Abstract
In this study, we determined the contents of glucosinolate, polyphenol, and flavonoid, and the antioxidant activities of uncooked, steamed, and boiled cauliflower. Eight glucosinolate peaks were detected, representing glucoiberin, progoitrin, glucoraphanin, sinigrin, gluconapin, glucoiberverin, glucobrassicin, and gluconasturtiin. Boiled cauliflower contained significantly lowered concentrations of glucosinolate, total polyphenol, and total flavonoid compared to uncooked or steamed cauliflower. These results clearly indicate that health-promoting compounds in cauliflower are significantly impacted by different cooking methods: uncooked> steamed> boiled. The amounts of total polyphenols and total flavonoids in uncooked cauliflower extracted with 80% ethanol were higher than extracts of steamed and boiled cauliflower. The highest antioxidant activity was observed in uncooked cauliflower extracted using 80% ethanol compared to those extracted with water at the same concentration. Steamed and boiled cauliflower extracts also showed lower antioxidant activity than uncooked extracts. Based on these results, fresh uncooked cauliflower contains higher contents of health-promoting compounds and elevated antioxidant activity. Moreover, steaming may be more desirable than boiling in order to minimize loss of glucosinolates when storing, pretreating, processing, and cooking cruciferous vegetables.
Keywords: cauliflower, glucosinolates, polyphenols, flavonoids, antioxidant
INTRODUCTION
Cruciferous vegetables, Brassicaceae, include cauliflower, broccoli, Brussels sprouts, kale, and mustard (Carlson et al., 1987). These vegetables contain various physiologically active substances such as glucosinolates, polyphenols, flavonoids, and various vitamins (Carlson et al., 1987; Picchi et al., 2012). Consumption of cruciferous vegetables is linked to suppression of many cancers including breast, prostate, lung, and colon cancer (Abdull Razis and Noor, 2013; Turati et al., 2015). The protective effect against risk of cancer can been accounted to glucosinolates, which are unique and important compounds naturally found in almost all cruciferous vegetables (Abdull Razis and Noor, 2013; Ishida et al., 2014). Glucosinolates are a group of sulfur-containing glucosides that are hydrolyzed by endogenous myrosinases (EC 3.2.1.147) into isothiocyanates, thiocyanates, and nitriles (Carlson et al., 1987; Dinkova-Kostova and Kostov, 2012). Both glucosinolates and isothiocyanates are of immense interest in food processing, given their bitter flavor, pungent taste, and aroma, and correlation with human health. Furthermore, the contents of glucosinolates and isothiocyanate formation are affected by food processing and preservation techniques (Rungapamestry et al., 2007). Depending on the type of cruciferous vegetable, storage condition, cooking method and time, cultivation environment, and different glucosinolate profiles may be obtained (Rungapamestry et al., 2007; Vicas et al., 2013; Cools and Terry, 2018).
Unlike other vegetables, cruciferous vegetables are often consumed after cooking by heat treatment. During the cooking process, the glucosinolate-myrosinase system may be modified through myrosinase inactivation, loss of cofactors, thermal breakdown, and glucosinolate leaching (Rungapamestry et al., 2007; Palermo et al., 2014). In addition, the hydrolytic products of glucosinolates are volatile. Boiling cruciferous vegetables resulted in a significant loss (90%) of glucosinolates due to leaching into the cooking water (Ciska et al., 2016; Hanschen et al., 2018). These phenomena are mostly influenced by the duration and method of cooking, the type of vegetable matrix and the extent of cellular disruption, and the chemical structure of the glucosinolates (Conaway et al., 2000; Oliviero et al., 2018). One report suggested that myrosinases from broccoli sprouts are more heat-resistant than those from mature broccoli (Matusheski et al., 2004). In this study, the enzymes in broccoli were thermally inactivated at approximately 70°C, whereas myrosinases in immature broccoli sprouts remained active at 100°C (Matusheski et al., 2004).
Glucosinolates may act as an antioxidant by increasing enzymatic activities that inhibit oxidation, such as those of glutathione-S-transferase and uridine diphosphate-glucuronosyltransferase (Williamson et al., 1998; Vig et al., 2009). Commonly known antioxidants, such as vitamin C, vitamin E, and carotenoids, directly inhibit oxidation by neutralizing free radicals before cellular oxidation; whereas glucosinolates and their hydrolyzed products are indirect antioxidants that function by increasing phase I and phase II detoxification enzymes, and show long-lasting antioxidant activities (Vig et al., 2009; Vicas et al., 2013).
The purpose of this study was to investigate the effects of different cooking procedures (steaming and boiling) on glucosinolate, total polyphenol, and total flavonoid concentrations in cauliflower. In addition, we compared the antioxidant activity of fresh uncooked cauliflower to steamed and boiled cauliflower, the main methods of preparing cauliflower prior to consumption.
