Abstract
Background:
There is a paucity of literature about the atypical clinical manifestations in children with atopic dermatitis (AD) in Asian setting, and colonization with methicillin-resistant Staphylococcus aureus (MRSA), and its association with disease severity, if any.
Objective:
To elucidate atypical clinical patterns of AD in children and to determine the MRSA isolation and its association with disease severity.
Methods:
We studied 55 pediatric patients from 2 months to 10 years of age, of either sex, diagnosed with AD based on the diagnostic criteria of Hanifin and Rajka. History, clinical examination (including atypical features), and severity score using SCORing Atopic Dermatitis (SCORAD) severity index were recorded. Swabs from the cutaneous lesion and anterior nares were collected from each case and processed. Statistical analysis was done by SPSS (V 17).
Observations and Results:
Atypical clinical features were seen in 52.7% of cases. Retroauricular fissures (among atypical features), oozing, crusting, darkening, early age at onset, and nipple eczema were found to be significantly associated with disease severity (P < 0.05). The majority of the cases (56.4%) fell in the moderate disease severity (mean SCORAD 32.02). MRSA showed an isolation frequency of 7.27% from the skin swabs and 10.90% from the nares. No significant association was found between Staphylococcus aureus isolates (including MRSA) and disease severity in our study. A high degree of fluoroquinolone resistance was noted in MRSA isolates.
Limitation:
Further characterization of S. aureus by superantigen profiling was not done.
Conclusion:
Patients with AD need to be evaluated for atypical features which may serve as markers of severe disease.
Keywords: Atopic dermatitis, methicillin-resistant Staphylococcus aureus, pediatric
Introduction
The term “atopy” represents an inherited group of conditions whereby individuals show a propensity for certain “shock organ” systems to manifest atopic reactions in response to antigen, such as hay fever or asthma in the respiratory tract, dermatitis or urticaria in the skin, and emesis and diarrhea in the gastrointestinal tract.[1] Atopic dermatitis (AD) (synonyms: atopic eczema, Besnier's prurigo, disseminated neurodermatitis) is a chronic relapsing eczematous inflammatory skin disease.[2,3,4] The rash is characterized by itchy papules (occasionally vesicles in infants) which become excoriated and lichenified.[2]
There are many atypical clinical manifestations of AD that are not part of the gold standard Hanifin and Rajka's criteria, namely, genital dermatitis, atopic feet, infra-auricular fissures, retroauricular fissures, infranasal fissures, erythroderma, and eyelid eczema.[5] Also, when compared to the Western world, we encounter a less severe form of AD. In this study, the severity of the disease was graded using a standard SCORing Atopic Dermatitis (SCORAD) severity index, to know the current pattern of the disease severity in our setting.[6]
Until now, Staphylococcus aureus infection has been implicated in the disease flares. The concept is changing in the recent times, with these microorganisms believed to function in much larger bacterial communities, the microbiota, and change in its diversity and composition is believed to be a trigger for disease flares.[7] This pathogen produces many proinflammatory chemokines that trigger the cutaneous immune response and may bring a flare in the disease. However, there is a paucity of data in our scenario regarding the current pattern and hospital prevalence of cutaneous and nasal colonization with S. aureus in children with AD, especially with reference to methicillin-resistant Staphylococcus aureus (MRSA)and also whether this has a significant association with a more severe form of the disease. This would also enable us to use proper antibiotics in affected patients.
Methods
This study was a hospital-based cross-sectional prospectively conducted study carried out in Department of Dermatology, and Department of Microbiology of our Institute. The protocol was approved by the Institutional Ethical Committee. The study group consisted of 55 pediatric patients ranging from 2 months to 10 years of age, and either sex, diagnosed with AD based on the diagnostic criteria of Hanifin and Rajka. Patients who had received antibiotics/corticosteroids (topical/systemic) in the past 7 days or those suffering from severe systemic illness (other than organs of atopy) were excluded from the study. A predesigned proforma for recording the history and clinical examination (including atypical features, viz, infra-auricular fissure, retroauricular fissure, infranasal fissure, eyelid eczema, genital dermatitis, posterior thigh eczema, juvenile plantar dermatosis, and follicular variant) was used. Disease severity was graded using SCORAD severity index. Photography of characteristic lesions was done.
