Abstract
Leprosy has a predilection for peripheral nerves, but rarely does it involve the central nervous system (CNS). There is a single study of CNS involvement in leprosy showing vacuolar changes of motor neurons in medulla oblongata and spinal cord in autopsy findings. Besides this, there has been only one case report providing direct histopathological and molecular evidence of CNS involvement by leprosy in a living patient. Segmental necrotizing granulomatous neuritis (SNGN) is a rare condition affecting the peripheral nerves in leprosy usually seen as a complication of tuberculoid (TT) and borderline tuberculoid (BT) leprosy. We report the case of a 23-year-old male patient, a case of Hansen's disease (BT) who developed CNS involvement in the form of partial Horner's syndrome (right) and SNGN while on treatment. Magnetic resonance imaging of the brain revealed T2 hyperintense lesion on the dorsal aspect of left pontomedullary junction, suggestive of vacuolar degeneration of leprosy. Histopathology of greater auricular nerve (right) revealed SNGN.
Keywords: Granulomatous neuritis, Horner's syndrome, leprosy, segmental necrotizing, vacuolar degeneration
Introduction
Few studies have focused on central nervous system (CNS) involvement in leprosy. A postmortem study of 67 cases of CNS involvement in leprosy revealed vacuolar changes in medulla oblongata and spinal cord.[1] Brain cortex involvement is extremely rare and only one case report could be traced after thorough search on the internet.[2]
Segmental necrotizing granulomatous neuritis (SNGN), earlier reported as nerve abscess, is a rare complication of peripheral nerves in tuberculoid (TT)/borderline tuberculoid (BT) leprosy, with ulnar nerve being the most common involved nerve.[3] This case is being reported for its extremely rare association of partial Horner's syndrome with vacuolar degeneration of pontomedullary junction of the brain and SNGN of the greater auricular nerve (right).
Case History
A 23-year-old male presented with sudden-onset symmetrical erythematous hypoaesthetic plaques involving both cheeks and ears, as well as grade-1 thickened and nontender supraorbital nerve (right) and thickened greater auricular nerves on both sides. Slit skin smear for Acid Fast Bacilli (Leprosy) (AFB(L)) revealed bacteriological index (BI) of zero, and histopathology of the plaque was suggestive of Hansen's disease (BT) in type 1 reaction. He was treated with multidrug therapy (multibacillary) and tab. prednisolone (1 mg/kg). Steroids were gradually tapered off over the next 4 months with satisfactory resolution of patches.
At this point, the patient again developed erythema and edema of previous patches over the face and ears and nerves became grade-3 thickened [Figure 1]. Repeat BI was zero. Simultaneously, he also developed partial Horner's syndrome (right) with ptosis, lid lag, and miosis. Magnetic resonance imaging (MRI) of the brain revealed a T2 hyperintense lesion on the dorsal aspect of left pontomedullary junction measuring 6.6 × 8.0 × 8.7 mm with no post contrast enhancement, suggestive of vacuolar degeneration of leprosy [Figure 2]. Nerve biopsy from the thickened greater auricular nerve (right) revealed intact nerve with perineurium with large areas of caseous necrosis surrounded by multiple ill-formed granulomas bordered by epithelioid cells, lymphocytes, and giant cells, characteristic of SNGN. Modified Ziehl–Neelsen stain did not reveal any AFB(L) [Figure 3a and b].
Figure 1.
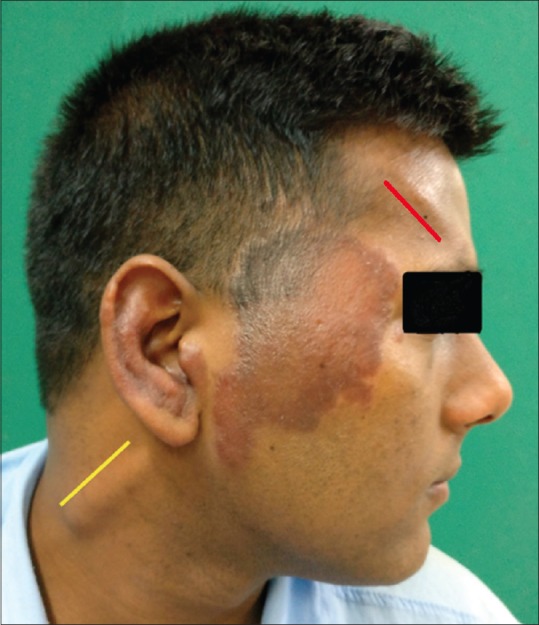
Malar region (Rt) and ear showing well-defined erythematous edematous plaque, Grade 3 thickened (Rt) supraorbital (red line), and greater auricular nerve (yellow line)
Figure 2.