MATERIALS AND METHODS
Materials
Glucosinolate standards (glucoraphanin, sinigrin, gluconapin, glucobrassicin, and gluconasturtiin) were purchased from Extrasynthese (Genay, France). Aryl sulfates (type H-1, EC 3.1.6.1), diethyl aminoethyl-Sephadex A-25, and high-pressure liquid chromatography (HPLC)- grade acetonitrile were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). All other chemicals were of analytical grade.
Cauliflower preparation
Fresh cauliflower (Brassica oleracea L. spp. botrytis) grown in Jeju, Korea was purchased from a local market, washed with tap water, and air-dried. The effect of controlled steaming and boiling on the content of bioactive compounds and antioxidant activities in cauliflower relative to uncooked cauliflower was then studied. For steaming, 300 g of cauliflower was placed in a steamer pot (Kitchen Art, Ltd., Gimpo, Korea) containing hot water for approximately 2 min. Thereafter, the cauliflower was removed and cooled to room temperature before storage at −80 °C. For boiling, 300 g of cauliflower was placed in boiling water for 3 min and then cooled to room temperature before storage at −80°C. Fresh (uncooked) cauliflower was drained of water and stored at −80°C. All samples were frozen at −80°C and then freeze-dried. Freeze-dried samples were finely ground with a food grinder (Hanil Electric, Seoul, Korea) and stored at −80°C until further analysis.
Extraction and desulfation of glucosinolates
The glucosinolate concentration in cauliflower was determined using the method of Grosser and van Dam (2017). First, 0.5 g of powdered sample was mixed with 2 mL of boiling 70% methanol, extracted in a water bath at 95°C for 5 min, and centrifuged at 12,000 rpm for 10 min at 4°C. The pellet was then re-extracted and the supernatants were pooled. The crude glucosinolate extract was loaded onto a Mini Bio-Spin chromatography column (Bio-Rad Laboratories, Hercules, CA, USA) containing 1 mL of cross-linked dextran gel (type G-25) anion exchange resin, which was pre-activated with 20 mM sodium acetate (pH 5.5). Next, the sample was subjected to desulfation through addition of 75 μL of purified aryl sulfatase (EC 3.1.6.1, type H-1 from Helix pomatia). The column was capped, incubated at room temperature for 24 h, and the desulfo-glucosinolates were eluted with 1.5 mL of distilled water, filtered through a 0.2-μm syringe filter, and injected into the column for HPLC.
HPLC analysis was carried out using an Agilent 1200 HPLC system coupled with a photodiode array detector (Agilent Technologies, Memphis, TN, USA), containing a Waters symmetry 300 C18 chromatographic column (75 mm×4.6 mm, i.d. with 3.5 μm particle diameter, Waters, Franklin, MA, USA) at 40°C. The mobile phase composed of A (water) and B (acetonitrile) with gradient elutions of 0 min (0% B), 0~1 min (2% B), 2~35 min (2~35% B), 35~40 min (35~2% B), and 41 min (0% B). Sample were injected in volumes of 20 μL, and the flow rate was set at 0.5 mL/min. Peaks were detected at 229 nm.
Authentic glucosinolate standards, expressed as micromoles per gram (μmol/g), were desulfated and used for identification and quantification of the peaks. For glucoiberin, progoitrin, and glucoiberverin, for which standards were unavailable, the retention time and peaks were identified and confirmed by liquid chromatography-tandem mass spectrometry (ThermoQuest; Thermo Finnigan, San Jose, CA, USA) and quantified by relative comparison based on the sinigrin content, using the method reported in ISO-9167-1 (International Organization for Standardization, 1992). Quantification of individual glucosinolates was carried out using the response factors shown in Table 1.
Table 1.
High-pressure liquid chromatography identification of glucosinolates in cauliflower cooked in different ways
| No | Retention time (min) | Trivial name | Semi-systemic name | Response factor |
|---|---|---|---|---|
| 1 | 3.03 | Glucoiberin | 3-Methylsulfinylpropylglucosinolate | 0.80 |
| 2 | 4.71 | Progoitrin | 2-Hydroxy-3-butenylglucosinolate | 1.09 |
| 3 | 7.05 | Sinigrin (Std)1) | 2-Propenylglucosinolate | 1.00 |
| 4 | 8.20 | Glucoraphanin (Std)1) | 4-Methylsulfinylbutylglucosinolate | 0.90 |
| 5 | 12.00 | Gluconapin (Std)1) | 3-Butenylglucosinolate | 1.11 |
| 6 | 14.65 | Glucoiberverin | 3-Methylthiopropylglucosinolate | 0.80 |
| 7 | 19.01 | Glucobrassicin (Std)1) | 3-Indolylmethylglucosinolate | 0.29 |
| 8 | 20.65 | Gluconasturtiin (Std)1) | 2-Phenylethylglucosinolate | 0.95 |
Std, standard.