Swabs from the cutaneous lesion (eczematous lesion from typical site affected, viz, popliteal/cubital fossa in children more than 1 year and facial lesion in infants with no clinical evidence of secondary infection) and anterior nares were collected under all aseptic precautions from each case and immediately inoculated into the tubes of Mannitol Salt Broth which was transported to the Department of Microbiology for further processing [Figure 1]. After overnight incubation at 37°C, subcultures were made onto 10% sheep blood agar and MacConkey's medium and incubated again at 37°C and read the following day.
Figure 1.

Mannitol Salt Broth. Yellow indicating fermentation of mannitol by presumptive pathogenic Staphylococci
The staphylococcal isolates obtained from the skin lesions and anterior nares were identified by standard recommended techniques.[8]
All strains of S. aureus isolated were tested for methicillin resistance by the cefoxitin disc method as recommended by the Clinical and Laboratory Standards Institute.[9] Since existing literature shows that MRSA identification by cefoxitin disc method is comparable to polymerase chain reaction, hence, this method was resorted to in a resource-constraint setting.[10] Also, all the isolates of S. aureus were subjected to antimicrobial susceptibility testing to standard panel of antibiotics using the modified Stokes’ technique[11] [Figure 2]. The interpretation of the susceptibility was given as sensitive (S), intermediate susceptible (IS), or resistant (R) in accordance with standard recommendation.[8]
Figure 2.
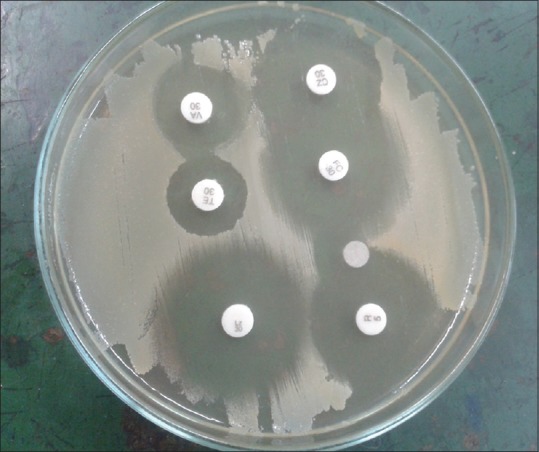
Antimicrobial susceptibility testing by disc diffusion (modified Stokes’ technique)
Data were analyzed using Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA; version 17.0 for Windows). Continuous data were given as mean ± standard deviation and range. Normality of quantitative data was checked by measures of Kolmogorov–Smirnov tests of normality. Discrete categorical data were presented as percentages (%); for categorical data, comparisons were made by Pearson's Chi-square test or Fisher's exact test as appropriate. All statistical tests were two-sided and were performed at a significance level of P = 0.05.
Results
Out of all cases, 22 (40%) were toddlers, followed by preschool children who comprised 13 cases (23.63%), followed by school children with 12 cases (21.81%); the lowest percentage was comprised of infants (8 cases, i.e., 14.54%). Maximum cases had their onset in infancy with 24 cases (43.64%). Only 5.45% cases had their disease onset at or after 6 years of age. A slight male preponderance was noted (52.7% cases). The mean age (at presentation) was 3.64 ± 2.49 years and the mean age of onset (as noticed by the caregivers) was 2.13 ± 2.10 years.
The distribution of lesions as per site and the distribution of major and minor features of Hanifin and Rajka as seen in our study are given in Tables 1-3, respectively. The distribution of cases with history of atopy is given in Table 4. Oozing, crusting, and darkening were significantly associated with disease severity (P ≤ 0.05). Also, early age at onset and nipple eczema were found to be significantly associated with disease severity (P < 0.05). The correlation analysis of age at onset with the severity score showed Pearson's correlation coefficient of − 0.370 with a P value of 0.005 (statistically significant). Hence, the age at onset has significant negative correlation with disease severity.
Table 1.
Distribution of lesions according to the sites in infants and children (>1 year)
| Site | AD in infants (n=8) | AD in children (>1 year of age), n=47 | ||
|---|---|---|---|---|
| No. of cases (n) | Percentage (100 n/N) | No. of cases (n) | Percentage (100 n/N) | |
| Face | 7 | 87.5 | 33 | 70.21 |
| Extensors | 6 | 75 | 31 | 65.96 |
| Flexors | 1 | 12.5 | 18 | 38.30 |
| Nape of neck | 0 | 0 | 7 | 14.89 |
| B/L cubital fossa | 0 | 0 | 13 | 27.66 |
| B/L popliteal fossa | 1 | 12.5 | 12 | 25.53 |
| Axilla | 0 | 0 | 2 | 4.25 |
| Groins | 0 | 0 | 1 | 2.13 |
AD=Atopic dermatitis
Table 3.