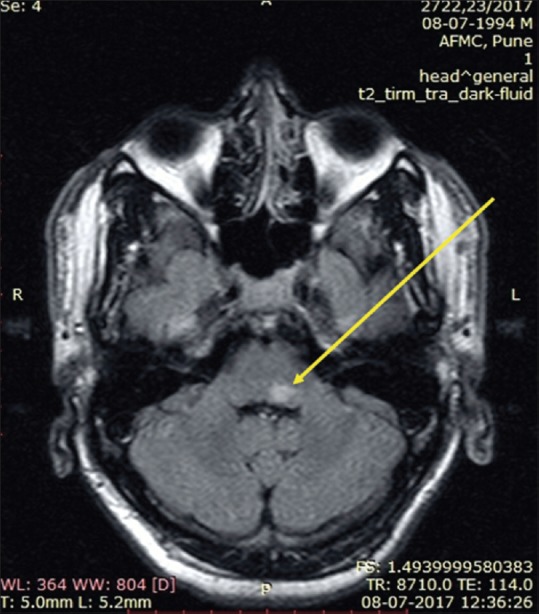
Magnetic resonance imaging of the brain revealing T2 hyperintense lesion on the dorsal aspect of left pontomedullary junction measuring 6.6 × 8 × 8.7 mm with no post contrast enhancement (yellow arrow)
Figure 3.
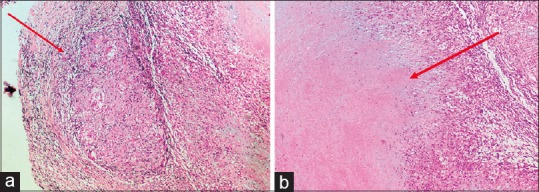
(a) Nerve biopsy of greater auricular nerve (Rt) showing intact nerve within perineurium with multiple ill-formed granulomas bordered by epithelioid cells, lymphocytes, and giant cell reaction (10×, H and E stain). (b) Nerve biopsy revealing large areas of caseous necrosis surrounded by granuloma characteristic of segmental necrotizing granulomatous neuritis (10×, H and E stain)
Discussion
Mycobacterium leprae has been shown to cross blood–brain barrier (BBB) in experimental animals. In addition, M. leprae antigen has been reported in the cerebrospinal fluid (CSF) of leprosy patients in few case reports.[4,5] The route of entry of M. leprae into motor neurons is controversial.
Vaidya et al. showed that M. leprae crossed BBB and was present in the gray and white matter of the brain and spinal cord in thymectomized, irradiated mice, possibly due to amyloid angiopathy of blood vessels, which can weaken tight junctions leading to impaired BBB.[2,4] Nonetheless, there has been no direct evidence of M. leprae penetrating through BBB in humans.
The only histopathological evidence of CNS involvement in leprosy has been provided by Aung et al. in a study of 67 postmortem lepromatous cases, where 44 cases (67%) showed vacuolar changes of motor neurons either in medulla oblongata or spinal cord and 19 cases (28%) in both. These vacuolar areas were positive for M. leprae PGL-1 antibodies and PCR for M. leprae DNA. The more frequent identification of M. leprae DNA in the spinal cord (74%) than in the medulla oblongata (41%) suggests CNS involvement by bacilli through upward invasion from the periphery to the central region through motor axons. The more common occurrence of vacuolated changes in the cervical and lumbar cords than thoracic cord may also suggest bacterial invasion through extremities rather than the trunk of the patients.[1]
Lee et al. gave histopathological and molecular evidence of CNS involvement by leprosy in a living patient with cystic lesion in the frontal region of the cortex with presence of foamy histiocytes, granulomas, and fragmented bacilli on Fite Faraco stain. PCR analysis of this lesional tissue revealed intact M. leprae genome DNA.[2]
In our patient, MRI brain revealed a T2 hyperintense lesion on the dorsal aspect of the left pontomedullary junction with no postcontrast enhancement, suggestive of vacuolar degeneration of leprosy.