The compounds were identified by comparing the chromatograph with the authentic standards.
Determination of total phenolic and flavonoid contents
The total phenolic content in cauliflower was determined using the Folin-Ciocalteu method (Thi and Hwang, 2014). Lyophilized cauliflower powder was extracted with 25 volumes of ethanol for 24 h at room temperature. The ethanol extract was filtered through Whatman No.1 filter paper and concentrated using a rotary vacuum evaporator (EYELA 400 series, EYELA, Tokyo, Japan). Total polyphenol and flavonoid contents were analyzed in dried extracts. Briefly, 200 μL of the diluted sample was mixed with 400 μL of 2% 2 N Folin-Ciocalteu’s phenol reagent. After incubation at room temperature for 3 min, 800 μL of 10% Na2CO3 was added and the sample was mixed. The mixture was covered with aluminum foil and incubated in the dark at room temperature for 1 h. After vortexing, the absorbance was measured at 750 nm using a microplate reader (Infinite M200 Pro, Tecan Group Ltd., San Jose, CA, USA). Gallic acid was employed as a calibration standard and the results are expressed as gallic acid equivalents (GAE) per gram of dry weight (mg GAE /g).
The total flavonoid content of cauliflower was determined using the method of Thi and Hwang (2014). 500 μL of sample was mixed with 30 μL of 5% NaNO2, incubated for 6 min at room temperature, before 60 μL of 10 % AlCl3·6H2O was added and the sample was mixed. The mixture was maintained at room temperature for 6 min, after which 200 μL of 1.0 M NaOH was added, followed by 110 μL of distilled water. The absorbance of the colored flavonoid-aluminum complex was measured immediately at 510 nm using a microplate reader (Infinite M200 Pro, Tecan Group Ltd.). Catechin was used as a calibration standard and the results are expressed as catechin equivalents (CE) per gram of dry weight (mg CE/g).
Determination of antioxidant activity
Antioxidant properties were determined by evaluating 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical-scavenging activities and reducing powers, according to the method described by Thi and Hwang (2014). Samples for determining antioxidant activity were used following the same pretreatment steps as for the total polyphenol and total flavonoid measurements. Lyophilized cauliflower powder was extracted with 25 volumes of ethanol for 24 h at room temperature. The ethanol extract was filtered through Whatman No.1 filter paper and concentrated using a rotary vacuum evaporator (EYELA 400 series, EYELA). Dried extracts were used to determine antioxidant activity using DPPH, ABTS, and reducing power assays. For the DPPH assay, 100 μL of prepared sample was mixed with 2 mL of methanolic solution containing 0.1 mmol/L DPPH radicals. The mixture was shaken vigorously and incubated for 30 min in the dark, and absorbance was measured at 517 nm against a blank. Antioxidant activity was calculated based on the relative ratio between the samples and the control.
For the ABTS assay, ABTS radicals were produced by mixing the ABTS stock solution (7 mmol/L in water) with 2.45 mmol/L potassium persulfate. The solution was incubated at room temperature in the dark for 16 h before use. Once the radicals had formed, absorbance at 734 nm was adjusted to 0.7 by dilution with 95% ethanol. One milliliter of ABTS solution was added to 10 mL of sample and the reaction mixture was incubated at room temperature for 6 min. The absorbance at 734 nm was then measured immediately. The antioxidant activity was calculated based on the relative ratio between the samples and the control.
To calculate the reducing power, 0.25 mL of samples at various concentrations were mixed with 0.25 mL of phosphate buffer (0.2 M, pH 6.6) and 0.25 mL of potassium hexacyanoferrate [K3Fe(CN)6, 1% w/v]. After the mixture was incubated in a water bath at 50°C for 20 min, the reaction was stopped by adding 0.25 mL of trichloroacetic acid solution (10% w/v) and the mixture was centrifuged at 3,000 rpm for 10 min. Next, 0.5 mL of the supernatant was mixed with 0.5 mL of distilled water and 0.1 mL of ferric chloride (FeCl3) solution (0.1% w/v) for 10 min. The reducing power was calculated by immediately measuring the absorbance at 700 nm using a microplate reader (Infinite M200 Pro, Tecan Group Ltd.). Ascorbic acid was used as a standard with the final concentration ranging from 0~200 μg/mL.
Statistical analysis
All results are presented as mean±standard deviation (SD). Statistical analysis was performed using the SPSS software package, version 17.0 (SPSS Inc., Chicago, IL, USA). Data were compared using one-way analysis of variance; P<0.05 was considered statistically significant.