Distribution of minor features of Hanifin and Rajka’s criteria
| Minor clinical features | No. of cases (n) | Percentage |
|---|---|---|
| Xerosis | 55 | 100 |
| Ichthyosis/palmar hyperlinearity/KP | 6 (1 of ichthyosis, 4 of palmar hyperlinearity, 1 of KP) | 10.91 (1.82, 7.27, ad 1.82, respectively) |
| Type I skin reactivity | NT | NT |
| Elevated S. IgE levels | NT | NT |
| Early age at onset | 28 | 50.9 |
| Tendency toward skin infections/impaired CMI | 8 | 14.5 |
| Tendency toward nonspecific hand or foot dermatitis | 3 | 5.5 |
| Nipple eczema | 3 | 5.5 |
| Cheilitis | 6 | 10.9 |
| Recurrent conjunctivitis | 0 | 0 |
| Dennie–Morgan folds | 10 | 18.18 |
| Keratoconus | 0 | 0 |
| Anterior subcapsular cataracts | 0 | 0 |
| Orbital darkening | 1 | 1.8 |
| Facial pallor/erythema | 24 | 43.6 |
| Pityriasis alba | 5 | 9.1 |
| Anterior neck folds | 1 | 1.8 |
| Itch when sweating | 15 | 27.3 |
| Intolerance to wool or lipid solvents | 12 | 21.8 |
| Perifollicular accentuation | 8 | 14.5 |
| Food intolerance | 5 | 9.1 |
| Course influenced by environmental/emotional factors | 26 (winter exacerbation) | 47.3 |
| White dermographism | 8 | 14.54 |
KP=Keratosis pilaris; NT=Not tested
Table 4.
Distribution of cases with personal/family history of atopy
| History of atopy | Infants with AD (N=8) | Children (>1 year) with AD (N=47) | Total (N=55) | |||
|---|---|---|---|---|---|---|
| No. of cases (n) | Percentage (100 n/N) | No. of cases (n) | Percentage | No. of cases (n) | Percentage | |
| Personal | 1 | 12.5 | 9 | 19.15 | 10 | 18.18 |
| Family | 5 | 62.5 | 24 | 51.06 | 29 | 52.73 |
| Both | 0 | 0 | 2 | 4.26 | 2 | 3.63 |
AD=Atopic dermatitis
Table 2.
Distribution of major clinical features of Hanifin and Rajka’s criteria
| Major clinical features | No. of cases (n) | Percentage |
|---|---|---|
| Pruritus | 55 | 100 |
| Typical morphology and distribution | 52 | 94.5 |
| Chronic/chronically relapsing dermatitis | 36 | 65.5 |
Atypical clinical features were seen in 52.7% of cases; the most common were retroauricular fissures, eyelid eczema, and posterior thigh eczema [Figures 3-5]. The distribution of various atypical features seen is as shown in Table 5 [Figures 6 and 7]. Retroauricular fissures were found to be significantly associated with disease severity (P < 0.05).
Figure 3.
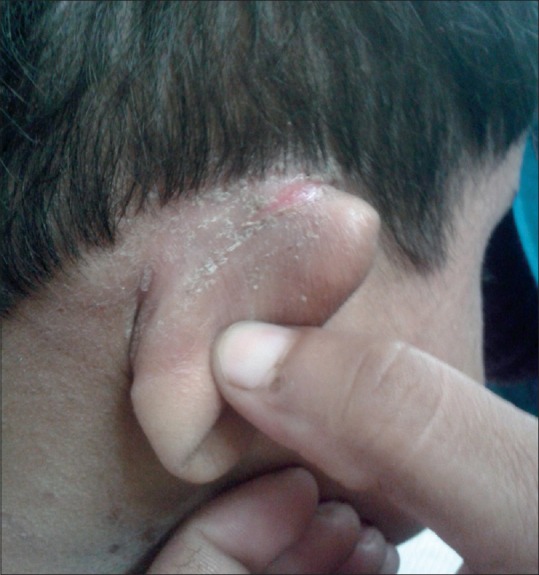
Retroauricular fissure
Figure 5.
