–146. doi: 10.1089/ars.2016.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar D, Sambandam G, Ramakrishna Rao M, Pugalendi KV. Modulation of blood pressure, lipid profiles and redox status in hypertensive patients taking different edible oils. Clin Chim Acta. 2005;355:97–104. doi: 10.1016/j.cccn.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Sekiya M, Yahagi N, Matsuzaka T, Najima Y, Nakakuki M, Nagai R, et al. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology. 2003;38:1529–1539. doi: 10.1053/jhep.2003.09028. [DOI] [PubMed] [Google Scholar]
- Teare JP, Punchard NA, Powell JJ, Lumb PJ, Mitchell WD, Thompson RP. Automated spectrophotometric method for determining oxidized and reduced glutathione in liver. Clin Chem. 1993;39:686–689. [PubMed] [Google Scholar]
- Wan Y, Li H, Fu G, Chen X, Chen F, Xie M. The relationship of antioxidant components and antioxidant activity of sesame seed oil. J Sci Food Agric. 2015;95:2571–2578. doi: 10.1002/jsfa.7035. [DOI] [PubMed] [Google Scholar]
- Yang R, Le G, Li A, Zheng J, Shi Y. Effect of antioxidant capacity on blood lipid metabolism and lipoprotein lipase activity of rats fed a high-fat diet. Nutrition. 2006;22:1185–1191. doi: 10.1016/j.nut.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Zhang R, Yu Y, Deng J, Zhang C, Zhang J, Cheng Y, et al. Sesamin ameliorates high-fat diet-induced dyslipidemia and kidney injury by reducing oxidative stress. Nutrients. 2016;8:E276. doi: 10.3390/nu8050276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Zheng S, Yang J, Wu Y, Ren Y, Kong X, et al. Suppression of TGF-β1/Smad signaling pathway by sesamin contributes to the attenuation of myocardial fibrosis in spontaneously hypertensive rats. PloS One. 2015;10:e0121312. doi: 10.1371/journal.pone.0121312. [DOI] [PMC free article] [PubMed] [Google Scholar]