RESULTS
Determination of glucosinolate content
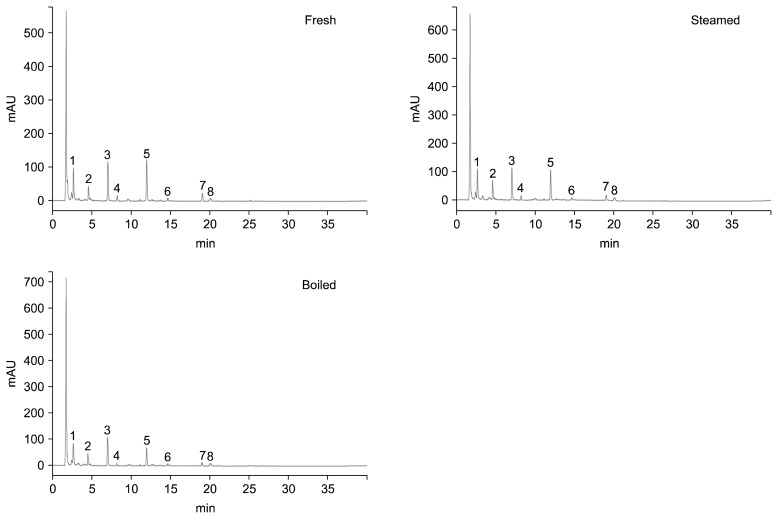
A typical HPLC chromatogram of glucosinolates isolated from cauliflower cooked in different ways is shown in Fig. 1. In the current study, eight glucosinolates (glucoiberin, progoitrin, glucoraphanin, sinigrin, gluconapin, glucoiberverin, glucobrassicin, and gluconasturtiin) were detected (Table 1). The glucosinolate contents (μmol/g dry weight) of fresh, steamed, and boiled cauliflower are shown in Table 2. Progoitrin (0.756 μmol) was the main glucosinolate identified in fresh cauliflower, followed by gluconapin (0.733 μmol), sinigrin (0.694 μmol), and glucoiberverin (0.474 μmol). The concentrations of glucoiberin, progoitrin, glucoraphanin, sinigrin, gluconapin, and glucoiberverin were decreased in steamed and boiled cauliflower compared with uncooked cauliflower; this decrease was greatest in boiled cauliflower. The progoitrin content of uncooked cauliflower was 0.756 μmol/g dry weight, which was decreased to 0.545 and 0.452 μg/g dry weight (72.09% and 59.79%) after steaming and boiling, respectively. The glucoiberverin content of uncooked cauliflower was 0.474 μmol/g dry weight, which decreased to 0.341 and 0.284 μg/g dry weight (71.94% and 59.92%) after steaming and boiling, respectively. The concentrations of glucoraphanin and gluconasturtiin did not significantly differ between uncooked, steamed, and boiled cauliflower.
Fig. 1.
Representative high-pressure liquid chromatography chromatogram of glucosinolates isolated from fresh, steamed, and boiled cauliflower. Peak identification 1: glucoiberin, 2: progoitrin, 3: sinigrin, 4: glucoraphanin, 5: gluconapin, 6: glucoiberverin, 7: glucobrassicin, 8: gluconasturtiin.
Table 2.
Glucosinolate contents in fresh, steamed, and boiled cauliflower
| (unit: μmol/g dry weight) | |||
|---|---|---|---|
|
| |||
| Glucosinolates | Fresh | Steamed | Boiled |
| Glucoiberin | 0.452±0.005b | 0.326±0.001ab | 0.271±0.001a |
| Progoitrin | 0.756±0.008b | 0.545±0.001b | 0.452±0.001a |
| Sinigrin (Std)1) | 0.694±0.008b | 0.500±0.001a | 0.415±0.001a |
| Glucoraphanin (Std)1) | 0.021±0.001ns | 0.019±0.001 | 0.018±0.006 |
| Gluconapin (Std)1) | 0.733±0.008c | 0.528±0.001b | 0.439±0.001a |
| Glucoiberverin | 0.474±0.005b | 0.341±0.001ab | 0.284±0.001a |
| Glucobrassicin (Std)1) | 0.187±0.004b | 0.109±0.001a | 0.105±0.001a |
| Gluconasturtiin (Std)1) | 0.033±0.001ns | 0.031±0.001 | 0.032±0.001 |
Data represent the mean±SD of three separate experiments.
Values with different letters (a–c) within the same row are significantly different at P<0.05.
Std, standard.
The compounds were identified by comparing the chromatograph with the authentic standards.
Not significant.