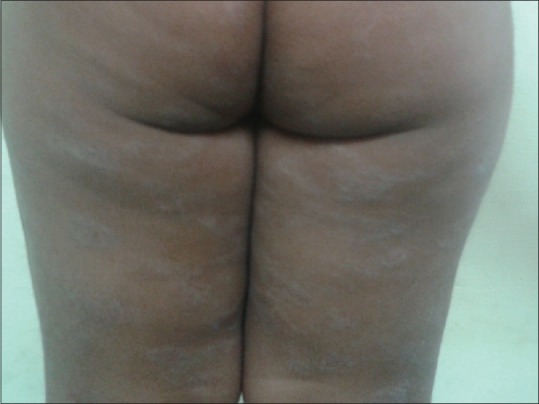
Posterior thigh eczema
Table 5.
Distribution and association of atypical clinical features and disease severity
| Atypical clinical features | No. of cases | Disease grade | Pearson’s Chi-square (P) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| mild | Moderate | Severe | |||||||
| n | Percentage | n | Percentage | n | Percentage | n | Percentage | ||
| Infra-auricular fissure | 2 | 3.63 | 0 | 0 | 1 | 50 | 1 | 50 | 0.165 |
| Retroauricular fissure | 9 | 16.36 | 1 | 11.1 | 5 | 55.6 | 3 | 33.3 | 0.039# |
| Infranasal fissure | 2 | 3.63 | 0 | 0 | 1 | 50 | 1 | 50 | 0.165 |
| Eyelid eczema | 9 | 16.36 | 2 | 22.2 | 6 | 66.7 | 1 | 11.1 | 0.754 |
| Genital dermatitis | 5 | 9.09 | 1 | 20 | 3 | 60 | 1 | 20 | 0.704 |
| Posterior thigh eczema | 9 | 16.36 | 0 | 0 | 7 | 77.8 | 2 | 22.2 | 0.059 |
| Juvenile plantar dermatoses | 1 | 1.82 | 0 | 0 | 1 | 100 | 0 | 0 | 0.674 |
| Follicular variant | 4 | 7.27 | 1 | 25 | 2 | 50 | 1 | 25 | 0.639 |
#P≤0.05 considered significant
Figure 6.
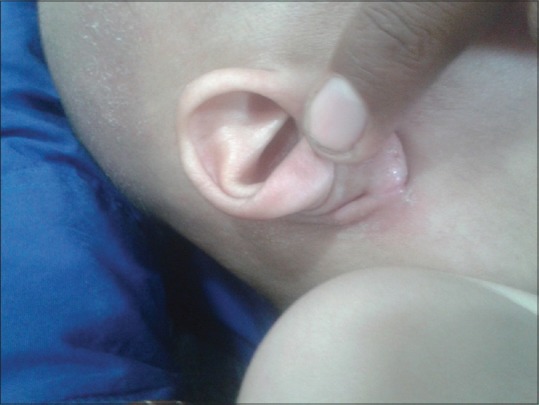
Infra-auricular fissure
Figure 7.
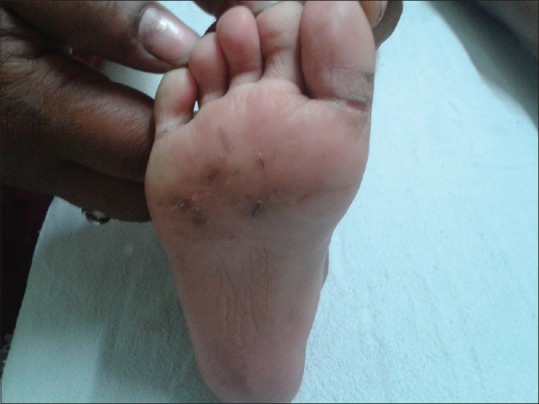
Juvenile plantar dermatosis
Figure 4.