Horner's syndrome, characterized by triad of miosis, partial ptosis and hemifacial anhidrosis, results from interruption of sympathetic nerve supply to eye. It can arise from lesion of primary neuron, preganglionic or postganglionic neurons, brachial plexus trauma, and tumors of lung apex or brainstem. Our patient had partial Horner's syndrome with miosis and ptosis without anhidrosis. A thorough literature search revealed no earlier reported case of leprosy with concomitant Horner's syndrome, which is unique to our case.
Occurrence of necrotizing nodular lesions in peripheral nerves is an uncommon complication of leprosy frequently mistaken as nerve abscess. Chandi et al., in their retrospective study of nerve biopsies in 30 cases, described segmental involvement of peripheral nerves with necrotizing granulomatous inflammation showing central area of caseous necrosis surrounded by epithelioid cell granulomas but lacking collection of neutrophils, as typically seen in abscess. Hence, the terminology SNGN.[3]
SNGN is usually seen in association with BT and TT leprosy followed by the pure neuritic variant. SNGN may be considered as type 1 upgrading reaction where high levels of TNF-alpha mRNA and TNF-alpha protein generated in the macrophages of the peripheral nerve produce severe local tissue damage resulting in caseating necrosis.[6,7,8] Although it is considered as a type 1 reaction,[8] no report exists regarding its response to oral corticosteroids.
Chandi et al. demonstrated AFB in the lesion of 77% of the cases.[3] However, as SNGN is seen in association with higher immunological status, chance of detecting bacilli is rare on routine staining methods. Jayalakshmy et al. described similar findings in two patients of BT and pure neuritic leprosy, but without demonstration of AFB.[8] In our patient, greater auricular nerve (right) was involved, however AFB(L) could not be demonstrated.
The intent of this case report is to highlight the involvement of CNS in leprosy which is rarely reported in literature. CNS involvement by leprosy has remained enigmatic mostly due to the lack of histological evidence. Leprosy being more prevalent in underprivileged areas, cases of CNS involvement may have been easily overlooked due to resource constraints. SNGN is a rare complication of peripheral nerves of leprosy which is separate from nerve abscess, as previously described.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Aung T, Kitajima S, Nomoto M, En J, Yonezawa S, Arikawa I, et al. Mycobacterium leprae in neurons of the medulla oblongata and spinal cord in leprosy. J Neuropathol Exp Neurol. 2007;66:284–94. doi: 10.1097/nen.0b013e31803d597e. [DOI] [PubMed] [Google Scholar]
- 2.Lee KH, Moon KS, Yun SJ, Won YH, Lee JH, Lee MC, et al. Brain involvement by leprosy presenting as a frontal cystic lesion. J Neurosurg. 2014;121:184–8. doi: 10.3171/2014.1.JNS131429. [DOI] [PubMed] [Google Scholar]
- 3.Chandi SM, Chacko CJG, Fritschi EP, Job CK. Segmental necrotizing granulomatous neuritis of leprosy. Int J Lepr Other Mycobact Dis. 1980;48:41–7. [PubMed] [Google Scholar]
- 4.Vaidya MC, Palmer E, Weddell G, Rees RJ. A note on the presence of Mycobacterium leprae in the central nervous system of a mouse with lepromatous leprosy. J Med Microbiol. 1970;3:194–6. doi: 10.1099/00222615-3-1-194. [DOI] [PubMed] [Google Scholar]
- 5.Patil SA, Tyagi P, Katoch K. Antigens of Mycobacterium leprae in the cerebrospinal fluid of leprosy patients: Detection by monoclonal-antibody-based sandwich immunoradiometric assay and avidin/biotin immunoblotting. Clin Exp Immunol. 1991;84:515. [PMC free article] [PubMed] [Google Scholar]
- 6.Khanolkar-Young S, Rayment N, Brickell PM, Katz DR, Vinayakumar S, Colston MJ, et al. Tumour necrosis factor-alpha (TNF-alpha) synthesis is associated with the skin and peripheral nerve pathology of leprosy reversal reactions. Clin Exp Immunol. 1995;99:196–202. doi: 10.1111/j.1365-2249.1995.tb05532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucas S. Bacterial Diseases. In: Elder DE, editor. Skin LHOT. 10th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2008. p. 564. [Google Scholar]
- 8.Jayalakshmy PS, Prasad PH, Kamala V V, Aswathy R, Pratap P. “Segmental necrotizing granulomatous neuritis”: A rare manifestation of Hansen Disease—Report of 2 cases. Case Rep Dermatol Med. 2012;2012:758093. doi: 10.1155/2012/758093. [DOI] [PMC free article] [PubMed] [Google Scholar]