Total polyphenol and flavonoid contents
The total polyphenol and flavonoid contents in cauliflower subjected to different cooking methods are presented in Table 3. Uncooked samples extracted with 80% ethanol showed the highest total polyphenol content of 35.01 mg GAE/g dry weight. In contrast, boiled samples exhibited the lowest total polyphenol content of only 15.57 mg GAE/g dry weight. Uncooked cauliflower extracted in 80 % ethanol contained higher total flavonoid contents both steamed and boiled samples. The total flavonoid contents of cauliflower after steaming and boiling were 5.15 and 1.58 mg CE/g dry weight, respectively, both lower than that of uncooked samples.
Table 3.
Total polyphenol and total flavonoid contents in fresh, steamed, and boiled cauliflower
| Cooking method | Total polyphenols (mg GAE/g) | Total flavonoids (mg CE/g) |
|---|---|---|
| Fresh | 35.01±3.04c | 6.06±1.11b |
| Steamed | 20.65±1.39b | 5.15±0.83b |
| Boiled | 15.57±0.77a | 1.58±0.21a |
Data represent the mean±SD of four separate experiments.
Values with different letters (a–c) within the same column are significantly different at P<0.05.
GAE, gallic acid equivalent; CE, catechin equivalent.
Antioxidant activity
The antioxidant activities of cauliflower extracts were determined using the widely used in vitro DPPH, ABTS, and reducing power assays. As the concentration of cauliflower extracts increased (500 and 1,000 μg/mL), the radical-scavenging activity increased significantly (P< 0.05). Overall, cauliflower samples extracted with an aqueous solvent of 80% ethanol showed higher radical-scavenging activities than those extracted with distilled water.
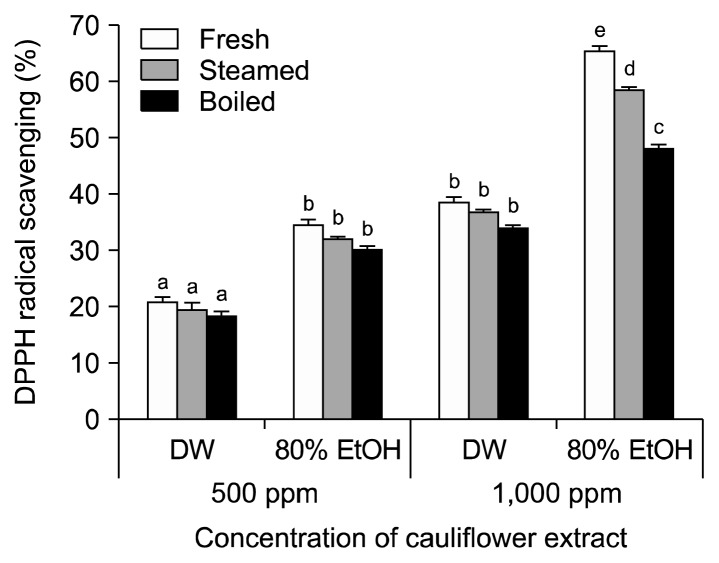
The DPPH radical-scavenging activity of cauliflower increased in 80% in ethanol extracts and with higher concentration (Fig. 2). The average inhibition of DPPH radical formation in 500 μg/mL fresh cauliflower was 34.59% compared with 32.23% and 30.21% in steamed and boiled cauliflower extracted with 80% ethanol, respectively. At 1,000 μg/mL, the DPPH radical-scavenging activity of the uncooked sample was 65.44%, which was higher than that of the steamed and boiled samples (58.63% and 48.20%, respectively). Inhibition of DPPH radical formation was higher in uncooked cauliflower extracted with 80% ethanol compared with water when at the same concentration.
Fig. 2.
DPPH radical-scavenging activity in cauliflower cooked in different ways. Each value is expressed as the mean±SD of three separate experiments. Different letters (a–e) indicate that the means are significantly different from the control (P <0.05).