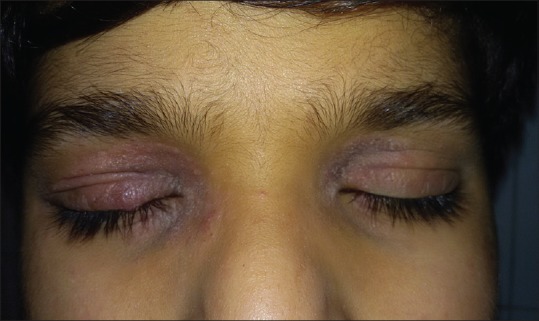
Eyelid eczema
The majority of the cases fell in the moderate disease severity (56.4%). Only six cases had severe AD by SCORAD comprising 10.9% as mentioned in Figure 8. The mean SCORAD score was 32.02 ± 12.16 (range 12.9–61.0).
Figure 8.
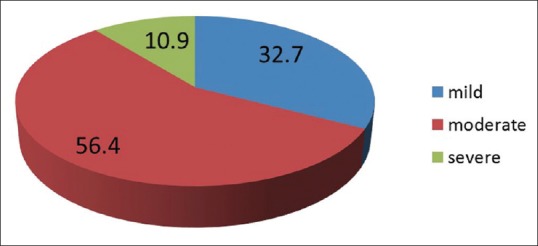
SCORAD grading of the disease severity
The bacterial isolation of MRSA from the lesion and anterior nares as obtained from our study is as mentioned in Figure 9. MRSA was isolated from 7.27% and 10.9% of cases from the lesion and anterior nares, respectively, while the total S. aureus isolation was 29.09 and 27.27%, respectively.
Figure 9.
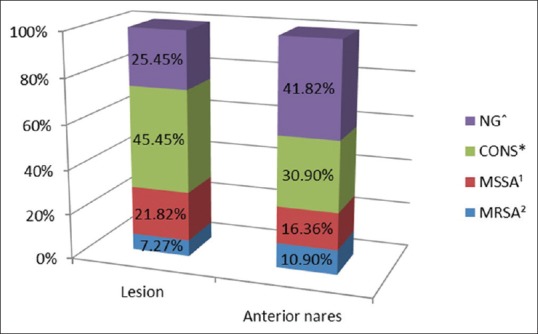
Proportion of various organisms isolated in the lesion and anterior nares
No significant association was found between S. aureus isolates (total/MSSA/MRSA) (lesion or nares) and disease severity, where a P value of >0.05 was noted.
All strains of S. aureus were resistant to penicillin regardless of their methicillin sensitivity. Also, a high resistance to fluoroquinolones was noted among MRSA from both the lesions and anterior nares.
No resistance was noted in S. aureus lesional/anterior nares isolates to cefazolin, fusidic acid, mupirocin, rifampicin, gentamycin, amikacin, teicoplanin, linezolid, and vancomycin (regardless of their methicillin sensitivity). Some resistance was noted for clindamycin in both groups.
Discussion
Atypical features such as retroauricular or infranasal fissuring, fingertip eczema, atopic feet, and genital dermatitis were seen in 52.7% of the cases. In 1998, Dhar and Kanwar noted juvenile plantar dermatoses in 6.28% of cases,[12] while in 2011, Yazganoglu and Ozkaya noted that nearly 60% of the patients had nontypical morphological variants such as follicular atopic eczema and other features.[13] Besides, a time-related increase in the number of minor criteria was seen. The knowledge regarding the prevalence of such atypical features will help in diagnosing incipient cases of AD.
Disease severity encountered in Asia is less than the west. The study by Dae et al. reported mild AD in a majority of the cases (67.9%),[14] and prior studies from India, namely, by Dhar and Kanwar, Dhar et al., and Jagadeesan et al., also reported most of the cases in the mild to moderate grade.[15,16,17] Our study showed 56.4% of cases in the moderate grade, and only 10.9% of cases in severe grade, similar to previous trends in India.
Several studies have demonstrated increased carriage rate of S. aureus in both the skin and nasal mucosa in patients with AD.[18] In the Western world, approximately, 93% of patients with AD have positive cultures for S. aureus from the affected skin.[18,19,20,21] The studies from other Asian nations, namely, by Gong et al. (from China), Chung et al. (from Republic of Korea), and Higaki et al. (from Japan) showed the rates of S. aureus isolation as 79.8%, 75.4%, and 5.7%, respectively.[22,23,24] In an Indian study by Dhar et al., the positivity of S. aureus was 50% from the eczematous skin and 34% from anterior nares.[25] Our study yielded isolation of S. aureus from the lesions in 29.09% cases, whereas 27.29% of cases demonstrated positivity from the anterior nares, contrary to the West. Also, no significant association was noted between S. aureus isolation and disease severity in our study, consistent with a recent study from South India.[17] A possible explanation for the lack of association in our study being the fact that it is the colonization density of S. aureus and superantigen profile which are positively correlated with the severity,[23,26,27] and which are not evaluated as part of this study. Superantigens augment allergen-induced skin inflammation, induce the CLA (cutaneous lymphocyte associated antigen) skin-homing receptor on T cells, and induce corticosteroid resistance.
Our findings of S. aureus isolation from nares are similar to that of Dhar et al.[25] S. aureus is considered as a commensal in anterior nares.[26] We could not find published data from the industrialized nations on S. aureus isolation from the anterior nares in AD, especially MRSA.
Regarding MRSA, the frequency of colonization varies with the geographical area.[17] The available meager data suggests that MRSA colonization is infrequent among general AD patients.[28] In a study from Canada, the incidence of MRSA was 0.5% in pediatric population with AD; a study from New Zealand reported a prevalence of 2%; whereas in the United States, the colonization rate of MRSA in patients with AD was as high as 18.3%, and the higher colonization rates there may reflect the higher overall prevalence or different management strategies including antibiotic use.[20,22,29,30] Two Asian studies found the MRSA rate in children with AD to be 13.9% and 14.3%, respectively.[22,31] In a recent study from South India, MRSA colonization of 25.2% (highest so far) was reported in atopic patients.[17] From our study, MRSA colonization from the lesions was found to be 7.27%, whereas from anterior nares it was found to be 10.9%, which is lower than other Asian studies. No significant association was found between MRSA isolates and disease severity in our study, in contrast to a recent study from South India. Although MRSA strains are considered more virulent as many produce Panton-Valentine-Leucocidin (PVL) toxin, not produced by methicillin-sensitive strains, no role of PVL toxin has been described in AD till date. MRSA poses challenges in devising adequate antibiotic therapy. Also, while individual microbes causative of skin infections in AD have been studied, it is now clear that such individual microbes function within larger bacterial communities termed microbiota.[7] S. aureus production of antibacterial compounds including bacteriocins and antimicrobial peptides contribute to a relative decrease in Streptococcus, Corynebacterium, and Propionicacterium species, and such shifts in microbiome are associated with the AD flares.[32] Emerging concepts state that clinical effectiveness of AD treatments does not rely on S. aureus elimination, rather they act to diversify the skin microbiome.
It was noted in the study that in cases where S. aureus was isolated from both the lesion and anterior nares, the antimicrobial susceptibility of the two isolates was the same; more genetic data are required to know whether they belong to the same strain. Nasal colonization in such children may serve as a source for exacerbations/relapses of the disease. Targeting the nasal colonization in such cases with appropriate antibiotics may prevent frequent exacerbations/relapses in patients with AD.
Some limitations of the study being relatively small sample size and adolescents were not in the inclusion criteria. A control group could have been included to demonstrate MRSA colonization frequency. Further characterization of S. aureus by phage typing to know the strain and their superantigen profiling is required.
We concluded that early age of onset, nipple eczema, and retroauricular fissures are the features which are significantly associated with disease severity. The majority of the cases belong to the moderate grade of the disease. The frequency of cutaneous isolation of S. aureus from the lesions in children with AD is less in our study when compared with the West. Nasal isolation in children with AD has even a higher proportion of MRSA from the nasal isolates of S. aureus. Both the lesional and nasal isolates of S. aureus (including MRSA) have no significant association with disease severity. All the isolates of S. aureus whether from the lesion or anterior nares are resistant to penicillin, regardless of their methicillin sensitivity, in our study. A high degree of fluoroquinolone resistance is noted in MRSA isolates (from the lesion and nares).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors thank Mr. R. C. Goel, the statistician, from PGI Chandigarh, for helping in statistical analysis.