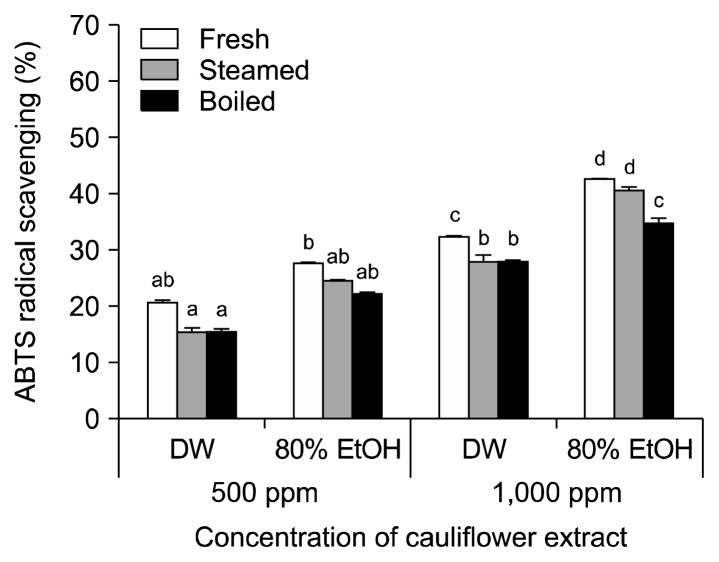
The ABTS radical-scavenging activities of cauliflower cooked in various ways are shown in Fig. 3. The ABTS radical-scavenging activity was significantly increased (P<0.05) in 80% ethanol extracts compared to water extracts of the same concentration; higher antioxidant activities were also shown with increased concentration. At 500 μg/mL, uncooked cauliflower showed 20.77% ABTS radical-scavenging activity when extracted with water and 27.21% when extracted with 80% ethanol. Steamed cauliflower extracts showed similar patterns, with ABTS radical-scavenging activities of 15.63% in water and 24.71 % in 80% ethanol. At 1,000 μg/mL, uncooked, steamed, and boiled cauliflower showed higher ABTS radical-scavenging activities at 42.74%, 40.74%, and 34.89%, respectively. The highest ABTS-scavenging activity in uncooked cauliflower compared with steamed and boiled cauliflower was observed in 80% ethanol extracts.
Fig. 3.
ABTS radical-scavenging activity in cauliflower cooked in different ways. Each value is expressed as the mean±SD of three separate experiments. Different letters (a–d) indicate that the means are significantly different from the control (P <0.05).
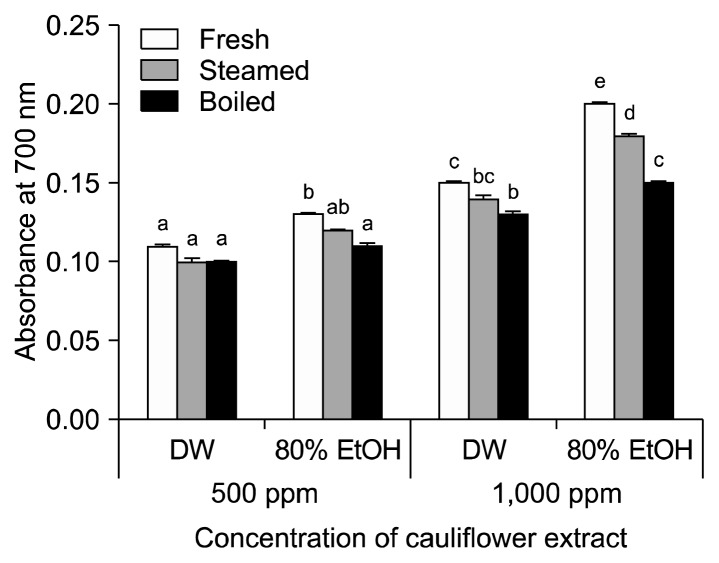
The reducing powers of cauliflower cooked in different ways, where higher absorbances correspond to higher reducing powers, are shown in Fig. 4. The highest absorbance at 700 nm was measured in uncooked cauliflower extracted with 80% ethanol, while the lowest absorbance was obtained in boiled cauliflower extracted with distilled water. The absorbance values of uncooked, steamed, and boiled cauliflower extracted with water were 0.15, 0.14, and 0.13 at 1,000 μg/mL, respectively; these values were slightly increased to 0.20, 0.18, and 0.15, respectively, when extracted with 80% ethanol.
Fig. 4.
The reducing power activity in cauliflower cooked in different ways. Each value is expressed as the mean±SD of three separate experiments. Different letters (a–e) indicate that the means are significantly different from the control (P <0.05).
DISCUSSION
Cauliflower possesses very low glucosinolate contents compared with other cruciferous vegetables such as broccoli and Brussels sprouts (Carlson et al., 1987; Lafarga et al., 2018). The types and contents of glucosinolates in cruciferous vegetables vary depending on storage condition, growth stage, tissues, and cooking method (Rungapamestry et al., 2007; Volden et al., 2009; López-Cervantes et al., 2013). Bhandari et al. (2015) identified several glucosinolates from the seeds, sprouts, shoots, and roots of cauliflower and found that the amount of glucosinolates varied in the different parts. For example, different concentrations of glucoiberin (0.32~12.77 μmol/g dry weight), progoitrin (0.05~0.92 μmol/g dry weight), epiprogoitrin (0~0.12 μmol/g dry weight), glucoraphanin (0.01~0.31 μmol/g dry weight), sinigrin (1.22~34.09 μmol/g dry weight), glucobrassicin (0.73~2.56 μmol/g dry weight), and gluconasturtiin (0~52.08 μmol/g dry weight) were identified.
During the cooking of cruciferous vegetables, myrosinases are partially or totally inactivated and altered (Fahey et al., 2012). The changes in enzymatic activity may be due to glucosinolates decomposition by heat, loss of enzymatic cofactors, leaching, volatilization, and thermal decomposition (Dekker et al., 2000; Fahey et al., 2012). In a previous study, glucosinolate contents were analyzed in broccoli, Brussels sprouts, cauliflower, and green cabbage. Steaming and microwave oven cooking did not significantly decrease glucosinolate contents; however, glucosinolates were significantly decreased by boiling, following which approximately 90% of glucosinolates were detected in the cooking water (Song and Thornalley, 2007). Glucosinolates are water-soluble substances; the content of glucosinolates are significantly reduced when vegetables are washed before cooking or immersed in hot water. In general, pouring over boiling water reduces glucosinolate contents by 40~60%, and longer cooking times cause higher amounts of glucosinolates to be eluted (Song and Thornalley, 2007). However, since steaming is a steam-cooking procedure, the content of glucosinolates eluted into the water is relatively low compared to that of boiling.
Glucosinolate contents depend on the specificity of the physiologically active substance contained in the extraction solvent. To efficiently extract a substance from plant materials, it is preferable to use a solvent with a polarity similar to that of the target substance (Thi and Hwang, 2014). In this study, 80% ethanol was more effective than water in extracting polyphenol and flavonoid compounds from cauliflower. Antioxidant activity was found to be proportional to glucosinolate, total polyphenol, and total flavonoid contents. Correspondingly, uncooked cauliflower contained higher glucosinolate, total polyphenol, and total flavonoid contents, and higher antioxidant activity, than that of steamed or boiled cauliflower. In addition, the 80% ethanol extracts showed higher antioxidant activities than the water extracts.
Based on these results, it may be desirable to use steaming rather than boiling to minimize the loss of glucosinolates when storing, pretreating, processing, and cooking cruciferous vegetables.
ACKNOWLEDGEMENTS
This work was supported by a research grant from Hankyong National University for an academic exchange program in 2017.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The author declares no conflict of interest.
REFERENCES
- Abdull Razis AF, Noor NM. Cruciferous vegetables: dietary phytochemicals for cancer prevention. Asian Pac J Cancer Prev. 2013;14:1565–1570. doi: 10.7314/APJCP.2013.14.3.1565. [DOI] [PubMed] [Google Scholar]
- Bhandari SR, Jo JS, Lee JG. Comparison of glucosinolate profiles in different tissues of nine Brassica crops. Molecules. 2015;20:15827–15841. doi: 10.3390/molecules200915827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson DG, Daxenbichler ME, VanEtten CH. Glucosinolates in crucifer vegetables: broccoli, brussels sprouts, cauliflower, collards, kale, mustard greens, and kohlrabi. J Amer Soc Hort Sci. 1987;112:173–178. [Google Scholar]
- Ciska E, Drabińska N, Narwojsz A, Honke J. Stability of glucosinolates and glucosinolate degradation products during storage of boiled white cabbage. Food Chem. 2016;203:340–347. doi: 10.1016/j.foodchem.2016.02.079. [DOI] [PubMed] [Google Scholar]
- Conaway CC, Getahun SM, Liebes LL, Pusateri DJ, Topham DK, Botero-Omary M, et al. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr Cancer. 2000;38:168–178. doi: 10.1207/S15327914NC382_5. [DOI] [PubMed] [Google Scholar]
- Cools K, Terry LA. The effect of processing on the glucosinolate profile in mustard seed. Food Chem. 2018;252:343–348. doi: 10.1016/j.foodchem.2018.01.096. [DOI] [PubMed] [Google Scholar]
- Dekker M, Verkerk R, Jongen WMF. Predictive modelling of health aspects in the food production chain: a case study on glucosinolates in cabbage. Trends Food Sci Technol. 2000;11:174–181. doi: 10.1016/S0924-2244(00)00062-5. [DOI] [Google Scholar]