References
- 1.Williams HC. Is the prevalence of atopic dermatitis increasing? Clin Exp Dermatol. 1992;17:124–91. doi: 10.1111/j.1365-2230.1992.tb00244.x. [DOI] [PubMed] [Google Scholar]
- 2.Friedmann PS, Ardern-Jones MR, Holden CA. Atopic dermatitis. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. Oxford: Wiley-Blackwell; 2010. [Google Scholar]
- 3.Sarkar R, Kanwar AJ. Clinico-epidemiological profile and factors affecting severity of atopic dermatitis in north Indian children. Indian J Dermatol. 2004;49:117–22. [Google Scholar]
- 4.Laughter D, Istvan JA, Tofte SJ, Hanifin JM. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;19:649–55. doi: 10.1067/mjd.2000.107773. [DOI] [PubMed] [Google Scholar]
- 5.Rolando EJG, Luz OC, Carola DM, Carolina PL, Ramon RM, Marimar S. Less common clinical manifestations of atopic dermatitis: Prevalence by age. Pediatr Dermatol. 2012;29:580–3. doi: 10.1111/j.1525-1470.2012.01739.x. [DOI] [PubMed] [Google Scholar]
- 6.European Task Force on Atopic dermatitis. Severity scoring of atopic dermatitis: The SCORAD index: Consensus report of the European Task force on atopic dermatitis. Dermatology. 1993;186:23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- 7.Cogen AL, Yamasaki K, Sanchez KM, Dorschner RA, Lai Y, MacLeod DT, et al. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol. 2010;130:192–200. doi: 10.1038/jid.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collee JG, Marmion BP, Fraser AG, Simmons A, editors. 14th ed. New York: Churchill Livingstone; 1996. Mackie and McCartney Practical Microbiology. [Google Scholar]
- 9.Performance standards for antimicrobial susceptibility testing. National Committee for clinical laboratory standards twentieth informational supplement. 2010;30:M100–S20. [Google Scholar]
- 10.Anand KB, Agrawal P, Kumar S, Kapila K. Comparison of cefoxitin disc diffusion test, oxacillin screen agar, and PCR for mecA gene for detection of MRSA. Indian J Med Microbiol. 2009;27:27–9. [PubMed] [Google Scholar]
- 11.Stokes EJ, Ridgway GL, Wren MWD, editors. 7th ed. London: Edward Arnold; 1993. Clinical Microbiology. [Google Scholar]
- 12.Dhar S, Kanwar AJ. Epidemiology and clinical pattern of atopic dermatitis in a north Indian pediatric population. Pediatr Dermatol. 1998;15:347–51. doi: 10.1046/j.1525-1470.1998.1998015347.x. [DOI] [PubMed] [Google Scholar]
- 13.Yazganoglu KD, Ozkaya E. Non-typical morphology and localization in Turkish atopic dermatitis patients with onset before the age of 18 years. Indian J Dermatol Venereol Leprol. 2011;77:23–7. doi: 10.4103/0378-6323.74969. [DOI] [PubMed] [Google Scholar]
- 14.Dae SK, Ju HL, Kwang HL, Min GL. Prevalence and severity of atopic dermatitis in Jeju Island: A cross-sectional study of 1628 Korean elementary school children by physical examination utilizing the three-item severity score. Acta Derm Venereol. 2012;92:472–4. doi: 10.2340/00015555-1410. [DOI] [PubMed] [Google Scholar]
- 15.Dhar S, Kanwar AJ. Grading the severity of atopic dermatitis in north Indian children. Indian J Dermatol. 1995;16:67–72. [Google Scholar]
- 16.Dhar S, Mandal B, Ghosh A. Epidemiology and clinical pattern of atopic dermatitis in 100 children seen in a city hospital. Indian J Dermatol. 2002;47:202–4. [Google Scholar]
- 17.Jagadeesan S, Kurien G, Divakaran MV, Sadanandan SM, Sobhanakumari K, Sarin A. Methicillin-resistant Staphylococcus aureus colonization and disease severity in atopic dermatitis: A cross-sectional study from South India. Indian J Dermatol Venereol Leprol. 2014;80:229–34. doi: 10.4103/0378-6323.132250. [DOI] [PubMed] [Google Scholar]
- 18.Leyden JJ, Marpes RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525–30. doi: 10.1111/j.1365-2133.1974.tb06447.x. [DOI] [PubMed] [Google Scholar]