- Dinkova-Kostova AT, Kostov RV. Glucosinolates and isothiocyanates in health and disease. Trends Mol Med. 2012;18:337–347. doi: 10.1016/j.molmed.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Fahey JW, Wehage SL, Holtzclaw WD, Kensler TW, Egner PA, Shapiro TA, et al. Protection of humans by plant glucosinolates: efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev Res. 2012;5:603–611. doi: 10.1158/1940-6207.CAPR-11-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosser K, van Dam NM. A straightforward method for glucosinolate extraction and analysis with high-pressure liquid chromatography (HPLC) J Vis Exp. 2017 doi: 10.3791/55425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanschen FS, Kühn C, Nickel M, Rohn S, Dekker M. Leaching and degradation kinetics of glucosinolates during boiling of Brassica oleracea vegetables and the formation of their break-down products. Food Chem. 2018;263:240–250. doi: 10.1016/j.foodchem.2018.04.069. [DOI] [PubMed] [Google Scholar]
- International Organization for Standardization. ISO 9167-1: 1992. 1992. Rapeseed—Determination of glucosinolates content—Part 1: Method using high-performance liquid chromatography; pp. 1–9. [Google Scholar]
- Ishida M, Hara M, Fukino N, Kakizaki T, Morimitsu Y. Glucosinolate metabolism, functionality and breeding for the improvement of Brassicaceae vegetables. Breed Sci. 2014;64:48–59. doi: 10.1270/jsbbs.64.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafarga T, Bobo G, Viñas I, Collazo C, Aguiló-Aguayo I. Effects of thermal and non-thermal processing of cruciferous vegetables on glucosinolates and its derived forms. J Food Sci Technol. 2018;55:1973–1981. doi: 10.1007/s13197-018-3153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Cervantes J, Tirado-Noriega LG, Sánchez-Machado DI, Campas-Baypoli ON, Cantú-Soto EU, Núñez-Gastélum JA. Biochemical composition of broccoli seeds and sprouts at different stages of seedling development. Int J Food Sci Technol. 2013;48:2267–2275. [Google Scholar]
- Matusheski NV, Juvik JA, Jeffery EH. Heating decreases epithio-specifier protein activity and increases sulforaphane formation in broccoli. Phytochemistry. 2004;65:1273–1281. doi: 10.1016/j.phytochem.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Oliviero T, Verkerk R, Dekker M. Isothiocyanates from Brassica vegetables—effects of processing, cooking, mastication, and digestion. Mol Nutr Food Res. 2018;62:e1701069. doi: 10.1002/mnfr.201701069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo M, Pellegrini N, Fogliano V. The effect of cooking on the phytochemical content of vegetables. J Sci Food Agric. 2014;94:1057–1070. doi: 10.1002/jsfa.6478. [DOI] [PubMed] [Google Scholar]
- Picchi V, Migliori C, Lo Scalzo R, Campanelli G, Ferrari V, Di Cesare LF. Phytochemical content in organic and conventionally grown Italian cauliflower. Food Chem. 2012;130:501–509. doi: 10.1016/j.foodchem.2011.07.036. [DOI] [PubMed] [Google Scholar]
- Rungapamestry V, Duncan AJ, Fuller Z, Ratcliffe B. Effect of cooking brassica vegetables on the subsequent hydrolysis and metabolic fate of glucosinolates. Proc Nutr Soc. 2007;66:69–81. doi: 10.1017/S0029665107005319. [DOI] [PubMed] [Google Scholar]
- Song L, Thornalley PJ. Effect of storage, processing and cooking on glucosinolate content of Brassica vegetables. Food Chem Toxicol. 2007;45:216–224. doi: 10.1016/j.fct.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Thi ND, Hwang E. Bioactive compound contents and antioxidant activity in aronia (Aronia melanocarpa) leaves collected at different growth stages. Prev Nutr Food Sci. 2014;19:204–212. doi: 10.3746/pnf.2014.19.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turati F, Rossi M, Pelucchi C, Levi F, La Vecchia C. Fruit and vegetables and cancer risk: a review of southern European studies. Br J Nutr. 2015;113:S102–S110. doi: 10.1017/S0007114515000148. [DOI] [PubMed] [Google Scholar]
- Vicas SI, Teusdea AC, Carbunar M, Socaci SA, Socaciu C. Glucosinolates profile and antioxidant capacity of Romanian Brassica vegetables obtained by organic and conventional agricultural practices. Plant Foods Hum Nutr. 2013;68:313–321. doi: 10.1007/s11130-013-0367-8. [DOI] [PubMed] [Google Scholar]
- Vig AP, Rampal G, Thind TS, Arora S. 2009. Bio-protective effects of glucosinolates—a review. LWT-Food Sci Technol. 2009;42:1561–1572. doi: 10.1016/j.lwt.2009.05.023. [DOI] [Google Scholar]
- Volden J, Bengtsson GB, Wicklund T. Glucosinolates, L-ascorbic acid, total phenols, anthocyanins, antioxidant capacities and colour in cauliflower (Brassica oleracea L. ssp. botrytis); effects of long-term freezer storage. Food Chem. 2009;112:967–976. doi: 10.1016/j.foodchem.2008.07.018. [DOI] [Google Scholar]
- Williamson G, Faulkner K, Plumb GW. Glucosinolates and phenolics as antioxidants from plant foods. Eur J Cancer Prev. 1998;7:17–21. [PubMed] [Google Scholar]