- 19.Hoeger PH, Lenz W, Boutonnier A, Fournier JM. Staphylococcal skin colonization in children with atopic dermatitis: Prevalence, persistence and transmission of toxigenic and nontoxigenic strains. J Infect Dis. 1992;165:1064–8. doi: 10.1093/infdis/165.6.1064. [DOI] [PubMed] [Google Scholar]
- 20.Breuer K, Haussler S, Kapp A, Werfel T. Staphylococcus aureus: Colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br J Dermatol. 2002;147:55–61. doi: 10.1046/j.1365-2133.2002.04872.x. [DOI] [PubMed] [Google Scholar]
- 21.Suh L, Coffin S, Leckerman KM, Gelfand JM, Honig PJ, Yan AC. Methicillin–resistant Staphylococcus aureus colonisation in children with atopic dermatitis. Pediatr Dermatol. 2008;25:528–34. doi: 10.1111/j.1525-1470.2008.00768.x. [DOI] [PubMed] [Google Scholar]
- 22.Gong JQ, Lin L, Lin T, Hao F, Zeng FQ, Bi ZG, et al. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: A double-blind multicentre randomized controlled trial. Br J Dermatol. 2006;155:680–7. doi: 10.1111/j.1365-2133.2006.07410.x. [DOI] [PubMed] [Google Scholar]
- 23.Chung HJ, Jeon HS, Sung H, Kim MN, Hong SJ. Epidemiological characteristics of methicillin-resistant Staphylococcus aureus isolates from children with eczematous atopic dermatitis lesions. J Clin Microbiol. 2008;1:991–5. doi: 10.1128/JCM.00698-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higaki S, Morohashi M, Yamagishi T, Hasegawa Y. Comparative study of staphylococci from the skin of atopic dermatitis patients and from healthy subjects. Int J Dermatol. 1999;38:265–9. doi: 10.1046/j.1365-4362.1999.00686.x. [DOI] [PubMed] [Google Scholar]
- 25.Dhar S, Kanwar AJ, Kaur S, Sharma P, Ganguly NK. Role of bacterial flora in the pathogenesis and management of atopic dermatitis. Indian J Med Res. 1992;95:234–8. [PubMed] [Google Scholar]
- 26.Nada HA, Gomaa NI, Elakhras A, Wasfy R, Baker RA. Skin colonization by superantigen-producing Staphylococcus aureus in Egyptian patients with atopic dermatitis and its relation to disease severity and serum interleukin-4 level. Int J Infect Dis. 2012;16:29–33. doi: 10.1016/j.ijid.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Yeung M, Balma-Mena A, Shear N, Simor A, Pope E, Walsh S, et al. Identification of major clonal complexes and toxin producing strains among Staphylococcus aureus associated with atopic dermatitis. Microbes Infect. 2011;13:189–97. doi: 10.1016/j.micinf.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 28.Nishijima S, Namura S, Nakagawa M, Kurokawa I, Kawabata S. Sensitivity to antibacterials of Staphylococcus aureus isolated from different types of skin infections. J Int Med Res. 1997;25:1–7. doi: 10.1177/030006059702500101. [DOI] [PubMed] [Google Scholar]
- 29.Balma-Mena A, Lara-Corrales I, Zeller J, Richardson S, McGavin MJ, Weinstein M, et al. Colonization with community acquired methicillin-resistant Staphylococcus aureus in children with atopic dermatitis: A cross-sectional study. Int J Dermatol. 2011;50:682–8. doi: 10.1111/j.1365-4632.2010.04751.x. [DOI] [PubMed] [Google Scholar]
- 30.Hill SE, Yung A, Rademaker M. Prevalence of Staphylococcus aureus and antibiotic resistance in children with atopic dermatitis: A New Zealand experience. Australas J Dermatol. 2011;52:27–31. doi: 10.1111/j.1440-0960.2010.00714.x. [DOI] [PubMed] [Google Scholar]
- 31.Tang CS, Wang CC, Huang CF, Chen SJ, Tseng MH, Lo WT. Antimicrobial susceptibility of Staphylococcus aureus in children with atopic dermatitis. Pediatr Int. 2011;53:363–7. doi: 10.1111/j.1442-200X.2010.03227.x. [DOI] [PubMed] [Google Scholar]
- 32.Iwase T, Uehara Y, Shinji H, Tajima A, Seo H, Takada K, et al. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature. 2010;465:346–9. doi: 10.1038/nature09074. [DOI] [PubMed] [Google Scholar]


